Abstract
Euphausia superba, commonly known as krill, is a small marine crustacean from the Antarctic Ocean that plays an important role in the marine ecosystem, serving as feed for most fish. It is a known source of highly bioavailable omega-3 polyunsaturated fatty acids (eicosapentaenoic acid and docosahexaenoic acid). In preclinical studies, krill oil showed metabolic, anti-inflammatory, neuroprotective and chemo preventive effects, while in clinical trials it showed significant metabolic, vascular and ergogenic actions. Solvent extraction is the most conventional method to obtain krill oil. However, different solvents must be used to extract all lipids from krill because of the diversity of the polarities of the lipid compounds in the biomass. This review aims to provide an overview of the chemical composition, bioavailability and bioaccessibility of krill oil, as well as the mechanisms of action, classic and non-conventional extraction techniques, health benefits and current applications of this marine crustacean.
1. Introduction
Euphausia superba, commonly known as krill, is a small marine crustacean from the Antarctic Ocean that plays an important role in the marine ecosystem, serving as feed for most fish [1]. Although measuring krill biomass is difficult, it has been estimated at approximately 379 million metric tons. The Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) has set a catch limit of 620,000 tons per year to protect the marine ecosystem [2]. Nevertheless, the annual catch is around 250,000 tons, indicating use below the established limits, which is probably due to the difficulty in conserving krill and its fragility [3].
In fact, krill is commonly used in the sport fishing market as well as in the aquaculture industry. However, in recent years, krill has been successfully investigated for its role as a nutritional supplement to improve human health. This is because krill is rich in nutrients, including vitamins A and E, minerals, n-3 polyunsaturated fatty acids (n-3 PUFAs), phospholipids (PLs), astaxanthin and flavonoids [4].
Particular attention has been paid to lipid content (0.5% to 3.6%) [5], including phospholipids (30–65%) and triglycerides, while fish oil is only comprised of triglycerides. The main phospholipid in krill oil is phosphatidylcholine, with 40% of the total fatty acids bound to phosphatidylcholine being eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [6]. The EPA and DHA omega-3 fatty acids found in krill oil have shown several useful pharmacological properties in the management of numerous chronic dysfunctions, including cardiovascular, neurological and inflammatory diseases, as well as the prevention of cancer and promoting gut microbiota health [7,8,9,10]. In this regard, supplementation with krill polyunsaturated fatty acids may be a natural way to relieve the symptoms of these conditions, potentially in combination with conventional therapies [11]. EPA and DHA from krill oil have also shown higher bioaccessibility than other forms of n-3 PUFAs (ethyl-ester and re-esterified omega 3), demonstrating similar benefits, but at smaller dosages [12].
Krill oil was authorized in 2008 by the U.S. Food and Drug Administration (FDA) as GRAS (Generally Recognized as Safe), was approved in Europe by EFSA as a novel food in 2009 and was also approved in China in 2014. Finally, krill oil was authorized by EFSA for pregnant and lactating women in 2014.
This review aims to provide an overview of the chemical composition, bioavailability and bioaccessibility, mechanism of actions, classic and non-conventional extraction techniques, health benefits and current applications of krill oil underlying the future perspectives of this nutraceutical.
2. Krill Oil Composition
Krill oil composition provides a large variety of substances beneficial to health which justifies its use as a novel food ingredient for pharmacological and nutraceutical applications in different pathological conditions, such as cardiometabolic and neurodegenerative diseases. Generally, although the content of the different classes of nutritional components is influenced by the extraction technologies, krill oil contains a high level of n-3 PUFAs and phospholipids (PLs), and minor components such as vitamins, minerals, astaxanthin and flavonoids [13,14].
2.1. Lipid Fraction
The analysis of the lipid component of krill oil revealed a very complex composition which is characterized by the presence of polar lipids representing the major lipid class in krill oil, followed by triacylglycerols (TAG) [15,16]. Many factors have been reported to influence the specific composition of the lipid fraction, for example interannual environmental changes, seasonal variation, krill sample variety and sexual maturity of krill samples as well as transportation process, storage conditions and pretreatment methods [17]. For example, larger amounts of FFAs have been obtained by dehydrating krill through hot air [18]. Many studies have analyzed krill oil composition through different methods of analysis [19,20]. The range of krill oil PLs ranges between 39.9% found in gravid females of Euphausia superba in South Georgia and 80.7% in krill oil found in Aker BioMarine (Table 1). This content varies depending on analysis methods and sample variety. Other differences in krill oil composition have been found considering feeding behavior, krill age and regions. Higher PL levels have been found in ovarian tissues and in gravid females compared to muscle tissues. Another important factor leading to variation in krill composition is extraction methods. A larger amount of PL can be obtained by using ethanol and isopropanol rather than acetone and hexane. PE and phosphatidylcholine (PC) results in the most abundant types of phospholipids ranging from 44.58–99.80%, with the low end of this range reported by Araujo and colleagues using HPTLC as the analysis method and the high end of the range described by Castro-Gomez and coworkers in 2015 using HPLC-ELSD as the analysis method. Phosphatidylethanolamine (PE), even if less abundant, has been found as 0.20% to 24.74% of total PLs [21]. Interestingly, in studies that reported a minor presence of PC, a higher amount of lysophosphatidylcholine (LPC) has been described ranging from 43.3–44.4%, probably due to PC hydrolysis caused by inappropriate storage or inadequate treatment of krill samples [22]. Other important components have been described sometimes in small amounts (less than 10% of total PLs) including phosphatidylglycerol, sphingomyelin, cardiolipin phosphatidylserine and phosphatidic acid. Due to the high content of PC, krill oil is now considered a very promising marine supply of PLs and an alternative to PLs deriving from vegetable oils, egg yolk and dairy products [23].

Table 1.
The different content of polar lipids, monoacylglycerols, diacylglycerols, sterols, free fatty acids and triacylglycerols that have been found in different krill samples expressed as percent of total lipids.
It is worth noting that the PL fraction obtained from the krill lipid had much higher percentages of PUFA and n-3 PUFA [24,25]. In particular, 31.13% of EPA and 14.87% of DHA were measured in the PL fraction, while only 3.17% of EPA and 1.5% of DHA were found in the TAG fraction. However, as reported by Paluchová and colleagues, the TAG lipid class containing esterified DHA proved to be the best substrate for a better bioavailability of DHA for polyunsaturated fatty acid esters of hydroxy fatty acids (FAHFA) synthesis. This is crucial for selection of novel food sources, which could stimulate endogenous synthesis of functional lipids from a nutritional point of view [26].
2.2. Fatty Acid Composition
As reported by the Codex Alimentarius Commission (STANDARD FOR FISH OILS CXS 32 9-2017 Adopted in 2017), the most abundant fatty acids in krill oil that have been described are C14:0 myristic acid (5.0–13.0), C16:0 palmitic acid (17.0–24.6), C16:1 (n-7) palmitoleic acid (2.5–9.0), C18:1 (n-7) vaccenic acid (4.7–8.1), C18:1 (n-9) oleic acid (6.0–14.5), C20:5 (n-3) eicosapentaenoic acid (14.3–28.0) and C22:6 (n-3) docosahexaenoic acid (7.1–15.7) (Table 2). DHA and EPA are known as n-3 PUFAs and play a fundamental role in mediating beneficial effects in different mammalian systems [29]. In general, consuming fish oil represents a daily practice for increasing EPA and DHA intake. Since EPA and DHA krill oil content are similar to other common fish oils (anchovy, tuna or salmon), consuming krill oil may represent a potential alternative for a nutritional approach as a dietary supplement [31]. Interestingly, krill lipid fraction is characterized by a higher amount of n-3 PUFA and very low levels of saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA) than TAG in fish oil [32]. Indeed, EPA and DHA (respectively 31.13% and 14.87%) have been mainly found in PLs fraction, while only 3.17% of EPA and 1.5% of DHA were present in the TAG fraction [33]. This composition has been confirmed by many studies, which demonstrated that n-3 PUFAs in the PLs fraction are characterized by a higher quantity of EPA and DHA, significantly improving the bioavailability of these two pharmacological and nutraceutical components, compared to EPA and DHA contained in TAG fraction [34,35].

Table 2.
The table reports the fractions expressed as % of total fatty acid characterized in krill samples.
2.3. Astaxanthin
One of the most important minor components of krill oil is represented by astaxanthin, a carotenoid that has been characterized in different algae and marine animals [36]. This compound is responsible for the typical dark red color of krill oil and is endowed with potent antioxidant properties, even more than other carotenoids such as zeaxanthin, lutein, canthaxanthin, β-carotene and α-tocopherol [37]. Generally, the amount of astaxanthin in krill oil ranges from 40 to 5000 mg/kg and depends on intrinsic features of krill (for example raw material, krill species) or can vary due to extraction and analysis methods [38]. Indeed, as reported in the below section, a high concentration of astaxanthin can be achieved by using acetone as the extraction solvent [39]. Chemically, astaxanthin can be found in krill oil as a fatty acid ester. In particular, 51% of total astaxanthin is present as diester, 43% as monoesters and only 6% as free astaxanthin [40]. The main fatty acids that are conjugated with astaxanthin are myristic acid, palmitic acid, palmitoleic acid, vaccenic acid, arachidic acid, eicosapentaenoic acid and docosahexaenoic acid [41]. Furthermore, three different isomers of astaxanthin have been identified by Grynbaum and colleagues, such as (all-trans) astaxanthin, which represents the most abundant isomers, (13-cis) astaxanthin, and (9-cis) astaxanthin [41].
2.4. Sterols
Krill oil also contains an important fraction of sterols that range between 2.3–3.9% of total lipids [42]. Desmosterol and cholesterol represent the most abundant sterols of the total sterols [30]. Desmosterol is the precursor of cholesterol and represents 1.70–18.63% of total sterols [17]. The component variation is due to the different food intake of krill since crustaceans are not able to endogenously synthetize sterols but are supplied by diet and phytosterol dealkylation. Cholesterol has been reported as 81.33–82.34% of total sterols with a concentration of 18.95 to 31.96 mg/g of oil [43]. Compared to other fish oil, cholesterol amount in krill oil is even higher than hoki oil, which contains 5.15 mg/g of oil, 2.04 mg/g of tuna oil and 11.81 mg/g of egg yolk oil [17]. Considering the potential nutraceutical application of krill oil, a higher content of cholesterol may represent a matter of concern due to the onset of cardiovascular diseases, for example atherosclerosis, in a cholesterol rich diet. However, extraction methods could limit the content of total cholesterol in krill oil as reported by Bruheim and Cameron who demonstrated that using pure ethanol instead of ethanol mixed with water significantly reduced the cholesterol concentration. Minor percentages are represented by other sterols found in krill samples in 1977, such as 24-nordehydrocholesterol (0.1–1.7%), trans-dehydrocholesterol (1.1–1.5%), brassicasterol (0.5–1.7%), 24-methylenecholesterol (0.1–0.4%), and two stanols (0.1–0.2%) [44].
2.5. Vitamins
Krill oil contains a high amount of α-tocopherol (Vitamin E) which is characterized by a potent antioxidant effect. The concentration of this isoform ranges between 14.74 to 63.0 mg/100 g of oil and represents 90% of total tocopherols, while the other homologues of tocopherols (β-, γ-, δ-) are present only in traces (γ (0.25 to 3.67 mg/100 g of oil) and δ-tocopherol (0 to 0.65 mg/100 g of oil)) [45]. Besides vitamin E, vitamin A has also been found in frozen krill (about 0.11 mg/100 g of wet weight) [21]. The presence of vitamin A in krill samples represents an important feature contributing to making krill oil a very promising food supplement for the regulation of human immune function and for counteracting some infectious diseases. Depending on extraction methods, the vitamin A content of krill oil ranges between 16.4 to 28.5 mg per 100 g of krill oil, a concentration which is significantly higher than tuna oil (11.1 mg/100 g of oil) [45].
2.6. Flavonoids
In general, flavonoids represent a large variety of compounds with a similar chemical structure and are endowed with antioxidant activities, antibacterial and immunomodulatory effects. They also exert cardio protection and reduce the production of proinflammatory mediators. Krill oil has been reported to contain a particular flavonoid whose chemical structure is similar to 6,8-di-C-glucosyl luteolin. As reported by Sampalis and colleagues, by using specific extraction methods, a krill extract containing a significative level of flavonoid (7 mg/100 mL) can be obtained and used for protecting the skin from ultraviolet B (UVB) radiation [46]. Although some studies report that the C-glycosylation of flavonoids in krill oil could improve their antioxidant and anti-diabetic effects, there are no direct data on the use of krill oil for this purpose.
2.7. Minerals
Other than the organic compounds responsible for the pharmacological activity of krill oil, there is also a large quantity of minerals that have been characterized in some krill samples. Indeed, one of the most abundant minerals contained in krill is calcium (1322 mg/100 g) which can be exploited for bone health, phosphorus (1140 mg/100 g), and magnesium (360 mg/100 g) [30]. Other minerals are contained in minor quantities such as zinc, selenium and potassium. Besides these essential elements, krill is characterized by a large quantity of fluoride (2400 mg/kg dry matter) which may cause skeletal fluorosis if its intake is high. However, the major quantity of fluoride is contained in the krill exoskeleton which could be removed during krill oil extraction to avoid excessive fluoride content reaching low fluoride levels (<0.5 ppm) [47].
3. Mechanism of Action
Due to the complex composition of krill oil, which contains structurally different chemical compounds such as PUFAs, flavonoids, astaxanthin and vitamins, the pharmacological effects that have been described are ascribable to multiple mechanisms of action. Krill oil is characterized by a high quantity of (n-3) PUFA (mainly EPA and DHA) which are natural PPAR’s ligands responsible for the activation of PPAR [33]. These transcription factors play a fundamental role in regulating cell and tissue behavior to different stimuli. Generally, PPAR forms a heterodimer with the retinoic-X-receptor whose ligand is represented by cis-9-retinoic acid [48]. PPARα and PPARγ are the most investigated isoforms of PPAR. PPARα is mainly expressed in hepatic cells and regulates lipid accumulation [49], while PPARγ has been mainly described in adipose tissues where it promotes insulin sensitivity, adipocyte differentiation and regulates metabolic responses, fat storage and energy homeostasis [50]. Furthermore, PPARγ has also been described in inflammatory cells, where it controls the release of proinflammatory mediators and promotes anti-inflammatory effects [51]. The PPARγ activation occurs in the cells and the uptake of EPA and DHA seems to be due to the expression of FAT/CD36 (a transmembrane fatty acid transporter) [52]. Intriguingly, PPARγ also regulates the expression of FAT/CD36 itself, indicating that n3-PUFA can increase their own uptake inside the adipocytes, promoting the production of adiponectin [53]. The involvement of PPARγ have been demonstrated using PPARγ antagonists (e.g., bisphenol-A-diglycidyl ether or GW9662) that suppressed the secretion of adiponectin [54]. Furthermore, n-3 PUFA are able to activate PPAR through a non-covalent interaction, promoting the reduction of inflammatory responses with a consequent reduction of the release of TNFα and IL-6 after LPS stimulation [55].
Another important target mediating the pharmacological effects of krill oil is represented by G protein-coupled transmembrane receptors (GPCRs) which are involved in the regulation of many metabolic processes. In particular, EPA and DHA activate GPR120 (also known as FFA receptor 4; FFAR4) leading to the increase of intracellular cAMP level and Ca2+ concentrations, consequently promoting the phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) [56]. Since EPA and DHA are involved in the regulation of inflammatory processes mainly in adipose tissue, the involvement of GPR120 has been investigated. In particular, DHA inhibited the IKK (Inhibitor of κB kinase) complex activation and JNK phosphorylation resulting in a reduction of TNF-α release in macrophages treated with LPS [57]. The involvement of GPR120 has been confirmed by GPR120 knockdown. In addition, DHA has been reported to facilitate the formation of GPR120 and β-arrestin2 complex (GPR120-βarr2) that blocks the pro-inflammatory stimulus due to LPS exposure [58]. The GPR120-mediated anti-inflammatory effects of DHA and EPA have also been confirmed in 3T3-L1 adipocytes, resulting in a significant reduction in MCP-1, IL-1β and TNF-α gene expression [58].
The inflammatory process is mainly regulated by NF-κB. Once this transcription factor is activated after IκB phosphorylation due to external stimuli such as UV radiation, endotoxins, oxidative stress, saturated fatty acids, it is able to translocate into the nucleus. It can then promote the production of several pro-inflammatory mediators, adhesion molecules, COX-2, and inducible NO synthase [59]. EPA and DHA, as reported above, reduce the production of a variety of pro inflammatory molecules, such as TNFα, IL-1, IL-6, IL-8, and IL-12 and limit the transcription of those enzymes involved in the inflammatory process including inducible NO synthase and COX-2 in different cell lines (for example endothelial cells, macrophages and monocytes) [60]. This effect seems to involve the reduction of IκB phosphorylation and consequently the reduction of the activation of NF-κB in a GPR120 and PPARγ dependent manner. Indeed, PPAR physically interacts with NF-κB, avoiding its translocation into the nucleus. Furthermore, NF-κB activity is also related to GPR120 since DHA strongly inhibited IKK activity via GPR120 in both stimulated macrophages and adipocytes [61].
In addition, treatment with n3-PUFA limited NF-κB DNA binding activity in adipocytes, macrophages and THP-1 monocytes stimulated with LPS, which prevents the production of IL-6, IL-1β and TNF-α [62]. Intriguingly, as along with NF-κB DNA binding activity being reduced, the authors demonstrated that PPARγ DNA binding activity was also significantly abolished, providing evidence of the tight connection between PPARγ and NF-κB activity in regulating the inflammatory processes. Alongside the reduction of pro inflammatory mediators, treatment with EPA and DHA promoted the release of IL10 in 3T3-L1 adipocytes [63]. IL10 is an important interleukin that is involved in the anti-inflammatory response and inhibits IKK, preventing NF-κB DNA binding activity and PPARγ binding motif. This demonstrates that n3-PUFA may regulate NF-κB activity by inducing IL-10 expression in a PPARγ dependent manner [64].
Besides the high content of EPA and DHA, krill oil also contains potent antioxidant molecules. In particular, many studies have demonstrated that the presence of astaxanthin is responsible for the potent antioxidant effect and potentially the well-known anti-inflammatory properties of EPA and DHA. Oxidative stress is the leading cause of many pathological conditions by triggering the activation of important proinflammatory intracellular pathways which feed a vicious circle. This is especially true in neurodegenerative processes and in cardiovascular diseases characterized by endothelial dysfunction. Preventing the excessive production of reactive oxygen species (ROS) through a nutraceutical approach may be a promising strategy to manage several pathological conditions. In this context, Nrf-2 is one of the main transcriptional factors controlling antioxidant machinery. Indeed, its activation is reported to exert beneficial effects through the increased production of direct antioxidant molecules as well as the hyper activation of antioxidant enzymes SOD, CAT, and GPX [65]. Nrf-2 is one of the main targets of the antioxidant effect of astaxanthin, which induces Nrf2–ARE-mediated antioxidant enzymes in different in vitro models. Astaxanthin reduced oxidative stress in neuronal cells exposed to doxorubicin results in an increase in cell viability and reduction of pro inflammatory mediators [66]. Similarly, astaxanthin protected human mesangial cells exposed to high glucose exert an anti-inflammatory and anti-oxidant effect [67].
Oxidative stress, beyond promoting the inflammatory response, is also responsible for insulin resistance due to the activation of several kinases, among which JNK promotes the phosphorylation of IRS-1, inhibiting its activity and preventing its interaction with the insulin receptor. In addition, elevated levels of ROS cause the degradation of the GLUT4 vesicle, dramatically decreasing glucose uptake [68]. The potent antioxidant effect of astaxanthin facilitates insulin secretion, accelerates glucose metabolism and improves insulin sensitivity, IRS-1 activation, Akt phosphorylation, and GLUT4 translocation in skeletal muscle. This leads to increased insulin sensitivity and a decrease in blood glucose level, which in turn paves the way for using the astaxanthin nutraceutical supply for the management of type 2 diabetes [69] (Table 3).

Table 3.
Experimental studies in which krill supplementation has been tested. Primary endpoints have been reported, as well as design and duration of the studies. (TG = triglycerides).
4. Krill Oil Extraction Technologies
Krill oil can be extracted from different biomasses of fresh krill and dried krill [70,71]. However, fresh krill contains high levels of proteolytic enzymes that induce the rapid autolysis of the crustacean. For this reason, it is necessary to process krill immediately after it is caught [72]. The extraction techniques (Figure 1) can be divided into classic and non-conventional methods. Conventional techniques include several solvent extractions, while the innovative methods include non-solvent extractions, super- and sub-critical fluid extractions and enzyme-assisted extractions.
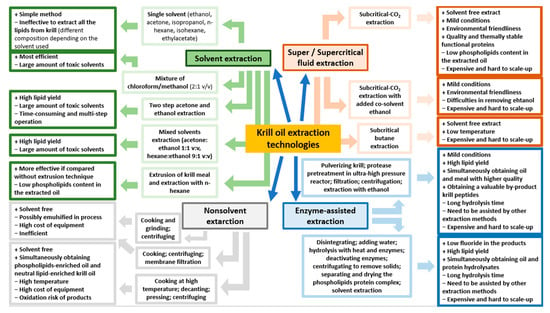
Figure 1.
The advantages and disadvantages of krill oil extraction technologies.
4.1. Conventional Extraction Techniques
Solvent extraction is the most conventional method to obtain krill oil. However, different solvents must be used to extract all the lipids from krill because of the diversity of the polarities of the lipid compounds in the biomass. As reported in a study by Xie et al., ethanol and isopropanol can extract most of the phospholipids, although the obtained krill oil is lacking in other lipid components [21]. Hexane is the most common solvent for vegetable oil production due to its low cost, high extraction efficiency and because it is easily eliminated and leaves few traces of residues in food sources. Nevertheless, data on the use of hexane for krill oil production has demonstrated that this solvent only has moderate PL-extracting ability [28,73].
Acetone has proven to be effective for the extraction of minor molecules from krill, but not for PLs. In this regard, a combination of nonpolar and polar solvents is a good compromise for the extraction of both the PLs and other molecules from krill [74]. Two-step acetone and ethanol extraction techniques are the most common methods used for krill [71], as other methods, including the Folch method (which is popular for lipid extraction from animal tissues), are not commercially feasible because of the toxicity of the solvents used (e.g., chloroform and methanol) [75]. Similar results have been obtained using a single-step extraction method with a mixture of acetone and ethanol as the solvents (1:1, v/v) [76]. Moreover, lipid efficiency can be improved by combining the extrusion pre-treatment of krill meal with the solvent extraction technique [77].
Environmental concerns, which have a real impact even in krill oil production, are one of the main limitations with the use of solvents. In addition, this technique is time-consuming and labor intensive because of the multiple extraction and evaporation steps. However, it remains the most economical and easily scalable.
Krill oil can also be extracted using non-solvent techniques including mechanical pressing, which is commonly used for oilseed extraction, such as in sesame oil and sunflower oil [78]. However, this method is inefficient compared to solvent extraction, for two reasons in particular: the low lipid content present in fresh krill (0.5–3.6%) and the difficulty of the mechanical processing used for krill [77].
Non-solvent sequential procedures, such as cooking, decanting, pressing and centrifuging, are able to successfully separate krill oil from the mixture (Figure 2). In fact, the release of PLs from the lipid fraction of fresh or defrosted krill—after conventional grinding into a slurry which involves mechanical disruption procedures and centrifugation—does not facilitate the complete separation of the oil fraction due to the amphipathic nature of PLs, which act as emulsifying agents in the formed slurry [79]. Katevas et al., have developed a method that can extract both the PL fraction and the neutral lipid-enriched krill oil. In this method, the first cooking step is conducted at 90° C without grinding and agitation, thus avoiding the emulsification process. The same authors concluded that fresh krill is a better option than defrosted krill as it was able to avoid or reduce the emulsification process, which may occur in defrosted krill due to the formation of ice crystals (after freezing) and the consequent disruption of krill tissue [70].
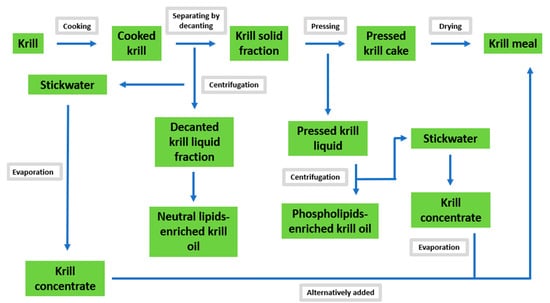
Figure 2.
An overview of non-solvent extraction technique (Adapted from Katevas et al. [70].
Although the non-solvent extraction technique avoids the use of solvents, making it safer and more eco-friendly than solvent-extraction methods, it presents many drawbacks. First, it is poorly scalable because of the high investment in equipment and the high costs of energy required. Secondly, the cooking procedure at high temperatures may induce lipid oxidation in the products. Last but not least, this technique has only been observed to give a total lipid yield of 2.2% and does not extract all of the available oil from krill. For these reasons, mechanical separation can be used to initially obtain part of the krill oil, but also to produce krill meal, which can be subsequently treated with other techniques including supercritical fluid extraction and solvent extraction [80].
4.2. Unconventional Extraction Techniques
The supercritical fluid extraction method is an unconventional extraction technique that can be used for krill oil extraction and is known to be solvent-free [81]. It is considered environmentally friendly as it uses supercritical carbon dioxide, which is safe, non-toxic and chemically inert. Nevertheless, carbon dioxide is not an ideal molecule for the extraction of all the lipid content from krill as it fails to extract PLs [82]. For this reason, the addition of 5–20% of ethanol to supercritical carbon dioxide extraction has been tested and demonstrated to improve PL solubility and thus lipid recovery [83]. The use of this technique presents some issues, including its limited processing capacity, the high cost of the equipment and the risk of de-solvation by the liquid state of ethanol at 25 °C.
Subcritical fluid extraction with compressed butane and propane, performed at low temperature and pressure, is commonly used for oil extraction [84]. In a study conducted by Xie et al., the use of subcritical butane (30 °C, 0.3 to 0.8 MPa) gave results that are comparable to those of hexane in terms of yield and krill oil quality, although it was faster and the extraction process required less solvent [73]. In addition, in a study by Sun et al., subcritical butane extraction was proven to give the extract that was richest in astaxanthin and tocopherol, and the lowest in oxidation degree, compared to the solvent extraction technique [85]. However, even in this case, the high cost of equipment and limited processing capacity are the main issues for a successful scale-up process.
Enzyme-assisted extraction is a novel pre-treatment technique than can increase lipid yield, as it can release the bound molecules using specific enzymes (e.g., amylase, protease, glucanase, cellulase and pectinase) [86]. These enzymes can selectively enhance the extractability of lipid compounds, improving the breakdown of cell walls and destroying lipid bodies. The high quality of the extracted oil and the mild process conditions also make this technique attractive for the extraction of krill oil [75]. Bruheim et al., have obtained satisfactory results with enzyme-assisted extraction for krill oil. After an initial krill-disintegration process, the small particles were treated with water and heat and the hydrolytic enzymes were then added to hydrolyze the material and improve lipid extraction. Finally, the enzymes were deactivated and, after removal of the solid material, the PL-protein complex was separated and dried, and the krill oil was extracted [75]. Finally, in a study by Lee et al., a combination of an enzyme-assisted extraction process and an ultrahigh-pressure (10 to 300 MPa) reactor made krill easily liquefiable and ensured full contact with the proteases [87].
The high costs and longer hydrolysis times are still challenging for the use of this technique in large-scale industrial applications.
5. Bioavailability and Bioaccessibility of Krill Oil Omega-3
Several studies have investigated the effects of krill oil supplementation on health, and these effects appear to be superior to those of fish oil [88,89]. The superiority of krill oil has been attributed to the higher bioavailability of EPA and DHA, which are in the form of PL [90]. Nevertheless, most of the RCTs conducted to date did not use the same doses of EPA and DHA (for the same outcomes) from krill and fish oil and ignored the differences between the bioactive substances in fish and krill oil [91].
In fact, the minor components contained in krill oil, such as astaxanthin, alpha-tocopherol, vitamin A and flavonoids, can exert pleiotropic activities and have a positive impact on health, in addition to probably improving the bioaccessibility of EPA and DHA. In this regard, a study by Kohler et al., s reported that EPA and DHA from krill meal had lower bioavailability than krill oil, but the same as fish oil. This study underlines the potential role of minor lipophilic molecules in improving the bioaccessibility, and thus the bioavailability, of EPA and DHA from krill oil [92]. However, the mechanisms of action by which krill oil appears to be superior remain unknown and seem to be closely related to the extract in its entirety and the extraction method.
Long-term RCTs are needed to determine the differences in efficacy and performance of krill oil compared to fish oil. This information is currently incomplete and needs clarification, starting from the role of minor components in the extracts. In addition, EPA and DHA supplemented from fish and krill oils in comparative studies should be at the same dosages and ratio. In fact, in a study by Ramprasath et al., which reported the superiority of krill oil over fish oil (expressed as concentration of n-3 PUFA plasmatic levels), supplementation with EPA and DHA was 777 mg for the krill oil group and 664 mg for the fish oil group [91]. Ulven et al., have demonstrated a change in the EPA/DHA ratio after supplementation with 543 mg of krill oil and 864 mg of fish oil (EPA/DHA ratio: 1.74 for krill group and 1.12 for fish oil group) [12]. Weather this improvement of EPA/DHA ration is an advantage in terms of health effect it is yet to be fully demonstrated.
6. Krill and Metabolic Disorders
Dietary supplementation with omega-3 fatty acids has been demonstrated to be beneficial for the prevention and/or treatment of cardiovascular diseases (CVD) [93] and possibly other inflammatory and neurological disorders [94,95]. Moreover, increased consumption of EPA and DHA may also be of clinical significance in the prevention and reversal of insulin resistance [96].
The American Heart Association (AHA) recommends a daily intake of at least 1000 mg of omega-3 fatty acids to minimize risk factors associated with CVD, even for patients at high risk of developing CVD. Therefore, despite the great abundance of omega-3 fatty acids available in fish oil, the delivery of fatty acids and presence of astaxanthin in krill oil may provide superior health benefits and meet the AHA recommendations [97].
Krill oil is endowed with a unique chemical composition. Unlike fish oil, it is rich in omega-3 fatty acids present in the form of phospholipids rather than triglycerides. This may be biologically and therapeutically significant, since phospholipids are well-absorbed by the intestine and they are readily incorporated into cell membranes, suggesting that they could be endowed with a more favorable pharmacokinetic profile [98]. Moreover, supplementation with krill oil gives the advantage of not only supplying n-3 PUFAs, but also choline, which is an essential nutrient, since that it is needed in the synthesis of neurotransmitters (acetylcholine) and phospholipids and is important in the transport of lipids and reduction of homocysteine [23]. In addition, krill contains several endogenous antioxidants including astaxanthin (which is responsible for the deep red color), the preservation of krill oil against oxidation and it has potential health-promoting properties [99].
Maki and colleagues observed that a supplementation for four weeks with Antarctic krill oil (2 g/day) increased plasma concentration of EPA and DHA [100]. This suggests it could be used similar to the way fish and fish oil are used, as it is also rich in long-chain omega-3 polyunsaturated fatty acids which contains cardiovascular risk. In this context, supplementation with krill can also represent a promising approach to ameliorate obesity and obesity-associated diseases. To date, preclinical evidence has been collected on the positive impact of krill oil in conditions of metabolic disorders.
It has been also demonstrated that plasma EPA level was significantly greater following krill oil treatment when compared to fish oil treatment. Significant remodeling of the plasma lipidome was observed after four weeks of treatment with krill oil (containing 1.27 g/day of long-chain omega-3 polyunsaturated fatty acids) if compared to fish oil (containing 1.44 g/day of long-chain omega-3 polyunsaturated fatty acids), with a clear differentiation in their effects on different plasma lipids species. In particular the authors reported that more than 38% of the lipids species increased following krill treatment, while only 12% increased when fish oil was used as supplementation [101]. Similar results have been obtained in healthy women over a five hour postprandial period. Clear differences between krill oil and fish oil supplementations in the postprandial period were reported. The most noticeable changes were revealed in diacyl-phospholipids and ether-phospholipids [102].
Finally, subchronic toxicity and genotoxicity studies in rats confirm that krill oil is well-tolerated and seems to be safe with a daily supplementation of 5% [103].
In male Sprague Dawley rats who were fed a high-fat diet (HFD) for two weeks, the consumption of krill oil significantly reduced serum lipids, and the two highest krill oil doses (100 and 200 g/L) also significantly increased HDL levels [104].
In 2009 Cohn’s group demonstrated that supplementation of krill oil to HFD mice for eight weeks, dose-dependently reduced hepatic triglycerides, cholesterol and serum, as well as total cholesterol and glucose. Unfortunately, this study was unconclusive regarding the putative mechanism. The peroxisome proliferator-activated receptor (PPAR)α which is supposed to be critical to promote β-oxidation at the liver level, did not significantly change which means it was not involved [105].
The intake of a powder isolated from Antarctic krill showed to ameliorate the hepatic metabolism in a transgenic mouse model of chronic inflammation, for expressing the human tumor necrosis factor-alpha (hTNFα) gene. Lower hepatic and plasma triacylglycerol levels, as well as hepatic gene expression of sterol regulatory element binding transcription factor 2 (SREBP2) and enzymes involved in cholesterol synthesis were found. In addition, genes involved in lipogenesis and glycerolipid synthesis were down-regulated and β-oxidation was promoted, confirming the capability of this product to increase the hepatic lipid catabolism and suppress lipidogenesis. Finally, krill powder reduced endogenous TNFα in the liver, indicating anti-inflammatory effects [106].
In 2020, Saito et al. evaluated the effects of 8-HEPE-concentrated material from Pacific krill on dyslipidemia and hepatic steatosis in low-density lipoprotein (LDL) receptor-deficient (LDLR-KO) mice. Very interestingly, they observed that over 18 weeks a supplementation of a typical western diet with Pacific krill (8-HEPE (100 mg/kg), but not EPA or DHA) improved the lipidic profile (reducing LDL and total cholesterol levels and increasing HDL levels) and reduced hepatic triglyceride levels [107]. This led to the hypothesis that eicosapentaenoic acid (EPA) and 8-hydroxyeicosapentaenoic acid (8 HEPE) have more positive effects on the metabolic syndrome by activating the peroxisome proliferator activated receptor (PPAR α) in the liver.
In addition, in dyslipidemic and diabetic non-human primates, the daily intake omega-3 phospholipids purified from krill showed a positive impact on CVD risk factors by reducing total cholesterol, LDL-cholesterol and triglycerides and increasing HDL-cholesterol, if used at the dose equal to 150 mg/Kg/day [108].
Runbland and colleagues reported a randomized control study carried out on 36 individuals who were divided in three groups: fish group, krill group and control group. For eight weeks, krill and control groups received capsules containing oil, while the fish group was invited to consume lean and fatty fishes based on dietary guidelines. As expected, the levels of EPA and DHA increased in the fish group and krill group, whereas docosapentaenoic acid (DPA) increased only in the krill group. However, the overall differences between the three intervention groups were significant for EPA (p < 0.0001), DPA (p < 0.001) and DHA (p < 0.001). In general, the authors observed a great variability among the participants, however a tendency towards a decrease in total lipids and triacylglycerols was observed in the fish and krill groups with the largest VLDL levels. In agreement with previous works in animal models, in which a down-regulation of the genes involved in gluconeogenesis has been demonstrated [109,110], as well as in clinical studies [111], they reported a significant reduction in fasting blood glucose in the krill group compared with the control group. This result is strongly predictive of a reduction of CVD risk [112].
In humans with borderline or high triglyceride levels (range between 150–499 mg/dL), the treatment with krill oil at the dose of 0.5, 1, 2, or 4 g/day for 6 and 12 weeks could be efficient to reduce triglycerides. Nevertheless, the great heterogeneity of the selected sample impeded to have a clear view of the real nutritional value [113].
Cicero et al., published a randomized cross-over clinical trial in which 25 moderately hypertriglyceridemic subjects (150–500 mg/dL) were treated with omega 3 ethyl ester (2000 mg/day) or krill oil (1000 mg/day) for four weeks. Only the krill oil treatment significantly improved HDL and apolipoprotein AI levels, compared to the values measured both at baseline (p < 0.05) and at end of treatment in the group supplemented with esterified omega 3 (p < 0.05). Both treatments were able to significantly reduce high-sensitivity C-reactive protein (hs-CRP) levels from the baseline (p < 0.05), but krill oil improved it more efficaciously than esterified omega 3 ethyl ester group (p < 0.05) [114].
A reduction in body weight because of krill oil supplementation in obesity models has been reported in some animal studies. Sun and colleagues observed that an Antarctic krill oil, extracted from dry krill using an innovative procedure of hot pump dehydration combined with freezing-drying, was endowed with anti-obesity effects in metabolic disorder conditions. In particular, supplementation for 12 weeks improved dyslipidemia, fatty liver and glucose metabolism in C57BL/6J mice fed with HFD. Krill oil also reduced body weight gain, reduced fat accumulation in adipose and liver tissue, lowered serum density of lipoprotein-cholesterol (LDL-C) content and ameliorated glucose tolerance. In addition, krill oil feeding also reduced oxidative damage in the liver [115].
In another study, rats were fed a control diet, an HFD or an HFD supplemented with 2.5% krill oil for 12 weeks. Krill oil significantly prevented increased body weight in the HFD group [105].
An improvement of insulin sensitivity and secretion after administration of krill oil (600 mg/day) has also been seen in an obesity model of castrated male New Zealand white rabbits [110]. Expression levels of key enzymes involved in the β-oxidation and lipogenesis were different after krill oil feeding for 8 weeks, compared to placebo, which ultimately led to decreased fasting blood glucose and improved glucose tolerance in the rabbits.
Further evidence suggested that omega-3 phospholipids of krill oil enhanced intestinal fatty acid oxidation and could contribute to the anti-steatotic effects in obese mice, meaning there could exist an axis microbiota-intestine-liver [116]. Indeed, omega-3 alleviated hepatic steatosis in various rodent models of obesity even in exacerbated hepatic steatosis conditions, in which an HFD was combined with thermoneutral animal housing (i.e., ambient temperature approximately 30 °C) [117].
Several authors highlighted that omega-3 supplemented by krill oil is even more effective, mainly phosphatidylcholine -rich phospholipids when compared to the same dose of the triacylglycerol form. It has been observed that omega-3 phospholipids contribute more effectively to improve glucose intolerance and insulin resistance in dietary obese mice when compared to their triacylglycerol form [116]. The reason for this is likely due to the amelioration of bioavailability; EPA and DHA present in fish and fish oil are almost exclusively in triacylglycerol form, while in krill oil up to 65% of EPA and DHA occur in phospholipids [118].
Krill oil was also found to directly influence cardiac remodeling and function in an experimental myocardial infarction (MI). In such experimental conditions, rats were randomized in krill oil or control groups for 14 days before the induction of MI. Seven weeks after the MI induction, the echocardiography showed a significant attenuation of left ventricular (LV) dilation in the group pre-treated with krill oil. Attenuated heart and lung hypertrophy and reduced mRNA levels encoded classical markers of LV stress, including matrix remodeling and inflammation [119].
Krill oil has been also associated with moderate improvement in endothelial dysfunction and HDL, two known CVD risk factors, in patients with type 2 diabetes. In 34 participants with type 2 diabetes, an improvement of their endothelial function and a reduction in blood C peptide levels and HOMA scores were reported after four weeks of supplementation with krill oil (1 g/day in PUFA) when compared to the olive oil group. There were differences in weight loss between krill oil and olive oil after 17 weeks, though if compared with their respective baseline measurements, the participants of each group had a statistically significant improvement in endothelium [120].
A close relationship between krill oil consumption and reduction of circulating levels of endocannabinoids 2-arachidonoylglycerol (2-AG) and N-arachidonoyl-ethanolamine (AEA) has been highlighted [116].
In obese subjects, the endocannabinoids are elevated in the blood and this phenomenon appears to be due to changes in expression of adipose tissue metabolizing enzymes. Moreover, endocannabinoids are made by enzymatic reactions from arachidonic acid; hence, the more omega-6 arachidonic acid (ARA) available, the more endocannabinoids can be made. On the other hand, an increased intake of omega-3 might help to counterbalance a disturbed omega-3 to omega-6 ratio and result in lower endocannabinoid levels that may positively affect membrane signaling and energy metabolism. Recently, Di Marzo and Silvestri brought to light the existence of a triangle among the lifestyle–gut microbiome–endocannabinoid system and its crucial role in the development of metabolic syndrome [121].
Interestingly, krill oil supplementation (dose ranging 1.25–5%) led to a significant decrease in AEA, as compared to controls [122]. According to previous preclinical evidence, Berge and colleagues demonstrated that besides a reduction of plasma triglycerides, supplementation with krill powder (4 g/day per os) contributed to a reduction in 11 obese men’s anthropometric parameters and blood endocannabinoid (AEA and 2-AG), whose levels were correlated with high levels of triglycerides and were responsible for hyperactivity of the cannabinoid system which feeds metabolic dysfunction. Indeed, the endocannabinoid system is deeply involved in the regulation of the homeostasis of body composition by regulating food intake and energy expenditure; therefore, this could be another mechanism through which krill may have beneficial effects on metabolism [123]. According to a previous paper, a significant reduction of the 2-AG levels has been highlighted with 2 g/day of krill oil (providing 309 mg/day of EPA/DHA 2:1), although no significant effect on anthropometric parameters has been observed [124].
7. Krill and Inflammatory Bowel Diseases and Gut Microbiota
It is well known that obesity and its complications, such as insulin resistance, hyperlipidemia and atherosclerosis caused by HFD are often accompanied with alteration in gut microbiota, in particular an increase of pro-inflammatory/pathogenic bacteria. In 2017, Cui et al. observed that treatment with fish oil (600 μg/g/day), krill oil (600 μg/g/day) and their mixture (300 + 300 μg/g/day) for 12 weeks led to obesity alleviation, as well as gut microbiota modulation. In fact, they reported a decrease of body weight gain, adiposity index and liver index. They also reported an increase in the abundance of some positive phyla in the gut, including Bacteroides and Lactobacilli [125]. Likewise, Lu and colleagues demonstrated that supplementation with krill oil shifted the gut microbiota composition and that it was associated with the alleviation of hyperlipidemia. According to an experimental model, mice fed for 12 weeks with a high fat and high sugar diet showed obesity and hyperlipidemia. Treatment with a high dose (600 µg/g/day) of krill oil, but not with lower doses (100 µg/g/day or 200 µg/g/day) improved the microbiotic alteration and cardiometabolic parameters [126].
Krill oil treatments for seven weeks at different dosages (100, 200 and 600 mg/kg) decreased the abundance of tyrosine consumers and increased the abundance of Lactobacillus spp. and short-chain fatty acids producers [127].
Moreover, other research points out beneficial effects of krill supplementation against inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease, which share common symptoms such as bleeding, diarrhea and weight loss. In these cases, the integrity of the intestinal barrier layer and the gut microbiota play a critical role. In this regard, a mixture composed of krill oil plus probiotic Lactobacillus reuteri plus vitamin D has demonstrated to significantly improve clinical and histological scores, restore epithelial restitution and reduce proinflammatory cytokines in an experimental model of colitis induced by dextran sulphate sodium (DSS) treatment [128]. Of note, krill oil also appears to attenuate inflammation in an experimental model of ulcerative colitis in rats. In male rats submitted to treatment with dextran sulphate sodium (DSS), the supplementation with krill oil (5%) for 30 days preserved the colon length, which was significantly shortened in the DSS-treated compared to control animals, in line with oedema and inflammation in the colonial mucosa. Moreover, typical factors such as disease activity index (DAI) and TNF-α and IL-1β levels were positively affected by krill oil compared to DSS administration alone [129].
Suppression of the pro-inflammatory cytokines TNF and IL6 and the systemic levels of endotoxin, a marker of IBD, were also found. Recently innovative krill oil-entrapped liposomes were developed and their efficacy in an IBD model was demonstrated. The authors observed that liposomes were incorporated into the impaired enterocyte membrane, which contributed to re-establish the hydrophobic protective barrier in the inflamed/impaired region and decrease the permeation typical of IBD [130].
Liu and colleagues suggested that krill oil could contribute both to attenuating the inflammatory pathway and modulating gut microbiota through the reduction of Rickettsiales and several species of Lactobacillus [131].
8. Inflammation
Arthritic Diseases
Based on pre-clinical studies carried out on mice experimental models of inflammatory arthritis, krill oil was supposed to have a positive effect in reducing joint inflammation more so than fish oil. In a rodent model of rheumatoid arthritis, mice fed with a krill oil diet, in which EPA and DHA were 0.44 g/100 g of krill oil diet, exhibited a decreased infiltration of inflammatory cells at the joint level, and decreased hyperplasia at the synovial layer, compared to controls [132]. Moreover, in mice transgenic for human TNF-α, while the fish oil and krill oil had a similar effect on cholesterol levels, only krill oil reduced markers of fatty acid oxidation [133]. This is consistent with the observation that fish oil and krill oil are both rich in EPA and DHA, but only krill oil naturally contains antioxidants agents like astaxanthin [134].
In a previous randomized, double blind, placebo controlled clinical trials carried out on 90 patients affected by cardiovascular diseases and/or osteoarthritis and/or rheumatoid arthritis, the effect of krill oil on C-reactive protein (CRP) and on arthritic symptoms were evaluated. Patients received krill oil 300 mg/day or placebo for 30 days and CRP and osteoarthritis symptoms were recorded at baseline and at days 7, 14 and 30. Despite the short treatment, krill oil significantly reduced CRP levels even after 7 days (about 20% of CRP reduction versus an increase of about 16% in the placebo group), reaching a higher reduction (about 30% versus an increase of CRP levels of about 25% in the placebo arm) after 30 days. Moreover, krill oil significantly reduced symptoms such as pain by about 29%, stiffness by about 20% and functional impairment by about 23%, suggesting that a 300 mg daily dose of krill oil could represent a good strategy to counteract arthritic symptoms and inflammation period [135].
In a more recent randomized, double-blind, parallel-group, placebo-controlled study, 50 adult patients affected by mild knee pain received 2 g/day of krill oil or placebo for 30 days. After 30 days of treatment, patients treated with krill oil exhibited a significant reduction of knee pain both in sleeping and in standing and the range of motion in both knees was improved [136].
These promising pre-clinical and clinical studies led Laslett and co-workers to design and start a clinical trial named “KARAOKE” (Krill oil for OA of the knee), focused on the use of krill oil to improve knee osteoarthritis (OA). This study is currently ongoing and 260 Australian patients affected by knee OA characterized by pain, synovitis and effusion will be recruited and randomized to receive 2 g/day of krill oil or placebo every day for six months. Symptoms, functionality and knee structural abnormalities will be monitored at the beginning of the study and after 6 months using validated clinical methods and magnetic resonance imaging. In particular, the primary outcomes of this study are change in knee pain and in size of knee synovitis/effusion over 24 weeks. The secondary outcomes are improvement in knee pain over 4, 8, 12, 16 and 20 weeks [11].
9. Neuroprotection
9.1. Neurodegeneration and Alzheimer Disease
The astaxanthin content present in krill oil was evaluated as a peculiar feature which gives krill oil more antioxidant properties than fish oil. In particular, the first results demonstrated that astaxanthin was able to protect the human neuronal SH-SY5Y cell line from an oxidative stimulus via its ability to act as a mitochondria protective agent [137]. In the same neuronal cell line, astaxanthin induction increased expression of the antioxidant enzyme heme oxygenase-1 (HO-1) by activation of the ERK1/2 signal pathways. This mechanism could account for the neuroprotection induced by astaxanthin against the cellular apoptosis induced by beta-amyloid (Aβ25-35). This protection was abolished by the administration of a specific ERK inhibitor [138]. Finally, astaxanthin (250–1000 nM) in primary culture of cortical neurons prevents the H2O2-induced (50 µM) reduction of cell-viability, restores mitochondrial potential and inhibits apoptosis. In a rat model of focal cerebral ischemia, in vivo, astaxanthin which was administered intragastrically at 80 mg/kg twice (5 h and 1 h before the induction of cerebral ischemia), induced a significant protection against infarct volume, improving the neurological deficit [139].
Several pre-clinical studies investigated the role of krill oil on cognitive function. In particular, Lee and colleagues evaluated the potential improvement in memory and learning due to the administration of phosphatidylserine isolated from krill (PK) in aged rats through the Morris water maze experimental model. Their results demonstrated that the administration of PK 20 or 50 mg/kg/day for 7 days significantly improved the escape latency for finding the platform in the Morris water maze compared with rats which received the 50 mg/kg/day of phosphatidylserine isolated from soybean. Moreover, the treatment with PK also improved the loss of cholinergic immunoreactivity, muscarinic receptors and choline transporters typically observed in the hippocampus of aged rats, demonstrating a neuroprotective role for PK [140]. The examination of these results on different components naturally contained in krill oil, taken together with the presence of omega-3, led Barros and colleagues to hypothesize a perspective for the use of krill oil as a neuroprotective supplement [141].
After the above reported studies demonstrating the neuroprotective effect of krill oil, more specific studies on neurodegenerative diseases were carried out on recognized animal models. Among them, Choi and co-workers administered krill oil 80 mg/kg/day for one month to a mice model of Alzheimer obtained through lipopolysaccharide (LPS) injections, 250 µg/kg, seven times daily. The team found that krill oil induced a general reduction in oxidative and inflammatory markers. Krill oil administration prevented the LPS-induced expression of the inducible isoform of nitric oxide synthase (iNOS) and of cyclooxygenase-2 (COX-2), inhibited IkB degradation suppressing the NFkB pro-inflammatory signaling and induced a decrease of ROS levels and malondialdehyde levels. Moreover, krill oil also suppressed amyloid beta (1–42) peptide generation, demonstrating a multitarget mechanism on the three main aspects which support Alzheimer disease: oxidation, inflammation and amyloid beta production [142].
A recent study, carried out on a more specific animal model of neurodegenerative diseases, represented by the senescence-accelerated prone mouse strain 8 (SAMP8) mice, demonstrated that krill oil improved both cognitive function and anxiety. In particular, the administration of a diet enriched with 1% of krill oil for 12 weeks improved SAMP-8 performances tested through the Morris water maze, the open-field test and the Barnes maze test, resulting in reduction of memory deficit and learning improvement. Moreover, by examining the hippocampus it was clear that krill oil reduced β-amyloid Aβ42 accumulation. This effect was linked to a mechanism involving an increase in activity of glutathione peroxidase and superoxide dismutase and a contemporary decrease in malondialdehyde and 7,8-dihydro-8-oxoguanine levels [143]. Interesting findings about possible use of krill oil in prevention and treatment of Alzheimer disease were recorded by Kim and co-workers through the employment of a mouse model of Alzheimer obtained by injection of amyloid Aβ25-35 in mice. After the Aβ25-35 injection, mice developed cognitive impairments but the mice receiving an oral administration (gavage) of 100, 200 or 500 mg/kg/day of krill oil for 14 days showed shorter latency in the Morris water maze test, downregulation of Bax/Bcl-2 ratio in the brain and reduced levels of ROS, malondialdehyde and NO [89].
Despite the evidence obtained in pre-clinical studies, the clinical trials studying the effect of krill oil on human brain are very poor. In particular, a randomized, double-blind clinical trial was performed on 45 healthy elderly males (61–72 years-old), treated for 12 weeks with placebo (represented by medium-chain triglycerides), sardine-oil (abundant in n-3 polyunsaturated fatty acids-PUFAs- incorporated in triglycerides) or krill oil (abundant in n-3 PUFAs incorporated in phosphatidylcholine). The results of this study indicated that during the working memory task, both in the sardine and in the krill group the oxyhemoglobin concentrations in the cerebral cortex were significantly increased compared to the placebo group. In the calculation task, only the krill oil evoked an increase in oxyhemoglobin significantly different from the placebo group. Only the krill group showed a significantly lower differential value for P300 latency when compared to the placebo group [144,145].
9.2. Depression
The effect of krill oil supplementation was evaluated on cognition and depression-like behaviors both in pre-clinical and clinical studies.
One of the first studies in this field was carried out for seven weeks in rats, which received krill oil 0.2 g/rat/day, or imipramine 20 mg/kg/day (used as an anti-depressant reference drug) or placebo. At the end of the treatment, the cognitive abilities were tested by the Aversive Light Stimulus Avoidance Test (ALSAT) while the potential anti-depressant effect was tested by the Unavoidable Aversive Light Stimulus (UALST) and the Forced Swimming Test (FST). The results showed that krill oil treated rats had a significant ability to discriminate between the active and the inactive levers in the ALSAT test since the first day of training. Moreover, rats treated with krill oil and impramine exhibited significant improvement in behavior features such as lower levels in the UALST test since day three, and shorter immobility time in the FST test. The investigation also involved the expression of brain-derived neurotrophic factor (Bdnf) which was found to be upregulated in the hippocampus of krill oil treated rats [146].
Similar results were obtained by Zadeh-Ardabili and colleagues on mice treated with fish oil, krill oil, vitamineB12, imipramine or saline 5 mL/kg once per day for 14 days starting after one week of the Chronic Unpredictable Stress (CUS) paradigm overnight procedure. During CUS procedures, light was used to stress the mice overnight with an illumination of 10 W LED, 15 Hz for 12 hours for 3 weeks. The potential antidepressant effect of the treatments was tested by tail suspension test (TST) and FST. After animal sacrifice, the presence of oxidation markers was evaluated in the brain tissue. Both fish oil and krill oil significantly reduced the immobility factors and increased the time of climbing and swimming, similar to imipramine, when compared with the control group. Both the fish oil and the krill oil led to decreased malondialdehyde and hydrogen peroxide levels, decreased catalase activity, increased glutathione peroxidase levels and increased superoxide dismutase activities and glutathione levels in hippocampal tissue [147].
Another pre-clinical study, by Mendoza et al., investigated the role of krill oil on restraint stress in mice after reduced mobility. After two weeks of acclimation and handling, mice were immobilized for three weeks followed by a week dedicated to behavioral tests. During the four weeks of the study, the mice orally received PBS or cotinine (a nicotine-derivative) at 5 mg/kg, or cotinine plus krill oil 143 mg/kg. The results showed that cotinine alone reduced both the loss in cerebral synaptic density, memory deficits, anxiety and depression-like behaviors, but that the co-administration of cotinine plus krill oil was more effective than cotinine alone in reducing depression-like behaviors linked with reduced mobility. This confirms krill oil plays a role in depression mechanisms [148].
These encouraging pre-clinical data encouraged van der Wurff and colleagues to design and carry out a year long randomized, controlled, double-blind clinical trial on the effects of a krill oil supplementation on adolescent behaviors linked to learning, cognition, visual processing and mental well-being. The study included 264 adolescents between the ages of 13 and 15 years, who were divided in two cohorts. Cohort I started with 400 mg/day of EPA + DHA or placebo, and after three months the dose was increased to 800 mg of EPA + DHA per day. Cohort II started with 800 mg of EPA+DHA per day. The effects of these treatments were assessed by Omega-3 Index finger-prick blood measurements, Centre for Epidemiologic Studies Depression Scale evaluation and the Rosenberg Self Esteem questionnaire. The authors concluded that there was no evidence of an effect of krill oil in reducing depressive feelings or in inducing higher self-esteem. However, the authors reported that the results were affected by low adherence and drop-out, and for these reasons they suggest caution when interpreting the data [149,150].
10. Cancer
Only a few studies carried out with respect to krill oil and cancer cell lines are available. In a screening study carried out on several cancer cell lines such as cells derived from histiocytic lymphoma (U937), leukemia (K562, HL60), human hepatocarcinoma (SMMC-7721), bone metastasis of pancreatic cancer (PC3) and breast cancer (MDA-MB-231, MCF-7), a general inhibition of cancer cells proliferation by krill oil was observed [151].
In a comparison with fish oil, EPA and DHA incubated in human osteosarcoma cells for 24, 48 and 72 h, found only krill oil induced a significant inhibition of cancer cells proliferation (23, 50 and 64% of inhibition, respectively). Fish oil did not change the proliferation observed in control cells except for an increase observed after 24 h. On the contrary, EPA and DHA promoted cell proliferation and cell migration [152].
Some researchers have focused their attention on human colorectal cancer cells, in particular, Jayathilake and co-workers treated HCT-15, SW-480 and Caco-2 cells for 48 h with free fatty acid (FFA) extract from krill oil and fish oil. Their results indicated that krill oil and fish oil extracts inhibited cell proliferation in a similar manner but only an increase in mitochondrial membrane potential and consequent cell apoptosis was only observed with krill oil [153]. The same research group further investigated the antiproliferative effect of free fatty acid extract (FFAE) of krill oil on other human (DLD-1, HT-29 and LIM-2405) and murine (CT-26) colorectal cancer cells, in comparison with EPA, DHA (after 24 and 48 h) and oxaliplatin (after 24 h). Osteosarcoma, with colorectal cancer FFAE of krill oil, EPA and DHE inhibited cell proliferation and ROS formation in a similar way to oxaliplatin. FFAE of krill oil also induced a significant increase in caspase 3 and 9 levels which are markers of apoptosis [154]. An investigation of potential mechanisms of action was then carried out on DLD-1 and HT-29 cell lines treated with FFAE of krill oil at 8 and 24 h. From the evaluation of epidermal growth factor receptor (EGFR) signaling, the results indicated that FFAE of krill oil, at 0.03 and 0.12 µL/100 µL, induced reduction in EGFR, pEGFR, pERK1/2 and pAKT expression without any changes in total ERK1/2 and AKT levels. The expression of the ligand PD-L1 was significantly inhibited by FFAE of krill oil [155].
11. Exercise Performance
Krill oil has been associated with improvement of exercise and antioxidant/anti-inflammatory markers and several clinical trials have been carried out. The first was a small double-blind study carried out on 17 members of the Polish National Rowing Team. The rowers were divided in two groups: one received 1 g/day of krill oil for six weeks and one received placebo. The parameter of athletes was tested before, after 1 min and after 24 h, the latter of which was deemed maximum effort whereby participants had rowed 2000 m. Exercise induced an increase in erythrocytes or serum levels of some markers collected from rowers, such as superoxide dismutase, TNF-α and thiobarbituric acid reactive substances (TBARS, a marker of lipid peroxidation). While the other parameters did not differ from control and krill oil supplemented group, during recovery time TBARS continued to increase in the control group while the krill oil supplemented group showed significantly lower levels of lipid peroxidation. This suggests that krill oil could reduce the effort-associated free radical mediated injuries [156].
The effects of krill oil were also investigated to see its ability to influence exercise performance and post-effort immune function. In a small randomized clinical trial, 37 young (25.8 ± 5.3 years) athletes were divided into two groups: one received 2 g/day of krill oil for six weeks and the other received a placebo. A cycling time test was performed before and at the end of the supplementation period, where blood samples were collected before supplementation and immediately after exercise, or after 1 or 3 h, or at rest. The results showed that after six weeks of supplementation, the levels of peripheral blood mononuclear cell IL-2 production and natural killer cell cytotoxic activity 3 h post-exercise were significantly increased in krill oil supplemented athletes [157]. On this basis, other authors investigated the ability of krill oil to increase the body mass and the potential mechanism action behind this effect. To investigate the mechanism of action, they used C2C12 rat myoblasts (skeletal muscle) treated with krill oil or phosphatidylcholine derived from soy or control and observed that only krill oil was able to stimulate the mTOR pathway. In the clinical part of the study, a double-blind, placebo-controlled clinical trial was performed on resistance trained athletes receiving 3 g/day of krill oil or placebo during the resistance training program of eight weeks. At the end of the study, no difference in comprehensive metabolic panel, complete blood count or urine analysis were recorded between the two groups. However, krill oil was able to induce a significant increase in the lean body mass from baseline of about 2.1% [158].
Moreover, a particular mixed formulation, named ESPO-572® composed of 75% PCSO-524®, which is green-lipped mussel oil and 25% krill oil, was effective in mitigation of exercise-induced muscle damage and cytokine-induced tissue degradation when administered for 26-day, 600 mg/day, in untrained men who underwent a running test [159]. As the levels of choline are known to maintain muscle function and exercise performance, a decrease in choline levels were recorded after high-resistance or high-intensity exercises. Storsve and colleagues performed a clinical trial to evaluate a possible protective effect by krill oil on this loss of choline. There were 47 triathletes placed randomly in two groups, one receiving 4 g/day of a particular formulation of krill oil named SuperbaBoostTM for five weeks before the race and another group receiving placebo. Blood samples were collected pre-, immediately post-race and one day after the race and the serum choline and the choline metabolites were evaluated. As expected, the choline levels significantly decreased after the race, but significantly higher choline levels were found in athletes of the krill oil group compared with athletes who received placebo. These results seem to suggest that a krill oil supplement could prevent choline levels from falling and could avoid impairment in exercise performance, especially during high-resistance efforts [160].
12. Discussion and Future Perspectives
Considering the increased market of n-3 PUFA containing dietary supplements, supported by increased clinical evidence, there is a constant search for new n-3 PUFA sources and formulations [13].
Krill oil possesses several health benefits in clinical practice, in particular in cardiovascular disease risk factor management and in neurological diseases and inflammation [161]. It is commercialized in both the nutraceutical and pharmaceutical market in different dosage forms including soft gels, gummies, capsules and tablets.
However, despite many activities and functionalities that have been attributed to krill oil, the molecular pathways of actions are still in part unclear because few studies of pharmacodynamic are available and few have provided detailed information about molecular mechanisms of krill components such as astaxanthin, vitamin A, tocopherols, flavonoids, and minerals [162]. Most published RCTs do not provide any information regarding krill oil composition (except for the EPA and DHA content) [93]. In this regard, further studies are necessary to emphasize the relationship between krill oil components, mechanisms of action, health benefits and diversifying the different composition of krill oils for specific applications.
The importance of knowing the actions of the active components of krill oil is fundamental for the future development of new extraction techniques which could give rise to new chemical extract compositions for certain pathological conditions. To date, solvent and non-solvent extraction, super and subcritical fluid extraction and enzyme-assisted pre-treatment extraction represent the main technologies used for krill oil extraction, each of which have both advantages and disadvantages [163].
Among the active ingredients contained in krill oil, EPA and DHA constitute the main title of the products studied in clinical trials. EPA and DHA from krill oil are attached to phospholipids and to phosphatidylcholine. This composition promotes the efficiency of absorption of fatty acids into the blood when compared with omega-3 from fish oil [4]. However, the minor components contained in krill oil such as astaxanthin, alpha-tocopherol, vitamin A and flavonoids could exert pleiotropic activities and improve the bioaccessibility of EPA and DHA, even if data need to be clarified. Many studies on krill rarely detail the concentration in minor components, so it is hard to estimate their contribution to the final observed effects.
Krill oil products can be associated with other nutritional supplements to provide more benefits. Alvarez-Ricartes et al. demonstrated the efficacy of krill oil in addition to cotinine in the treatment of depressive symptoms in posttraumatic stress disorder people [164]. A study by Costanzo et al. found an association of krill oil with Lactobacillus reuteri, and vitamin D showed to reduce gut inflammation, reducing gut dysbiosis as well as increasing the epithelial restitution [135].
Currently, supplementation with krill oil is considered safe and well tolerated. Side effects are minimal or absent, and may include bloating, diarrhea and flatulence [165]. However, the available evidence is limited and further long-term RCTs, including many people, are needed to confirm both safety and efficacy of this nutraceutical. In addition, a cost/benefit analysis is necessary to better understand the implication of krill oil supplementation on health.
In conclusion, preliminary clinical data suggest that krill oil represent a valid supplement in the treatment of several conditions including CVDs, osteoarthritis, premenstrual syndrome and dysmenorrhea. Innovative technologies applied to improve krill oil purification and concentration could improve its cost-efficacy ratio.
Author Contributions
Conceptualization, A.F.G.C. and A.C.; methodology, A.F.G.C. and A.C.; writing—original draft preparation, A.C., G.C., V.C., A.M., L.T., A.F.G.C.; writing—review and editing, A.C., G.C., V.C., A.M., L.T., A.F.G.C.; supervision, A.F.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge Elisa Grandi for her support in the paper preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicol, S.; Endo, Y. Krill fisheries: Development, management and ecosystem implications. Aquat. Living Resour. 1999, 12, 105–120. [Google Scholar] [CrossRef]
- Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR). Krill Fisheries and Sustainability. Available online: https://www.ccamlr.org/en/fisheries/krill-fisheries-and-sustainability (accessed on 23 May 2021).
- Nicol, S.; Foster, J.; Kawaguchi, S. The fishery for Antarctic krill—Recent developments. Fish Fish. 2011, 13, 30–40. [Google Scholar] [CrossRef]
- Cicero, A.F.; Colletti, A. Krill oil: Evidence of a new source of polyunsaturated fatty acids with high bioavailability. Clin. Lipidol. 2015, 10, 1–4. [Google Scholar] [CrossRef]
- Grantham, G.J. The Southern Ocean: The Utilization of Krill; Southern Ocean Fisheries Survey Programme GLO/SO/7/3; Food and Agriculture Organization: Rome, Italy, 1977; pp. 1–61. [Google Scholar]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013, 12, 178. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Ertek, S.; Borghi, C. Omega-3 polyunsaturated fatty acids: Their potential role in blood pressure prevention and management. Curr. Vasc. Pharmacol. 2009, 7, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; De Sando, V.; Parini, A.; Borghi, C. Polyunsaturated fatty acids application in internal medicine: Beyond the established cardiovascular effects. Arch. Med. Sci. 2012, 8, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-S.; Hu, X.-J.; Zhao, Y.-M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013, 346, f3706. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010, 376, 540–550. [Google Scholar] [CrossRef]
- Laslett, L.L.; Antony, B.; Wluka, A.E.; Hill, C.; March, L.; Keen, H.I.; Otahal, P.; Cicuttini, F.M.; Jones, G. KARAOKE: Krill oil versus placebo in the treatment of knee osteoarthritis: Protocol for a randomised controlled trial. Trials 2020, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic Effects of Krill Oil are Essentially Similar to Those of Fish Oil but at Lower Dose of EPA and DHA, in Healthy Volunteers. Lipids 2010, 46, 37–46. [Google Scholar] [CrossRef]
- Kim, M.G.; Yang, I.; Lee, H.S.; Lee, J.-Y.; Kim, K. Lipid-modifying effects of krill oil vs fish oil: A network meta-analysis. Nutr. Rev. 2020, 78, 699–708. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2019, 19, 64–123. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nelson, M.M.; Mooney, B.D.; Nichols, P.D. Interannual and between species comparison of the lipids, fatty acids and sterols of Antarctic krill from the US AMLR Elephant Island survey area. Comp. Biochem. Physiol. Part B 2002, 131, 733–747. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef]
- Gang, K.-Q.; Wu, Z.-X.; Zhou, D.-Y.; Zhao, Q.; Zhou, X.; Lv, D.-D.; Rakariyatham, K.; Liu, X.-Y.; Shahidi, F. Effects of hot air drying process on lipid quality of whelks Neptunea arthritica cumingi Crosse and Neverita didyma. J. Food Sci. Technol. 2019, 56, 4166–4176. [Google Scholar] [CrossRef]
- Bottino, N.R. Lipid composition of two species of antarctic krill: Euphausia superba and E. crystallorophias. Comp. Biochem. Physiol. Part B 1975, 50, 479–484. [Google Scholar] [CrossRef]
- Han, X.; Liu, D. Detection and analysis of 17 steroid hormones by ultra-high-performance liquid chromatography-electrospray ionization mass spectrometry (UHPLC-MS) in different sex and maturity stages of Antarctic krill (Euphausia superba Dana). PLoS ONE 2019, 14, e0213398. [Google Scholar] [CrossRef]
- Xie, D.; Jin, J.; Sun, J.; Liang, L.; Wang, X.; Zhang, W.; Wang, X.; Jin, Q. Comparison of solvents for extraction of krill oil from krill meal: Lipid yield, phospholipids content, fatty acids composition and minor components. Food Chem. 2017, 233, 434–441. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, B.H.; Kim, I.-H.; Lee, M.-W. Modeling and optimization of phospholipase A1-catalyzed hydrolysis of phosphatidylcholine using response surface methodology for lysophosphatidylcholine production. Biotechnol. Prog. 2014, 31, 35–41. [Google Scholar] [CrossRef]
- Burri, L.; Johnsen, L. Krill Products: An Overview of Animal Studies. Nutrients 2015, 7, 3300–3321. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A. The biochemical composition of krill, Euphausia superba Dana, from South Georgia. J. Exp. Mar. Biol. Ecol. 1980, 43, 221–236. [Google Scholar] [CrossRef]
- WHO, Food and Agriculture Organization of the United Nations. Codex Standard for Fish Oils. Codex Alimentarius Commission; Codex Standard 329-2017; WHO: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Quebec, QC, Canada, 2017. [Google Scholar]
- Paluchova, V.; Vik, A.; Cajka, T.; Brezinova, M.; Brejchova, K.; Bugajev, V.; Draberova, L.; Draber, P.; Buresova, J.; Kroupova, P.; et al. Triacylglycerol-Rich Oils of Marine Origin are Optimal Nutrients for Induction of Polyunsaturated Docosahexaenoic Acid Ester of Hydroxy Linoleic Acid (13-DHAHLA) with Anti-Inflammatory Properties in Mice. Mol. Nutr. Food Res. 2020, 64, e1901238. [Google Scholar] [CrossRef] [PubMed]
- Kołakowska, A. The influence of sex and maturity stage of krill (Euphausia superba Dana) upon the content and composition of its lipid. Pol. Polar Res. 1991, 12, 73–78. [Google Scholar]
- Li, D.-M.; Zhou, D.-Y.; Zhu, B.-W.; Chi, Y.-L.; Sun, L.-M.; Dong, X.-P.; Qin, L.; Qiao, W.-Z.; Murata, Y. Effects of krill oil intake on plasma cholesterol and glucose levels in rats fed a high-cholesterol diet. J. Sci. Food Agric. 2013, 93, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar]
- Skubic, C.; Vovk, I.; Rozman, D.; Križman, M. Simplified LC-MS Method for Analysis of Sterols in Biological Samples. Molecules 2020, 25, 4116. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; A Napier, J. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.W.; Huang, P.C. Effects of the ratio of polyunsaturated and monounsaturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentrations. Lipids 1998, 33, 481–487. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and Distribution of Omega-3 Fatty Acids in South Pacific Fish and Shellfish Species. Foods 2020, 9, 233. [Google Scholar] [CrossRef]
- Murru, E.; Banni, S.; Carta, G. Nutritional Properties of Dietary Omega-3-Enriched Phospholipids. BioMed Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; Von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—A comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef]
- Šimat, V.; ElAbed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S.; Matsui, K.; Nakamura, M.; Muramatsu, M.; Hanada, S. Fatty acids of astaxanthin esters in krill determined by mild mass spectrometry. Comp. Biochem. Physiol. Part B 2003, 136, 317–322. [Google Scholar] [CrossRef]
- Sun, W.; Shi, B.; Xue, C.; Jiang, X. The comparison of krill oil extracted through ethanol–hexane method and subcritical method. Food Sci. Nutr. 2019, 7, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Grynbaum, M.D.; Hentschel, P.; Putzbach, K.; Rehbein, J.; Krucker, M.; Nicholson, G.; Albert, K. Unambiguous detection of astaxanthin and astaxanthin fatty acid esters in krill (Euphausia superba Dana). J. Sep. Sci. 2005, 28, 1685–1693. [Google Scholar] [CrossRef]
- Alessandri, J.M.; Goustard, B.; Guesnet, P.; Durand, G. Docosahexaenoic acid concentrations in retinal phospholipids of piglets fed an infant formula enriched with long-chain polyunsaturated fatty acids: Effects of egg phospholipids and fish oils with different ratios of eicosapentaenoic acid to docosahexaenoic acid. Am. J. Clin. Nutr. 1998, 67, 377–385. [Google Scholar] [CrossRef][Green Version]
- Araujo, P.; Zhu, H.; Breivik, J.F.; Hjelle, J.I.; Zeng, Y. Determination and Structural Elucidation of Triacylglycerols in Krill Oil by Chromatographic Techniques. Lipids 2013, 49, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Souchet, N.; Laplante, S. Seasonal and geographical variations of sterol composition in snow crab hepatopancreas and pelagic fish viscera from Eastern Quebec. Comp. Biochem. Physiol. Part B 2007, 147, 378–386. [Google Scholar] [CrossRef]
- Bruheim, I.; Cameron, J. Flowable Concentrated Phospholipid Krill Oil Composition. U.S. Patent 20170020928 Al, 26 January 2017. [Google Scholar]
- Dunlap, W.C.; Fujisawa, A.; Yamamoto, Y.; Moylan, T.J.; Sidell, B.D. Notothenioid fish, krill and phytoplankton from Antarctica contain a vitamin E constituent (α-tocomonoenol) functionally associated with cold-water adaptation. Comp. Biochem. Physiol. Part B 2002, 133, 299–305. [Google Scholar] [CrossRef]
- Sampalis, F.; Bunea, R.; Pelland, M.F.; Kowalski, O.; Duguet, N.; Dupuis, S. Evaluation of the effects of Neptune Krill Oil on the management of premenstrual syndrome and dysmenorrhea. Altern. Med. Rev. 2003, 8, 171–179. [Google Scholar] [PubMed]
- Peng, Y.; Ji, W.; Zhang, D.; Ji, H.; Liu, S. Composition and content analysis of fluoride in inorganic salts of the integument of Antarctic krill (Euphausia superba). Sci. Rep. 2019, 9, 7853. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.I.; Xia, Z. The retinoid X receptors and their ligands. Biochim. Biophys. Acta 2012, 1821, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the Roles of PPARgamma in Adipocytes via Dynamic Change of Transcription Complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.; Badr, M. Role of Peroxisome Proliferator-Activated Receptors in Inflammation Control. J. Biomed. Biotechnol. 2004, 2004, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 35–45. [Google Scholar] [CrossRef]
- Marechal, L.; Laviolette, M.; Rodrigue-Way, A.; Sow, B.; Brochu, M.; Caron, V.; Tremblay, A. The CD36-PPARgamma Pathway in Metabolic Disorders. Int. J. Mol. Sci. 2018, 19, 1529. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome Proliferator-Activated Receptor Targets for the Treatment of Metabolic Diseases. Mediat. Inflamm. 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef]
- Son, S.-E.; Kim, N.-J.; Im, D.-S. Development of Free Fatty Acid Receptor 4 (FFA4/GPR120) Agonists in Health Science. Biomol. Ther. 2021, 29, 22–30. [Google Scholar] [CrossRef]
- Si, T.-L.; Liu, Q.; Ren, Y.-F.; Li, H.; Xu, X.-Y.; Li, E.-H.; Pan, S.-Y.; Zhang, J.-L.; Wang, K.-X. Enhanced anti-inflammatory effects of DHA and quercetin in lipopolysaccharide-induced RAW264.7 macrophages by inhibiting NF-κB and MAPK activation. Mol. Med. Rep. 2016, 14, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, M.M.D.; Warner, G.M.; Cheng, J.; Gray, C.E.; Nath, K.A.; Grande, J.P. n-3 Fatty acids block TNF-α-stimulated MCP-1 expression in rat mesangial cells. Am. J. Physiol. Physiol. 2011, 300, F1142–F1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, F. N-3 Polyunsaturated Fatty Acids and Inflammation in Obesity: Local Effect and Systemic Benefit. BioMed Res. Int. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARgamma as a molecular target of EPA anti-inflammatory activity during TNF-alpha-impaired skeletal muscle cell differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef]
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- Tiedge, M.; Lortz, S.; Munday, R.; Lenzen, S. Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes 1998, 47, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tao, J.; Xie, X. Astaxanthin Promotes Nrf2/ARE Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs. Mar. Drugs 2018, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Solinas, G.; Becattini, B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Y.; Guo, N.; Huang, P.; Mi, Y. Effects of Astaxanthin on Inflammation and Insulin Resistance in a Mouse Model of Gestational Diabetes Mellitus. Dose-Response 2020, 18, 1559325820926765. [Google Scholar] [CrossRef]
- Katevas, D.S.; Toro Guerra, R.R.; Chiong Lay, M. Solvent-Free Process for Obtaining Phospholipids and Neutral Enriched Krill Oils. U.S. Patent 8865236 B2, 6 March 2014. [Google Scholar]
- Beaudoin, A.; Martin, G. Method of Extracting Lipids from Marine and Aquatic Animal Tissues. U.S. Patent 6800299 B1, 5 October 2004. [Google Scholar]
- Yoshitomi, B.; Shigematsu, Y. Process for Making Dried Powdery and Granular Krill. U.S. Patent 20030113432 A1, 16 June 2003. [Google Scholar]
- Xie, D.; Mu, H.; Tang, T.; Wang, X.; Wei, W.; Jin, J.; Wang, X.; Jin, Q. Production of three types of krill oils from krill meal by a three-step solvent extraction procedure. Food Chem. 2018, 248, 279–286. [Google Scholar] [CrossRef]
- Ronen, L.; Ran, N.; Ben-Dror, G. Krill Oil Preparations with Optimal Mineral and Metal Composition, Low Impurities and Low and Stable TMA Levels. US Patent 20170354694 Al, 14 December 2017. [Google Scholar]
- Bruheim, I.; Griinari, M.; Ervik, J.R.; Remoy, S.R.; Remoy, E.; Cameron, J. Method for Processing Crustaceans to Produce Low Fluoride/Low Trimethyl Amine Products Thereof. U.S. Patent 20160345616 Al, 2 June 2016. [Google Scholar]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Yin, F.-W.; Liu, X.-Y.; Fan, X.-R.; Zhou, D.-Y.; Xu, W.-S.; Zhu, B.-W.; Murata, Y.-Y. Extrusion of Antarctic krill (Euphausia superba) meal and its effect on oil extraction. Int. J. Food Sci. Technol. 2014, 50, 633–639. [Google Scholar] [CrossRef]
- Khan, L.M.; Hanna, M.A. Expression of oil from oilseeds—A review. J. Agric. Eng. Res. 1983, 28, 495–503. [Google Scholar] [CrossRef]
- Larsen, P.M.; Fey, S.J.; Breuning, J.; Ludvigsen, B. A Method for the Extraction of Lipid Fractions from Krill. WO Patent 2007080514 A2, 15 January 2007. [Google Scholar]
- Tilseth, S.; Høstmark, Ø. New Method for Making Krill Meal. U.S. Patent 20150050403 A1, 16 January 2015. [Google Scholar]
- Friedrich, J.P.; Pryde, E.H. Supercritical CO2 extraction of lipid-bearing materials and characterization of the products. J. Am. Oil Chem. Soc. 1984, 61, 223–228. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Miki, W.; Toriu, N.; Kondo, Y.; Murakami, M.; Konosu, S. The composition of carotenoid pigments in the Antarctic krill (Euphausia superba). Nippon Suisan Gakkaishi 1983, 49, 1411–1415. [Google Scholar] [CrossRef]
- Bruheim, I.; Tilseth, S.; Mancinelli, D. Bioeffective Krill Oil Compositions. U.S. Patent 9889163 B2, 13 February 2018. [Google Scholar]
- Liu, H.-M.; Wang, F.-Y.; Li, H.-Y.; Wang, X.-D.; Qin, G.-Y. Subcritical Butane and Propane Extraction of Oil from Rice Bran. Bioresources 2015, 10, 4652–4662. [Google Scholar] [CrossRef]
- Sun, D.; Cao, C.; Li, B.; Chen, H.; Cao, P.; Li, J.; Liu, Y. Study on combined heat pump drying with freeze-drying of Antarctic krill and its effects on the lipids. J. Food Process. Eng. 2017, 40, e12577. [Google Scholar] [CrossRef]
- Domínguez, H.; Núñez, M.; Lema, J. Enzymatic pretreatment to enhance oil extraction from fruits and oilseeds: A review. Food Chem. 1994, 49, 271–286. [Google Scholar] [CrossRef]
- Lee, S. Krill Oil and Method for Manufacturing the Same. U.S. Patent 8624046 B2, 7 January 2014. [Google Scholar]
- Bunea, R.; El Farrah, K.; Deutsch, L. Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern. Med. Rev. 2004, 9, 420–428. [Google Scholar]
- Konagai, C.; Yanagimoto, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Koga, Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clin. Interv. Aging 2013, 8, 1247–1257. [Google Scholar] [CrossRef]
- Laidlaw, M.; Cockerline, C.A.; Rowe, W.J. A randomized clinical trial to determine the efficacy of manufacturers’ recommended doses of omega-3 fatty acids from different sources in facilitating cardiovascular disease risk reduction. Lipids Health Dis. 2014, 13, 99. [Google Scholar] [CrossRef]
- Salem, N.; Kuratko, C.N. A reexamination of krill oil bioavailability studies. Lipids Health Dis. 2014, 13, 1–6. [Google Scholar] [CrossRef]
- Köhler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects–a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Harris, W.S.; Miller, M.; Tighe, A.P.; Davidson, M.H.; Schaefer, E.J. Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis 2008, 197, 12–24. [Google Scholar] [CrossRef]
- Cleland, L.G.; James, M.J.; Proudman, S.M. The Role of Fish Oils in the Treatment of Rheumatoid Arthritis. Drugs 2003, 63, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Boudrault, C.; Bazinet, R.P.; Ma, D.W. Experimental models and mechanisms underlying the protective effects of n-3 polyun-saturated fatty acids in Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 1–10. [Google Scholar] [CrossRef]
- Fedor, D.; Kelley, D.S. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.S.; Wat, E.; Kamili, A.; Tandy, S. Dietary phospholipids, hepatic lipid metabolism and cardiovascular disease. Curr. Opin. Lipidol. 2008, 19, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Nakagawa, T.; Goto, H.; Shimada, Y.; Matsumoto, K.; Sankawa, U.; Watanabe, H. Astaxanthin ameliorates features of metabolic syndrome in SHR/NDmcr-cp. Life Sci. 2007, 80, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Griinari, M.; Berge, K.; Vik, H.; Hubacher, R.; Rains, T.M. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr. Res. 2009, 29, 609–615. [Google Scholar] [CrossRef]
- Sung, H.H.; Sinclair, A.J.; Huynh, K.; Smith, A.A.T.; Mellett, N.A.; Meikle, P.J.; Su, X.Q. Krill Oil Has Different Effects on the Plasma Lipidome Compared with Fish Oil Following 30 Days of Supplementation in Healthy Women: A Randomized Con-trolled and Crossover Study. Nutrients 2020, 12, 2804. [Google Scholar] [CrossRef]
- Sung, H.H.; Sinclair, A.J.; Huynh, K.; Smith, A.T.; Mellett, N.A.; Meikle, P.J.; Su, X.Q. Differential plasma postprandial lipidomic responses to krill oil and fish oil supplementations in women: A randomized crossover study. Nutrients 2019, 65, 191–201. [Google Scholar] [CrossRef]
- Robertson, B.; Burri, L.; Berge, K. Genotoxicity test and subchronic toxicity study with Superba™ krill oil in rats. Toxicol. Rep. 2014, 1, 764–776. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Shi, J.-H.; Qian, W.-B.; Cai, Z.-Z.; Li, D. Effects of Krill Oil on serum lipids of hyperlipidemic rats and human SW480 cells. Lipids Health Dis. 2008, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Tandy, S.; Chung, R.W.S.; Wat, E.; Kamili, A.; Berge, K.; Griinari, M.; Cohn, J.S. Dietary Krill Oil Supplementation Reduces Hepatic Steatosis, Glycemia, and Hypercholesterolemia in High-Fat-Fed Mice. J. Agric. Food Chem. 2009, 57, 9339–9345. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, B.; Vik, R.; Brattelid, T.; Vigerust, N.F.; Burri, L.; Bohov, P.; Nygård, O.; Skorve, J.; Berge, R.K. Krill powder increases liver lipid catabolism and reduces glucose mobilization in tumor necrosis factor-alpha transgenic mice fed a high-fat diet. Metabolism 2012, 61, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Ishida, N.; Yamada, H.; Ibi, M.; Hirose, M. 8-HEPE-Concentrated Materials from Pacific Krill Improve Plasma Cho-lesterol Levels and Hepatic Steatosis in High Cholesterol Diet-Fed Low-Density Lipoprotein (LDL) Receptor-Deficient Mice. Biol. Pharm. Bull. 2020, 43, 919–924. [Google Scholar] [CrossRef]
- Hals, P.-A.; Wang, X.; Xiao, Y.-F. Effects of a purified krill oil phospholipid rich in long-chain omega-3 fatty acids on cardiovascular disease risk factors in non-human primates with naturally occurring diabetes type-2 and dyslipidemia. Lipids Health Dis. 2017, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Conte, A.; Burri, L.; Berge, K.; De Nuccio, F.; Giudetti, A.M.; Zara, V. A Krill Oil Supplemented Diet Suppresses Hepatic Steatosis in High-Fat Fed Rats. PLoS ONE 2012, 7, e38797. [Google Scholar] [CrossRef]
- Ivanova, Z.; Bjørndal, B.; Grigorova, N.; Roussenov, A.; Vachkova, E.; Berge, K.; Burri, L.; Berge, R.; Stanilova, S.; Milanova, A.; et al. Effect of fish and krill oil supplementation on glucose tolerance in rabbits with experimentally induced obesity. Eur. J. Nutr. 2015, 54, 1055–1067. [Google Scholar] [CrossRef]
- Rundblad, A.; Holven, K.B.; Bruheim, I.; Myhrstad, M.C.; Ulven, S.M. Effects of krill oil and lean and fatty fish on cardiovascular risk markers: A randomised controlled trial. J. Nutr. Sci. 2018, 7, e3. [Google Scholar] [CrossRef]
- Berge, K.; Musa-Veloso, K.; Harwood, M.; Hoem, N.; Burri, L. Krill oil supplementation lowers serum triglycerides without in-creasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Nutr. Res. 2014, 34, 126–133. [Google Scholar] [CrossRef]
- Cicero, A.F.; Rosticci, M.; Morbini, M.; Cagnati, M.; Grandi, E.; Parini, A.; Borghi, C. Lipid-lowering and anti-inflammatory effects of omega 3 ethyl esters and krill oil: A randomized, cross-over, clinical trial. Arch. Med. Sci. 2016, 3, 507–512. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, L.; Chen, H.; Feng, R.; Cao, P.; Liu, Y. Effects of Antarctic krill oil on lipid and glucose metabolism in C57BL/6J mice fed with high fat diet. Lipids Health Dis. 2017, 16, 218. [Google Scholar] [CrossRef]
- Sistilli, G.; Kalendova, V.; Cajka, T.; Irodenko, I.; Bardova, K.; Oseeva, M.; Zacek, P.; Kroupova, P.; Horakova, O.; Lackner, K.; et al. Krill Oil Supplementation Reduces Exacerbated Hepatic Steatosis Induced by Thermoneutral Housing in Mice with Diet-Induced Obesity. Nutrients 2021, 13, 437. [Google Scholar] [CrossRef] [PubMed]
- Kroupova, P.; Van Schothorst, E.M.; Keijer, J.; Bunschoten, A.; Vodicka, M.; Irodenko, I.; Oseeva, M.; Zacek, P.; Kopecky, J.; Rossmeisl, M.; et al. Omega-3 Phospholipids from Krill Oil Enhance Intestinal Fatty Acid Oxidation More Effectively than Omega-3 Triacylglycerols in High-Fat Diet-Fed Obese Mice. Nutrients 2020, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, M.; Pavlisova, J.; Bardova, K.; Kalendova, V.; Buresova, J.; Kuda, O.; Kroupova, P.; Stankova, B.; Tvrzicka, E.; Fiserova, E.; et al. Increased plasma levels of palmitoleic acid may contribute to beneficial effects of Krill oil on glucose homeostasis in dietary obese mice. Biochim. Biophys. Acta 2020, 1865, 158732. [Google Scholar] [CrossRef] [PubMed]
- Fosshaug, L.E.; Berge, R.K.; Beitnes, J.O.; Berge, K.; Vik, H.; Aukrust, P.; Gullestad, L.; Vinge, L.E.; Oie, E. Krill oil attenuates left ventricular dilatation after myocardial infarction in rats. Lipids Health Dis. 2011, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Lobraico, J.M.; DiLello, L.C.; Butler, A.D.; Cordisco, M.E.; Petrini, J.R.; Ahmadi, R. Effects of krill oil on endothelial function and other cardiovascular risk factors in participants with type 2 diabetes, a randomized controlled trial. BMJ Open Diabetes Res. Care 2015, 3, e000107. [Google Scholar] [CrossRef]
- Di Marzo, V.; Silvestri, C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Carta, G.; Bisogno, T.; Murru, E.; Cordeddu, L.; Berge, K.; Tandy, S.; Cohn, J.S.; Griinari, M.; Banni, S.; et al. Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutr. Metab. 2011, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Berge, K.; Piscitelli, F.; Hoem, N.; Silvestri, C.; Meyer, I.; Banni, S.; Di Marzo, V. Chronic treatment with krill powder reduces plasma triglyceride and anandamide levels in mildly obese men. Lipids Health Dis. 2013, 12, 78. [Google Scholar] [CrossRef]
- Banni, S.; Carta, G.; Murru, E.; Cordeddu, L.; Giordano, E.; Sirigu, A.R.; Berge, K.; Vik, H.; Maki, K.C.; Di Marzo, V.; et al. Krill oil significantly decreases 2-arachidonoylglycerol plasma levels in obese subjects. Nutr. Metab. 2011, 8, 7. [Google Scholar] [CrossRef]
- Cui, C.; Li, Y.; Gao, H.; Zhang, H.; Han, J.; Zhang, D.; Li, Y.; Zhou, J.; Lu, C.; Su, X. Modulation of the gut microbiota by the mixture of fish oil and krill oil in high-fat diet-induced obesity mice. PLoS ONE 2017, 12, e0186216. [Google Scholar] [CrossRef]
- Lu, C.; Sun, T.; Li, Y.; Zhang, D.; Zhou, J.; Su, X. Modulation of the Gut Microbiota by Krill Oil in Mice Fed a High-Sugar High-Fat Diet. Front. Microbiol. 2017, 8, 905. [Google Scholar] [CrossRef]
- Jiang, Q.; Lu, C.; Sun, T.; Zhou, J.; Li, Y.; Ming, T.; Bai, L.; Wang, Z.J.; Su, X. Alterations of the Brain Proteome and Gut Microbiota in d-Galactose-Induced Brain-Aging Mice with Krill Oil Supplementation. J. Agric. Food Chem. 2019, 67, 9820–9830. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Cesi, V.; Palone, F.; Pierdomenico, M.; Colantoni, E.; Leter, B.; Vitali, R.; Negroni, A.; Cucchiara, S.; Stronati, L. Krill oil, vitamin D and Lactobacillus reuteri cooperate to reduce gut inflammation. Benef. Microbes 2018, 9, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, T.; Bjørndal, B.; Cacabelos, D.; Aasprong, O.G.; Janssen, E.A.; Omdal, R.; Svardal, A.; Hausken, T.; Bohov, P.; Portero-Otin, M.; et al. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scand. J. Gastroenterol. 2011, 47, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Hong, S.-S.; Lee, M.; Lee, E.-H.; Rhee, I.; Chang, S.-Y.; Lim, S.-J. Krill Oil-Incorporated Liposomes as an Effective Nanovehicle to Ameliorate the Inflammatory Responses of DSS-Induced Colitis. Int. J. Nanomed. 2019, 14, 8305–8320. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Smith, A.D.; Solano-Aguilar, G.; Wang, T.T.Y.; Pham, Q.; Beshah, E.; Tang, Q.; Urban, J.F., Jr.; Xue, C.; Li, R.W. Mechanistic insights into the attenuation of intestinal inflammation and modulation of the gut microbiome by krill oil using in vitro and in vivo models. Microbiome 2020, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheu-matoid arthritis. BMC Musculoskelet. Disord. 2010, 11, 136. [Google Scholar] [CrossRef]
- Vigerust, N.F.; Bjørndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Berge, R.K. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-α. Eur. J. Nutr. 2013, 52, 1315–1325. [Google Scholar] [CrossRef]
- Tou, J.C.; Jaczynski, J.; Chen, Y.-C. Krill for Human Consumption: Nutritional Value and Potential Health Benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef]
- Deutsch, L. Evaluation of the Effect of Neptune Krill Oil on Chronic Inflammation and Arthritic Symptoms. J. Am. Coll. Nutr. 2007, 26, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Fukushima, M.; Sakuraba, K.; Sawaki, K.; Sekigawa, K. Krill Oil Improves Mild Knee Joint Pain: A Randomized Control Trial. PLoS ONE 2016, 11, e0162769. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Osawa, T. Astaxanthin Protects Neuronal Cells against Oxidative Damage and Is a Potent Candidate for Brain Food. Forum Nutr. 2009, 61, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Sun, X.-B.; Xu, Y.-X.; Zhao, H.; Zhu, Q.-Y.; Zhu, C.-Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010, 1360, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-P.; Liu, S.-Y.; Sun, H.; Wu, X.-M.; Li, J.-J.; Zhu, L. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.-J.; Han, J.-J.; Shim, I.; Her, S.; Lee, H.-J.; Hahm, D.-H. Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2010, 34, 1085–1093. [Google Scholar] [CrossRef]
- Barros, M.P.; Poppe, S.C.; Bondan, E.F. Neuroprotective Properties of the Marine Carotenoid Astaxanthin and Omega-3 Fatty Acids, and Perspectives for the Natural Combination of Both in Krill Oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jang, J.S.; Son, D.J.; Im, H.-S.; Kim, J.Y.; Park, J.E.; Choi, W.R.; Han, S.-B.; Hong, J.T. Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef]
- Li, Q.; Wu, F.; Wen, M.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. The Protective Effect of Antarctic Krill Oil on Cognitive Function by Inhibiting Oxidative Stress in the Brain of Senescence-Accelerated Prone Mouse Strain 8 (SAMP8) Mice. J. Food Sci. 2018, 83, 543–551. [Google Scholar] [CrossRef]
- Kim, J.H.; Meng, H.W.; He, M.T.; Choi, J.M.; Lee, D.; Cho, E.J. Krill Oil Attenuates Cognitive Impairment by the Regulation of Oxidative Stress and Neuronal Apoptosis in an Amyloid β-Induced Alzheimer’s Disease Mouse Model. Molecules 2020, 25, 3942. [Google Scholar] [CrossRef]
- Andraka, J.M.; Sharma, N.; Marchalant, Y. Can krill oil be of use for counteracting neuroinflammatory processes induced by high fat diet and aging? Neurosci. Res. 2020, 157, 1–14. [Google Scholar] [CrossRef]
- Wibrand, K.; Berge, K.; Messaoudi, M.; Duffaud, A.; Panja, D.; Bramham, C.R.; Burri, L. Enhanced cognitive function and antidepressant-like effects after krill oil supplementation in rats. Lipids Health Dis. 2013, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Zadeh-Ardabili, P.M.; Rad, S.K.; Rad, S.K.; Movafagh, A. Antidepressant-like effects of fish, krill oils and Vit B12 against exposure to stress environment in mice models: Current status and pilot study. Sci. Rep. 2019, 9, 19953. [Google Scholar] [CrossRef]
- Mendoza, C.; Perez-Urrutia, N.; Alvarez-Ricartes, N.; Barreto, G.E.; Pérez-Ordás, R.; Iarkov, A.; Echeverria, V. Cotinine Plus Krill Oil Decreased Depressive Behavior, and Increased Astrocytes Survival in the Hippocampus of Mice Subjected to Restraint Stress. Front. Neurosci. 2018, 12, 952. [Google Scholar] [CrossRef]
- Van der Wurff, I.S.; von Schacky, C.; Berge, K.; Kirschner, P.A.; de Groot, R.H. A protocol for a randomised controlled trial in-vestigating the effect of increasing Omega-3 index with krill oil supplementation on learning, cognition, behaviour and visual processing in typically developing adolescents. BMJ Open 2016, 6, e011790. [Google Scholar] [CrossRef] [PubMed]
- Van der Wurff, I.; von Schacky, C.; Bergeland, T.; Leontjevas, R.; Zeegers, M.; Kirschner, P.; de Groot, R. Effect of one year krill oil supplementation on depressive symptoms and self-esteem of Dutch adolescents: A randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2020, 163, 102208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, X.; Cao, W.; Yang, B.; Mu, Y.; Dong, Y.; Xiu, Z. E-configuration structures of EPA and DHA derived from Euphausia superba and their significant inhibitive effects on growth of human cancer cell lines in vitro. Prostaglandins Leukot. Essent. Fat. Acids 2017, 117, 47–53. [Google Scholar] [CrossRef]
- Xiao, S.U.; Tanalgo, P.; Bustos, M.; Dass, C.R. The Effect of Krill Oil and n-3 Polyunsaturated Fatty Acids on Human Osteosarcoma Cell Proliferation and Migration. Curr. Drug Targets 2018, 19, 479–486. [Google Scholar] [CrossRef]
- Jayathilake, A.G.; Senior, P.V.; Su, X.Q. Krill oil extract suppresses cell growth and induces apoptosis of human colorectal cancer cells. BMC Complement. Altern. Med. 2016, 16, 328. [Google Scholar] [CrossRef]
- Jayathilake, A.G.; Kadife, E.; Luwor, R.B.; Nurgali, K.; Su, X.Q. Krill oil extract suppresses the proliferation of colorectal cancer cells through activation of caspase 3/9. Nutr. Metab. 2019, 16, 53. [Google Scholar] [CrossRef]
- Jayathilake, A.G.; Veale, M.F.; Luwor, R.B.; Nurgali, K.; Su, X.Q. Krill oil extract inhibits the migration of human colorectal cancer cells and down-regulates EGFR signalling and PD-L1 expression. BMC Complement. Med. Ther. 2020, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Skarpańska-Stejnborn, A.; Pilaczyńska-Szcześniak, L.; Basta, P.; Foriasz, J.; Arlet, J. Effects of Supplementation with Neptune Krill Oil (Euphasia Superba) on Selected Redox Parameters and Pro-Inflammatory Markers in Athletes during Exhaustive Exercise. J. Hum. Kinet. 2010, 25, 49–57. [Google Scholar] [CrossRef]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The Effect of Krill Oil Supplementation on Exercise Performance and Markers of Immune Function. PLoS ONE 2015, 10, e0139174. [Google Scholar] [CrossRef] [PubMed]
- Georges, J.; Sharp, M.H.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; Hornberger, T.A.; Harding, F.; Johnson, J.H.; Peele, D.M.; Jäger, R. The Effects of Krill Oil on mTOR Signaling and Resistance[tnq_nbsp] Exercise: A Pilot Study. J. Nutr. Metab. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Barenie, M.J.; Freemas, J.A.; Baranauskas, M.N.; Goss, C.S.; Freeman, K.L.; Chen, X.; Dickinson, S.L.; Fly, A.D.; Kawata, K.; Chapman, R.F.; et al. Effectiveness of a combined New Zealand green-lipped mussel and Antarctic krill oil supplement on markers of exercise-induced muscle damage and inflammation in untrained men. J. Diet Suppl. 2020, 1–26. [Google Scholar] [CrossRef]
- Storsve, A.B.; Johnsen, L.; Nyborg, C.; Melau, J.; Hisdal, J.; Burri, L. Effects of Krill Oil and Race Distance on Serum Choline and Choline Metabolites in Triathletes: A Field Study. Front. Nutr. 2020, 7, 133. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Malhi, H.; Gores, G.J. Animal Models of Nonalcoholic Steatohepatitis: Eat, Delete, and Inflame. Dig. Dis. Sci. 2015, 61, 1325–1336. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Morbini, M.; Borghi, C. Do we need ’new’ omega-3 polyunsaturated fatty acids formulations? Expert Opin. Pharmacother. 2015, 16, 285–288. [Google Scholar] [CrossRef]
- Kwantes, J.M.; Grundmann, O. A Brief Review of Krill Oil History, Research, and the Commercial Market. J. Diet. Suppl. 2014, 12, 23–35. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Rong, Y.; Yuan, Y.; Ding, Y.; Shi, W.; Wang, Z. Effects of different proteases enzymatic extraction on the lipid yield and quality of Antarctic krill oil. Food Sci. Nutr. 2019, 7, 2224–2230. [Google Scholar] [CrossRef]
- Alvarez-Ricartes, N.; Oliveros-Matus, P.; Mendoza, C.; Perez-Urrutia, N.; Echeverria, F.; Iarkov, A.; Barreto, G.E.; Echeverria, V. Correction to: Intranasal Cotinine Plus Krill Oil Facilitates Fear Extinction, Decreases Depressive-Like Behavior, and Increases Hippocampal Calcineurin a Levels in Mice. Mol. Neurobiol. 2018, 55, 7961. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wurff, I.S.; Von Schacky, C.; Bergeland, T.; Leontjevas, R.; Zeegers, M.P.; Jolles, J.; Kirschner, P.A.; De Groot, R.H. Effect of 1 Year Krill Oil Supplementation on Cognitive Achievement of Dutch Adolescents: A Double-Blind Randomized Controlled Trial. Nutrients 2019, 11, 1230. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).