Autotrophic vs. Heterotrophic Cultivation of the Marine Diatom Cyclotella cryptica for EPA Production
Abstract
:1. Introduction
2. Results and Discussion
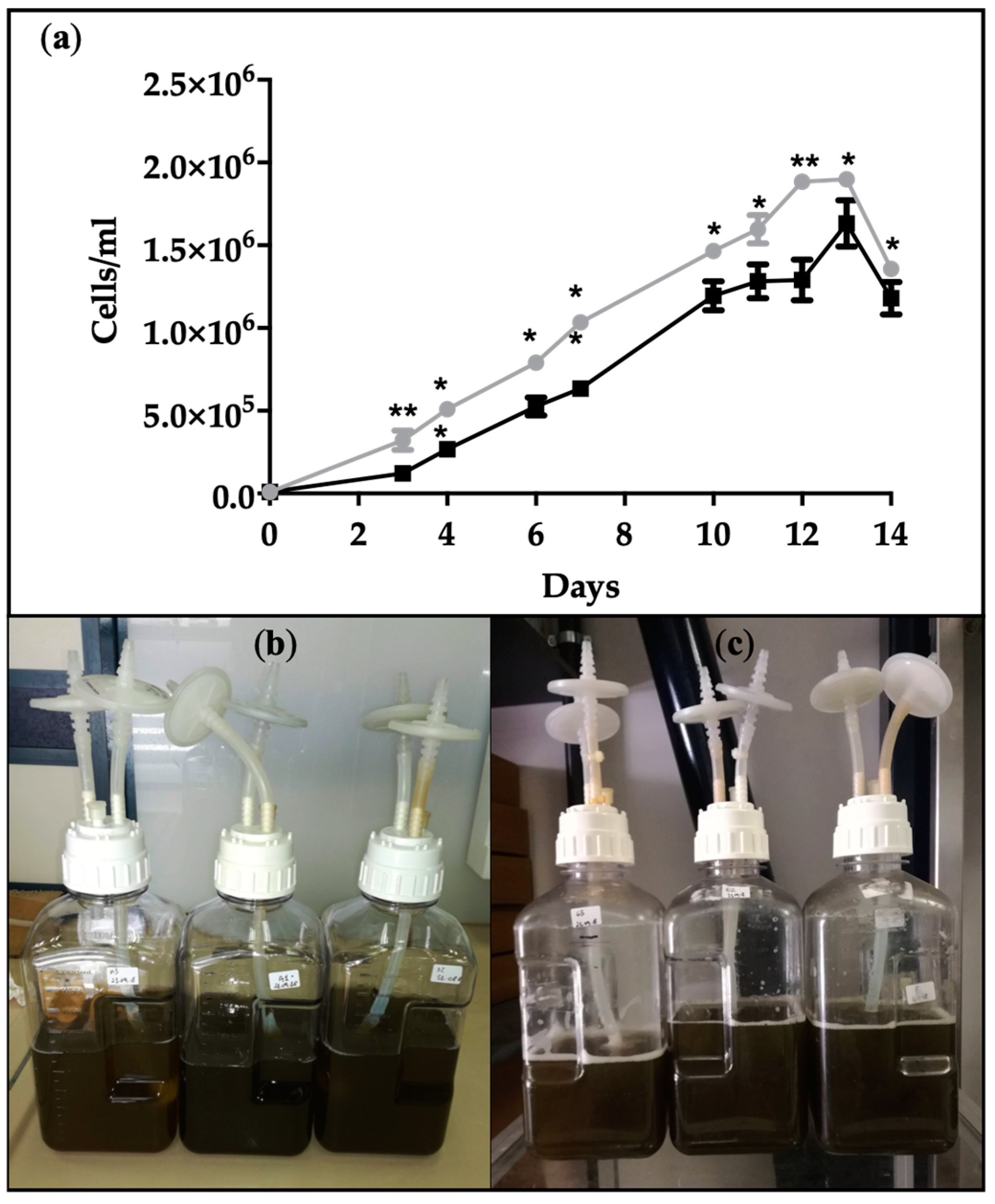
2.1. Growth Curves and Biomass Production by Cyclotella cryptica
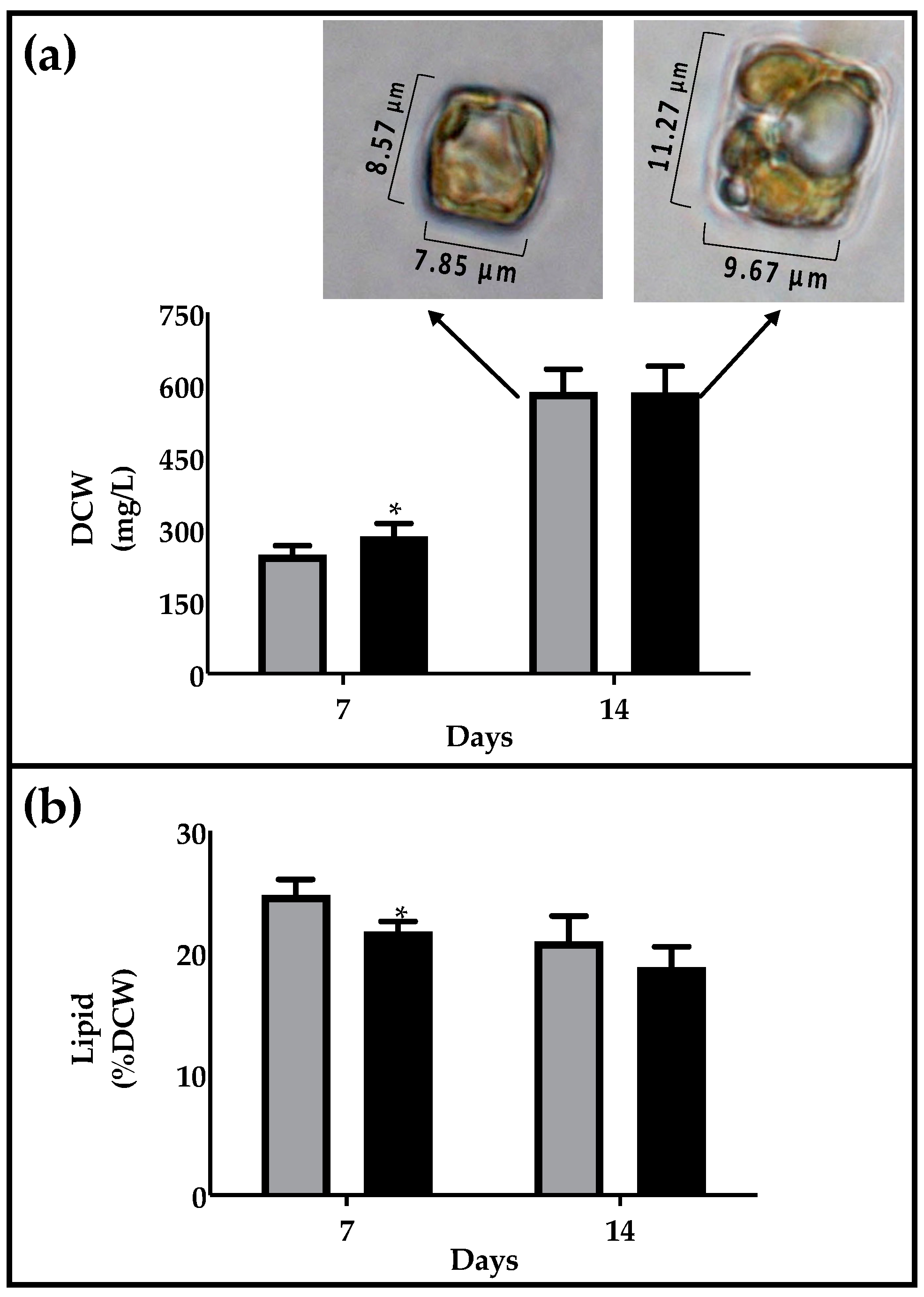
2.2. Biomass and Lipid Production
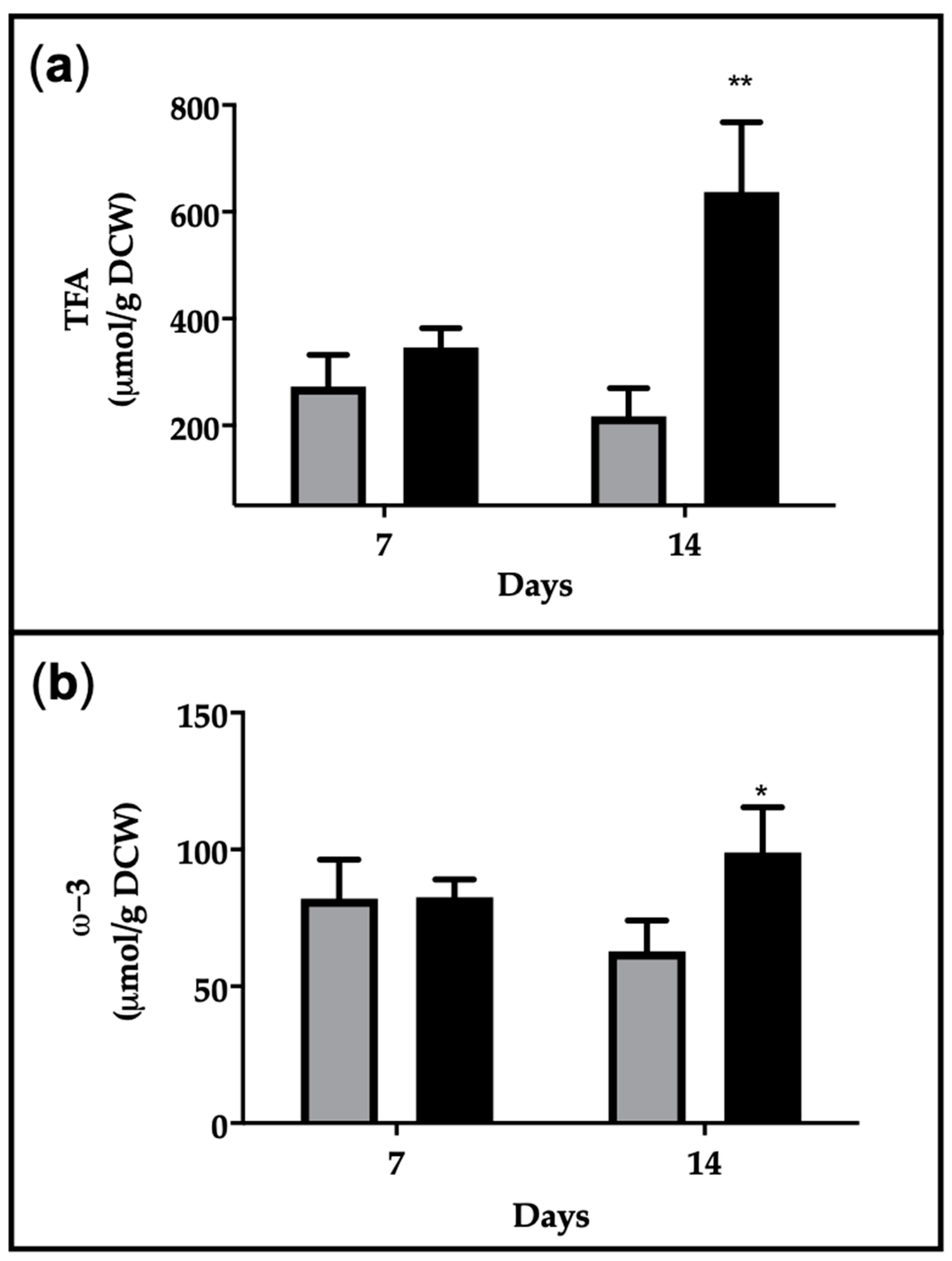
2.3. Fatty Acid Composition
2.4. EPA Production and Productivity
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Strain and Culture Conditions
4.3. Calculation
4.4. Biomass Content
4.5. Lipid Extraction
4.6. NMR Analysis of Lipid Extracts
4.7. GC–MS Analysis of Lipid Extracts
4.8. Assessment of EPA Productivity
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Asgharpour, M.; Rodgers, B.; Hestekin, J.A. Eicosapentaenoic acid from Porphyridium cruentum: Increasing growth and productivity of microalgae for pharmaceutical products. Energies 2015, 8, 10487–10503. [Google Scholar] [CrossRef] [Green Version]
- Dyerberg, J. Linolenate-derived Polyunsaturated Fatty Acids and Prevention of Atherosclerosis. Nutr. Rev. 1986, 44, 125–134. [Google Scholar] [CrossRef]
- Nomura, S.; Kanazawa, S.; Fukuhara, S. Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with Type 2 diabetes mellitus. J. Diabetes Complicat. 2003, 17, 153–159. [Google Scholar] [CrossRef]
- Lands, B. Historical perspectives on the impact of n-3 and n-6 nutrients on health. Prog. Lipid Res. 2014, 55, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Wanasundara, U.N. Omega-3 fatty acid concentrates: Nutritional aspects and production technologies. Trends Food Sci. Technol. 1998, 9, 230–240. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Molendi-Coste, O.; Legry, V. Why and how meet n-3 PUFA dietary recommendations? Gastroenterol. Res. Pract. 2010, 2011. [Google Scholar] [CrossRef] [Green Version]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [Green Version]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Sardo, A.; Paris, D.; Vella, F.M.; Adelfi, M.G.; Botte, P.; Gallo, C.; Fontana, A. Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol. Biofuels 2015, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cutignano, A.; Luongo, E.; Nuzzo, G.; Pagano, D.; Manzo, E.; Sardo, A.; Fontana, A. Profiling of complex lipids in marine microalgae by UHPLC/tandem mass spectrometry. Algal Res. 2016, 17, 348–358. [Google Scholar] [CrossRef]
- Sharma, J.; Sarmah, P.; Bishnoi, N.R. Market Perspective of EPA and DHA Production from Microalgae. In Nutraceutical Fatty Acids from Oleaginous Microalgae: A Human Health Perspective; Alok, K.P., Leonidas, M., Eds.; Wiley-Scrivener Publications, Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 281–297. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- De Vree, J.H.; Bosma, R.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Comparison of four outdoor pilot-scale photobioreactors. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. J. Chem. Technol. Biotechnol. 2017, 92, 925–936. [Google Scholar] [CrossRef]
- Sheehan, J.; Terri, D.; John, B.; Paul, R. A Look Back at the U.S. Department of Energy’s Aquatic Species. Eur. Phys. J. C 2012, 72, 14. [Google Scholar]
- Pérez-López, P.; González-García, S.; Allewaert, C.; Verween, A.; Murray, P.; Feijoo, G.; Moreira, M.T. Environmental evaluation of eicosapentaenoic acid production by Phaeodactylum tricornutum. Sci. Total Environ. 2014, 466–467, 991–1002. [Google Scholar] [CrossRef]
- Jónasdóttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef] [Green Version]
- Chauton, M.S.; Winge, P.; Brembu, T.; Vadstein, O.; Bones, A.M. Gene regulation of carbon fixation, storage, and utilization in the diatom phaeodactylum tricornutum acclimated to light/dark cycles. Plant Physiol. 2013, 161, 1034–1048. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.Y.; Kao, C.Y.; Tsai, M.T.; Ong, S.C.; Chen, C.H.; Lin, C.S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Johns, M.R. Screening of diatoms for heterotrophic eicosapentaenoic acid production. J. Appl. Phycol. 1996, 8, 59–64. [Google Scholar] [CrossRef]
- Wen, Z.-Y.; Chen, F. Production Potential of Eicosapentaenoic Acid by the Diatom Nitzschia Laevis. Biotechnol. Lett. 2000, 22, 727–733. [Google Scholar] [CrossRef]
- Hellebust, J.A. Kinetics of Glucose Transport and Growth of Cyclotella Cryptica Reimann, Lewin and Guillard. J. Phycol. 1971, 7, 1–4. [Google Scholar] [CrossRef]
- White, A.W. Growth of the two facultatively heterotrophic marine centric diatoms. J. Phycol. 1974, 10, 292–300. [Google Scholar]
- Gladue, R.M.; Maxey, J.E. Microalgal feeds for aquaculture. J. Appl. Phycol. 1994, 6, 131–141. [Google Scholar] [CrossRef]
- Laing, I.; Millican, P.F. Indoor nursery cultivation of juvenile bivalve molluscs using diets of dried algae. Aquaculture 1992, 102, 231–243. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lewis, D.M.; King, K.D.; Chen, F. Heterotrophic Production of the Microalgae Cyclotella Cryptica: Fed for Aquaculture. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2010. Volume 24, pp. 235–239. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lewis, D.M.; Chen, F.; King, K.D. Growth dynamics and the proximate biochemical composition and fatty acid profile of the heterotrophically grown diatom Cyclotella cryptica. J. Appl. Phycol. 2010, 22, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Pahl, S.L.; Lewis, D.M.; King, K.D.; Chen, F. Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): Effect of nitrogen source and concentration. J. Appl. Phycol. 2012, 24, 301–307. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of diatom strains and characterization of Cyclotella cryptica as a potential fucoxanthin producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef]
- Rosenberg, J.N.; Kobayashi, N.; Barnes, A.; Noel, E.A.; Betenbaugh, M.J.; Oyler, G.A. Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Stadnichuk, I.N.; Rakhimberdieva, M.G.; Bolychevtseva, Y.V.; Yurina, N.P.; Karapetyan, N.V.; Selyakh, I.O. Inhibition by glucose of chlorophyll a and phycocyanobilin biosynthesis in the unicellular red alga Galdieria partita at the stage of coproporphyrinogen III formation. Plant Sci. 1998, 136, 11–23. [Google Scholar] [CrossRef]
- Barone, R.; de Napoli, L.; Mayol, L.; Paolucci, M.; Volpe, M.G.; D’Elia, L.; Pollio, A.; Guida, M.; Gambino, E.; Carraturo, F.; et al. Autotrophic and heterotrophic growth conditions modify biomolecole production in the microalga galdieria sulphuraria (cyanidiophyceae, rhodophyta). Mar. Drugs 2020, 18, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajadian, S.F.; Morowvat, M.H.; Ghasemi, Y. Investigation of autotrophic, heterotrophic, and mixotrophic modes of cultivation on lipid and biomass production in Chlorella vulgaris. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 594–599. [Google Scholar] [CrossRef]
- Matos, Â.P.; Cavanholi, M.G.; Moecke, E.H.S.; Sant’Anna, E.S. Effects of different photoperiod and trophic conditions on biomass, protein and lipid production by the marine alga Nannochloropsis gaditana at optimal concentration of desalination concentrate. Bioresour. Technol. 2017, 224, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Chen, F. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol. Adv. 2003, 21, 273–294. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Chen, F. Heterotrophic production of eicosapentaenoid acid by the diatom Nitzschia laevis: Effects of silicate and glucose. J. Ind. Microbiol. Biotechnol. 2000, 25, 218–224. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef]
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Couto, D.; Melo, T.; Conde, T.A.; Costa, M.; Silva, J.; Domingues, M.R.M.; Domingues, P. Chemoplasticity of the polar lipid profile of the microalgae Chlorella vulgaris grown under heterotrophic and autotrophic conditions. Algal Res. 2021, 53. [Google Scholar] [CrossRef]
- Nuzzo, G.; Gallo, C.; D’Ippolito, G.; Cutignano, A.; Sardo, A.; Fontana, A. Composition and quantitation of microalgal lipids by ERETIC 1H NMR method. Mar. Drugs 2013, 11, 3742–3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.Y.; Chen, F. Optimization of nitrogen sources for heterotrophic production of eicosapentaenoic acid by the diatom Nitzschia laevis. Enzyme Microb. Technol. 2001, 29, 341–347. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana hustedt, and Detonula confervacea (CLEVE). Canadian Journal of Microbiology. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Everroad, R.C.; Wingard, L.M. Measuring growth rates in microalgal cultures. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier: Burlington, MA, USA, 2005; pp. 269–286. [Google Scholar]
| Day 7 | Day 14 | |||
|---|---|---|---|---|
| Fatty Acids | Autotrophy | Heterotrophy | Autotrophy | Heterotrophy |
| 14:0 | 5.3 ± 0.1 | 2.9 ± 0.5 * | 5.5 ± 0.03 | 2.5 ± 0.3 ** |
| 16:4 ω-1 | 0.3 ± 0.01 | 0 * | 1.1 ± 0.2 | 0 ** |
| 16:3 ω-4 | 22.5 ± 1.9 | 1.0 ± 0.01 ** | 21.0 ± 2.3 | 1.1 ± 0.2 ** |
| 16:2 ω-4 | 7.9 ± 0.1 | 0 ** | 6.4 ± 0.9 | 0 ** |
| 16:1 ω-7 | 23.5 ± 2.7 | 52.0 ± 0.1 ** | 28.6 ± 2.6 | 60.7 ± 2.3 ** |
| 16:0 | 12.2 ± 0.7 | 17.2 ± 0.3 ** | 11.4 ± 1.2 | 16.6 ± 0.3 * |
| 18:4 ω-3 | 1.4 ± 0.1 | 1.2 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.1 |
| 18:3 ω-3 | 0.1 ± 0.01 | 0.3 ± 0.01 ** | 0.1 ± 0.01 | 0.1 ± 0.001 * |
| 18:2 ω-6 | 0.2 ± 0.03 | 1.0 ± 0.1 ** | 0.2 ± 0.003 | 0.4 ± 0.1 |
| 18:1 ω-9 | 0.4 ± 0.03 | 1.0 ± 0.05 ** | 0.5 ± 0.05 | 1.0 ± 0.1 * |
| 18:0 | 0.8 ± 0.6 | 0.3 ± 0.03 | 0.19 ± 0.03 | 0 * |
| 20:5 ω-3 | 23.3 ± 0.6 | 21.2 ± 0.2 * | 19.4 ± 1.0 | 15.1 ± 1.7 * |
| 22:6 ω-3 | 2.5 ± 0.3 | 1.9 ± 0.03 | 4.8 ± 0.3 | 1.6 ± 0.2 ** |
| SFA (%TFA) | 18.3 ± 0.03 | 20.4 ± 0.2 ** | 17.1 ± 1.3 | 19.2 ± 0.1 |
| MUFA (%TFA) | 23.9 ± 2.6 | 53.0 ± 0.2 ** | 29.1 ± 2.7 | 61.7 ± 0.2 ** |
| PUFA (%TFA) | 58.4 ± 3.2 | 26.6 ± 0.03 ** | 53.8 ± 3.9 | 19.2 ± 2.3 ** |
| EPA | ||||
|---|---|---|---|---|
| Day 7 | Day 14 | |||
| Autotrophy | Heterotrophy | Autotrophy | Heterotrophy | |
| Content (% DCW) | 2.2 ± 2.5 | 2.2 ± 1.2 | 1.6 ± 1.5 | 2.7 ± 1.9 ** |
| Yield (mg L−1) | 7.3 ± 1.4 | 7.1 ± 1.0 | 12.2 ± 1.3 | 18.0 ± 0.7 ** |
| Productivity (mg L−1 day−1) | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.3 ± 0.1 * |
| Algal Species | Volume (L) | Cultivation Period (days) | Glucose (g/L) | Biomass (g/L) | EPA (% DCW) | EPA (% in TFA) | Reference |
|---|---|---|---|---|---|---|---|
| Cyclotella cryptica | 0.1 | 3 | 10 | 1 | - | 18–22 | [30] |
| Nitzschia laevis | 0.2 | 12 | 20 | 5.5 | 1.9 | 10.7 | [41] |
| Nitzschia laevis | 0.2 | 8 | 5 | 2.2 | 1.7 | 14.9 | [24] |
| Nitzschia laevis | 0.1 | - | 10 | - | 1.7 | 23.2 | [23] |
| Navicula incerta | 0.1 | - | 10 | - | 0.8 | 7.2 | [23] |
| Navicula pelliculosa | 0.1 | - | 10 | - | 0.5 | 4.6 | [23] |
| Cyclotella cryptica | 2 | 14 | 2 | 0.58 | 2.7 | 15.1 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cupo, A.; Landi, S.; Morra, S.; Nuzzo, G.; Gallo, C.; Manzo, E.; Fontana, A.; d’Ippolito, G. Autotrophic vs. Heterotrophic Cultivation of the Marine Diatom Cyclotella cryptica for EPA Production. Mar. Drugs 2021, 19, 355. https://doi.org/10.3390/md19070355
Cupo A, Landi S, Morra S, Nuzzo G, Gallo C, Manzo E, Fontana A, d’Ippolito G. Autotrophic vs. Heterotrophic Cultivation of the Marine Diatom Cyclotella cryptica for EPA Production. Marine Drugs. 2021; 19(7):355. https://doi.org/10.3390/md19070355
Chicago/Turabian StyleCupo, Adelaide, Simone Landi, Salvatore Morra, Genoveffa Nuzzo, Carmela Gallo, Emiliano Manzo, Angelo Fontana, and Giuliana d’Ippolito. 2021. "Autotrophic vs. Heterotrophic Cultivation of the Marine Diatom Cyclotella cryptica for EPA Production" Marine Drugs 19, no. 7: 355. https://doi.org/10.3390/md19070355
APA StyleCupo, A., Landi, S., Morra, S., Nuzzo, G., Gallo, C., Manzo, E., Fontana, A., & d’Ippolito, G. (2021). Autotrophic vs. Heterotrophic Cultivation of the Marine Diatom Cyclotella cryptica for EPA Production. Marine Drugs, 19(7), 355. https://doi.org/10.3390/md19070355










