Pacific-Ciguatoxin-2 and Brevetoxin-1 Induce the Sensitization of Sensory Receptors Mediating Pain and Pruritus in Sensory Neurons
Abstract
:1. Introduction
2. Results
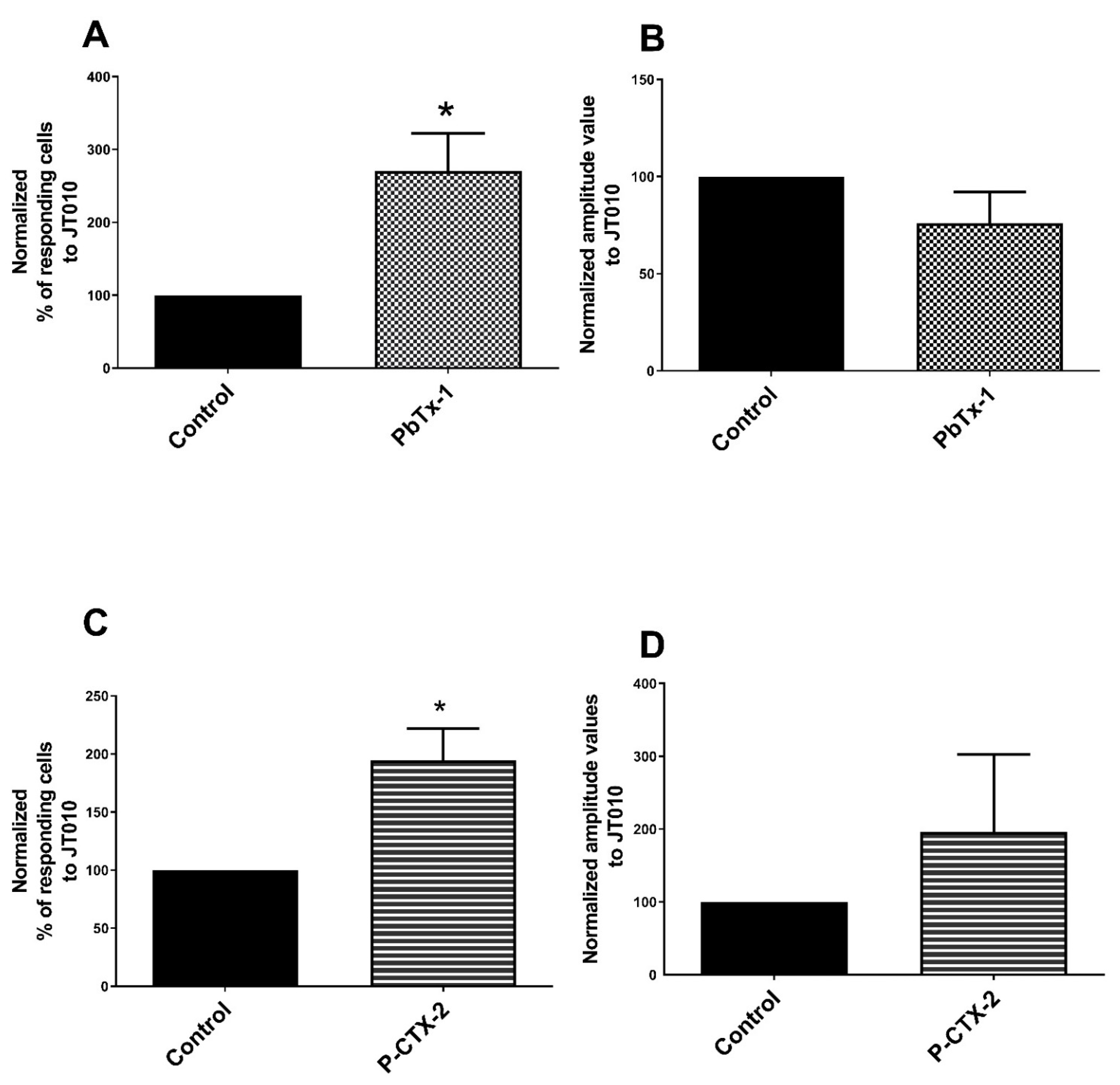
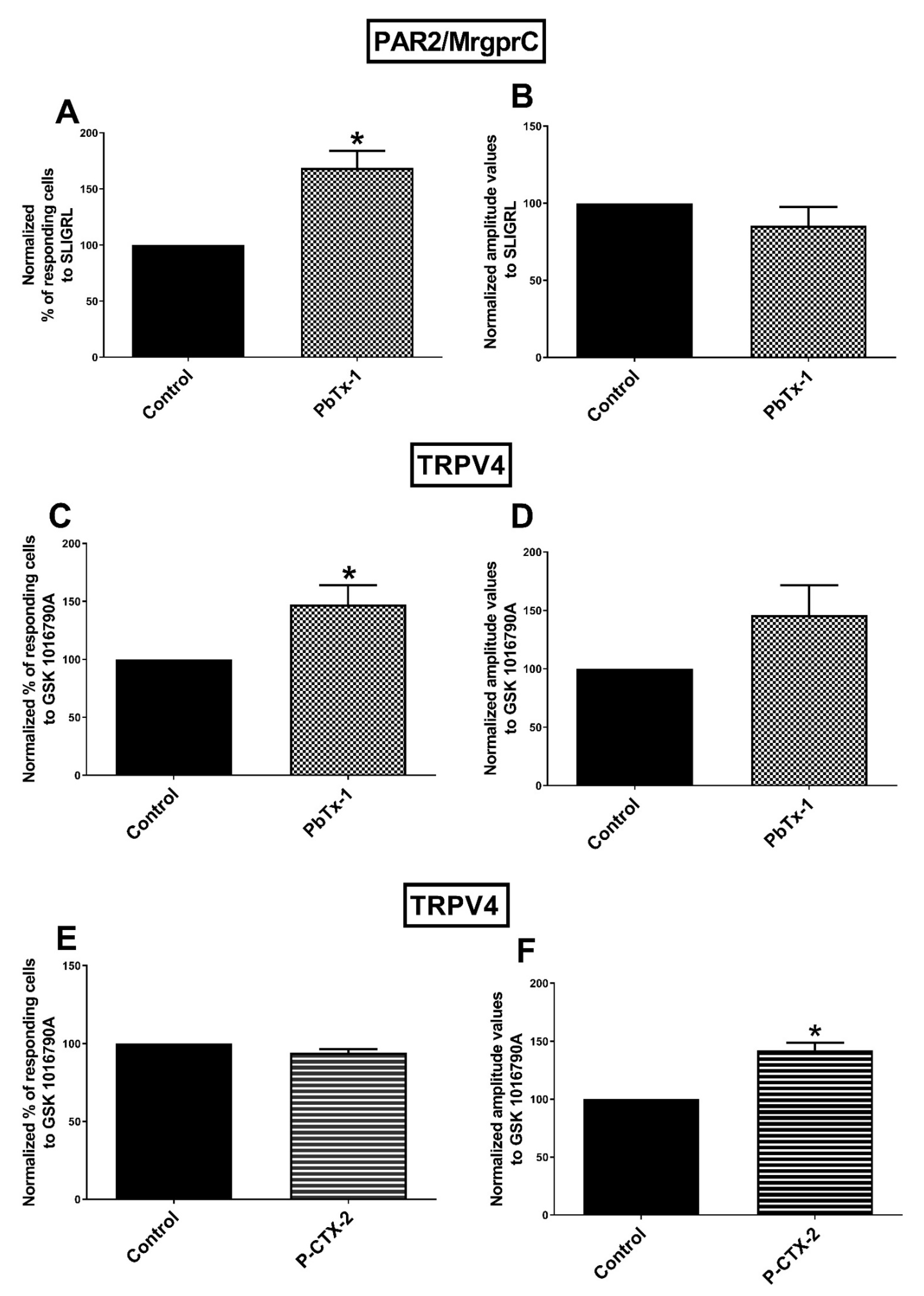
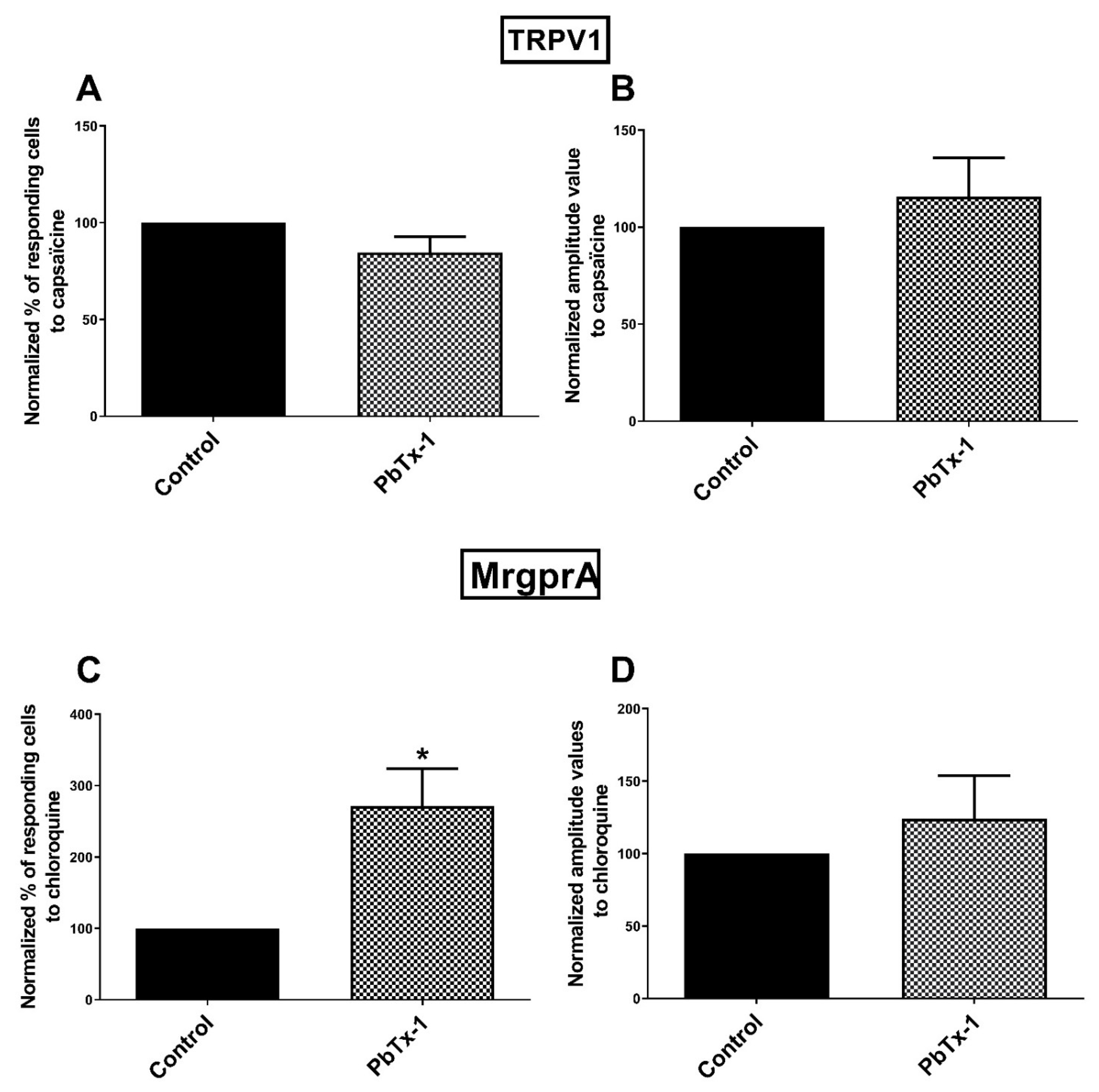
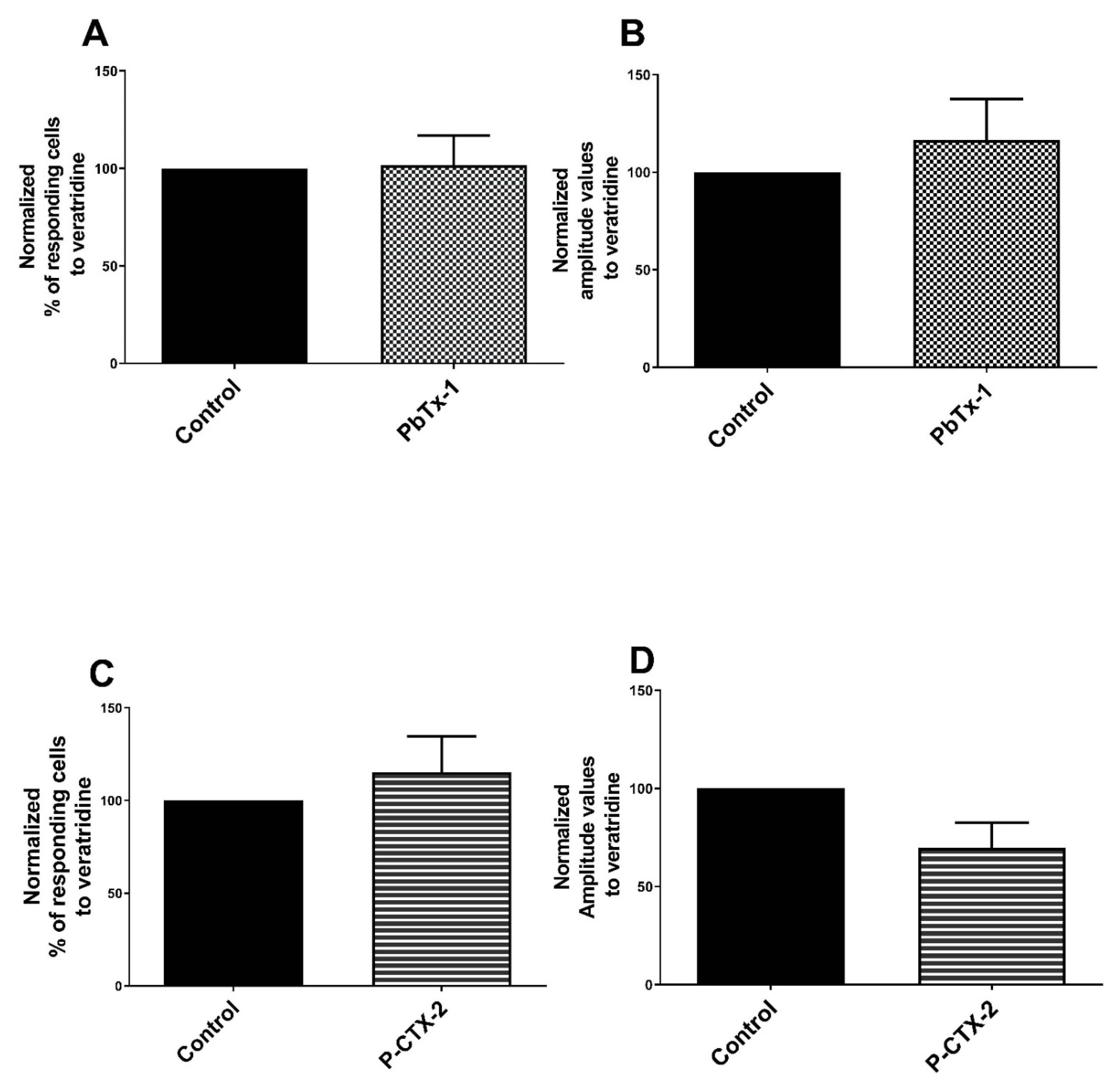
2.1. Potentiation of the Calcium Response of Sensory Receptors/Channels to their Agonist after PbTx-1 or P-CTX-2 Pretreatment
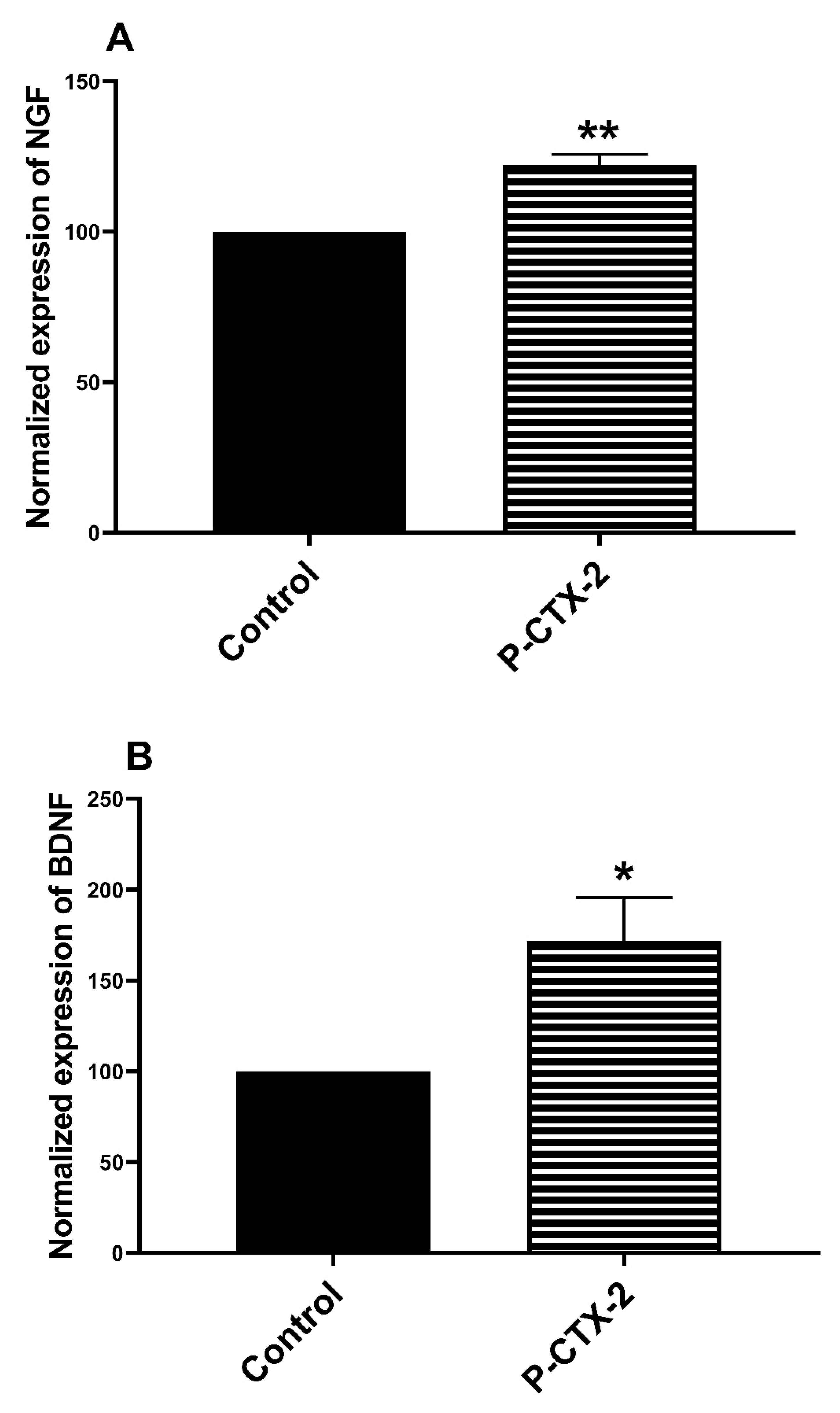
2.2. Increase in the release of NGF and BDNF in the Supernatant of Homologue Co-Culture Treated by P-CTX-2
3. Discussion
3.1. TRPA1 Channel Is Sensitized by PbTx-1 and P-CTX-2 in Rat Sensory Neurons
3.2. Sensitization of PAR2 and/MrgprC by PbTx-1 in Rat Sensory Neurons
3.3. Sensitization of TRPV4 Channels by PbTx-1 and P-CTX-2 in Rat Sensory Neurons
3.4. MrgprA Is Sensitized by PbTx-1 in Rat Sensory Neurons
3.5. TRPV1 Channels Is Not Sensitized by PbTx-1 in Rat Sensory Neurons
3.6. TTX-rNaV Channels Are Sensitized by PbTx-1 and P-CTX-2 in Rat Sensory Neurons
3.7. Increase of NGF and BDNF Levels in Co-Culture Model
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Single-Cell Calcium Video Imaging
4.4. Magnetic Bead-Based NGF and BDNF Immunoassays
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, R.J.; Holmes, M.J. Origin and Transfer of Toxins Involved in Ciguatera. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Bagnis, R.; Chanteau, S.; Chungue, E.; Hurtel, J.M.; Yasumoto, T.; Inoue, A. Origins of Ciguatera Fish Poisoning: A New Dinoflagellate, Gambierdiscus Toxicus Adachi and Fukuyo, Definitively Involved as a Causal Agent. Toxicon 1980, 18, 199–208. [Google Scholar] [CrossRef]
- Yasumoto, T.; Nakajima, I.; Bagnis, R.; Adachi, R. Finding of a Dinoflagellate as a Likely Culprit of Ciguatera. Nippon Suisan Gakkaishi 1977, 43, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa Species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef]
- FAO/WHO. Report of the Expert Meeting on Ciguatera Poisoning; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Lewis, R.J. The Changing Face of Ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar] [CrossRef]
- Bagnis, R.; Kuberski, T.; Laugier, S. Clinical Observations on 3009 Cases of Ciguatera (Fish Poisoning) in the South Pacific. Am. J. Trop. Med. Hyg. 1979, 28, 1067–1073. [Google Scholar] [CrossRef]
- Lawrence, D.N. Ciguatera Fish Poisoning in Miami. J. Am. Acad. Dermatol. 1980, 244, 254. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent Advances but the Risk Remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Pearn, J. Neurology of Ciguatera. J. Neurol. Neurosurg Psychiatry 2001, 70, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.; Deuis, J.R.; Inserra, M.C.; Collins, L.S.; Namer, B.; Cabot, P.J.; Reeh, P.W.; Lewis, R.J.; Vetter, I. Analgesic Treatment of Ciguatoxin-Induced Cold Allodynia. Pain 2013, 154, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Russell, F.E. Ciguatera Poisoning: A Report of 35 Cases. Toxicon 1975, 13, 383–385. [Google Scholar] [CrossRef]
- Adams, M.J. An Outbreak of Ciguatera Poisoning in a Group of Scuba Divers. J. Wilderness Med. 1993, 4, 304–311. [Google Scholar] [CrossRef]
- Cameron, J.; Capra, M.F. The Basis of the Paradoxical Disturbance of Temperature Perception in Ciguatera Poisoning. J. Toxicol. Clin. Toxicol. 1993, 31, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Geller, R.J.; Olson, K.R.; Senécal, P.E. Ciguatera Fish Poisoning in San Francisco, California, Caused by Imported Barracuda. West. J. Med. 1991, 155, 639–642. [Google Scholar]
- Butera, R.; Prockop, L.D.; Buonocore, M.; Locatelli, C.; Gandini, C.; Manzo, L. Mild Ciguatera Poisoning: Case Reports with Neurophysiological Evaluations. Muscle Nerve 2000, 23, 1598–1603. [Google Scholar] [CrossRef]
- Hampton, M.T.; Hampton, A.A. Ciguatera Fish Poisoning. J. Am. Acad. Dermatol. 1989, 20, 510–511. [Google Scholar] [CrossRef]
- L’Herondelle, K.; Talagas, M.; Mignen, O.; Misery, L.; Le Garrec, R. Neurological Disturbances of Ciguatera Poisoning: Clinical Features and Pathophysiological Basis. Cells 2020, 9, 2291. [Google Scholar] [CrossRef]
- Vucic, S.; Kiernan, M.C. Normal Axonal Ion Channel Function in Large Peripheral Nerve Fibers Following Chronic Ciguatera Sensitization. Muscle Nerve 2008, 37, 403–405. [Google Scholar] [CrossRef]
- Dechraoui, M.-Y.; Naar, J.; Pauillac, S.; Legrand, A.-M. Ciguatoxins and Brevetoxins, Neurotoxic Polyether Compounds Active on Sodium Channels. Toxicon 1999, 37, 125–143. [Google Scholar] [CrossRef]
- Benoit, E.; Legrand, A.M.; Dubois, J.M. Effects of Ciguatoxin on Current and Voltage Clamped Frog Myelinated Nerve Fibre. Toxicon 1986, 24, 357–364. [Google Scholar] [CrossRef]
- Birinyi-Strachan, L.C.; Gunning, S.J.; Lewis, R.J.; Nicholson, G.M. Block of Voltage-Gated Potassium Channels by Pacific Ciguatoxin-1 Contributes to Increased Neuronal Excitability in Rat Sensory Neurons. Toxicol. Appl. Pharmacol. 2005, 204, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inserra, M.C.; Israel, M.R.; Caldwell, A.; Castro, J.; Deuis, J.R.; Harrington, A.M.; Keramidas, A.; Garcia-Caraballo, S.; Maddern, J.; Erickson, A.; et al. Multiple Sodium Channel Isoforms Mediate the Pathological Effects of Pacific Ciguatoxin-1. Sci. Rep. 2017, 7, 42810. [Google Scholar] [CrossRef] [Green Version]
- Strachan, L.C.; Lewis, R.J.; Nicholson, G.M. Differential Actions of Pacific Ciguatoxin-1 on Sodium Channel Subtypes in Mammalian Sensory Neurons. J. Pharmacol. Exp. Ther. 1999, 288, 379–388. [Google Scholar]
- L’Herondelle, K.; Pierre, O.; Fouyet, S.; Leschiera, R.; Le Gall-Ianotto, C.; Philippe, R.; Buscaglia, P.; Mignen, O.; Talagas, M.; Lewis, R.J.; et al. PAR2, Keratinocytes, and Cathesin S Mediate the Sensory Effects of Ciguatoxins Reponsible for Ciguatera Poisoning. J. Investig. Dermatol. 2020, in press. [Google Scholar]
- Le Garrec, R.; L’Herondelle, K.; Le Gall-Ianotto, C.; Lebonvallet, N.; Leschiera, R.; Buhe, V.; Talagas, M.; Vetter, I.; Lewis, R.J.; Misery, L. Release of Neuropeptides from a Neuro-Cutaneous Co-Culture Model: A Novel in Vitro Model for Studying Sensory Effects of Ciguatoxins. Toxicon 2016, 116, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, O.; Fouchard, M.; Buscaglia, P.; Le Goux, N.; Leschiera, R.; Mignen, O.; Fluhr, J.W.; Misery, L.; Le Garrec, R. Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling. Cells 2020, 9, 2704. [Google Scholar] [CrossRef]
- Touska, F.; Sattler, S.; Malsch, P.; Lewis, R.J.; Reeh, P.W.; Zimmermann, K. Ciguatoxins Evoke Potent CGRP Release by Activation of Voltage-Gated Sodium Channel Subtypes NaV1.9, NaV1.7 and NaV1.1. Mar. Drugs 2017, 15, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.E.; Di Nardo, A. Skin Neurogenic Inflammation. Semin. Immunopathol. 2018, 40, 249–259. [Google Scholar] [CrossRef]
- Carstens, E.E.; Carstens, M.I.; Simons, C.T.; Jinks, S.L. Dorsal Horn Neurons Expressing NK-1 Receptors Mediate Scratching in Rats. Neuroreport 2010, 21, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, T.; Carstens, E. Neural Processing of Itch. Neuroscience 2013, 250, 697–714. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, T.; Tominaga, M.; Davoodi, A.; Nagamine, M.; Blansit, K.; Horwitz, A.; Carstens, M.I.; Carstens, E. Roles for Substance P and Gastrin-Releasing Peptide as Neurotransmitters Released by Primary Afferent Pruriceptors. J. Neurophysiol. 2012, 109, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seybold, V.S. The Role of Peptides in Central Sensitization. In Sensory Nerves; Canning, B.J., Spina, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 194, pp. 451–491. ISBN 978-3-540-79089-1. [Google Scholar]
- Gold, M.S.; Gebhart, G.F. Nociceptor Sensitization in Pain Pathogenesis. Nat. Med. 2010, 16, 1248–1257. [Google Scholar] [CrossRef]
- Kress, M. Nociceptor Sensitization by Proinflammatory Cytokines and Chemokines. Open Pain J. 2010, 3, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Chemokines and Cytokines in Neuroinflammation Leading to Neuropathic Pain. Curr. Opin. Pharmacol. 2012, 12, 55–61. [Google Scholar] [CrossRef]
- Jankowski, M.P.; Koerber, H.R. Neurotrophic Factors and Nociceptor Sensitization. In Translational Pain Research: From Mouse to Man; Kruger, L., Light, A.R., Eds.; Frontiers in Neuroscience; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 31–51. ISBN 978-1-4398-1209-9. [Google Scholar]
- Camprubí-Robles, M.; Ferrer-Montiel, A.; Planells-Cases, R. Contribution of Ion Channel Trafficking to Nociceptor Sensitization. Open Pain J. 2010, 3, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Dubin, A.E.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Nociceptive Signals Induce Trafficking of TRPA1 to the Plasma Membrane. Neuron 2009, 64, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Hensellek, S.; Brell, P.; Schaible, H.-G.; Bräuer, R.; Segond von Banchet, G. The Cytokine TNFα Increases the Proportion of DRG Neurones Expressing the TRPV1 Receptor via the TNFR1 Receptor and ERK Activation. Mol. Cell. Neurosci. 2007, 36, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Lewin, G.R.; Mendell, L.M. Nerve Growth Factor and Nociception. Trends Neurosci. 1993, 16, 353–359. [Google Scholar] [CrossRef]
- Rukwied, R.; Mayer, A.; Kluschina, O.; Obreja, O.; Schley, M.; Schmelz, M. NGF Induces Non-Inflammatory Localized and Lasting Mechanical and Thermal Hypersensitivity in Human Skin. Pain 2010, 148, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Takamori, K. Sensitization of Itch Signaling: Itch Sensitization—Nerve Growth Factor, Semaphorins. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; Frontiers in Neuroscience; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2014; pp. 293–305. ISBN 978-1-4665-0543-8. [Google Scholar]
- Bennett, D.L.H. Neurotrophic Factors: Important Regulators of Nociceptive Function. Neuroscientist 2001, 7, 13–17. [Google Scholar] [CrossRef]
- Vetter, I.; Touska, F.; Hess, A.; Hinsbey, R.; Sattler, S.; Lampert, A.; Sergejeva, M.; Sharov, A.; Collins, L.S.; Eberhardt, M.; et al. Ciguatoxins Activate Specific Cold Pain Pathways to Elicit Burning Pain from Cooling. EMBO J. 2012, 31, 3795–3808. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.L.; Narahashi, T. Differential Properties of Tetrodotoxin-Sensitive and Tetrodotoxin-Resistant Sodium Channels in Rat Dorsal Root Ganglion Neurons. J. Neurosci. 1992, 12, 2104–2111. [Google Scholar] [CrossRef]
- Gold, M.S.; Levine, J.D.; Correa, A.M. Modulation of TTX-R NaV by PKC and PKA and Their Role in PGE 2 -Induced Sensitization of Rat Sensory Neurons in Vitro. J. Neurosci. 1998, 18, 10345–10355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Moriyama, T.; Higashi, T. Proteinase-Activated Receptor 2-Mediated Potentiation of Transient Receptor Potential Vanilloid Subfamily 1 Activity Reveals a Mechanism for Proteinase-Induced Inflammatory Pain. J. Neurosci. 2004, 24, 4293–4299. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of TRPV1 by EP1 and IP Reveals Peripheral Nociceptive Mechanism of Prostaglandins. Mol. Pain. 2005, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, X.; Mendell, L.M. Acute Sensitization by NGF of the Response of Small-Diameter Sensory Neurons to Capsaicin. J. Neurophysiol. 2001, 86, 2931–2938. [Google Scholar] [CrossRef]
- Wang, Y. The Functional Regulation of TRPV1 and Its Role in Pain Sensitization. Neurochem. Res. 2008, 33, 2008–2012. [Google Scholar] [CrossRef]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-Activated Receptor 2 Sensitizes the Transient Receptor Potential Vanilloid 4 Ion Channel to Cause Mechanical Hyperalgesia in Mice. J. Physiol. 2007, 578, 715–733. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Tominaga, M.; Yamamoto, S.; Fukuoka, T.; Higashi, T.; Kobayashi, K.; Obata, K.; Yamanaka, H.; Noguchi, K. Sensitization of TRPA1 by PAR2 Contributes to the Sensation of Inflammatory Pain. J. Clin. Investig. 2007, 117, 1979–1987. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, C.; Wang, Z.J. Proteinase-Activated Receptor 2 Sensitizes Transient Receptor Potential Vanilloid 1, Transient Receptor Potential Vanilloid 4, and Transient Receptor Potential Ankyrin 1 in Paclitaxel-Induced Neuropathic Pain. Neuroscience 2011, 193, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Gupta, R.; Jordt, S.-E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2017, 34, 120–142. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.; Vergnolle, N.; Young, S.H.; Tognetto, M.; Amadesi, S.; Ennes, H.S.; Trevisani, M.; Hollenberg, M.D.; Wallace, J.L.; Caughey, G.H.; et al. Agonists of Proteinase-Activated Receptor 2 Induce Inflammation by a Neurogenic Mechanism. Nat. Med. 2000, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Lerner, E.A.; Carstens, E. Protease-Activated Receptors and Itch. Handb. Exp. Pharmacol. 2015, 226, 219–235. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, Z.-F.; Liao, M.-F.; Yao, W.-L.; Wang, J.; Wang, X.-R. Blocking PAR2 Attenuates Oxaliplatin-Induced Neuropathic Pain via TRPV1 and Releases of Substance P and CGRP in Superficial Dorsal Horn of Spinal Cord. J. Neurol. Sci. 2015, 352, 62–67. [Google Scholar] [CrossRef]
- Tian, L.; Fan, T.; Zhou, N.; Guo, H.; Zhang, W. Role of PAR2 in Regulating Oxaliplatin-Induced Neuropathic Pain via TRPA1. Transl. Neurosci. 2015, 6, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Gao, D.; Li, J. Inhibition of PAR2 and TRPA1 Signals Alleviates Neuropathic Pain Evoked by Chemotherapeutic Bortezomib. J. Biol. Regul. Homeost. Agents 2017, 31, 977–983. [Google Scholar] [PubMed]
- Amadesi, S. Protease-Activated Receptor 2 Sensitizes the Capsaicin Receptor Transient Receptor Potential Vanilloid Receptor 1 to Induce Hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X. The Role of the Mrgpr Receptor Family in Itch. Handb. Exp. Pharmacol. 2015, 226, 71–88. [Google Scholar] [CrossRef]
- Akiyama, T.; Tominaga, M.; Davoodi, A.; Nagamine, M.; Blansit, K.; Horwitz, A.; Carstens, M.I.; Carstens, E. Cross-Sensitization of Histamine-Independent Itch in Mouse Primary Sensory Neurons. Neuroscience 2012, 226, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Takaya, J.; Mio, K.; Shiraishi, T.; Kurokawa, T.; Otsuka, S.; Mori, Y.; Uesugi, M. A Potent and Site-Selective Agonist of TRPA1. J. Am. Chem. Soc. 2015, 137, 15859–15864. [Google Scholar] [CrossRef] [Green Version]
- Nystedt, S.; Emilsson, K.; Wahlestedt, C.; Sundelin, J. Molecular Cloning of a Potential Proteinase Activated Receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 9208–9212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Weng, H.-J.; Patel, K.N.; Tang, Z.; Bai, H.; Steinhoff, M.; Dong, X. The Distinct Roles of Two GPCRs, MrgprC11 and PAR2, in Itch and Hyperalgesia. Sci. Signal. 2011, 4, ra45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Wu, Z.; Chen, L.; Jaimes, J.; Collins, D.; Walters, E.T.; O’Neil, R.G. Determinants of TRPV4 Activity Following Selective Activation by Small Molecule Agonist GSK1016790A. PLoS ONE 2011, 6, e16713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, S.R.; Nelson, A.M.; Batia, L.; Morita, T.; Estandian, D.; Owens, D.M.; Lumpkin, E.A.; Bautista, D.M. The Ion Channel TRPA1 Is Required for Chronic Itch. J. Neurosci. 2013, 33, 9283–9294. [Google Scholar] [CrossRef] [Green Version]
- Bader, M.; Alenina, N.; Andrade-Navarro, M.A.; Santos, R.A. Mas and Its Related G Protein–Coupled Receptors, Mrgprs. Pharmacol. Rev. 2014, 66, 1080–1105. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Z.; Surdenikova, L.; Kim, S.; Patel, K.N.; Kim, A.; Ru, F.; Guan, Y.; Weng, H.-J.; Geng, Y.; et al. Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus. Cell 2009, 139, 1353–1365. [Google Scholar] [CrossRef] [Green Version]
- Terracina, M.; Franchi, J.; Bonté, F.; Romagnoli, G.; Maurelli, R.; Failla, C.M.; Dumas, M.; Marconi, A.; Fila, C.; Pincelli, C. Expression and Function of Neurotrophins and Their Receptors in Cultured Human Keratinocytes. J. Investig. Dermatol. 2003, 121, 1515–1521. [Google Scholar] [CrossRef] [Green Version]
- Botchkarev, V.A.; Metz, M.; Botchkareva, N.V.; Welker, P.; Lommatzsch, L.; Renz, R. Brain-Derived Neurotrophic Factor, Neurotrophin-3, and Neurotrophin-4 Act as “Epitheliotrophins” in Murine Skin. Lab. Investig. 1999, 79, 557–572. [Google Scholar]
- Pincelli, C.; Sevignani, C.; Manfredini, R.; Grande, A.; Fantini, F.; Bracci-Laudiero, L.; Aloe, L.; Ferrari, S.; Cossarizza, A.; Giannetti, A. Expression and Function of Nerve Growth Factor and Nerve Growth Factor Receptor on Cultured Keratinocytes. J. Investig. Dermatol. 1994, 103, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 Is Required for Histamine-Independent, Mas-Related G Protein–Coupled Receptor–Mediated Itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- L’Herondelle, K.; Misery, L.; Le Gall-Ianotto, C.; Philippe, R.; Talagas, M.; Mignen, O.; Lewis, R.J.; Le Garrec, R. Ciguatera Poisoning: The Role of High-Voltage-Activated and Store-Operated Calcium Channels in Ciguatoxin-Induced Sensory Effects. ITCH 2020, 5, e43. [Google Scholar] [CrossRef]
- Diogenes, A.; Akopian, A.N.; Hargreaves, K.M. NGF Up-Regulates TRPA1: Implications for Orofacial Pain. J. Dent. Res. 2007, 86, 550–555. [Google Scholar] [CrossRef]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Tominaga, M.; Noguchi, K. TRPA1 Induced in Sensory Neurons Contributes to Cold Hyperalgesia after Inflammation and Nerve Injury. J. Clin. Investig. 2005, 115, 2393–2401. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.-J.; Yamanaka, H.; Obata, K.; Dai, Y.; Kobayashi, K.; Kozai, T.; Tokunaga, A.; Noguchi, K. Expression of MRNA for Four Subtypes of the Proteinase-Activated Receptor in Rat Dorsal Root Ganglia. Brain Res. 2005, 1041, 205–211. [Google Scholar] [CrossRef]
- Böhm, S.K.; Khitin, L.M.; Grady, E.F.; Aponte, G.; Payan, D.G.; Bunnett, N.W. Mechanisms of Desensitization and Resensitization of Proteinase-Activated Receptor-2. J. Biol. Chem. 1996, 271, 22003–22016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Liu, Y.-P.; Yue, D.-M.; Liu, G.-J. Protease-Activated Receptor 2 in Dorsal Root Ganglion Contributes to Peripheral Sensitization of Bone Cancer Pain: Protease-Activated Receptor 2 Contribute to Bone Cancer Pain. Eur. J. Pain 2014, 18, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Frederick, J.; Buck, M.E.; Matson, D.J.; Cortright, D.N. Increased TRPA1, TRPM8, and TRPV2 Expression in Dorsal Root Ganglia by Nerve Injury. Biochem. Biophys. Res. Commun. 2007, 358, 1058–1064. [Google Scholar] [CrossRef]
- Liu, T.-T.; Bi, H.-S.; Lv, S.-Y.; Wang, X.-R.; Yue, S.-W. Inhibition of the Expression and Function of TRPV4 by RNA Interference in Dorsal Root Ganglion. Neurol. Res. 2010, 32, 466–471. [Google Scholar] [CrossRef]
- Poole, D.P.; Amadesi, S.; Veldhuis, N.A.; Abogadie, F.C.; Lieu, T.; Darby, W.; Liedtke, W.; Lew, M.J.; McIntyre, P.; Bunnett, N.W. Protease-Activated Receptor 2 (PAR-2) Protein and Transient Receptor Potential Vanilloid 4 (TRPV4) Protein Coupling Is Required for Sustained Inflammatory Signaling. J. Biol. Chem. 2013, 288, 5790–5802. [Google Scholar] [CrossRef] [Green Version]
- Rasband, M.N.; Park, E.W.; Vanderah, T.W.; Lai, J.; Porreca, F.; Trimmer, J.S. Distinct Potassium Channels on Pain-Sensing Neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 13373–13378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Wu, G.; Wang, Z.; Yang, N.; Shi, H.; He, Q.; Zhu, C.; Yang, Y.; Yu, G.; Wang, C.; et al. Voltage-Gated Potassium Channels Involved in Regulation of Physiological Function in MrgprA3-Specific Itch Neurons. Brain Res. 2016, 1636, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Harriott, A.M.; Gold, M.S. Contribution of Primary Afferent Channels to Neuropathic Pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuypers, E.; Yanagihara, A.; Rainier, J.D.; Tytgat, J. TRPV1 as a Key Determinant in Ciguatera and Neurotoxic Shellfish Poisoning. Biochem. Biophys. Res. Commun. 2007, 361, 214–217. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.-R.; Samad, T.A.; Jin, S.-X.; Schmoll, R.; Woolf, C.J. P38 MAPK Activation by NGF in Primary Sensory Neurons after Inflammation Increases TRPV1 Levels and Maintains Heat Hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF Rapidly Increases Membrane Expression of TRPV1 Heat-gated Ion Channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Galoyan, S.M.; Petruska, J.C.; Oxford, G.S.; Mendell, L.M. A Developmental Switch in Acute Sensitization of Small Dorsal Root Ganglion (DRG) Neurons to Capsaicin or Noxious Heating by NGF. J. Neurophysiol. 2004, 92, 3148–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, J.A.; Dib-Hajj, S.; McNabola, K.; Jeste, S.; Rizzo, M.A.; Kocsis, J.D.; Waxman, S.G. Spinal Sensory Neurons Express Multiple Sodium Channel A-Subunit MRNAs. Brain Res. Mol. Brain Res. 1996, 15, 117–131. [Google Scholar] [CrossRef]
- Dib-Hajj, S.; Black, J.A.; Cummins, T.R.; Waxman, S.G. NaN/Nav1.9: A Sodium Channel with Unique Properties. Trends Neurosci. 2002, 25, 253–259. [Google Scholar] [CrossRef]
- Cang, C.-L.; Zhang, H.; Zhang, Y.-Q.; Zhao, Z.-Q. PKCε-Dependent Potentiation of TTX-Resistant Nav1.8 Current by Neurokinin-1 Receptor Activation in Rat Dorsal Root Ganglion Neurons. Mol. Pain 2009, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Kerr, B.J.; Souslova, V.; McMahon, S.B.; Wood, J.N. A Role for the TTX-Resistant Sodium Channel Nav 1.8 in NGF-Induced Hyperalgesia, but Not Neuropathic Pain. Neuroreport 2001, 12, 3077–3080. [Google Scholar] [CrossRef]
- Hudmon, A.; Choi, J.-S.; Tyrrell, L.; Black, J.A.; Rush, A.M.; Waxman, S.G.; Dib-Hajj, S.D. Phosphorylation of Sodium Channel Nav1.8 by P38 Mitogen-Activated Protein Kinase Increases Current Density in Dorsal Root Ganglion Neurons. J. Neurosci. 2008, 28, 3190–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salio, C.; Ferrini, F. BDNF and GDNF Expression in Discrete Populations of Nociceptors. Ann. Anat. 2016, 207, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Michael, G.J.; Averill, S.; Nitkunan, A.; Rattray, M.; Bennett, D.L.H.; Yan, Q.; Priestley, J.V. Nerve Growth Factor Treatment Increases Brain-Derived Neurotrophic Factor Selectively in TrkA-Expressing Dorsal Root Ganglion Cells and in Their Central Terminations within the Spinal Cord. J. Neurosci. 1997, 17, 8476–8490. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.G.; Rush, R.A.; Zhou, X.F. Ultrastructural Localization of Brain-Derived Neurotrophic Factor in Rat Primary Sensory Neurons. Neurosci. Res. 2001, 39, 377–384. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kim, J.-K.; Park, H.-C.; Kim, J.-K.; Kim, D.-S.; Ha, S.-O.; Hong, H.-S. Changes in Brain-Derived Neurotrophic Factor Immunoreactivity in Rat Dorsal Root Ganglia, Spinal Cord, and Gracile Nuclei Following Cut or Crush Injuries. Exp. Neurol. 1998, 154, 224–230. [Google Scholar] [CrossRef]
- Obata, K.; Yamanaka, H.; Fukuoka, T.; Yi, D.; Tokunaga, A.; Hashimoto, N.; Yoshikawa, H.; Noguchi, K. Contribution of Injured and Uninjured Dorsal Root Ganglion Neurons to Pain Behavior and the Changes in Gene Expression Following Chronic Constriction Injury of the Sciatic Nerve in Rats. Pain 2003, 101, 65–77. [Google Scholar] [CrossRef]
- Obata, K.; Yamanaka, H.; Dai, Y.; Mizushima, T.; Fukuoka, T.; Tokunaga, A.; Yoshikawa, H.; Noguchi, K. Contribution of Degeneration of Motor and Sensory Fibers to Pain Behavior and the Changes in Neurotrophic Factors in Rat Dorsal Root Ganglion. Exp. Neurol. 2004, 188, 149–160. [Google Scholar] [CrossRef]
- Obata, K.; Yamanaka, H.; Dai, Y.; Mizushima, T.; Fukuoka, T.; Tokunaga, A.; Noguchi, K. Differential Activation of MAPK in Injured and Uninjured DRG Neurons Following Chronic Constriction Injury of the Sciatic Nerve in Rats. Eur. J. Neurosci. 2004, 20, 2881–2895. [Google Scholar] [CrossRef]
- Obata, K.; Noguchi, K. BDNF in Sensory Neurons and Chronic Pain. Neurosci. Res. 2006, 55, 1–10. [Google Scholar] [CrossRef]
- Fukuoka, T.; Kondo, E.; Dai, Y.; Hashimoto, N.; Noguchi, K. Brain-Derived Neurotrophic Factor Increases in the Uninjured Dorsal Root Ganglion Neurons in Selective Spinal Nerve Ligation Model. J. Neurosci. 2001, 21, 4891–4900. [Google Scholar] [CrossRef]
- Quintão, N.L.M.; Santos, A.R.S.; Campos, M.M.; Calixto, J.B. The Role of Neurotrophic Factors in Genesis and Maintenance of Mechanical Hypernociception after Brachial Plexus Avulsion in Mice. Pain 2008, 136, 125–133. [Google Scholar] [CrossRef]
- Rubiolo, J.A.; Vale, C.; Boente-Juncal, A.; Hirama, M.; Yamashita, S.; Camiña, M.; Vieytes, M.R.; Botana, L.M. Transcriptomic Analysis of Ciguatoxin-Induced Changes in Gene Expression in Primary Cultures of Mice Cortical Neurons. Toxins 2018, 10, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciobanu, C.; Reid, G.; Babes, A. Acute and Chronic Effects of Neurotrophic Factors BDNF and GDNF on Responses Mediated by Thermo-Sensitive TRP Channels in Cultured Rat Dorsal Root Ganglion Neurons. Brain Res. 2009, 1284, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Dembo, T.; Braz, J.M.; Hamel, K.A.; Kuhn, J.A.; Basbaum, A.I. Primary Afferent-Derived BDNF Contributes Minimally to the Processing of Pain and Itch. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Nicholas, R.S.; Winter, J.; Wren, P.; Bergmann, R.; Woolf, C.J. Peripheral Inflammation Increases the Capsaicin Sensitivity of Dorsal Root Ganglion Neurons in a Nerve Growth Factor-Dependent Manner. Neuroscience 1999, 91, 1425–1433. [Google Scholar] [CrossRef]
- Winter, J.; Alistair Forbes, C.; Sternberg, J.; Lindsay, R.M. Nerve Growth Factor (NGF) Regulates Adult Rat Cultured Dorsal Root Ganglion Neuron Responses to the Excitotoxin Capsaicin. Neuron 1988, 1, 973–981. [Google Scholar] [CrossRef]
- Malin, S.; Molliver, D.; Christianson, J.A.; Schwartz, E.S.; Cornuet, P.; Albers, K.M.; Davis, B.M. TRPV1 and TRPA1 Function and Modulation Are Target Tissue Dependent. J. Neurosci. 2011, 31, 10516–10528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averill, S.; McMahon, S.B.; Clary, D.O.; Reichardt, L.F.; Priestley, J.V. Immunocytochemical Localization of TrkA Receptors in Chemically Identified Subgroups of Adult Rat Sensory Neurons. Eur. J. Neurosci. 1995, 7, 1484–1494. [Google Scholar] [CrossRef]
- McMahon, S.B.; Armanini, M.P.; Ling, L.H.; Phillips, H.S. Expression and Coexpression of Trk Receptors in Subpopulations of Adult Primary Sensory Neurons Projecting to Identified Peripheral Targets. Neuron 1994, 12, 1161–1171. [Google Scholar] [CrossRef]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggström, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased Classification of Sensory Neuron Types by Large-Scale Single-Cell RNA Sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Joachim, R.A.; Kuhlmei, A.; Dinh, Q.T.; Handjiski, B.; Fischer, T.; Peters, E.M.J.; Klapp, B.F.; Paus, R.; Arck, P.C. Neuronal Plasticity of the “Brain–Skin Connection”: Stress-Triggered Up-Regulation of Neuropeptides in Dorsal Root Ganglia and Skin via Nerve Growth Factor-Dependent Pathways. J. Mol. Med. 2007, 85, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Kim, S.-Y.; Park, M.-J.; Kim, D.-S.; Kim, J.-K.; Chu, M.-Y. Expression of MRNA for Brain-Derived Neurotrophic Factor in the Dorsal Root Ganglion Following Peripheral Inflammation. Brain Res. 1997, 749, 358–362. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, S.-J.; Cho, H.-J. Differential Usage of Multiple Brain-Derived Neurotrophic Factor Promoter in Rat Dorsal Root Ganglia Following Peripheral Nerve Injuries and Inflammation. Mol. Brain Res. 2001, 92, 167–171. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.Y.; Choi, J.Y.; Park, M.J.; Kim, D.S. Nerve Growth Factor Activates Brain-Derived Neurotrophic Factor Promoter IV via Extracellular Signal-Regulated Protein Kinase 1/2 in PC12 Cells. Mol. Cells 2006, 21, 237–243. [Google Scholar] [PubMed]
- Shiers, S.; Klein, R.M.; Price, T.J. Quantitative Differences in Neuronal Subpopulations between Mouse and Human Dorsal Root Ganglia Demonstrated with RNAscope in Situ Hybridization. Pain 2020. [Google Scholar] [CrossRef]
- Chang, W.; Berta, T.; Kim, Y.H.; Lee, S.; Lee, S.-Y.; Ji, R.-R. Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel. Neurosci. Bull. 2018, 34, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; MacLeod, J.K.; Sheil, M.M. Purification and Characterization of Ciguatoxins from Moray Eel (Lycodontis Javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- Barnes, S.; Hille, B. Veratridine Modifies Open Sodium Channels. J. Gen. Physiol. 1988, 91, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Huang, R.; Tang, F.; Lin, Z.; Cheng, N.; Han, X.; Li, S.; Zhou, P.; Deng, S.; Huang, H.; et al. Transient Receptor Potential Ankyrin 1 Contributes to Lysophosphatidylcholine-Induced Intracellular Calcium Regulation and THP-1-Derived Macrophage Activation. J. Membr. Biol. 2020, 253, 43–55. [Google Scholar] [CrossRef]
- Wood, J.N.; Winter, J.; James, I.F.; Rang, H.P.; Yeats, J.; Bevan, S. Capsaicin-Induced Ion Fluxes in Dorsal Root Ganglion Cells in Culture. J. Neurosci. 1988, 8, 3208–3220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) Receptors and Mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar] [PubMed]
- Le Gall-Ianotto, C.; Andres, E.; Hurtado, S.P.; Pereira, U.; Misery, L. Characterization of the First Coculture between Human Primary Keratinocytes and the Dorsal Root Ganglion-Derived Neuronal Cell Line F-11. Neuroscience 2012, 210, 47–57. [Google Scholar] [CrossRef] [PubMed]

| Target(s) | Agonist/Antagonist | Concentration Used | References |
|---|---|---|---|
| NaV | Veratridine | 30 µM | [12,123] |
| TTX-r NaV | Veratridine/TTX | 30 µM/300 nM | [47,93,123] |
| PAR2/MrgprC | SLIGRL | 100 µM | [66,67] |
| TRPA1 | JT010 | 10 µM | [65,124] |
| TRPV1 | Capsaicin | 200 nM | [125,126] |
| TRPV4 | GSK-1016790A | 10 nM | [68] |
| MrgprA | Chloroquine | 2 mM | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierre, O.; Fouchard, M.; Le Goux, N.; Buscaglia, P.; Leschiera, R.; Lewis, R.J.; Mignen, O.; Fluhr, J.W.; Misery, L.; Le Garrec, R. Pacific-Ciguatoxin-2 and Brevetoxin-1 Induce the Sensitization of Sensory Receptors Mediating Pain and Pruritus in Sensory Neurons. Mar. Drugs 2021, 19, 387. https://doi.org/10.3390/md19070387
Pierre O, Fouchard M, Le Goux N, Buscaglia P, Leschiera R, Lewis RJ, Mignen O, Fluhr JW, Misery L, Le Garrec R. Pacific-Ciguatoxin-2 and Brevetoxin-1 Induce the Sensitization of Sensory Receptors Mediating Pain and Pruritus in Sensory Neurons. Marine Drugs. 2021; 19(7):387. https://doi.org/10.3390/md19070387
Chicago/Turabian StylePierre, Ophélie, Maxime Fouchard, Nelig Le Goux, Paul Buscaglia, Raphaël Leschiera, Richard J. Lewis, Olivier Mignen, Joachim W. Fluhr, Laurent Misery, and Raphaële Le Garrec. 2021. "Pacific-Ciguatoxin-2 and Brevetoxin-1 Induce the Sensitization of Sensory Receptors Mediating Pain and Pruritus in Sensory Neurons" Marine Drugs 19, no. 7: 387. https://doi.org/10.3390/md19070387
APA StylePierre, O., Fouchard, M., Le Goux, N., Buscaglia, P., Leschiera, R., Lewis, R. J., Mignen, O., Fluhr, J. W., Misery, L., & Le Garrec, R. (2021). Pacific-Ciguatoxin-2 and Brevetoxin-1 Induce the Sensitization of Sensory Receptors Mediating Pain and Pruritus in Sensory Neurons. Marine Drugs, 19(7), 387. https://doi.org/10.3390/md19070387









