The Prospective Use of Brazilian Marine Macroalgae in Schistosomiasis Control
Abstract
1. Introduction
2. Results and Discussion
2.1. Biological Activity
2.1.1. Schistosoma Mansoni
2.1.2. Biomphalaria glabrata
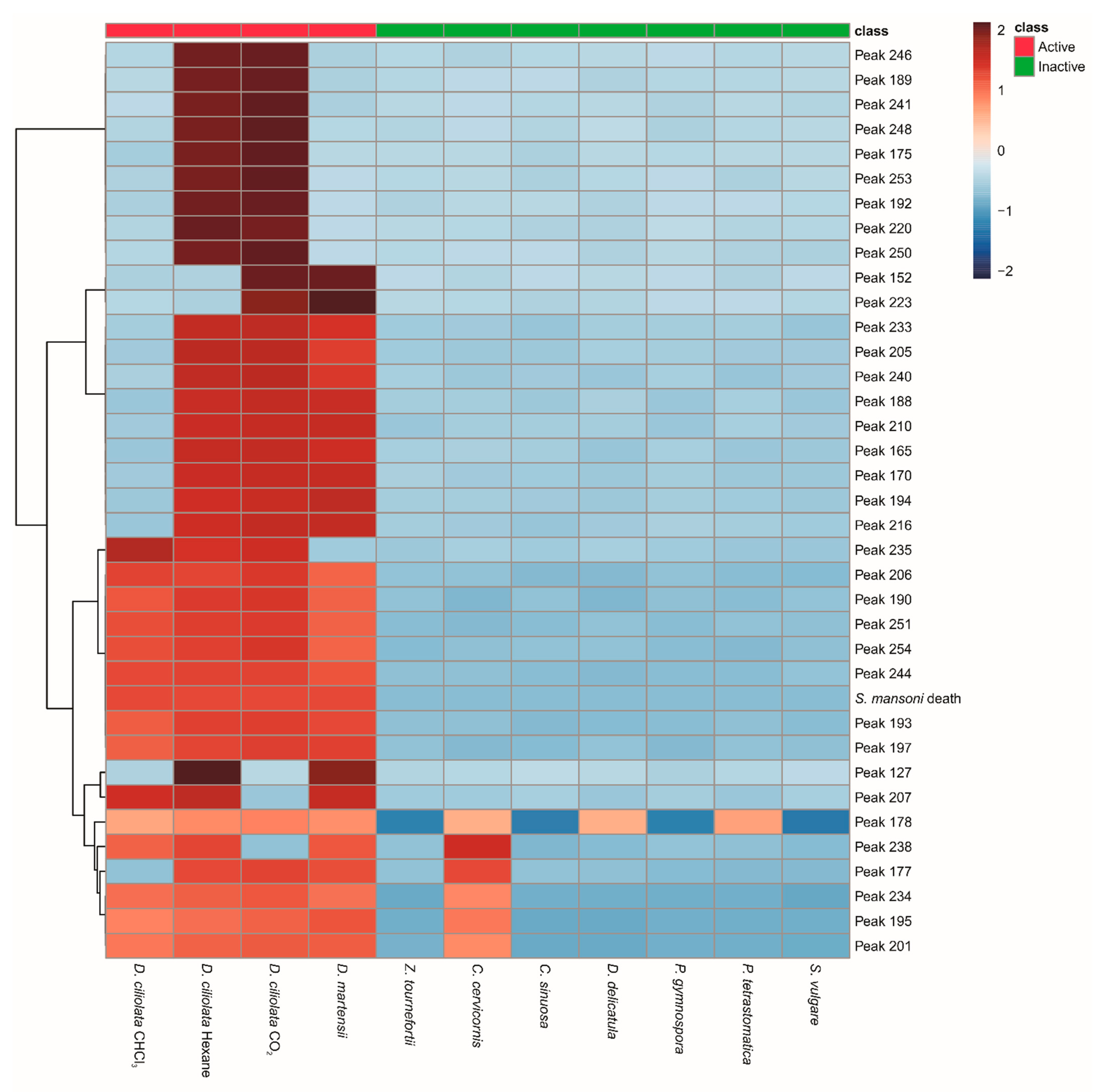
2.2. Metabolomic Analysis
2.2.1. Laurencia/Laurenciella Set
2.2.2. Ochrophyta Set
3. Materials and Methods
3.1. Seaweed Samples and Extracts Preparation
3.2. Schistosomicidal Activity Screening
3.3. Molluscicidal Activity Screening in Biomphalaria Glabrata Embryos
3.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
3.5. Sample Selection for Statistical Analysis
3.6. Data Processing, Correlation Analysis, and Compound Identification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Schistosomiasis—Situation and Trends. 2016. Available online: http://www.who.int/gho/neglected_diseases/schistosomiasis/en/ (accessed on 8 January 2018).
- Doenhoff, M.J.; Kimani, G.; Cioli, D. Praziquantel and the control of schistosomiasis. Parasitol. Today 2000, 16, 364–366. [Google Scholar] [CrossRef]
- Abreu, F.C.; Goulart, M.O.F.; Brett, A.M.O. Detection of the damage caused to DNA by niclosamide using an electrochemical DNA-biosensor. Biosens. Bioelectron. 2002, 17, 913–919. [Google Scholar] [CrossRef]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrin, Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J. Ethnopharmacol. 2008, 120, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kladi, M.; Vagias, C.; Roussis, V. Volatile halogenated metabolites from marine red algae. Phytochem. Rev. 2004, 3, 337–366. [Google Scholar] [CrossRef]
- Falkenberg, M.; Nakano, E.; Zambotti-Villela, L.; Zatelli, G.A.; Philippus, A.C.; Imamura, K.B.; Velasquez, A.M.A.; Freitas, R.P.; Tallarico, L.D.; Colepicolo, P.; et al. Bioactive compounds against neglected diseases isolated from macroalgae: A review. J. Appl. Phycol. 2019, 31, 797–823. [Google Scholar] [CrossRef]
- Fernandes, D.R.P.; de Oliveira, V.P.; Valentin, Y.Y. Seaweed biotechnology in Brazil: Six decades of studies on natural products and their antibiotic and other biological activities. J. Appl. Phycol. 2014, 26, 1923–1937. [Google Scholar] [CrossRef]
- Freile-Pelegrin, Y.; Robledo, D.; Chan-Bacab, M.J.; Ortega-Morales, B.O. Antileishmanial properties of tropical marine algae extracts. Fitoterapia 2008, 79, 374–377. [Google Scholar] [CrossRef]
- Stout, E.P.; Prudhomme, J.; Le Roch, K.; Fairchild, C.R.; Franzblau, S.G.; Aalbersberg, W.; Hay, M.E.; Kubanek, J. Unusual antimalarial meroditerpenes from tropical red macroalgae. Bioorg. Med. Chem. Lett. 2010, 20, 5662–5665. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Pelizzaro-Rocha, K.J.; Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Silva, S.O.; Sudatti, D.B.; Bianco, E.M.; Pereira, R.C.; Nakamura, C.V. In vitro anti-trypanosomal activity of elatol isolated from red seaweed Laurencia dendroidea. Parasitology 2010, 137, 1661–1670. [Google Scholar] [CrossRef]
- Vonthron-Senecheau, C.; Kaiser, M.; Devambez, I.; Vastel, A.; Mussio, I.; Rusig, A.-M. Antiprotozoal Activities of Organic Extracts from French Marine Seaweeds. Mar. Drugs 2011, 9, 922–933. [Google Scholar] [CrossRef]
- Nara, T.; Kamei, Y.; Tsubouchi, A.; Annoura, T.; Hirota, K.; Iizumi, K.; Dohmoto, Y.; Ono, T.; Aoki, T. Inhibitory action of marine algae extracts on the Trypanosoma cruzi dihydroorotate dehydrogenase activity and on the protozoan growth in mammalian cells. Parasitol. Int. 2005, 54, 59–64. [Google Scholar] [CrossRef] [PubMed]
- de Felício, R.; de Albuquerque, S.; Young, M.C.; Yokoya, N.S.; Debonsi, H.M. Trypanocidal, leishmanicidal and antifungal potential from marine red alga Bostrychia tenella J. Agardh (Rhodomelaceae, Ceramiales). J. Pharm. Biomed. Anal. 2010, 52, 763–769. [Google Scholar] [CrossRef]
- dos Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Dias, B.P.; Sudatti, D.B.; Bianco, E.M.; Pereira, R.C.; Nakamura, C.V. Effect of Elatol, Isolated from Red Seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef]
- Galle, J.-B.; Attioua, B.; Kaiser, M.; Rusig, A.-M.; Lobstein, A.; Vonthron-Senecheau, C. Eleganolone, a Diterpene from the French Marine Alga Bifurcaria bifurcata Inhibits Growth of the Human Pathogens Trypanosoma brucei and Plasmodium falciparum. Mar. Drugs 2013, 11, 599–610. [Google Scholar] [CrossRef]
- Capon, R.J.; Barrow, R.A.; Rochfort, S.; Jobling, M.; Skene, C.; Lacey, E.; Gill, J.H.; Friedel, T.; Wadsworth, D. Marine nematocides: Tetrahydrofurans from a southern Australian brown alga, Notheia anomala. Tetrahedron 1998, 54, 2227–2242. [Google Scholar] [CrossRef]
- Wishart, D.S. Emerging applications of metabolomics indrug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Borges, D.G.L.; Echeverria, J.T.; de Oliveira, T.L.; Heckler, R.P.; de Freitas, M.G.; Damasceno-Junior, G.A.; Carollo, C.A.; Borges, F.A. Discovery of potential ovicidal natural products using metabolomics. PLoS ONE 2019, 14, e0211237. [Google Scholar] [CrossRef]
- Stein, E.M.; Machado, L.P.; Roffato, H.K.; Miyasato, P.A.; Nakano, E.; Colepicolo, P.; Andreguetti, D.X. Antischistosomal activity from Brazilian marine algae. Rev. Bras. Farmacogn. 2015, 25, 663–667. [Google Scholar] [CrossRef]
- Pica-Mattoccia, L.; Ruppel, A.; Xia, C.M.; Cioli, D. Praziquantel and the benzodiazepine Ro 11-3128 do not compete for the same binding sites in schistosomes. Parasitology 2008, 135, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.T.; Lage, R.C.G.; Anderson, L.; Venancio, T.M.; Nakaya, H.I.; Miyasato, P.A.; Rofatto, H.K.; Zerlotini, A.; Nakano, E.; Oliveira, G.; et al. Synergy of Omeprazole and Praziquantel In Vitro Treatment against Schistosoma mansoni Adult Worms. PLoS Negl. Trop. Dis. 2015, 9, e0004086. [Google Scholar] [CrossRef] [PubMed]
- Hines-Kay, J.; Cupit, P.M.; Sanchez, M.C.; Rosenberg, G.H.; Hanelt, B.; Cunningham, C. Transcriptional analysis of Schistosoma mansoni treated with praziquantel in vitro. Mol. Biochem. Parasitol. 2012, 186, 87–94. [Google Scholar] [CrossRef]
- Davyt, D.; Fernandez, R.; Suescun, L.; Mombru, A.W.; Saldana, J.; Dominguez, L.; Coll, J.; Fujii, M.T.; Manta, E. New sesquiterpene derivatives from the red alga Laurencia scoparia. Isolation, structure determination, and anthelmintic activity. J. Nat. Prod. 2001, 64, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Davyt, D.; Fernandez, R.; Suescun, L.; Mombru, A.W.; Saldana, J.; Dominguez, L.; Fujii, M.T.; Manta, E. Bisabolanes from the red alga Laurencia scoparia. J. Nat. Prod. 2006, 69, 1113–1116. [Google Scholar] [CrossRef]
- Guedes, E.A.; de Carvalho, C.M.; Ribeiro Junior, K.A.; Lisboa Ribeiro, T.F.; de Barros, L.D.; de Lima, M.R.; Prado Moura, F.e.B.; Goulart Sant’ana, A.E. Larvicidal Activity against Aedes aegypti and Molluscicidal Activity against Biomphalaria glabrata of Brazilian Marine Algae. J. Parasitol. Res. 2014, 2014, 501328. [Google Scholar] [CrossRef]
- Patel, A.V.; Wright, D.C.; Romero, M.A.; Blunden, G.; Guiry, M.D. Molluscicidal polyphenols from species of Fucaceae. Nat. Prod. Commun. 2008, 3, 245–249. [Google Scholar] [CrossRef]
- Saad, A.E.-H.A.; Ragab, F.M.A.; Abdel Fatah, H.M.; Abdel-Wareth, M.T.A.; Ibrahim, N.K. Effect of Cystoseira barbata and Dictyota dichotoma-algae on reproduction and protein pattern of Biomphalaria alexandrina snails. Molluscan Res. 2019, 39, 82–88. [Google Scholar] [CrossRef]
- Saad, A.A.; Ragab, F.M.A.; Abdel Fatah, H.M.; Abdel-Wareth, M.T.A.; Ibrahim, N.K. Effect of two algae; Cystoseira barbata and Dictyota dichotoma on digestive gland of Biomphalaria alexandrina snails. J. Environ. Sci. 2017, 37, 37–59. [Google Scholar]
- Gressler, V.; Stein, E.M.; Dörr, F.; Fujii, M.T.; Colepicolo, P.; Pinto, E. Sesquiterpenes from the essential oil of Laurencia dendroidea (Ceramiales, Rhodophyta): Isolation, biological activities and distribution among seaweeds. Rev. Bras. Farmacogn. 2011, 21, 248–254. [Google Scholar] [CrossRef]
- Stein, E.M. Avaliação das Atividades Biológicas e Composição Química dos Extratos de algas Vermelhas do Gênero Laurencia (Rhodomelaceae, Ceramiales) do litoral do Espírito Santo, Brasil; Universidade de São Paulo: São Paulo, Brasil, 2011. [Google Scholar]
- Stein, E.M. Barcode e Bioprospecção de Metabólitos das Algas Marinhas Laurencia aldingensis, L. Dendroidea e Laurenciella sp. (Ceramiales, Rhodophyta); Universidade de São Paulo: São Paulo, Brasil, 2015. [Google Scholar]
- da Silva Machado, F.L.; Pacienza-Lima, W.; Rossi-Bergmann, B.; de Souza Gestinari, L.M.; Fujii, M.T.; Campos de Paula, J.; Costa, S.S.; Lopes, N.P.; Kaiser, C.R.; Soares, A.R. Antileishmanial sesquiterpenes from the Brazilian red alga Laurencia dendroidea. Planta Med. 2011, 77, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.L.; Kelecom, A. A chemotaxonomic study of diterpenes from marine brown algae of the genus Dictyota. Sci. Total Environ. 1988, 75, 271–283. [Google Scholar] [CrossRef]
- Teixeira, V.L. Produtos Naturais de Algas Marinhas Bentônicas. Rev. Virtual Química 2013, 5, 343–362. [Google Scholar]
- Freitas, O.d.S.P.; de Oliveira, A.S.; De-Paula, J.C.; Pereira, R.C.; Cavalcanti, D.N.; Teixeira, V.L. Chemical Variation in the Diterpenes from the Brazilian Brown Alga Dictyota Mertensii (Dictyotaceae, Phaeophyta). Nat. Prod. Commun. 2007, 2, 1934578X0700200104. [Google Scholar] [CrossRef]
- De-Paula, J.C.; Lopes-Filho, E.A.P.; Salgueiro, F.; Yoneshigue-Valentin, Y.; Cavalcanti, D.N.; Villaça, R.C.; Teixeira, V.L. Diterpenes content of the brown alga Dictyota ciliolata (Dictyotales, Phaeophyceae) and recognition of a Brazilian haplotype based on psbA sequences, New Zealand. J. Bot. 2018, 56, 415–429. [Google Scholar] [CrossRef]
- World Health Organization. Research Priorities for Helminth Infections: Technical Report of the TDR Disease Reference Groupon Helminth Infections; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Nixon, S.A.; Welz, C.; Woods, D.J.; Costa-Junior, L.; Zamanian, M.; Martin, R.J. Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 8–16. [Google Scholar] [CrossRef]
- Tallarico, L.F.; Miyasato, P.A.; Nakano, E. Rearing and Maintenance of Biomphalaria glabrata (Say, 1818): Adults and Embryos under Laboratory Conditions. Ann. Aquac. Res. 2016, 3, 1–4. [Google Scholar]
- World Health Organization. Guidelines for Laboratory and Field Testing of Molluscicides for Control of Schistosomiasis; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Molluscicide screening and evaluation. Wold Health Org. Technol. Rep. Ser. 1961, 214, 44. [Google Scholar]
- Lommen, A. MetAlign: Interface-Driven, Versatile Metabolomics Tool for Hyphenated Full-Scan Mass Spectrometry Data Preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Tikunov, Y.M.; Laptenok, S.; Hall, R.D.; Bovy, A.; de Vos, R.C. MSClust: A tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics 2012, 8, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]

| Algae Species | Extract | Schistosoma mansoni | Biomphalaria glabrata embryos | |||||
|---|---|---|---|---|---|---|---|---|
| Death Ratio (%) | Couple Separation (%) | Eggs (Average) | Death Ratio (%) | |||||
| Female | Male | Total | Blastula | Veliger | ||||
| Rhodophyta | ||||||||
| Amphiroa fragilissima | Chloroform | 0 | 0 | 0 | 80 | 40 | 100 | 0 |
| Bostrychia tennela | Dichlorometane | 0 | 80 | 40 | 100 | 69 | 0 | 0 |
| Botryocladia occidentalis | Dichlorometane | 0 | 0 | 0 | 0 | 379 | 100 | 0 |
| Bryothamnion seaforthii | Chloroform | 0 | 0 | 0 | 0 | 188 | 0 | 0 |
| Ceratodictyon variabile | Dichlorometane | 0 | 0 | 0 | 0 | 186 | 0 | 0 |
| Cryptonenia crenulata | Chloroform | 0 | 0 | 0 | 100 | 108 | 100 | 0 |
| Cryptonenia seminervis | Chloroform | 0 | 0 | 0 | 20 | 157 | 100 | 0 |
| Dichotomaria marginata | Chloroform | 0 | 0 | 0 | 100 | 0 | 100 | 0 |
| Gracilaria cf. intermedia | Chloroform | 0 | 0 | 0 | 0 | 301 | 0 | 0 |
| Gracilaria domingensis | Chloroform | 0 | 0 | 0 | 40 | 47 | 1 | 5 |
| Hypnea nigrescens | Chloroform | 0 | 0 | 0 | 20 | 223 | 0 | 0 |
| Jania rubens | Chloroform | 0 | 0 | 0 | 100 | 25 | 100 | 0 |
| Laurencia aldingensis | Chloroform | 0 | 40 | 20 | 100 | 0 | 100 | 11 |
| Laurencia aldingensis | Methanol | 0 | 0 | 0 | 0 | 190 | 57 | 2 |
| Laurencia aldingensis | Hexane | 100 | 100 | 100 | 80 | 0 | 100 | 100 |
| Laurencia catarinensis | Chloroform | 0 | 0 | 0 | 100 | 0 | 100 | 21 |
| Laurencia dendroidea | Hexane | 100 | 100 | 100 | 80 | 0 | 100 | 100 |
| Laurencia dendroidea | Chloroform | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| Laurencia dendroidea | Methanol | 0 | 0 | 0 | 60 | 116 | 100 | 100 |
| Laurenciella sp. | Hexane | 0 | 0 | 0 | 100 | 2 | 100 | 100 |
| Laurenciella sp. | Chloroform | 100 | 100 | 100 | 100 | 19 | 71 | 6 |
| Laurenciella sp. | Methanol | 0 | 0 | 0 | 20 | 200 | 100 | 70 |
| Octhodes secundiramea | Chloroform | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| Palisada perforata | Chloroform | 0 | 0 | 0 | 0 | 291 | 0 | 0 |
| Palisada flagellifera | Dichlorometane | 0 | 0 | 0 | 0 | 149 | 100 | 0 |
| Porphyra spiralis | Chloroform | 100 | 100 | 100 | 100 | 37 | 6 | 0 |
| Pterocladiella capillacea | Chloroform | 0 | 0 | 0 | 0 | 191 | - | - |
| Solieria filiformis | Chloroform | 0 | 0 | 0 | 100 | 0 | 100 | 0 |
| Spyridia aculeata | Chloroform | 0 | 0 | 0 | 100 | 35 | 100 | 100 |
| Tricleocarpa cylindrica | Chloroform | 0 | 0 | 0 | 100 | 13 | 100 | 0 |
| Vidalia obtusiloba | Chloroform | 0 | 0 | 0 | 100 | 74 | 56 | 0 |
| Ochrophyta | ||||||||
| Canistrocarpus cervicornis | Dichlorometane | 0 | 0 | 0 | 100 | 0 | 100 | 0 |
| Colpomenia sinuosa | Chloroform | 0 | 0 | 0 | 0 | 89 | 0 | 0 |
| Dictyota ciliolata | Hexane | 100 | 100 | 100 | 100 | 1 | 100 | 11 |
| Dictyota ciliolata | Chloroform | 100 | 100 | 100 | 100 | 0 | 100 | 40 |
| Dictyota ciliolata | Supercritical fluid | 100 | 100 | 100 | 100 | 0 | - | - |
| Dictyota mertensii | Supercritical fluid | 100 | 100 | 100 | 100 | 0 | 100 | 10 |
| Padina gymnospora | Chloroform | 0 | 0 | 0 | 0 | 284 | 0 | 0 |
| Padina tetrastomatica | Chloroform | 0 | 0 | 0 | 0 | 306 | 100 | 0 |
| Sargassum vulgare | Chloroform | 0 | 0 | 0 | 20 | 120 | 0 | 0 |
| Zonaria tournefortii | Chloroform | 0 | 0 | 0 | 0 | 168 | 0 | 0 |
| Chlorophyta | ||||||||
| Caulerpa cupressoides | Chloroform | 0 | 0 | 0 | 0 | 211 | 0 | 0 |
| Caulerpa racemosa | Chloroform | 0 | 0 | 0 | 80 | 12 | 100 | 0 |
| Caulerpa sertularioides | Chloroform | 0 | 0 | 0 | 100 | 4 | 0 | 0 |
| Codium isthmocladum | Chloroform | 0 | 20 | 10 | 100 | 108 | 100 | 0 |
| Controls | ||||||||
| PQZ (positive control) | 40 | 100 | 80 | 0 | 0 | - | - | |
| DMSO (negative control) | 0 | 0 | 0 | 0 | 266 | 0 | 0 | |
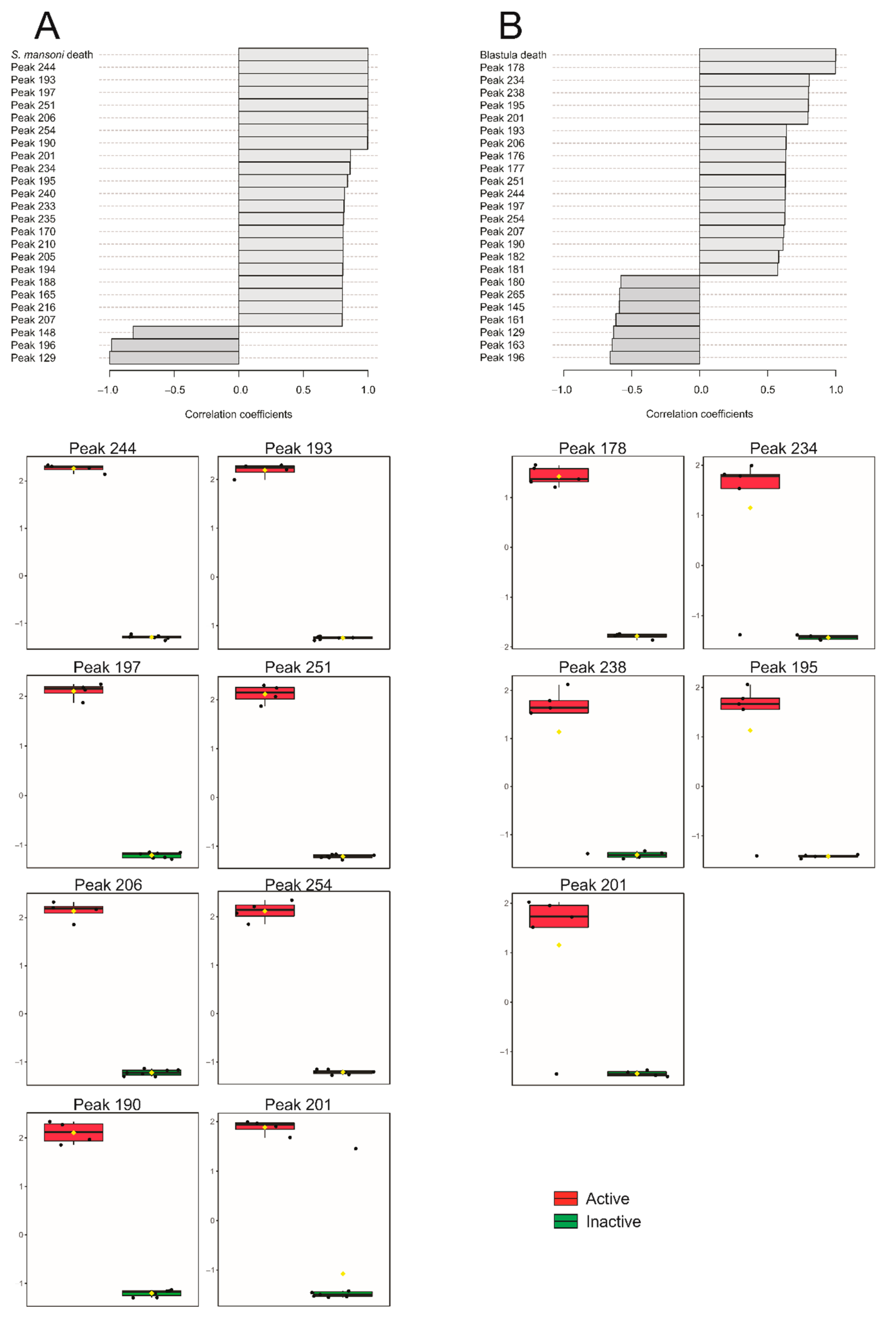
| Peak | S. mansoni | Blastula | ||
|---|---|---|---|---|
| Pearson Correlation | p-Value | Pearson Correlation | p-Value | |
| 127 | 0.61809 | 0.042685 | - | - |
| 152 | 0.60424 | 0.048964 | - | - |
| 165 | 0.80143 | 0.003019 | - | - |
| 170 | 0.80758 | 0.002645 | - | - |
| 175 | 0.60873 | 0.04686 | - | - |
| 177 | 0.61303 | 0.04491 | - | - |
| 178 | 0.63281 | 0.036648 | 0.99671 | <0.00001 |
| 188 | 0.80212 | 0.002975 | - | - |
| 189 | 0.61548 | 0.043823 | - | - |
| 190 | 0.99667 | <0.00001 | - | - |
| 192 | 0.61426 | 0.044361 | - | - |
| 193 | 0.99896 | <0.00001 | - | - |
| 194 | 0.80438 | 0.002835 | - | - |
| 195 | 0.84093 | 0.001182 | 0.7983 | 0.009899 |
| 197 | 0.99821 | <0.00001 | - | - |
| 201 | 0.86399 | 0.000605 | 0.79584 | 0.010302 |
| 205 | 0.80558 | 0.002762 | - | - |
| 206 | 0.9975 | <0.00001 | - | - |
| 207 | 0.79946 | 0.003146 | - | - |
| 210 | 0.8075 | 0.00265 | - | - |
| 216 | 0.80123 | 0.003031 | - | - |
| 220 | 0.62674 | 0.039061 | - | - |
| 223 | 0.61416 | 0.044408 | - | - |
| 233 | 0.81422 | 0.002282 | - | - |
| 234 | 0.86116 | 0.000661 | 0.80394 | 0.009017 |
| 235 | 0.81016 | 0.002499 | - | - |
| 238 | - | - | 0.79904 | 0.009781 |
| 240 | 0.8183 | 0.002077 | - | - |
| 241 | 0.61611 | 0.043545 | - | - |
| 244 | 0.9995 | <0.00001 | - | - |
| 246 | 0.60917 | 0.046658 | - | - |
| 248 | 0.62201 | 0.041017 | - | - |
| 250 | 0.62764 | 0.038698 | - | - |
| 251 | 0.99785 | <0.00001 | - | - |
| 253 | 0.62485 | 0.039835 | - | - |
| 254 | 0.99737 | <0.00001 | - | - |
| Peak | Anotation | Retention Time (min) | ||
|---|---|---|---|---|
| Class | Sub-Class | Compound | ||
| Laurencia/Laurenciella set | ||||
| 24 | Sesquiterpene | Triquinane alcohol | Silphiperfolanol derivative | 27.39 |
| 25 | Sesquiterpene | Triquinane alcohol | Silphiperfolan-7β-ol (C15H26O) | 27.41 |
| 53 | Unknown | - | - | 43.98 |
| Ochrophyta set | ||||
| 190 | Diterpene | Prenylated guaiane (Group I) | Dictyol derivative | 51.53 |
| 193 | Diterpene | Prenylated guaiane (Group I) | Dictyol derivative | 52.49 |
| 197 | Unknown | - | - | 53.37 |
| 201 | Diterpene | Prenylated guaiane (Group I) | Dictyol derivative | 54.15 |
| 206 | Diterpene | Prenylated guaiane (Group I) | Dictyol derivative | 54.91 |
| 234 | Diterpene | Prenylated guaiane (Group I) | Dictyol derivative | 59.17 |
| 244 | Diterpene | Dichotomane (Group III) | 9-Acetoxydichotoma-2,13-diene-16,17-dial (C22H32O4) | 60.87 |
| 251 | Diterpene | Xeniane (Group III) | Xeniane derivative | 62.44 |
| 254 | Unknown | - | - | 62.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, E.M.; Tajú, S.G.; Miyasato, P.A.; de Freitas, R.P.; Tallarico, L.d.F.; dos Santos, G.S.; Luiz, G.L.F.; Rofatto, H.K.; da Silva, F.N.V.; Colepicolo, P.; et al. The Prospective Use of Brazilian Marine Macroalgae in Schistosomiasis Control. Mar. Drugs 2021, 19, 234. https://doi.org/10.3390/md19050234
Stein EM, Tajú SG, Miyasato PA, de Freitas RP, Tallarico LdF, dos Santos GS, Luiz GLF, Rofatto HK, da Silva FNV, Colepicolo P, et al. The Prospective Use of Brazilian Marine Macroalgae in Schistosomiasis Control. Marine Drugs. 2021; 19(5):234. https://doi.org/10.3390/md19050234
Chicago/Turabian StyleStein, Erika M., Sara G. Tajú, Patrícia A. Miyasato, Rafaela P. de Freitas, Lenita de F. Tallarico, Guilherme S. dos Santos, Giovana L. F. Luiz, Henrique K. Rofatto, Fábio N. V. da Silva, Pio Colepicolo, and et al. 2021. "The Prospective Use of Brazilian Marine Macroalgae in Schistosomiasis Control" Marine Drugs 19, no. 5: 234. https://doi.org/10.3390/md19050234
APA StyleStein, E. M., Tajú, S. G., Miyasato, P. A., de Freitas, R. P., Tallarico, L. d. F., dos Santos, G. S., Luiz, G. L. F., Rofatto, H. K., da Silva, F. N. V., Colepicolo, P., Macedo, A. L., Carollo, C. A., & Nakano, E. (2021). The Prospective Use of Brazilian Marine Macroalgae in Schistosomiasis Control. Marine Drugs, 19(5), 234. https://doi.org/10.3390/md19050234







