Abstract
A chemical study on the extracts of soft coral Lemnalia bournei resulted in the isolation and identification of six new bicyclic diterpene glycosides including three new lemnaboursides E–G (1–3), and three new lemnadiolboursides A–C (4–6), along with three known lemnaboursides (7–9). Their structures were elucidated by detailed spectroscopic analysis, ECD analysis, chemical methods, and comparison with the literature data. Lemnadiolboursides A–C (4–6) contained a lemnal-1(10)-ene-7,12-diol moiety compared with the lemnaboursides. All these compounds were evaluated for antibacterial activity; cell growth inhibition of A549, Hela, HepG2, and CCRF-CEM cancer cell lines; and inhibition of LPS-induced NO production in RAW264.7 macrophages. The results indicated that compounds 1, 2, and 4–6 exhibited antibacterial activity against Staphylococcus aureus and Bacillus subtilis (MIC 4–16 μg/mL); compounds 1–9 displayed low cytotoxicity on the CCRF-CEM cell lines (IC50 10.44–27.40 µM); and compounds 1, 2, and 5 showed weak inhibition against LPS-induced NO production (IC50 21.56–28.06 μM).
1. Introduction
In recent years, marine invertebrates have afforded numerous structurally diverse and biologically active secondary metabolites. Soft corals of the genus Lemnalia (Coelenterata, Octocorallia, Alcyonacea, and Nephtheidae) consist of more than 30 species, which are widely distributed in the South China Sea, Taiwan, and off the coast of Australia and Kenya [1]. About 15 species of Lemnalia have been chemically investigated including Lemnalia africana, Lemnalia flava, Lemnalia philippinensis, Lemnalia cervicornis, Lemnalia bournei, Lemnalia tenuis, Lemnalia laevis, Lemnalia humesi, Lemnalia carnosa, and other unidentified Lemnalia sp. [1]. More than 120 terpenoids with diverse chemical structures have been isolated and identified from the extracts of Lemnalia sp., which exhibit broad biological properties including cytotoxic, antiviral, antimicrobial, and anti-inflammatory activities [1,2,3,4,5,6].
The diterpene glycosides from genus Lemnalia are all biflorane-type glycosides such as lemnaboursides, lemnaflavosides, lemnalosides, and their acetate derivatives [7,8,9,10]. Previous chemical investigations of Lemnalia bournei only provided four diterpene glycosides including lemnabourside and lemnaboursides B–D [9,10,11]. Lemnabourside was characterized with a D-glucose attached to a diterpene aldehyde through an acetal linkage, and lemnaboursides B and C were two analogs of lemnaboursides with monoacetylation of the sugar residue at different hydroxyl groups [1]. Biologically, these three metabolites only showed weak cytotoxicity.
To explore the bioactive secondary metabolites from marine organisms, soft coral L. bournei were collected from the coast of Xisha Island (7 m deep) by SCUBA diving. The acetone extracts were chemically investigated, resulting in the discovery of six new bicyclic diterpene glycosides (1–6) and three known lemnaboursides (7–9) (Figure 1). Lemnabourside E (1) was 2′-O-acetate of lemnabourside (7), and lemnabourside F (2) and G (3) were ring D (Figure 1) opened derivatives of lemnabourside acetates. Moreover, lemnadiolboursides A−C (4−6) contained lemnal-1(10)-ene-7,12-diol moiety compared with lemnabourside (7). In this paper, we report the isolation, structure determination, and bioactivities of these compounds.
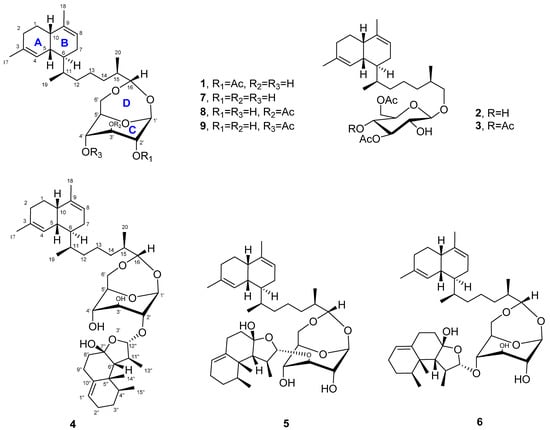
Figure 1.
Structures of compounds 1–9.
2. Results and Discussion
Lemnabourside E (1) was isolated as an amorphous solid. Its molecular formula was determined to be C28H44O7 by HRESIMS (m/z 515.2977, calcd for [M + Na]+ 515.2980), requiring seven degrees of unsaturation. The 1H NMR and HSQC spectra disclosed two trisubstituted olefinic bonds (δC/δH 134.6, 124.0/5.48 and δC/δH 136.7, 121.6/5.40), one sugar unit (δC/δH 98.2/4.93, 79.7/4.56, 74.5/3.75, 66.2/4.32, 79.6/3.88, 67.9/3.96/3.55), one acetyl (δC/δH 172.2, 21.1/2.16), another four methyls, six methylenes, and six methines. The spectral data of 1 were similar to those of diterpene glycosides isolated from the soft coral Lemnalia bournei (Table 1 and Table 2) with the differences reflecting the mono-acetylate position of the sugar residue [9]. Analysis of the COSY spectrum of 1 rapidly identified the sugar unit connection. In the HMBC experiment, the correlations from H-17 to C-3, and H-4 to C-17 suggested that C-3 is connected to C-17. The HMBC correlation between H-18 and C-10 indicated C-18 was located at C-9. In addition, the HMBC experiment showed correlations between H-10 and C-9, H-17 and C-2, H-20 and C-15, H-15 and C-1′, H-16 and C-6′, which indicated that C-9 was bonded to C-10, C-2 to C-3, C-20 to C-15, C-15 to C-16, and the presence of two acetal bonds. This evidence proved that 1 was a lemnabourside derivative, and the HMBC correlations from H-2′ to acetyl carbon δC 172.2 positioned the acetate group at C-2′ (Figure 2).

Table 1.
1H NMR spectroscopic data (600 MHz, CDCl3) for compounds 1–6.

Table 2.
13C NMR (150 MHz, CDCl3) spectroscopic data for compounds 1–6.
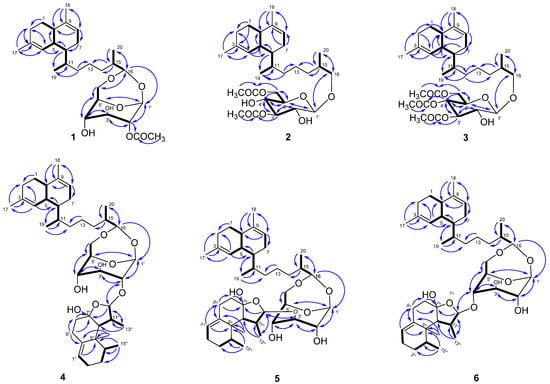
Figure 2.
Key COSY (bold) and HMBC (blue) correlations of compounds 1–6.
The configuration of the sugar unit was assigned after the hydrolysis of 1 with H2SO4. The hydrolysate reaction mixture was partitioned between CH2Cl2 and H2O. The CH2Cl2 yielded the decalin-type bicyclic diterpene aldehyde (see 1HNMR in Figure S56) according to the literature data [9]. The aqueous part was conducted with l-cysteine methyl ester and o-tolyl isothiocyanate and yielded methyl 2-(polyhydroxyalkyl)-3-(o-tolylthiocarbamoyl)-thiazolidine-4(R)-carboxylates, according to the reported method [12]. The HPLC retention time (Rt = 7.8 min) of the sugar derivative was compared with the standard sample prepared in the same manner (Figure S57). In this way, the sugar unit was determined to be D-glucose.
A cis-decalin configuration in the diterpene portion was deduced from the 13C chemical shifts in C-5 (δC 36.6) and C-10 (δC 39.7) as well as the small coupling constant (∼2 Hz) between H-1 and H-6, which were very similar to those of the separated known lemnaboursides (7–9). Moreover, H3-19/H-5 showed NOE interactions, but there were no NOE correlations between H3-19 and H-6, which suggested H3-19 and H-5 were positioned on the same orientation. The absolute configurations of the decalin part could also be deduced from the DFT/ECD calculations. The experimental ECD spectrum exhibited a negative Cotton effect (CE) at 202 nm, which was in good agreement with the calculated ECD spectra of (5R,6S,10S,11S)-1 (Figure S1). The β-configuration of the glucose was confirmed by the 1D-NOE method as the reported method, and it was in the boat form [10]. The torsion angle of H-1′ and H-2′ was close to 90°, and the magnitude of the coupling was generally the smallest (close to 0). Moreover, H3-20/H-16 and H3-20/H-1′ also showed NOE interactions, which positioned H3-20, H-16, and H-1′ on the same face.
Lemnabourside F (2) was also a white solid with the chemical formula of C30H48O8 as revealed by the HRESIMS ion peak, indicating seven degrees of unsaturation. Acid hydrolysis of 2 also yielded D-glucose. The 1H NMR spectra of 2 exhibited most of the structural features found in 1, with the major difference of ring D opened, and two acetyl groups (δC/δH 172.3, 21.1/2.17 and δC/δH 171.6, 20.9/2.11) and oxygenated methylene (δC/δH 75.7/3.67/3.38) rather than oxygenated methine were present. The HMBC correlation of H-3′/3′-OAc and H-6′/6′-OAc suggested that the C-3′ and C-6′ hydroxyl groups of the glucose were acetylated (Figure 2). The 1H-1H COSY, HSQC, and HMBC experiments allowed for the complete assignment for structure 2. Acid hydrolysis of 2 was discriminated in the same manner as 1. The CH2Cl2 part yielded the decalin-type bicyclic diterpene alcohol (see 1HNMR in Figure S56). The absolute configurations of 2 were also proposed to be the same as that of 1 based on their identical 13C chemical shifts of C-5, C-6, C-10, and C-11 as well as on biosynthetic considerations. The coupling constant of the anomeric proton was about 7 Hz, which suggested that ring C was in the chair form, and the torsion angle of H-1′ and H-2′ was close to 180°.
Compound 3, a white solid, had the molecular formula C32H50O9 as determined by HRESIMS. The 1H NMR spectra of 3 showed a high similarity to those of 2 except for the presence of three acetyl groups instead of two. These three acetyl groups were located at 3′-OH, 4′-OH, and 6′-OH, as confirmed by the HMBC correlation from H-3′ to 3′-OAc (δC 170.7), from H-4′ to 4′-OAc (δC 169.7), and from H-6′ to 6′-OAc (δC 170.7). The acid hydrolysis of 3 also yields D-glucose. 1H-1H COSY, HSQC, HMBC, and 1D-NOE experiments allowed for the complete assignment of the structure of 3. Compound 3 was given the name lemnabourside G.
Compound 4, a white solid named lemnadiolbourside A, had the molecular formula C41H64O8, established by HRESIMS and NMR data. The 13C and DEPT spectra exhibited a total of 41 carbon resonances (Table 2). Overall, the comparison of 1H and 13C NMR data of 4 and lemnabourside (7) revealed that 4 contained the structural unit of lemnabourside with 26 carbons [9]. The remaining 15 carbons belonged to a nardosinane-type sesquiterpenoid [13]. The analysis of the COSY spectrum of compound 4 revealed the presence of the lemnabourside unit and the nardosinane unit (Figure 2): (a) H-4/H-5/H-10/H2-1/H2-2, H-5/H-6/H2-7/H-8, H-6/H-11/H2-12/H2-13/H2-14/H-15/H-16, H-15/H3-20, H-11/H3-19, H-1′/H-2′/H-3′/H-4′/H-5′/H2-6′ and (b) H2-2″/H2-3″/H-4″/H3-15″, H2-8″/H2-9″, H-6″/H-11″/H-12″; H-11″/H3-13″. The HMBC correlations from H-2′ to C-12″ and H-12″ to C-2′ allowed us to identify the nardosinane located at 2′-OH of the lemnabourside unit (Figure 2). The acid hydrolysis of 4 was conducted following the above method, which yielded D-glucose and diterpene aldehyde, but failed to reveal the nardosinane moiety due to the inherent instability of lemnal-1(10)-ene-7,12-diol under strong acid and heating conditions.
The relative configuration of 4 was deduced based onNOE correlations (Figure 3). For the nardosinane unit, the NOE correlations of H3-15″/H3-14″, H3-15″/H3-13″, H3-15″/H-6″, H-12″/H3-13″, and H-12″/H-2′ suggested H-2′, H-6″, H-12″, H3-13″, H3-14″, and H3-15″ were assigned as the β-configuration. The chiral center at C-7″, as it is a hemiketal linkage, and no NOE could be detected. The 13C chemical shifts in C-6″ (δC 59.1) and C-8″ (δC 36.3), which were very similar to the chemical shifts C-6 and C-8 of the reported compound lemnal-1(10)-ene-7,12-diol (58.2 and 35.8, respectively) [13], revealed that the 7″S* relative configuration corresponded to 6″R*. The absolute configurations of the nardosinane unit were also the same as lemnal-1(10)-ene-7,12-diol for biogenetic consideration [13]. The cis-configuration for H-5 and H-10 in the diterpene portion was deduced as 1. The NOE correlations of H3-19/H-5, H-16/H3-20, and H-16/H-1′ were also observed. Given the same structural features of the lemnabourside, the absolute configurations of the lemnabourside unit in 4 werededuced to be lemnabourside (7), also on a biogenetic consideration.
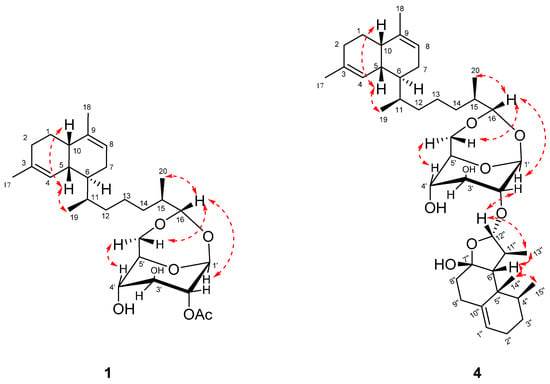
Figure 3.
Key NOE correlations (red) of compounds 1 and 4.
The HRESIMS spectra and NMR data of compounds 5 and 6 were very similar to 4 (Table 1 and Table 2), thus, suggesting they were an isomer of 4. The acid hydrolysis of 5 and 6 was conducted similar to 4 and the same hydrolysates were obtained. The major difference was that C-3′ (δC 86.9) of the glucose in 5 was shifted to a lower field, and C-2′ (δC 75.5) was shifted to an upper field compared with those in 4 (C-3′ (δC 86.9), C-2′ (δC 85.5)), which suggested that the C-3′ hydroxyl group of the glucose was substituted. The HMBC correlation of H-3′ (δH 3.38) to C-12″ (δC 110.6) and H-12″ (δH 4.78) to C-3′ further supported the above deduction. For compound 6, C-4′ (δC 75.8) of the glucose was shifted to a lower field, and C-2′ (δC 73.9) was shifted to an upper field compared with those in 4 (C-4′ (δC 65.4) and C-2′ (δC 85.5)), suggesting that the C-4′ hydroxyl group of the glucose was substituted. The HMBC correlation of H-4′ (δH 3.98) to C-12″ (δC 110.6) and H-12″ (δH 4.85) to C-4′ also supports this deduction. The 1H-1H COSY, HSQC, and HMBC experiments allowed for the complete assignment for structures 5 and 6, respectively (Figure 2).
All of the isolated compounds (1–9) were evaluated for antibacterial activity (against Bacillus subtilis, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and Salmonella paratyphi), cell growth inhibition (against A549, Hela, HepG2, and CCRF-CEM cancer cell lines), and inhibition of LPS-induced NO production in RAW264.7 macrophages. As shown in Table 3, compounds 1, 2, and 7–9 exhibited antibacterial activity against S. aureus and B. subtilis (MIC 4−16 μg/mL), compounds 1–9 displayed low cytotoxicity on the CCRF-CEM cell lines (IC50 10.44–27.40 µM); and compounds 1, 2, and 5 showed weak inhibition against LPS-induced NO production (IC50 21.56–28.06 μM). The antibacterial activity of lemnaboursides disappeared if the glucose was condensed with the nardosinane moiety. Moreover, lemnabourside G (tri-acetylation of the sugar at different hydroxyl groups) was also inactive. It can be concluded that steric hindrance may decrease the antibacterial activity of lemnaboursides, however, it seems to not affect the weak cytotoxic activity (against CCRF-CEM cell lines).

Table 3.
Antibacterial and antiviral activities of the isolated compounds.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured on a Jasco P-1010 Polarimeter (JASCO, Tokyo, Japan) in MeOH at 20 °C and UV spectra were measured on a ThermoFisher Evolution 201/220 spectrophotometer (Thermo Scientific, Waltham, MA, USA). NMR spectra were recorded on a Bruker AVANCE NEO 600 spectrometer (BrukerBiospin AG, Fällanden, Germany). 1H chemical shifts were referenced to the residual CDCl3 (7.26 ppm) and 13C chemical shifts were referenced to the CDCl3 (77.2 ppm) solvent peaks. High-resolution electrospray ionization mass spectra (HRESIMS) were performed on an Agilent 6230 TOF LC/MS system (Agilent Technologies Inc., Palo Alto, CA, USA) and Thermo Scientific TM Q Exactive PlusTM (Thermo Scientific, Waltham, MA, USA). Reversed-phase HPLC purifications were performed on a Waters 1525 binary HPLC pump attached to a Waters 2998 photodiode array detector (Waters) using a preparative Cosmosil ODS column (250 mm × 20.0 mm i.d., 5 µm, Cosmosil, Nakalai Tesque Co. Ltd., Kyoto, Japan). Column chromatography was performed on silica gel (Qingdao Haiyang Chemical Co. Ltd., Qingdao, China) and YMC reversed-phase silica gel (50 μm, YMC Co. Ltd., Kyoto, Japan). Precoated silica gel plates (HSGF-254, Qingdao Haiyang Chemical Co. Ltd., Qingdao, China) were used for analytical thin-layer chromatography (TLC). Spots were detected on TLC by heating after spraying with a sulfuric acid reagent.
3.2. Animal Material
Specimens of the soft coral Lemnalia bournei were collected from the coast of Xisha Island in the South China Sea by SCUBA diving at a depth of 7m, and frozen immediately after collection. The specimen was identified by Prof. Ping-Jyun Sung (National Museum of Marine Biology and Aquarium). The fresh sample was shown in Figure S58. The spicules from the cortex of the basal part of the stem, the cortex of the distal part of the stem, and the tentacles were extracted, then observed under a microscope (Figure S59). A voucher specimen (XSSC201915) was deposited at Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Research Center, Department of Marine Pharmacy, College of Food and Pharmaceutical Sciences, Ningbo University, Ningbo, China.
3.3. Extraction and Isolation
The frozen soft coral (wet mass 2.5 Kg) was cut into pieces and freeze-dried, then extracted with acetone six times under ultrasound condition. The acetone extract was concentrated, and then partitioned between Et2O and H2O. Evaporation of Et2O in vacuo yielded a residue of 60.3 g. The Et2O fraction (60.3 g) was subjected to silica gel column, eluting with a gradient of petroleum ether/EtOAc (from 50:0 to 1:1, v:v) to obtain five fractions (Fr.1–Fr.5). Fr.4 was mainly compound 7 (14.0 g). Fr.3 (30.0 g) was subjected to a ODS CC (MeOH/H2O, from 40:60 to 100:0, v/v) to give eight fractions. Fr.3.5 (6.0 g) was further separated on a silica gel CC (petroleum ether/EtOAc, from 10:1 to 1:1, v/v) to give nine fractions (Fr.3.5.1–Fr.3.5.9.). Fr.3.5.2 was purified by semipreparative HPLC (MeOH/H2O, 96:4, v/v) to yield 8 (16.0 mg) and 9 (13 mg). Fr.3.5.6 (124.0 mg) was purified by preparative HPLC (MeOH/H2O, 94:6) to afford 3 (33.0 mg). Fr.3.5.7 (75.0 mg) was purified by HPLC (MeOH/H2O, 95:5, v/v) to afford 2 (45.0 mg). Fr.3.5.8 (31.0 mg) was purified by semipreparative HPLC (MeOH/H2O, 94:6, v/v) to afford 1 (19.0 mg). Fr.3.7 (1.2 g) was subjected to a silica gel CC (petroleum ether/EtOAc, from 20:1 to 2:1, v/v) to give seven fractions, Fr.3.7.1–Fr.3.7.7. The subfraction Fr.3.7.4 (320.0 mg) was purified by preparative HPLC (MeCN/H2O, 97:3, v/v) to yield 3 (54.5 mg), 4 (20.0 mg), and 5 (32.0 mg).
Lemnabourside E (1): amorphous solid; { +33.0 (c 0.10, MeOH)}; UV (MeOH) λmax (log ε) 204 (1.85) nm; 1H and 13C NMR spectroscopic data, see Table 1 and Table 2; HRESIMS m/z 515.2977 [M + Na]+ (calcd for C28H44O7Na, 515.2980).
Lemnabourside F (2): amorphous solid; { +20.0 (c 0.10, MeOH)}; UV (MeOH) λmax (log ε) 204 (2.06) nm; 1H and 13C NMR spectroscopic data, Table 1 and Table 2; HRESIMS m/z 559.3246 [M + Na]+ (calcd for C30H48O8Na, 559.3242).
Lemnabourside G (3): amorphous solid; { +13.0 (c 0.10, MeOH)}; UV (MeOH) λmax (log ε) 203 (2.13) nm; 1H and 13C NMR spectroscopic data, Table 1 and Table 2; HRESIMS m/z 601.3336 [M + Na]+ (calcd for C32H50O9Na, 601.3348).
Lemnadiolbourside A (4): amorphous solid; { +46.0 (c 0.10, MeOH)}; UV (MeOH) λmax (log ε) 204 (2.53) nm; for 1H and 13C NMR spectroscopic data, see Table 1 and Table 2; HRESIMS m/z 707.4481 [M + Na]+ (calcd for C41H64O8Na, 707.4494).
Lemnadiolbourside B (5): amorphous solid; { +76.0 (c 0.10, MeOH)}; UV (MeOH) λmax (log ε) 204 (2.44) nm; 1H and 13C NMR spectroscopic data, Table 1 and Table 2; HRESIMS m/z 707.4483 [M + Na]+ (calcd for C41H64O8Na, 707.4494).
Lemnadiolbourside C (6): amorphous solid; { +25.0 (c 0.10, MeOH)}; UV (MeOH) λmax (log ε) 203 (2.28) nm; 1H and 13C NMR spectroscopic data, Table 1 and Table 2; HRESIMS m/z 707.4478 [M + Na]+ (calcd for C41H64O8Na, 707.4494).
Lemnabourside (7): white solid; { +35.0 (c 0.10, MeOH)}; this structure was deduced by comparison with 1H and 13C NMR literature data of the known compound as well as the sign of its specific rotation [9].
Lemnabourside B (8): white solid; { +27.0 (c 0.10, MeOH)}; this structure was deduced by comparison with 1H and 13C NMR literature data of the known compound as well as the sign of its specific rotation [9].
Lemnabourside C (9): white solid; { +33.0 (c 0.10, MeOH)}; this structure was deduced by comparison with the 1H and 13C NMR literature data of the known compound as well as the sign of its specific rotation [9].
3.4. Acid Hydrolysis of Compounds 1–6
A solution of compound (3.0 mg) dissolved in dioxane (0.5 mL) and in 1 N H2SO4 (0.5 mL) was refluxed (4 h), cooled, and extracted with CH2Cl2 (3 × 2 mL). The CH2Cl2 layer was condensed and washed with 5% NaHCO3 and H2O five times respectively, and then purified on Si gel preparative thin layer chromatography [petroleum ether/EtOAc, 30:1, v/v] to give a diterpene aldehyde (for 1, 4–6) or diterpene alcohol (for 2 and 3). The aqueous layer was neutralized with 5% NaOH to pH 7 and then condensed as white solids to yield D-glucose. The D-glucose product and L-cysteine methyl ester hydrochloride (5 mg) was dissolved in pyridine (0.5 mL) and heated at 60 °C for 60 min, and then phenylisothiocyanate (5 µL) was added to the mixture and heated at 60 °C for 60 min. The reaction mixture was analyzed by UPLC and detected at 250 nm, 25 °C, using a Waters BEH C18 (1.7 μm, 2.1 × 100 mm) column, eluting with MeCN/H2O (from 10:90 to 0:100, v/v, 0–10 min). Peaks of the glucose derivatives were detected by comparison with retention time. Standard d-glucose was treated in the same way as the sample.
3.5. Computational Methods
The theoretical electronic circular dichroism (ECD) spectra of the isolated compounds were calculated with the Gaussian09 program package based on the relative configurations determined by their 1D-NOE spectra (Gaussian Inc., Wallingford, CT, USA). Conformational analyses and density functional theory (DFT) calculations were used to generate and optimize the conformers at the B3LYP/6–31+G(d,p) level of theory as the method described in a reported article [14].
3.6. Antibacterial Assays
The antibacterial activities were evaluated with the broth dilution assay [15]. Five bacterial strains, Bacillus subtilis [CMCC (B) 63501], Staphylococcus aureus [CMCC (B) 26003], MRSA [ATCC43300]), Pseudomonas aeruginosa [CMCC (B) 10104], and Salmonella paratyphi A [CMCC (B) 50071] were used, and gentamicin as a positive control.
3.7. Cytotoxic Activity Assay
Cell lines were purchased from the American Type Cultural Collection (ATCC, Manassas, VA, USA). The cytotoxicity of the compounds was evaluated against the A549, HepG2, Hela, and CCRF-CEM with the Cell Counting Kit-8 (CCK-8) as the reported method [16]. Chidamide was used as the positive control.
3.8. Inhibition of Nitric Oxide Production Assay
The inhibition of the nitric oxide production assay was conducted as a previously reported protocol [17]. Dexamethasone in DMSO was used as the positive control.
4. Conclusions
In the course of the exploration of bioactive secondary metabolites from marine organisms, six new bicyclic diterpene glycosides (1–6) and three known lemnaboursides (7–9) were isolated and characterized from soft coral L. bournei. Based on the ECD spectroscopic data and the biogenetic consideration, the absolute configuration of the new compounds could be determined. In bioassay, compounds 1, 2, and 4–6 exhibited antibacterial activity against S. aureus and B. subtilis; compounds 1–9 displayed low cytotoxicity on CCRF-CEM cell lines; and compounds 1, 2, and 5 showed weak inhibition against LPS-induced NO production. The antibacterial activity of lemnabourside G (tri-acetylated glucose) and lemnadiolboursides disappeared, which indicated the steric hindrance may decrease the antibacterial activity of lemnaboursides. It is worth noting that the discovery of compounds 1–6, once again, enriched the chemical diversity and complexity of diterpene glycosides from marine organisms, and will stimulate further pharmacological studies due to their intriguing structural features and potent biological activities. As the main component of L. bournei, further pharmacological investigations of lemnabourside are still worth pursuing.
Supplementary Materials
The following are available online https://www.mdpi.com/article/10.3390/md19060339/s1. Figure S1: Calculated and experimental ECD spectra of compound 1; Figures S2–S48: HRESIMS, 1D and 2D NMR spectra of all new compounds 1–6; Figures S49–S54: 1D NMR spectra of known compounds 7–9. Figures S55–S56: 1H NMR data for the bicyclic diterpene aldehyde aglycon and bicyclic diterpene alcohol aglycon. Figure S57: HPLC chromatograms of the sugar derivatives of compounds 1–6 and the standard D-glucose. Figures S58 and S59: Photos of Lemnalia bournei.
Author Contributions
X.Y. (Xia Yan) performed the isolation, structure determination of the compounds, and wrote the manuscript. H.O. performed the cytotoxic, anti-inflammatory, and antimicrobial bioassays. T.L. and S.H. collected the soft coral by SCUBA. Y.S. performed the ECD calculations. S.H., B.W., and X.Y. (Xiaojun Yan) revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFC0310900), the National Natural Science Foundation of China (NSFC) (41906092), and Ningbo Municipal Natural Science Foundation (2019A610201).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials file associated with this article.
Acknowledgments
We thank Zhenhua Long, Daning Li, and Da Huo of the Xisha Marine Science Comprehensive Experimental Station, South China Sea Institute of Oceanology, Chinese Academy of Sciences for their assistance. We thank Xisha Marine Environment National Observation and Research Station. We thank BoRong Peng of the National Museum of Marine Biology and Aquarium for his help. We would also thank the MS Center and Fan Wu of the Institute of Drug Discovery Technology, Ningbo University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Q.; Sun, J.; Chen, J.; Zhang, H.; Guo, Y.-W.; Wang, H. Terpenoids from marine soft coral of the genus Lemnalia: Chemistry and biological activities. Mar. Drugs 2018, 16, 320. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, P.; Tang, X.; Luo, X.; Liu, K.; Zhang, Y.; Wang, Q.; Li, G. Lemnardosinanes A-I: New bioactive sesquiterpenoids from soft coral Lemnalia sp. J. Org. Chem. 2021, 86, 970–979. [Google Scholar] [CrossRef]
- Yang, F.; Li, S.-W.; Zhang, J.; Liang, L.-F.; Lu, Y.-H.; Guo, Y.-W. Uncommon nornardosinane, seconeolemnane and related sesquiterpenoids from Xisha soft coral Litophyton nigrum. Bioorg. Chem. 2020, 96, 103636. [Google Scholar] [CrossRef]
- Liu, M.; Li, P.; Luo, X.; van Ofwegen, L.; Tang, X.; Li, G. Sesquiterpenoids from the soft coral Lemnalia sp. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Gao, Y.; Zhang, M.-M.; Sheng, L.; Li, J.; Li, X.-W.; Wang, H.; Guo, Y.-W. New sesquiterpenoids from the South China Sea soft corals Clavularia viridis and Lemnalia flava. Beilstein J. Org. Chem. 2019, 15, 695–702. [Google Scholar] [CrossRef]
- Wu, Q.; Li, H.; Yang, M.; Jia, A.-Q.; Tang, W.; Wang, H.; Guo, Y.-W. Two new cembrane-type diterpenoids from the Xisha soft coral Lemnalia flava. Fitoterapia 2019, 134, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Rudi, A.; Levi, S.; Benayahu, Y.; Kashman, Y. Lemnaflavoside, a new diterpene glycoside from the soft coral Lemnalia flava. J. Nat. Prod. 2002, 65, 1672–1674. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Vidor, N.B.; Foss, A.P.; Chang, L.C. Lemnalosides A-D, decalin-type bicyclic diterpene glycosides from the marine soft coral Lemnalia sp. J. Nat. Prod. 2007, 70, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, Z. Two new diterpene glycosides from the soft coral Lemnalia bournei. J. Nat. Prod. 1998, 61, 1300–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Long, K.; Wu, H.; Ma, K. A novel diterpene glycoside from the soft coral of Lemnalia bournei. J. Nat. Prod. 1994, 57, 155–160. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Z.; Li, H.; Qiu, G. Novel diterpene glycoside from the soft coral Lemnalia bournei. Tianran Chanwu Yanjiu Yu Kaifa 2003, 15, 487–489. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J. Studies of Australian soft corals. XIX. Two new sesquiterpenes with the nardosinane skeleton from a Paralemnalia species. Aust. J. Chem. 1980, 33, 885–890. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Wu, M.; Li, X.; Li, W.; Ding, W.; She, Z.; Li, C. New antimicrobial cyclopentenones from Nigrospora sphaerica ZMT05, a fungus derived from Oxya chinensis Thunber. J. Agric. Food Chem. 2018, 66, 5368–5372. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, H.; Zhang, Q.; Yang, M.; Gu, Y.-C.; Liang, L.-F.; Tang, W.; Guo, Y.-W. Rare cembranoids from chinese soft coral Sarcophyton ehrenbergi: Structural and stereochemical studies. J. Org. Chem. 2019, 84, 5091–5098. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones O-T, new biscembranoids and cembranoid from soft coral Lobophytum pauciflorum. Mar. Drugs 2010, 8, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).