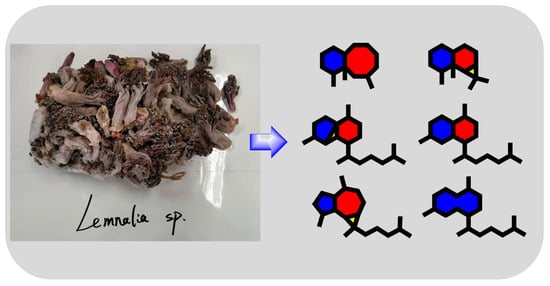
Antimicrobial Terpenoids from South China Sea Soft Coral Lemnalia sp.
Abstract
:1. Introduction
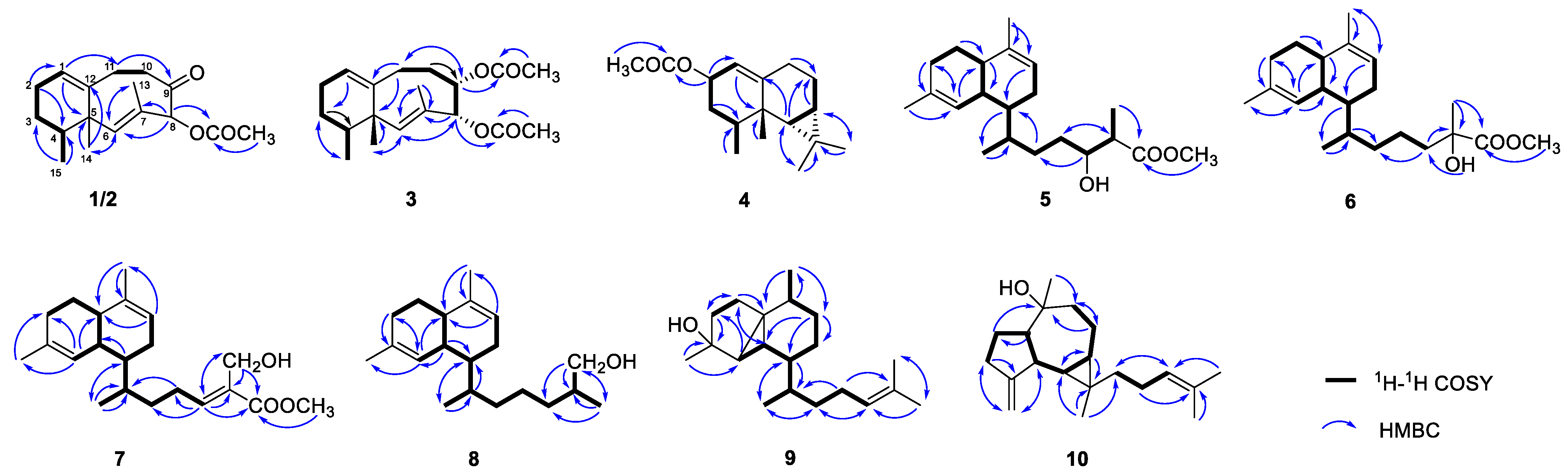
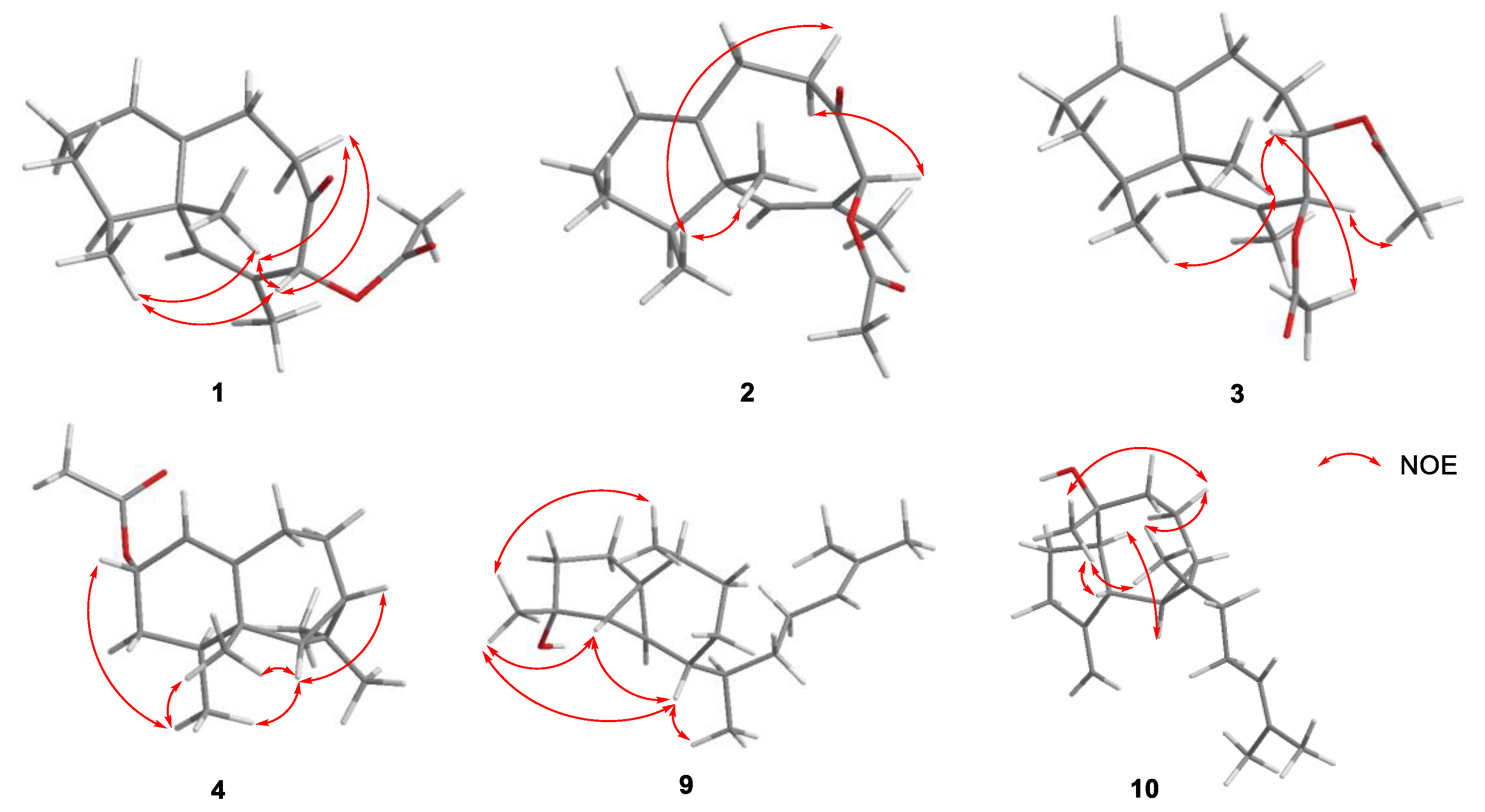
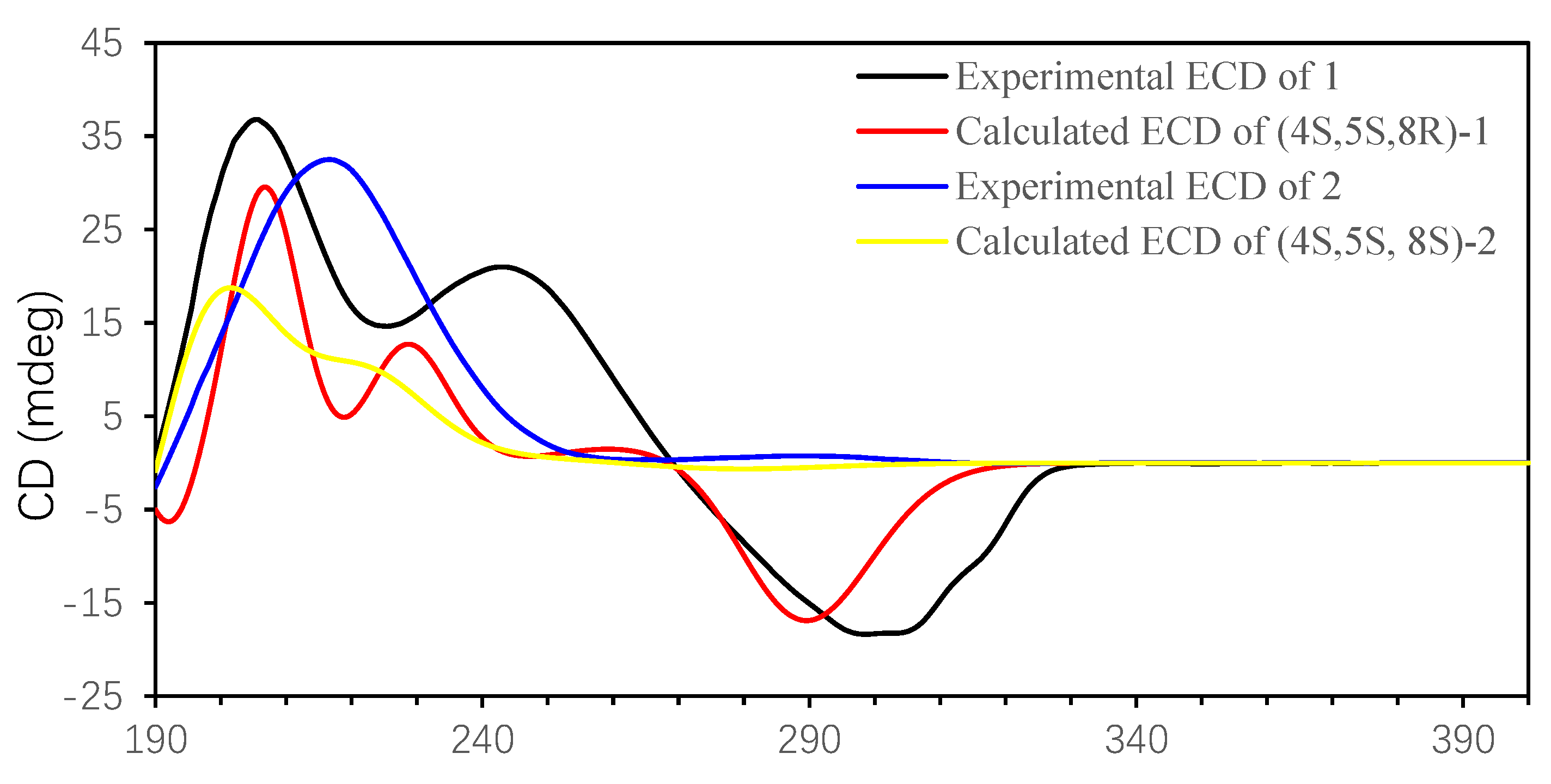
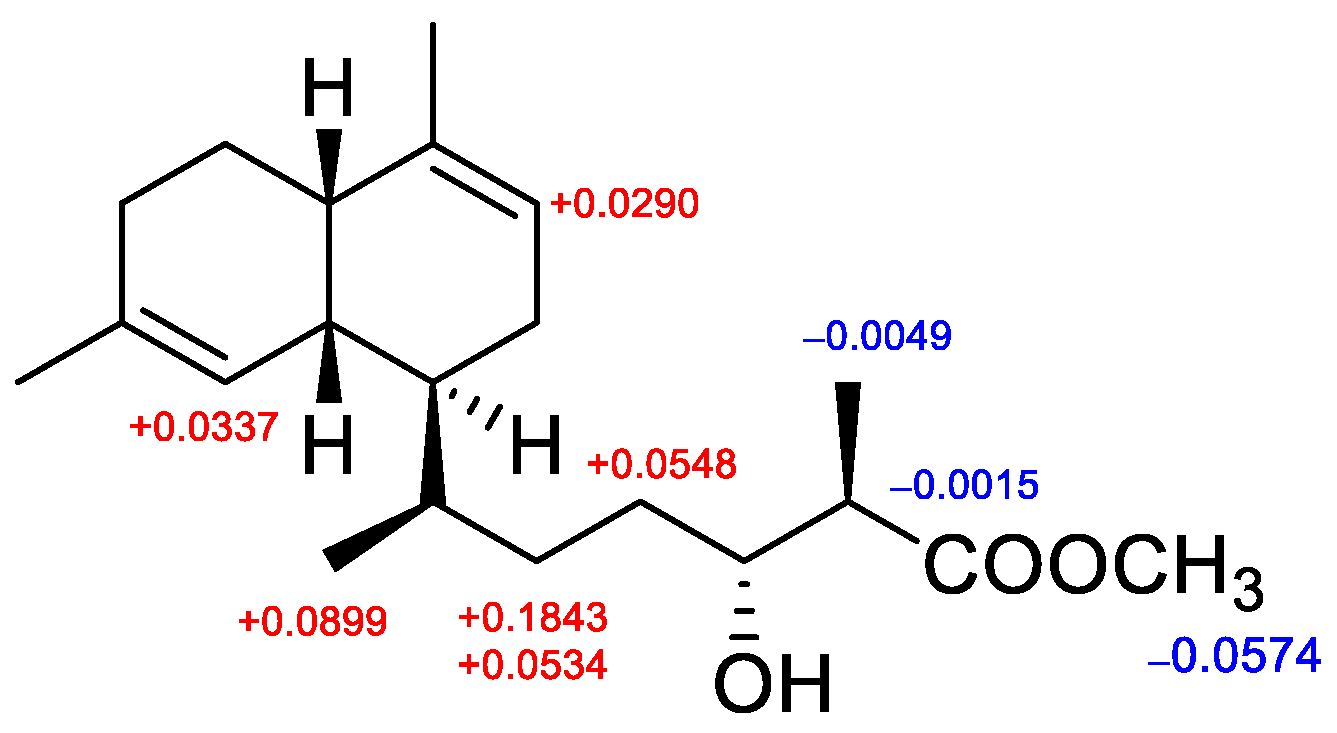
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Computational Methods
3.5. Cytotoxic Activity Assay
3.6. Antibacterial Assays
3.7. Antiviral Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef]
- Gong, K.-K.; Tang, X.-L.; Zhang, G.; Cheng, C.-L.; Zhang, X.-W.; Li, P.-L.; Li, G.-Q. Polyhydroxylated steroids from the South China Sea soft coral Sarcophyton sp. and their cytotoxic and antiviral activities. Mar. Drugs 2013, 11, 4788–4798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.-F.; Chen, W.-T.; Mollo, E.; Yao, L.-G.; Wang, H.-Y.; Xiao, W.; Guo, Y.-W. Sarcophytrols G–L, novel minor metabolic components from South China Sea soft coral Sarcophyton trocheliophorum Marenzeller. Chem. Biodivers. 2017, 14, 1700079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, X.; Liu, H.; Luo, X.; Sung, P.J.; Li, P.; Li, G. Clavukoellians G–K, new nardosinane and aristolane sesquiterpenoids with angiogenesis promoting activity from the marine soft coral Lemnalia sp. Mar. Drugs 2020, 18, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.-J.; Wang, H.; Jiang, C.-S.; Guo, Y.-W. New cembrane-type diterpenoids from the South China Sea soft coral Sinularia crassa and their α-glucosidase inhibitory activity. Bioorganic Chem. 2020, 104, 104281. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, J.; Chen, J.; Zhang, H.; Guo, Y.-W.; Wang, H. Terpenoids from marine soft coral of the genus Lemnalia: Chemistry and biological activities. Mar. Drugs 2018, 16, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Ye, F.; Li, X.-L.; Liang, L.-F.; Sun, J.; Sun, H.; Guo, Y.-W.; Wang, H. Uncommon polyoxygenated sesquiterpenoids from South China Sea soft coral Lemnalia flava. J. Org. Chem. 2019, 84, 3083–3092. [Google Scholar] [CrossRef]
- Izac, R.R.; Fenical, W.; Tagle, B.; Clardy, J. Neolemnane and eremophilane sesquiterpenoids from the pacific soft coral lemnalia africana. Tetrahedron 1981, 37, 2569–2573. [Google Scholar] [CrossRef]
- Yang, F.; Li, S.-W.; Zhang, J.; Liang, L.-F.; Lu, Y.-H.; Guo, Y.-W. Uncommon nornardosinane, seconeolemnane and related sesquiterpenoids from Xisha soft coral Litophyton nigrum. Bioorganic Chem. 2020, 96, 103636. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, A.A.H.; Chiu, E.P.; Li, C.-H.; Cheng, S.-Y.; Dai, C.-F.; Duh, C.-Y. Sesquiterpenoids and norsesquiterpenoids from the formosan soft coral Lemnalia laevis. J. Nat. Prod. 2005, 68, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Long, K.; Wu, H.; Ma, K. A novel diterpene glycoside from the soft coral of Lemnalia bournei. J. Nat. Prod. 1994, 57, 155–160. [Google Scholar] [CrossRef]
- Cao, F.; Shao, C.-L.; Liu, Y.-F.; Zhu, H.-J.; Wang, C.-Y. Cytotoxic serrulatane-type diterpenoids from the gorgonian Euplexaura sp. and their absolute configurations by vibrational circular dichroism. Sci. Rep. 2017, 7, 12548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjaneyulu, A.S.R.; Krishnamurthy, M.V.R.; Rao, G.V. Rare aromadendrane diterpenoids from a new soft coral species of Sinularia genus of the Indian Ocean. Tetrahedron 1997, 53, 9301–9312. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Wu, M.; Li, X.; Li, W.; Ding, W.; She, Z.; Li, C. New antimicrobial cyclopentenones from Nigrospora sphaerica ZMT05, a fungus derived from Oxya chinensis Thunber. J. Agric. Food Chem. 2018, 66, 5368–5372. [Google Scholar] [CrossRef] [PubMed]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, H.; Zhang, Q.; Yang, M.; Gu, Y.-C.; Liang, L.-F.; Tang, W.; Guo, Y.-W. Rare cembranoids from Chinese soft coral Sarcophyton ehrenbergi: Structural and stereochemical studies. J. Org. Chem. 2019, 84, 5091–5098. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Richter, M.; von Grafenstein, S.; Walther, E.; Xu, Z.; Schumann, L.; Grienke, U.; Mair, C.E.; Kramer, C.; Rollinger, J.M.; et al. Discovery and characterization of diazenylaryl sulfonic acids as inhibitors of viral and bacterial neuraminidases. Front. Microbiol. 2017, 8, 205. [Google Scholar] [CrossRef] [PubMed]


| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Position | δH, Mult. | δC, Type | δH, Mult. | δC, Type | δH, Mult. | δC, Type | δH, Mult. | δC, Type |
| 1 | 5.43, m | 123.4, CH | 5.49, m | 125.1, CH | 5.46, m | 123.9, CH | 5.17, br.s | 118.1, CH |
| 2a | 2.07, m | 26.1, CH2 | 2.09, m | 26.0, CH2 | 2.06, m | 26.0, CH2 | 5.35, m | 68.9, CH |
| 2b | 2.01, m | — | 2.09, m | — | 2.06, m | — | — | — |
| 3a | 1.49, m | 26.6, CH2 | 1.51, m | 26.1, CH2 | 1.48, m | 26.7, CH2 | 1.93, m | 42.6, CH2 |
| 3b | 1.37, m | — | 1.51, m | — | 1.41, m | — | 1.57, m | — |
| 4 | 2.26, ov | 36.0, CH | 2.41, m | 33.2, CH | 2.28, m | 35.3, CH | 1.95, m | 37.9, CH |
| 5 | — | 46.2, C | — | 45.3, C | — | 43.6, C | — | 51.7, C |
| 6 | 5.82, s | 138.6, CH | 5.22, s | 147.5, CH | 5.33, s | 132.4, CH | 0.85, d (6.5) | 38.8, CH |
| 7 | — | 127.8, C | — | 128.0, C | — | 137.4, C | 1.22, br.t (6.3) | 32.4, CH |
| 8a | 5.66, s | 85.5, CH | 5.16, s | 84.8, CH | 5.67, d (6.9) | 73.7, CH | 2.01, m | 26.8, CH2 |
| 8b | — | — | — | — | — | — | 1.74, m | — |
| 9a | — | 201.2, C | — | 203.2, C | 4.86, dd (9.9, 7.3) | 79.5, CH | 1.90, m | 33.0, CH2 |
| 9b | — | — | — | — | — | — | 1.59, m | — |
| 10a | 2.75, td (12.6, 3.8) | 40.8, CH2 | 2.38, m | 42.9, CH2 | 1.74, m | 32.2, CH2 | — | 145.9, C |
| 10b | 2.16, m | — | 2.08, m | — | 1.57, dd (15.1, 9.3) | — | — | — |
| 11a | 2.59, m | 32.6, CH2 | 2.33, m | 34.3, CH2 | 2.22, dd (15.2, 9.4) | 34.6, CH2 | — | 20.3, C |
| 11b | 2.30, ov | — | 2.32, m | — | 2.05, m | — | — | — |
| 12 | — | 140.4, C | — | 142.4, C | — | 143.8, C | 1.00, s | 28.8, CH3 |
| 13 | 1.51, s | 13.0, CH3 | 2.01, s | 21.2, CH3 | 1.72, s | 15.5, CH3 | 1.14, s | 17.1, CH3 |
| 14 | 1.13, s | 21.4, CH3 | 1.03, s | 21.0, CH3 | 1.05, s | 22.1, CH3 | 1.76, s | 20.4, CH3 |
| 15 | 0.99, d (6.9) | 17.8, CH3 | 0.95, d (6.9) | 17.5, CH3 | 0.91, d (6.9) | 17.6, CH3 | 1.07, d (7.1) | 17.0, CH3 |
| 7-OAc | — | — | — | — | — | — | — | 171.1, C |
| — | — | — | — | — | — | 2.04, s | 21.5, CH3 | |
| 8-OAc | — | 170.1, C | — | 170.6, C | — | 170.1, C | — | — |
| 2.18, s | 20.8, CH3 | 2.23, s | 20.9, CH3 | 2.01, s | 20.9, CH3 | — | — | |
| 9-OAc | — | — | — | — | — | 169.8, C | — | — |
| — | — | — | — | 2.12, s | 20.9, CH3 | — | — | |
| Position | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|
| δH, Mult. | δH, Mult. | δH, Mult. | δH, Mult. | δH, Mult. | δH, Mult. | |
| 1a | 1.85, m | 1.84, m | 1.78, m | 1.84, ov | 1.84, m | 1.88, ov |
| 1b | 1.36, ov | 1.36, m | 1.36, ov | 1.37, ov | 1.52, ov | 1.47, qd (11.5, 8.3) |
| 2a | 1.98, ov | 1.97, m | 1.99, ov | 1.98, ov | 1.53, ov | 2.40, m |
| 2b | 1.92, ov | 1.91, ov | 1.95, ov | 1.91, ov | 1.35, ov | 2.31, m |
| 4 | 5.47, br.s | 5.47, br.s | 5.45, br.s | 5.48, br.s | 0.87, ov | 1.71, ov |
| 5 | 2.04, dd (9.3, 4.7) | 2.02, m | 2.04, ov | 2.04, m | 0.80, ov | 0.54, br.t (9.5) |
| 6 | 1.52, ov | 1.48, m | 1.51, dt (7.4, 3.1) | 1.51, m | 1.14, m | 0.66, td (10.9, 10.1, 6.5) |
| 7a | 1.78, ov | 1.75, ov | 1.83, ov | 1.84, ov | 1.33, ov | 1.85, ov |
| 7b | 1.78, ov | 1.75, ov | 1.78, ov | 1.77, ov | 0.83, ov | 0.89, m |
| 8a | 5.39, br.s | 5.39, br.s | 5.39, br.s | 5.40, br.s | 1.57, ov | 1.75, dd (12.9, 7.1) |
| 8b | — | — | — | — | 0.51, m | 1.60, ov |
| 9 | — | — | — | — | 1.64, dd (11.9, 6.0) | — |
| 10 | 1.95, ov | 1.93, ov | 1.94, ov | 1.94, ov | — | 1.92, td (11.1, 6.5) |
| 11 | 1.78, ov | 1.78, ov | 1.83, ov | 1.79, ov | 1.51, ov | — |
| 12a | 1.38, ov | 1.18, ov | 1.36, ov | 1.30, ov | 1.46, m | 1.26, m |
| 12b | 1.22, ov | 1.18, ov | 1.36, ov | 1.21, ov | 1.21, m | 1.16, m |
| 13a | 1.51, m | 1.69, ov | 2.27, m | 1.19, m | 2.04, dd (13.9, 6.3) | 2.06, m |
| 13b | 1.36, ov | 1.62, ov | 2.27, m | 1.19, m | 1.92, dq (14.5, 7.5) | 2.06, m |
| 14a | 3.85, dd (8.0, 3.8) | 1.39, ov | 6.88, t (7.8) | 1.37, ov | 5.12, br.t (6.2) | 5.09, t (7.1) |
| 14b | — | 1.15, m | — | 1.08, ov | — | — |
| 15 | 2.54, qd (7.2, 3.5) | — | — | 1.61, m | — | — |
| 16a | — | — | — | 3.40, dd (10.4, 6.6) | 1.61, s | 1.60, s |
| 16b | — | — | — | 3.49, dd (10.5, 5.9) | — | — |
| 17 | 1.18, d (7.2) | 1.39, s | 4.33, br.s | 0.90, d (6.7) | 1.69, s | 1.67, s |
| 18 | 0.83, d (6.8) | 0.79, d (6.7) | 0.84, d (6.9) | 0.81, d (6.8) | 0.90, d (6.7) | 1.04, s |
| 19a | 1.68, s | 1.69, s | 1.70, s | 1.69, s | 1.28, s | 4.90, s |
| 19b | — | — | — | — | — | 4,79, s |
| 20 | 1.68, s | 1.68, s | 1.69, s | 1.69, s | 0.93, d (6.4) | 1.12, s |
| OMe | 3.71, s | 3.78, s | 3.78, s | — | — | — |
| Position | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|
| δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | |
| 1 | 24.6, CH2 | 24.6, CH2 | 24.5, CH2 | 24.7, CH2 | 29.5, CH2 | 26.3, CH2 |
| 2 | 30.8, CH2 | 30.8, CH2 | 30.7, CH2 | 30.8, CH2 | 36.3, CH2 | 32.1, CH2 |
| 3 | 134.7, C | 134.6, C | 134.8, C | 134.5, C | 80.4, C | 157.8, C |
| 4 | 123.6, CH | 123.8, CH | 123.4, CH | 123.8, CH | 39.0, CH | 41.6, CH |
| 5 | 36.3, CH | 36.4, CH | 36.2, CH | 36.3, CH | 23.1, CH | 32.7, CH |
| 6 | 39.1, CH | 38.9, CH | 38.7, CH | 39.0, CH | 42.5, CH | 26.6, CH |
| 7 | 24.6, CH2 | 24.6, CH2 | 24.5, CH2 | 24.6, CH2 | 25.3, CH2 | 20.4, CH2 |
| 8 | 121.2, CH | 121.4, CH | 121.0, CH | 121.4, CH | 31.7, CH2 | 44.6, CH2 |
| 9 | 136.7, C | 136.7, C | 136.6, C | 136.6, C | 30.9, CH | 75.1, C |
| 10 | 39.5, CH | 39.6, CH | 39.4, CH | 39.5, CH | 33.6, C | 57.9, CH |
| 11 | 31.8, CH | 35.6, CH | 31.6, CH | 31.7, CH | 38.3, CH | 23.2, C |
| 12 | 31.4, CH2 | 31.6, CH2 | 34.5, CH2 | 35.9, CH2 | 34.6, CH2 | 43.2, CH2 |
| 13 | 31.9, CH2 | 21.7, CH2 | 26.6, CH2 | 25.0, CH2 | 26.1, CH2 | 25.4, CH2 |
| 14 | 72.1, CH | 40.6, CH2 | 146.1, CH | 33.5, CH2 | 125.0, CH | 124.8, CH |
| 15 | 44.0, CH | 74.8, C | 130.4, C | 35.8, CH | 131.3, C | 131.1, C |
| 16 | 176.7, C | 177.9, C | 168.0, C | 68.4, CH2 | 17.7, CH3 | 17.6, CH3 |
| 17 | 10.6, CH3 | 26.0, CH3 | 57.2, CH2 | 16.6, CH3 | 25.7, CH3 | 25.7, CH3 |
| 18 | 13.2, CH3 | 13.3, CH3 | 13.1, CH3 | 13.3, CH3 | 16.1, CH3 | 13.7, CH3 |
| 19 | 23.9, CH3 | 24.0, CH3 | 23.8, CH3 | 24.0, CH3 | 27.9, CH3 | 105.5, CH2 |
| 20 | 21.7, CH3 | 21.8, CH3 | 21.6, CH3 | 21.7, CH3 | 18.8, CH3 | 20.5, CH3 |
| OMe | 51.7, CH3 | 52.7, CH3 | 51.7, CH3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Ouyang, H.; Wang, W.; Liu, J.; Li, T.; Wu, B.; Yan, X.; He, S. Antimicrobial Terpenoids from South China Sea Soft Coral Lemnalia sp. Mar. Drugs 2021, 19, 294. https://doi.org/10.3390/md19060294
Yan X, Ouyang H, Wang W, Liu J, Li T, Wu B, Yan X, He S. Antimicrobial Terpenoids from South China Sea Soft Coral Lemnalia sp. Marine Drugs. 2021; 19(6):294. https://doi.org/10.3390/md19060294
Chicago/Turabian StyleYan, Xia, Han Ouyang, Wei Wang, Jing Liu, Te Li, Bin Wu, Xiaojun Yan, and Shan He. 2021. "Antimicrobial Terpenoids from South China Sea Soft Coral Lemnalia sp." Marine Drugs 19, no. 6: 294. https://doi.org/10.3390/md19060294
APA StyleYan, X., Ouyang, H., Wang, W., Liu, J., Li, T., Wu, B., Yan, X., & He, S. (2021). Antimicrobial Terpenoids from South China Sea Soft Coral Lemnalia sp. Marine Drugs, 19(6), 294. https://doi.org/10.3390/md19060294