Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma
Abstract
1. Introduction
2. Results
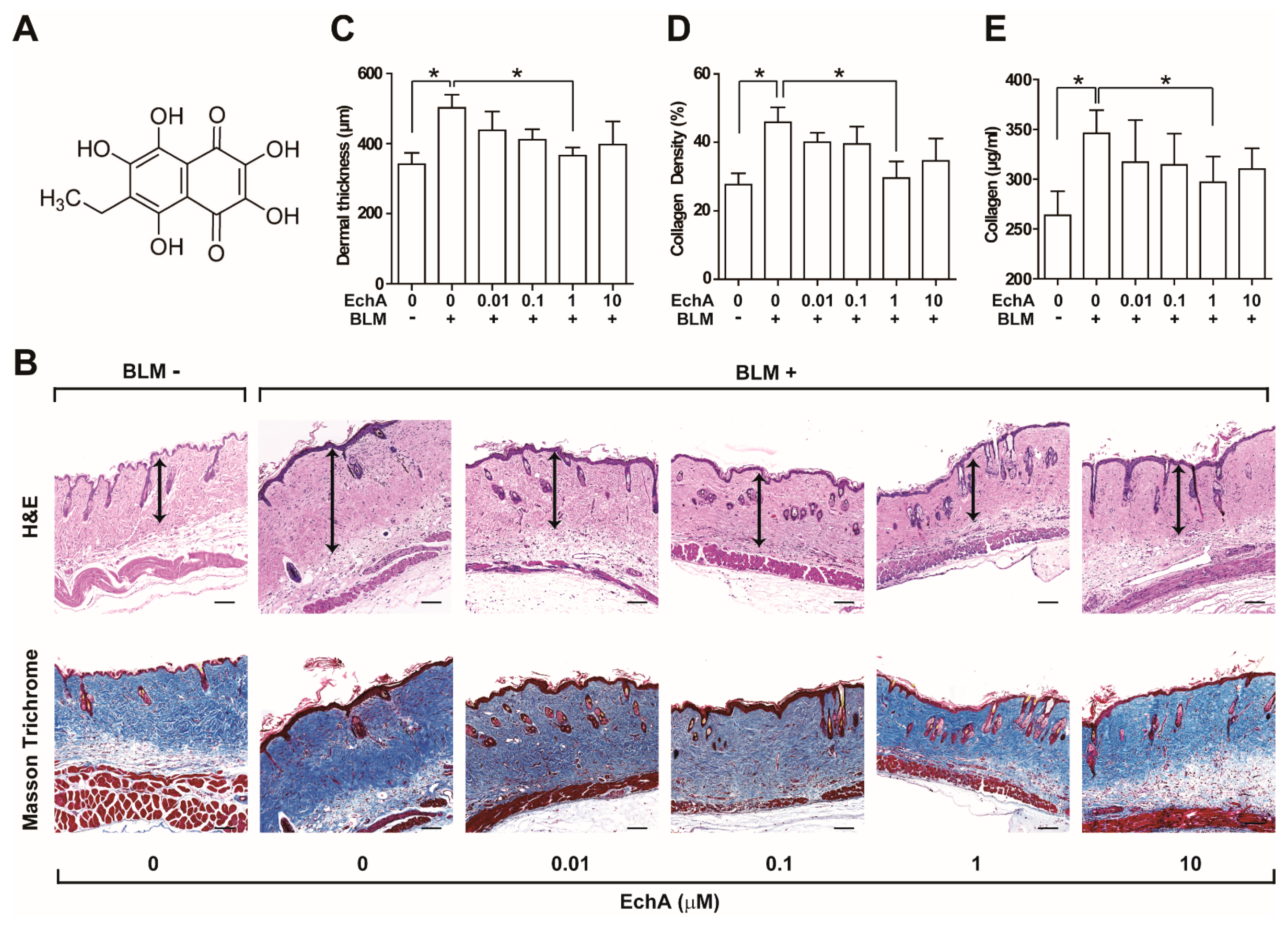
2.1. EchA Treatment Alleviates Fibrosis in Bleomycin-Induced Scleroderma
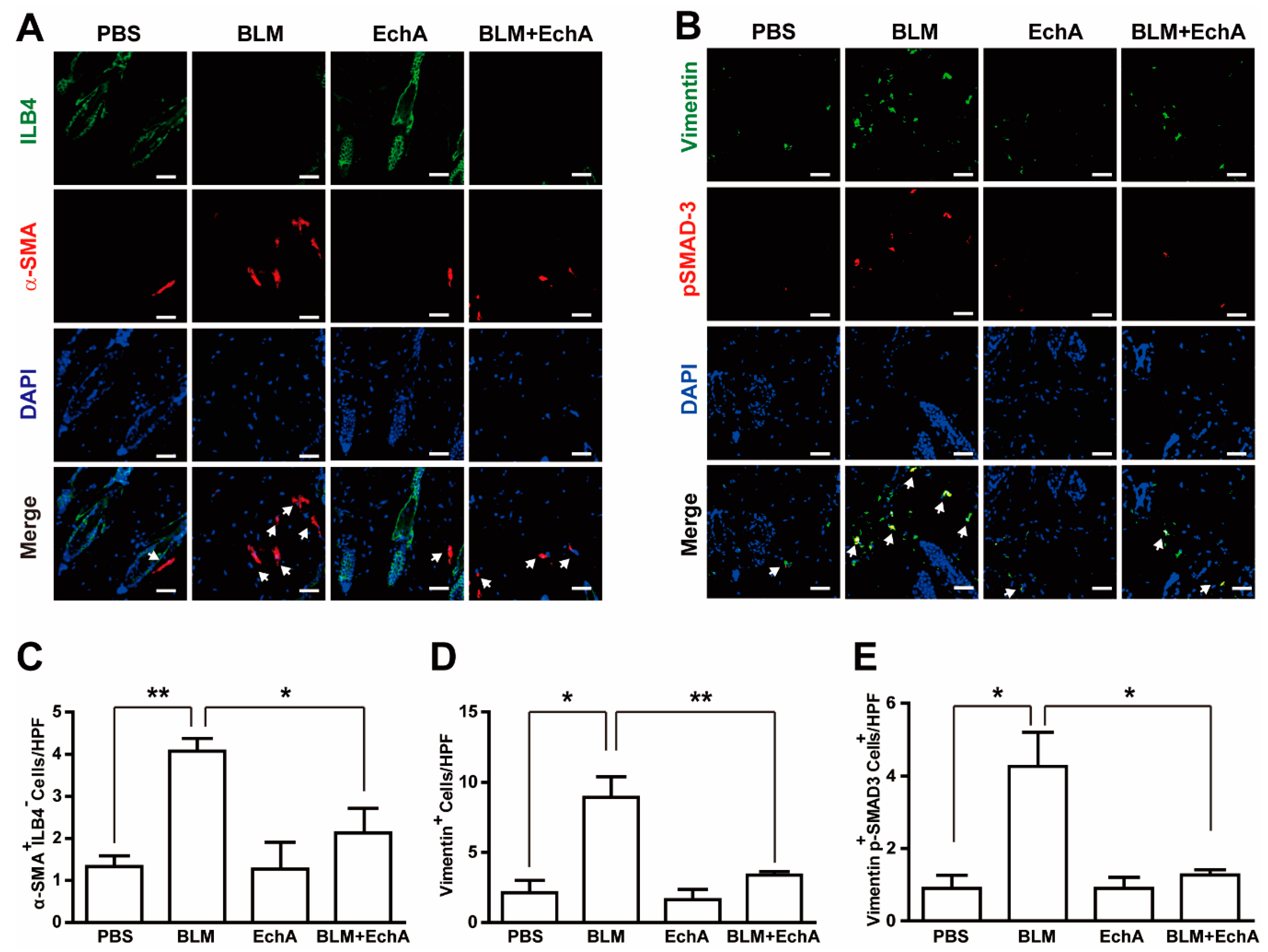
2.2. EchA Treatment Attenuates Inflammation in BLM-Induced Scleroderma
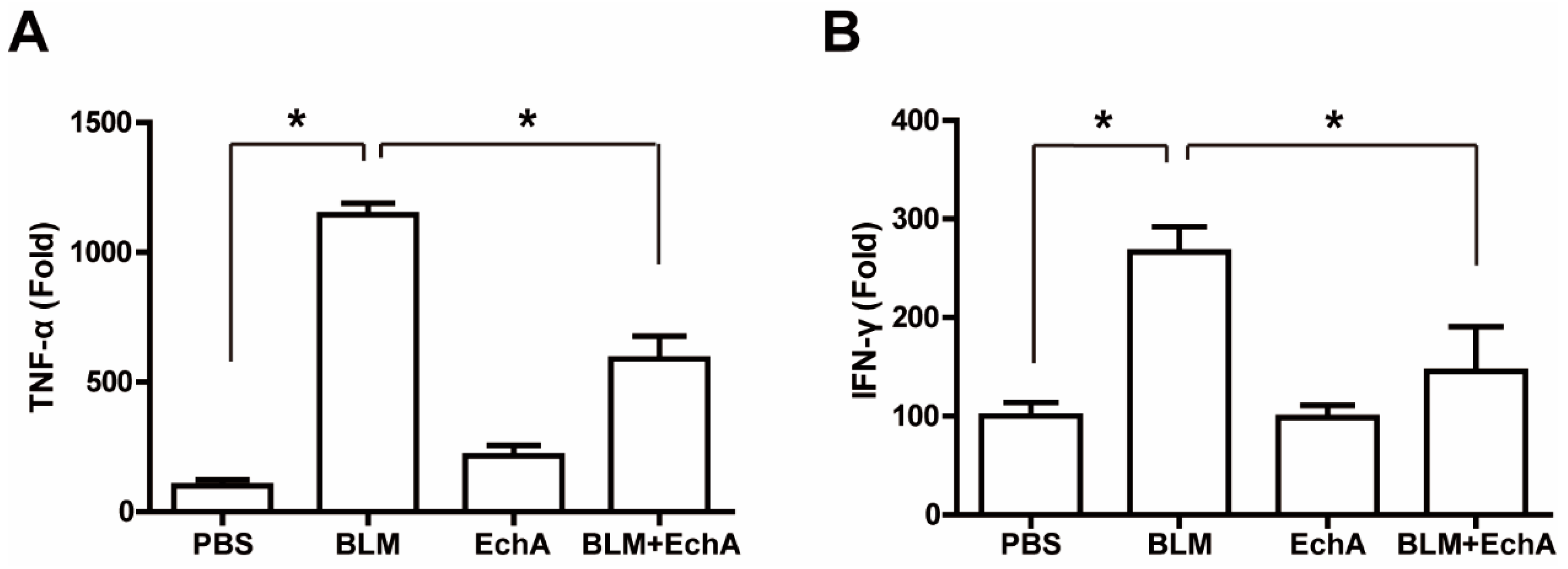
2.3. EchA Treatment Attenuates Systemic Inflammation in BLM-Induced Scleroderma
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation of EchA
4.3. Scleroderma Animal Model
4.4. Histological Analysis
4.5. Immunohistological Analysis
4.6. ELISA Assay
4.7. Autophagy Assay
4.8. Real-Time PCR (RT-PCR)
4.9. Western Blotting
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef]
- Fuschiotti, P. Current perspectives on the immunopathogenesis of systemic sclerosis. ImmunoTargets Ther. 2016, 5, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Toledo, D.M.; Pioli, P.A. Macrophages in Systemic Sclerosis: Novel Insights and Therapeutic Implications. Curr. Rheumatol. Rep. 2019, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Higashi-Kuwata, N.; Makino, T.; Inoue, Y.; Takeya, M.; Ihn, H. Alternatively activated macrophages (M2 macrophages) in the skin of patient with localized scleroderma. Exp. Dermatol. 2009, 18, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.; Mathieson, J.; Thomson, R. Distribution of spinochrome pigments in echinoids. Comp. Biochem. Physiol. 1969, 28, 333–345. [Google Scholar] [CrossRef]
- Kim, R.; Hur, D.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Chang, W. Echinochrome A Attenuates Cerebral Ischemic Injury through Regulation of Cell Survival after Middle Cerebral Artery Occlusion in Rat. Mar. Drugs 2019, 17, 501. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Sadek, S.A.; Hassanein, S.S.; Soliman, A.M. Hepatoprotective Effect of Echinochrome Pigment in Septic Rats. J. Surg. Res. 2019, 234, 317–324. [Google Scholar] [CrossRef]
- Sedova, K.; Bernikova, O.; Azarov, J.; Shmakov, D.; Vityazev, V.; Kharin, S. Effects of echinochrome on ventricular repolarization in acute ischemia. J. Electrocardiol. 2015, 48, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.K.; Song, I.-S.; Lee, S.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoryev, S.A.; Stonik, V.A.; et al. Echinochrome A Protects Mitochondrial Function in Cardiomyocytes against Cardiotoxic Drugs. Mar. Drugs 2014, 12, 2922–2936. [Google Scholar] [CrossRef] [PubMed]
- Lennikov, A.; Kitaichi, N.; Noda, K.; Mizuuchi, K.; Ando, R.; Dong, Z.; Fukuhara, J.; Kinoshita, S.; Namba, K.; Ohno, S.; et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol. Vis. 2014, 20, 171–177. [Google Scholar]
- Lebed’Ko, O.A.; Ryzhavskii, B.Y.; Demidova, O.V. Effect of Antioxidant Echinochrome A on Bleomycin-Induced Pulmonary Fibrosis. Bull. Exp. Biol. Med. 2015, 159, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Seo, Y.; Ahn, J.-S.; Shin, Y.Y.; Yang, J.W.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells. Mar. Drugs 2019, 17, 622. [Google Scholar] [CrossRef] [PubMed]
- Ammitzbøll, T.; Serup, J. Collagen metabolites in urine in localized scleroderma (morphoea). Acta Derm Venereol 1984, 64, 534–537. [Google Scholar] [PubMed]
- Lafyatis, R. Transforming growth factor beta—At the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Murray, V.; Chen, J.K.; Chung, L.H. The Interaction of the Metallo-Glycopeptide Anti-Tumour Drug Bleomycin with DNA. Int. J. Mol. Sci. 2018, 19, 1372. [Google Scholar] [CrossRef] [PubMed]
- Kappus, H. Oxidative Stress in Chemical Toxicity. Arch. Toxicol. 1992, 60, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.D.; Sadofsky, L.R.; Hart, S.P. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp. Lung Res. 2015, 41, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Batteux, F.; Kavian, N.; Servettaz, A. New insights on chemically induced animal models of systemic sclerosis. Curr. Opin. Rheumatology 2011, 23, 511–518. [Google Scholar] [CrossRef]
- Khalil, N.; O’Connor, R. The Role of TGF-β in Bleomycin Induced Pulmonary Fibrosis. In Transforming Growth Factor-β in Cancer Therapy; Humana Press: Totowa, NJ, USA, 2008; Volume I, pp. 581–594. [Google Scholar]
- Flanders, K.; Sato, M.; Ooshima, A.; Russo, A.; Roberts, A. Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 2004, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Ludwicka-Bradley, A.; Silver, R.M.; Bogatkevich, G.S. Coagulation and Autoimmunity in Scleroderma Interstitial Lung Disease. Semin. Arthritis Rheum. 2011, 41, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.M.; Peisach, J.; Horwitz, S.B. Mechanism of bleomycin action: In vitro studies. Life Sci. 1981, 28, 715–727. [Google Scholar] [CrossRef]
- Chambers, R.C. Procoagulant signalling mechanisms in lung inflammation and fibrosis: Novel opportunities for pharmaco-logical intervention? Br. J. Pharmacol. 2008, 153, S367–S378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.F.; Yu, J.F.; Zhang, J.X.; Jiang, T.; Xu, S.H.; Yu, Q.Y.; Zhu, Q.X. N-acetylcysteine attenuates subcutaneous administration of bleomycin-induced skin fibrosis and oxidative stress in a mouse model of scleroderma. Clin. Exp. Dermatol. 2013, 38, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, A.; Motegi, S.I.; Fujiwara, C.; Yamazaki, S.; Inoue, Y.; Uchiyama, A.; Akai, R.; Iwawaki, T.; Ishikawa, O. Inhibitory effect of kaempferol on skin fibrosis in systemic sclerosis by the suppression of oxidative stress. J. Dermatol. Sci. 2019, 96, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.V.; Levitskaya, E.L.; Tikhonova, E.V.; Ivanova, M.V. Antioxidant Properties, Autooxidation, and Mutagenic Activity of Echinochrome A Compared with Its Etherified Derivative. Biochemistry (Moscow) 2001, 66, 885–893. [Google Scholar] [CrossRef]
- Didier, K.; Giusti, D.; Le Jan, S.; Terryn, C.; Muller, C.; Pham, B.N.; Le Naour, R.; Antonicelli, F.D.; Servettaz, A. Neutrophil Extracellular Traps Generation Relates with Early Stage and Vascular Complications in Systemic Sclerosis. J. Clin. Med. 2020, 9, 2136. [Google Scholar] [CrossRef]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Pechkovsky, D.V.; Prasse, A.; Kollert, F.; Engel, K.M.; Dentler, J.; Luttmann, W.; Friedrich, K.; Müller-Quernheim, J.; Zissel, G. Alternatively activated alveolar macrophages in pulmonary fibrosis—mediator production and intracellular signal transduction. Clin. Immunol. 2010, 137, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Park, G.T.; Kwon, Y.W.; Lee, T.W.; Kwon, S.G.; Ko, H.-C.; Kim, M.B.; Kim, J.H. Formyl Peptide Receptor 2 Activation Ameliorates Dermal Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Front. Immunol. 2019, 10, 2095. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, R.; Wu, J.; Li, X. Self-eating: Friend or foe? The emerging role of autophagy in fibrotic diseases. Theranostics 2020, 10, 7993–8017. [Google Scholar] [CrossRef] [PubMed]
- Frech, T.; De Domenico, I.; Murtaugh, M.A.; Revelo, M.P.; Li, D.Y.; Sawitzke, A.D.; Drakos, S. Autophagy is a key feature in the pathogenesis of systemic sclerosis. Rheumatol. Int. 2013, 34, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Larson-Casey, J.L.; Deshane, J.S.; Ryan, A.J.; Thannickal, V.J.; Carter, A.B. Macrophage Akt1 Kinase-Mediated Mitophagy Modulates Apoptosis Resistance and Pulmonary Fibrosis. Immunity 2016, 44, 582–596. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.-T.; Yoon, J.-W.; Yoo, S.-B.; Song, Y.-C.; Song, P.; Kim, H.-K.; Han, J.; Bae, S.-J.; Ha, K.-T.; Mishchenko, N.P.; et al. Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Mar. Drugs 2021, 19, 237. https://doi.org/10.3390/md19050237
Park G-T, Yoon J-W, Yoo S-B, Song Y-C, Song P, Kim H-K, Han J, Bae S-J, Ha K-T, Mishchenko NP, et al. Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Marine Drugs. 2021; 19(5):237. https://doi.org/10.3390/md19050237
Chicago/Turabian StylePark, Gyu-Tae, Jung-Won Yoon, Sang-Bin Yoo, Young-Chul Song, Parkyong Song, Hyoung-Kyu Kim, Jin Han, Sung-Jin Bae, Ki-Tae Ha, Natalia P. Mishchenko, and et al. 2021. "Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma" Marine Drugs 19, no. 5: 237. https://doi.org/10.3390/md19050237
APA StylePark, G.-T., Yoon, J.-W., Yoo, S.-B., Song, Y.-C., Song, P., Kim, H.-K., Han, J., Bae, S.-J., Ha, K.-T., Mishchenko, N. P., Fedoreyev, S. A., Stonik, V. A., Kim, M.-B., & Kim, J.-H. (2021). Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Marine Drugs, 19(5), 237. https://doi.org/10.3390/md19050237








