Omega-3-Rich Oils from Marine Side Streams and Their Potential Application in Food
Abstract
1. Introduction
2. Marine Side Streams as Sources of Omega-3 Fatty Acids
| Fishbone | Gills | Guts | Head | Liver | Skin | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Alaska Pink Salmon (Oncorhynchus gorbuscha) | C18:3n-3 | - | - | 0.95 | 1.10 | - | - | [28] |
| C20:5n-3 | - | - | 10.93 | 7.56 | - | - | ||
| C22:5n-3 | - | - | 2.83 | 2.33 | - | - | ||
| C22:6n-3 | - | - | 17.32 | 11.77 | - | - | ||
| DHA/EPA | - | - | 1.59 | 1.56 | - | - | ||
| Alaska Walleye Pollock (Theragra chalcogramma) | C18:3n-3 | - | - | 0.47 | 0.34 | - | 0.33 | |
| C20:5n-3 | - | - | 14.99 | 12.47 | - | 16.85 | ||
| C22:5n-3 | - | - | 1.29 | 0.55 | - | 0.36 | ||
| C22:6n-3 | - | - | 6.41 | 11.82 | - | 12.89 | ||
| DHA/EPA | - | - | 0.43 | 0.95 | - | 0.77 | ||
| Black rockfish (Sebastes melanops) | C18:3n-3 | - | - | - | 0.51 | 0.14 | - | [29] |
| C20:5n-3 | - | - | - | 9.92 | 4.43 | - | ||
| C22:5n-3 | - | - | - | 1.65 | 1.38 | - | ||
| C22:6n-3 | - | - | - | 9.21 | 4.78 | - | ||
| DHA/EPA | - | - | - | 0.93 | 1.08 | - | ||
| Black Sea Anchovy (Engraulis encrasicholus) | C18:3n-3 | - | - | 1.31 | 1.62 | - | - | [30] |
| C20:5n-3 | - | - | 6.93 | 10.97 | - | - | ||
| C22:6n-3 | - | - | 18.88 | 21.34 | - | - | ||
| DHA/EPA | - | - | 2.72 | 1.95 | - | - | ||
| Pacific ocean perch (Sebastes alutus) | C20:5n-3 | - | - | 7.1 | 9.9 | - | - | [25] |
| C22:6n-3 | - | - | 3.3 | 4.7 | - | - | ||
| DHA/EPA | - | - | 0.47 | 0.48 | - | - | ||
| Sardine (Sardinella lemuru) | C20:5n-3 | - | - | 1.73 | 1.84 | 2.76 | - | [31] |
| C22:6n-3 | - | - | 11.87 | 15.95 | 12.97 | - | ||
| DHA/EPA | - | - | 6.86 | 8.67 | 4.70 | |||
| Salmon (Salmo salar) | C18:3n-3 | - | - | 1.14 | - | - | - | [32] |
| C20:5n-3 | - | - | 7.91 | - | - | - | ||
| C22:5n-3 | - | - | 3.48 | - | - | - | ||
| C22:6n-3 | - | - | 6.99 | - | - | - | ||
| DHA/EPA | - | - | 0.88 | - | - | - | ||
| Sea bream (Sparus aurata) | C18:3n-3 | 3.86 | 4.05 | 4.71 | 3.86 | 4.13 | 4.73 | [8] |
| C20:5n-3 | 2.77 | 1.92 | 1.83 | 2.78 | 1.91 | 2.03 | ||
| C22:5n-3 | 2.00 | 1.42 | 1.55 | 2.00 | 2.04 | 1.66 | ||
| C22:6n-3 | 4.58 | 4.09 | 3.51 | 5.00 | 4.90 | 3.98 | ||
| DHA/EPA | 1.65 | 2.13 | 1.91 | 1.80 | 2.57 | 1.96 | ||
| Sea bass (Dicentrarchus labrax) | C18:3n-3 | 3.77 | 2.70 | 3.30 | 3.69 | 2.00 | 3.10 | [33] |
| C20:5n-3 | 3.37 | 4.40 | 4.20 | 3.50 | 3.00 | 5.10 | ||
| C22:5n-3 | 1.06 | 0.96 | 1.10 | 1.20 | 0.84 | 1.20 | ||
| C22:6n-3 | 4.40 | 6.50 | 5.30 | 5.50 | 4.50 | 7.00 | ||
| DHA/EPA | 1.31 | 1.48 | 1.26 | 1.57 | 1.50 | 1.37 | ||
| Tuna (Euthynnus affinis) | C20:5n-3 | - | - | 2.71 | 1.48 | 1.70 | - | [34] |
| C22:6n-3 | - | - | 14.31 | 15.70 | 14.18 | - | ||
| DHA/EPA | - | - | 5.28 | 10.61 | 8.34 | - |
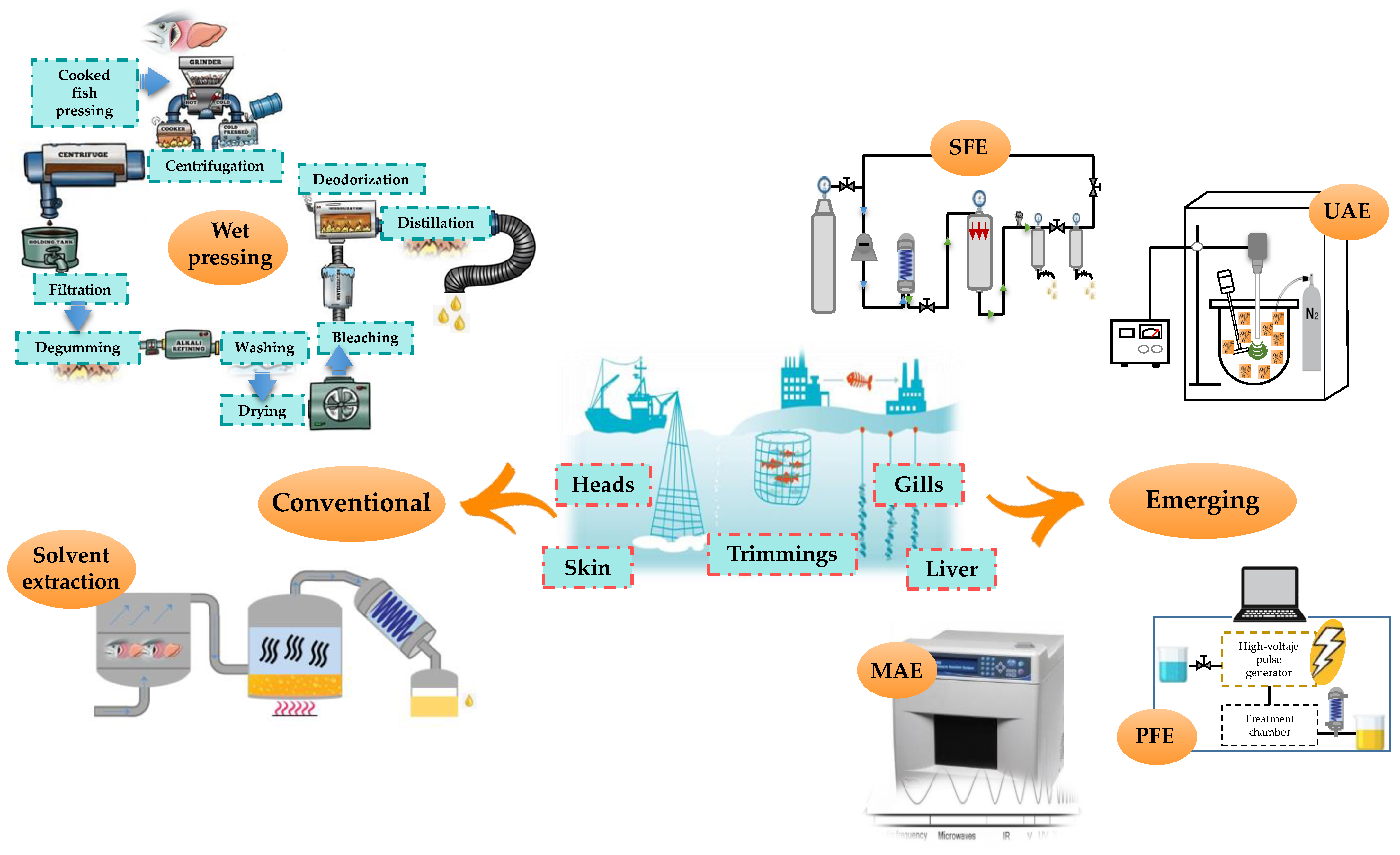
3. Extraction of Omega-3 Fatty Acids from Marine Side Streams
3.1. Conventional Extraction
3.2. Alternative Green Extraction Technologies
3.2.1. Supercritical Fluid Extraction (SFE)
| Side Stream | Source | SFE Conditions | Outcomes | Ref. |
|---|---|---|---|---|
| Caviar and viscera | Carp (Cyprinus carpio L.) | Temperature: 40, 50, and 60 °C Pressure: 200, 300, 350, and 400 bar CO2 flow: 0.194 kg/h Extraction time: 180 min | High yields (>50 g/100 g) in viscera, which are similar to those obtained with conventional methods | [51] |
| Head | Thunnus tonggol | Temperature: 65 °C Pressure: 40 MPa CO2 flow with ethanol: 3 mL/min Extraction time: 2 h | Co-solvent allowed to extract omega-3 after oil fractionations | [46] |
| Head, shells and tails | Northern shrimp (Pandalus borealis Kreyer) | Temperature: 40 °C Pressure: 35 MPa CO2 flow: 3–5 L/min Extraction time: 90 min | Lower yields (137 mg oil/g) than those obtained with solvent extraction. Higher fatty acid (795 mg/g), EPA (7.8%), and DHA (8%) contents | [49] |
| Heads and tails | Sardine | Temperature: 75 °C Pressure: 300 bar CO2 flow: 2.5 mL/min Extraction time: 45 min | Increased extraction yields: DHA (59%) and EPA (28%) | [47] |
| Liver | Rock lobsters (Jasus edwardsii) | Temperature: 50 °C Pressure: 35 MPa Continuous CO2 flow: 0.434 kg/h Extraction time: 4 h | Enrichment in PUFAs (DHA, EPA) vs. Soxhlet extraction Reduction of toxic heavy metals | [50] |
| Off-cuts | Hake (Merluccius capensis– Merluccius paradoxus) Orange roughy (Hoplostethus atlanticus) Salmon (Salmo salar) | Temperature: 313 K Pressure: 25 MPa CO2 flow: 880 kg/m3 | Increased fish oil stability Reduction of impurities Co-extraction of some endogenous volatile compounds | [48] |
| Liver | Jumbo squid (Dosidicus gigas) | |||
| Skins, scales and bones | Bigeye tuna (Thunnus obesus) | Temperature: 40 °C Pressure: 25 MPa CO2 flow: 10 kg/h | Recovery of 85.6, 83.2, and 87.7% of oil from skins, scales, and bones. EPA + DHA contents of 26.7–28.3% | [45] |
3.2.2. Other Alternative Green Analytical Techniques
| Side Stream | Source | Extraction Conditions | Outcomes | Ref. |
|---|---|---|---|---|
| By-products | Catfish (Pangasianodon gigas–Pangasianodon hypothalamus) | MAE: 110 W, 1 min Enzymatic hydrolysis: Alcalase 2%, 2 h, 120 rpm | Pretreatments with MAE improved extraction yield and oil quality (lower lipid oxidation). Omega-3 contents 7.54 and 8.62% for EPA and DHA. | [57] |
| Cephalothorax | Pacific white shrimp (Litopenaeus vannamei) | PEF: 16 kV/cm, 240 pulses UAE: 80% amplitude, 25 min | Improved lipid extraction yield (30.34 g/100 g). Higher content of PUFAs (40.99 g/100 g lipids) and reduction of lipid oxidation. Omega-3 contents 8.20 and 10.39 g/100 g lipids for EPA and DHA. | [56] |
| Head | Rohu (Labeo rohita) | UAE: 20 kHz, 40% amplitude, 5–15 min. MAE: 200 W, 50 °C, 5–15 min Enzymatic hydrolysis: Protamex ratio of 1:100 (w/w), 2 h, 150 rpm, 55 °C | Pretreatments with UAE and MAE improved the extraction yield (67.48 and 69.75%, respectively). Omega-3 contents 0.86–0.88 and 0.13–0.16% for EPA and DHA. | [54] |
| Liver | Cobia (Rachycentron canachum) | UAE: 40 kHz, 1 h | Omega-3 contents 4.45 and 16.09% for EPA and DHA. | [58] |
| Viscera | Bighead carp | UAE: 400 W, 50 °C, 57 min | Extraction yield of oil reached 94.82%. Oil within standards of super fine crude fish oil. | [59] |
4. Enrichment of Foods with Omega-3 Fatty Acids
4.1. Bakery Products
| Side Stream | Food Product | Dose and Incorporation | Storage Conditions | Outcomes | Ref. |
|---|---|---|---|---|---|
| Pacific whiteshrimp (Litopenaeus vannamei) hepatopancreas | Biscuits | Microencapsulates: 0, 3, 6, 9, and 12% (w/w) | 12 days at 30 °C | No adverse effects on quality and acceptability up to 6% No marked change in EPA and DHA contents were noticeable after 12 days of storage Dark storage ensures its oxidative stability | [72] |
| Bread | Microencapsulates: 0, 1, 3, and 5% (w/w) | 3 days | No adverse effect on quality and sensory acceptability were observed up to 3% Oxidation took place in bread fortified with 5% | [71] | |
| Sardine (Sardinella brasiliensis) head | Flour | 20% | Final product | Increased content of DHA and EPA High acceptability index | [73] |
| Sardine (Sardina pilchardus) gills and viscera | Wheat flour-based chips | 3.6% (v/w) | Improved nutritional (EPA 6.82% and DHA 8.27%) and health effects (antidiabetic, antihyperlipidemic, and histoprotective) | [74] | |
| Sea bass (Dicentrarchus labrax) trimmings and small pieces | Fresh pasta | 10% | 90 days at refrigerated storage | Improvement of nutritional values. Decrease in hardness and cooking time Some sensory changes observed, mainly in fishy odor, and decrease in intensity of the typical yellow color | [75] |
| Cod liver | Cream cheese | Emulsion with CAS, WPI, or MPL: 1.3% (w/w) | 20 weeks at 4.6 °C | Decreased oxidative stability (>5 weeks). MPL resulted in a more oxidative stable product | [76] |
| Pacific white shrimp (Litopenaeus vannamei) cephalothorax | Milk | Nanoliposomes: 0.05–0.2 g/100 mL | 15 days at 4 °C | Half of EPA and DHA were bioaccessible for adsorption by the body in the gastrointestinal tract | [77] |
| Fish oil | Yogurt | Nanoencapsulates: 2% (v/v) | 21 days at 4 °C | Higher DHA and EPA contents than yogurt with FFO. Reduction in acidity, syneresis, and PV. Sensory characteristics closer to control | [78] |
| Microcapsules: 0.15% (w/w) | 21 days at 6 °C | Improvement in health-promoting effect and consistency | [79] | ||
| Red salmon (Oncorhynchus nerka) heads | Strawberry-flavored yogurt | Microencapsulates: 2% (w/v) | 30 days at 4 °C | No significant modification of physicochemical characteristics (pH, color, and WHC). After 4 weeks, EPA (2.11% of TFA) and DHA (1.72% of TFA) were the main fatty acids | [80] |
| Cod liver oil | Chicken nuggets | EPA + DHA in BFO and MFO nuggets was 150 mg/100 g MFO: 5% (w/w) BFO: 0.5%(w/w) | Final product | MFO provides lipid (<0.5 mg MDA/kg; hexanal 35.03 AU × 106) and protein oxidation stability (≅3.6 nmol/mg). No effects of MFO on sensory attributes | [81] |
| Cooked and dry-cured meat products | Mo (2.75% w/w) and Mu (5.26% w/w) emulsions | 4 months at 0–5 °C | Enrichment in EPA and DHA: “source of ω-3 fatty acids”, without affecting main quality characteristics. | [82] |
4.2. Dairy Products
4.3. Meat Products
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djekic, I.; Nikolić, A.; Uzunović, M.; Marijke, A.; Liu, A.; Han, J.; Brnčić, M.; Knežević, N.; Papademas, P.; Lemoniati, K.; et al. Covid-19 pandemic effects on food safety—Multi-country survey study. Food Control 2021, 122, 107800. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Cantalapiedra, J.; Zapata, C.; Franco, J.M.; Franco, D. Aquaculture and by-products: Challenges and opportunities in the use of alternative protein sources and bioactive compounds. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2020; Volume 92, pp. 127–185. [Google Scholar]
- Stenmarck, Å.; Jensen, C.; Quested, T.; Moates, G.; FUSIONS. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016; pp. 1–79. [Google Scholar]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J.; Al Khawli, F.; et al. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P.E.S.; Domínguez, R.; Wang, M.; Barba, F.J.; Bermúdez, R.; Lorenzo, J.M. Nutritional profiling and the value of processing by-products from gilthead sea bream (Sparus aurata). Mar. Drugs 2020, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 amending Directive 2008/98/EC on waste. Off. J. Eur. Union 2018, L150, 109–140.
- European Commission EU Actions against Food Waste. Available online: https://ec.europa.eu/food/safety/food_waste/eu_actions_en#:~:text=TheRevisedEUWasteLegislation (accessed on 5 December 2020).
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Ahmad, T.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A. Marine waste utilization as a source of functional and health compounds. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: London, UK, 2019; pp. 187–254. [Google Scholar]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Pethybridge, H.R.; Parrish, C.C.; Morrongiello, J.; Young, J.W.; Farley, J.H.; Gunasekera, R.M.; Nichols, P.D. Spatial Patterns and Temperature Predictions of Tuna Fatty Acids: Tracing Essential Nutrients and Changes in Primary Producers. PLoS ONE 2015, 10, e0131598. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, M.I.; Sushchik, N.N.; Tolomeev, A.P.; Dgebuadze, Y.Y. Meta-analysis of factors associated with omega-3 fatty acid contents of wild fish. Rev. Fish Biol. Fish. 2018, 28, 277–299. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Enhancing and improving the extraction of omega-3 from fish oil. Sustain. Chem. Pharm. 2017, 5, 54–59. [Google Scholar] [CrossRef]
- Ucak, I.; Afreen, M.; Montesano, D.; Carrillo, C.; Tomasevic, I.; Simal-Gandara, J.; Barba, F.J. Functional and Bioactive Properties of Peptides Derived from Marine Side Streams. Mar. Drugs 2021, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Atef, M.; Ojagh, S.M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Bogue, J.; Collins, O.; Troy, A.J. Market analysis and concept development of functional foods. In Developing New Functional Food and Nutraceutical Products; Bagchi, D., Nair, S., Eds.; Elsevier: London, UK, 2017; pp. 29–45. ISBN 9780128027790. [Google Scholar]
- Grand View Research. Omega 3 Market Size, Share and Trends Analysis Peport by Type (EPA, DHA, ALA), by Source (Marine Source, Plant Source), by Application, by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/omega-3-market (accessed on 15 February 2021).
- Schneider, M. Marine phospholipids and their applications: Next generation omega-3 lipids. In Omega-6/3 Fatty Acids: Functions, Sustainability Strategies and Perspectives; De Meester, F., Watson, R.R., Zibadi, S., Eds.; Springer Science & Business Media: New York, NY, USA, 2012; pp. 297–308. [Google Scholar]
- Bechtel, P.J.; Morey, A.; Oliveira, A.C.M.; Wu, T.H.; Plante, S.; Bower, C.K. Chemical and nutritional properties of pacific ocean perch (Sebastes alutus) whole fish and by-products. J. Food Process. Preserv. 2010, 34, 55–72. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chem. 2006, 99, 83–91. [Google Scholar] [CrossRef]
- Shang, T.; Liu, L.; Zhou, J.; Zhang, M.; Hu, Q.; Fang, M.; Wu, Y.; Yao, P.; Gong, Z. Protective effects of various ratios of DHA/EPA supplementation on high-fat diet-induced liver damage in mice. Lipids Health Dis. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Oliveira, A.C.M.; Bechtel, P.J. Lipid composition of Alaska pink salmon (Oncorhynchus gorbuscha) and Alaska walleye pollock (Theragra chalcogramma) byproducts. J. Aquat. Food Prod. Technol. 2005, 14, 73–91. [Google Scholar] [CrossRef]
- Oliveira, A.C.M.; Bechtel, P.J.; Lapis, T.J.; Brenner, K.A.; Ellingson, R. Chemical composition of black rockfish (Sebastes melanops) fillets and byproducts. J. Food Process. Preserv. 2011, 35, 466–473. [Google Scholar] [CrossRef]
- Gencbay, G.; Turhan, S. Proximate composition and nutritional profile of the black sea anchovy (Engraulis encrasicholus) whole fish, fillets, and by-products. J. Aquat. Food Prod. Technol. 2016, 25, 864–874. [Google Scholar] [CrossRef]
- Khoddami, A.; Ariffin, A.A.; Bakar, J.; Ghazali, H.M. Fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Sardinella lemuru). World Appl. Sci. J. 2009, 7, 127–131. [Google Scholar]
- Sun, T.; Xu, Z.; Prinyawiwatkul, W. FA composition of the oil extracted from farmed Atlantic salmon (Salmo salar L.) viscera. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 615–619. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Zhou, J.; Barba, F.J.; Lorenzo, J.M. Nutritional Characterization of Sea Bass Processing By-Products. Biomolecules 2020, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Khoddami, A.; Ariffin, A.A.; Bakar, J.; Ghazali, H.M. Quality and fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Euthynnus affinis). Afr. J. Biotechnol. 2012, 11, 1683–1689. [Google Scholar] [CrossRef]
- FAO. Farmed Fish: A Major Provider or a Major Consumer of Omega-3 Oils? GLOBEFISH—Information and Analysis on World Fish Trade 2020. Available online: http://www.fao.org/in-action/globefish/fishery-information/resource-detail/es/c/338773/ (accessed on 15 February 2021).
- Gulzar, S.; Raju, N.; Chandragiri Nagarajarao, R.; Benjakul, S. Oil and pigments from shrimp processing by-products: Extraction, composition, bioactivities and its application—A review. Trends Food Sci. Technol. 2020, 100, 307–319. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Recovery of carotenoids from shrimp waste in organic solvents. Waste Manag. 2006, 26, 1092–1098. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Ultrasound Waves Increase the Yield and Carotenoid Content of Lipid Extracted From Cephalothorax of Pacific White Shrimp (Litopenaeus vannamei). Eur. J. Lipid Sci. Technol. 2018, 120, 1700495. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Fiori, L.; Manfrini, M.; Castello, D. Supercritical CO2 fractionation of omega-3 lipids from fish by-products: Plant and process design, modeling, economic feasibility. Food Bioprod. Process. 2014, 92, 120–132. [Google Scholar] [CrossRef]
- Rahimi, M.A.; Omar, R.; Ethaib, S.; Siti Mazlina, M.K.; Awang Biak, D.R.; Nor Aisyah, R. Microwave-assisted extraction of lipid from fish waste. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012096. [Google Scholar] [CrossRef]
- Haq, M.; Ahmed, R.; Cho, Y.J.; Chun, B.S. Quality Properties and Bio-potentiality of Edible Oils from Atlantic Salmon By-products Extracted by Supercritial Carbon Dioxide and Conventional Methods. Waste Biomass Valorization 2017, 8, 1953–1967. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, S.; Hu, W.; Zhang, J.; Ding, Y.; Liu, J. Extraction of Oil from High-Moisture Tuna Livers by Subcritical Dimethyl Ether: A Comparison with Different Extraction Methods. Eur. J. Lipid Sci. Technol. 2019, 121, 1–12. [Google Scholar] [CrossRef]
- Ahmed, R.; Haq, M.; Cho, Y.J.; Chun, B.S. Quality evaluation of oil recovered from by-products of bigeye tuna using supercritical carbon dioxide extraction. Turk. J. Fish. Aquat. Sci. 2017, 17, 663–672. [Google Scholar]
- Ferdosh, S.; Sarker, M.Z.I.; Rahman, N.N.N.A.; Akanda, M.J.H.; Ghafoor, K.; Kadir, M.O.A. Simultaneous Extraction and Fractionation of Fish Oil from Tuna By-Product Using Supercritical Carbon Dioxide (SC-CO2). J. Aquat. Food Prod. Technol. 2016, 25, 230–239. [Google Scholar] [CrossRef]
- Létisse, M.; Comeau, L. Enrichment of eicosapentaenoic acid and docosahexaenoic acid from sardine by-products by supercritical fluid fractionation. J. Sep. Sci. 2008, 31, 1374–1380. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of the omega-3 rich oil contained in hake (Merluccius capensis-Merluccius paradoxus) by-products: Study of the influence of process parameters on the extraction yield and oil quality. J. Supercrit. Fluids 2008, 47, 215–226. [Google Scholar] [CrossRef]
- Treyvaud Amiguet, V.; Kramp, K.L.; Mao, J.; McRae, C.; Goulah, A.; Kimpe, L.E.; Blais, J.M.; Arnason, J.T. Supercritical carbon dioxide extraction of polyunsaturated fatty acids from Northern shrimp (Pandalus borealis Kreyer) processing by-products. Food Chem. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W.; Barber, A.R.; Su, P.; He, S. Significant enrichment of polyunsaturated fatty acids (PUFAs) in the lipids extracted by supercritical CO2 from the livers of Australian rock lobsters (Jasus edwardsii). J. Agric. Food Chem. 2015, 63, 4621–4628. [Google Scholar] [CrossRef]
- Kuvendziev, S.; Lisichkov, K.; Zeković, Z.; Marinkovski, M.; Musliu, Z.H. Supercritical fluid extraction of fish oil from common carp (Cyprinus carpio L.) tissues. J. Supercrit. Fluids 2018, 133, 528–534. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Impact of pretreatment and atmosphere on quality of lipids extracted from cephalothorax of Pacific white shrimp by ultrasonic assisted process. Food Chem. 2020, 309, 125732. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Benjakul, S. Effect of pre-treatments on yield and properties of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasonic assisted process. LWT 2019, 100, 106–113. [Google Scholar] [CrossRef]
- Bruno, S.F.; Kudre, T.G.; Bhaskar, N. Impact of pretreatment-assisted enzymatic extraction on recovery, physicochemical and rheological properties of oil from Labeo rohita head. J. Food Process Eng. 2019, 42, e12990. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Benjakul, S. Impact of pulsed electric field pretreatment on yield and quality of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasound-assisted process. Int. J. Food Sci. Technol. 2020, 55, 619–630. [Google Scholar] [CrossRef]
- Chimsook, T.; Wannalangka, W. Effect of microwave pretreatment on extraction yield and quality of catfish oil in Northern Thailand. MATEC Web Conf. 2015, 35, 04001. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Liao, H.-Z.; Wang, Y.-H.; Wang, H.-M.D.; Shieh, C.-J.; Tseng, C.-Y. Highly efficient extraction of EPA/DHA-enriched oil from cobia liver using homogenization plus sonication. Eur. J. Lipid Sci. Technol. 2017, 119, 1600466. [Google Scholar] [CrossRef]
- Xiao, L.; Ji, Y.; Zhaoshuo, Y.; Wenjiao, L.; Xing, Z.; Chengjin, M. Ultrasound-assisted extraction of bighead carp viscera oil and its physiochemical properties. J. Jishou Univ. Nat. Sci. Ed. 2017, 38, 49. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal-Gandara, J. Advantages of techniques to fortify food products with the benefits of fish oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Feizollahi, E.; Hadian, Z.; Honarvar, Z. Food Fortification with Omega-3 Fatty Acids; Microencapsulation as an Addition Method. Curr. Nutr. Food Sci. 2018, 14, 90–103. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Agregán, R.; Lorenzo, J.M. Effect of the partial replacement of pork backfat by microencapsulated fish oil or mixed fish and olive oil on the quality of frankfurter type sausage. J. Food Sci. Technol. 2017, 54, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 116/2010 Regulation (EU) No 116/2010 of 9 February 2010, amending Regulation (EC) No. 1924/2006 of the European Parliament and of the Council with regard to the list of nutrition claims. Off. J. Eur. Union 2010, L37, 16–18.
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Ismail, A.; Bannenberg, G.; Rice, H.B.; Schutt, E.; MacKay, D. Oxidation in EPA- and DHA-rich oils: An overview. Lipid Technol. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Gómez, B.; Barba, F.J.; Domínguez, R.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Toldrá, F.; Lorenzo, J.M. Microencapsulation of antioxidant compounds through innovative technologies and its specific application in meat processing. Trends Food Sci. Technol. 2018, 82, 135–147. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Ishwarya, S.P. Spray drying for encapsulation. In Spray Drying Techniques for Food Ingredient Encapsulation; Anandharamakrishnan, C., Ishwarya, S.P., Eds.; John Wiley & Sons, Ltd.: Chicago, IL, USA, 2015; pp. 65–76. ISBN 9781118864197. [Google Scholar]
- Kadam, S.U.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Irshad, S.; Xiong, Z.; Xiong, H.; Shahbaz, H.M.; Siddique, F. Valorization of fisheries by-products: Challenges and technical concerns to food industry. Trends Food Sci. Technol. 2020, 99, 34–43. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; H-kittikun, A. Characteristics and oxidative stability of bread fortified with encapsulated shrimp oil. Ital. J. Food Sci. 2015, 27, 476–486. [Google Scholar]
- Takeungwongtrakul, S.; Benjakul, S. Biscuits fortified with micro-encapsulated shrimp oil: Characteristics and storage stability. J. Food Sci. Technol. 2017, 54, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.N.; Stevanato, F.B.; Visentainer, J.V. Food supplementation for workers: Flour enriched with omega-3. Acta Sci. Technol. 2015, 37, 133–139. [Google Scholar] [CrossRef]
- Benkhoud, H.; Baâti, T.; Njim, L.; Selmi, S.; Hosni, K. Antioxidant, antidiabetic, and antihyperlipidemic activities of wheat flour-based chips incorporated with omega-3-rich fish oil and artichoke powder. J. Food Biochem. 2020, e13297. [Google Scholar] [CrossRef] [PubMed]
- Ainsa, A.; Marquina, P.L.; Roncalés, P.; Beltrán, J.A.; Calanche M, J.B. Enriched Fresh Pasta with a Sea Bass By-Product, a Novel Food: Fatty Acid Stability and Sensory Properties throughout Shelf Life. Foods 2021, 10, 255. [Google Scholar] [CrossRef]
- Horn, A.F.; Green-Petersen, D.; Nielsen, N.S.; Andersen, U.; Hyldig, G.; Jensen, L.H.S.; Horsewell, A.; Jacobsen, C. Addition of Fish Oil to Cream Cheese Affects Lipid Oxidation, Sensory Stability and Microstructure. Agriculture 2012, 2, 359–375. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S.; Hozzein, W.N. Impact of β-glucan on debittering, bioaccessibility and storage stability of skim milk fortified with shrimp oil nanoliposomes. Int. J. Food Sci. Technol. 2020, 55, 2092–2103. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef]
- Tamjidi, F.; Nasirpour, A.; Shahedi, M. Rheological Characteristics of Yogurt Enriched with Microencapsulated Fish Oil. J. Agric. Sci. Technol. 2014, 16, 1073–1082. [Google Scholar]
- Estrada, J.D.; Boeneke, C.; Bechtel, P.; Sathivel, S. Developing a strawberry yogurt fortified with marine fish oil. J. Dairy Sci. 2011, 94, 5760–5769. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Palacios, T.; Ruiz-Carrascal, J.; Jiménez-Martín, E.; Solomando, J.C.; Antequera, T. Improving the lipid profile of ready-to-cook meat products by addition of omega-3 microcapsules: Effect on oxidation and sensory analysis. J. Sci. Food Agric. 2018, 98, 5302–5312. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Perez-Palacios, T. Evaluating the use of fish oil microcapsules as omega-3 vehicle in cooked and dry-cured sausages as affected by their processing, storage and cooking. Meat Sci. 2020, 162, 108031. [Google Scholar] [CrossRef]
- Stevanato, F.B.; Cottica, S.M.; Petenuci, M.E.; Matsushita, M.; Desouza, N.E.; Visentainer, J.V. Evaluation of processing, preservation and chemical and fatty acid composition of Nile tilapia waste. J. Food Process. Preserv. 2010, 34, 373–383. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 536/2013 of 11 June 2013 amending Regulation (EU) No 432/2012 establishing a list of permitted health claims made on foods other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1–40.
- Ferreira, V.L.P.; Almeida, T.C.A.; Pettineli, M.L.C.V.; Silva, M.A.A.P.; Chaves, J.B.P.; Barbosa, E.M.M. Análise Sensorial: Testes Discriminativos e Afetivos; Sociedade Brasileira de Ciência e Tecnologia de Alimentos: Campinas, Brasil, 2000. [Google Scholar]
- Rasti, B.; Erfanian, A.; Selamat, J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017, 230, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins; a systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077. [Google Scholar] [CrossRef]
- Hinriksdottir, H.H.; Jonsdottir, V.L.; Sveinsdottir, K.; Martinsdottir, E.; Ramel, A. Bioavailability of long-chain n-3 fatty acids from enriched meals and from microencapsulated powder. Eur. J. Clin. Nutr. 2015, 69, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Hermida, L.G.; Gallardo, G. Food applications of microencapsulated omega-3 oils. In Microencapsulation and Microspheres for Food Applications; Sagis, L., Ed.; Academic Press: London, UK, 2015; pp. 271–299. ISBN 9780128003503. [Google Scholar]
- Bakry, A.M.; Chen, Y.Q.; Liang, L. Developing a mint yogurt enriched with omega-3 oil: Physiochemical, microbiological, rheological, and sensorial characteristics. J. Food Process. Preserv. 2019, 43, e14287. [Google Scholar] [CrossRef]
- Tamjidi, F.; Nasirpour, A.; Shahedi, M. Physicochemical and sensory properties of yogurt enriched with microencapsulated fish oil. Food Sci. Technol. Int. 2012, 18, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Sanz, T.; Salvador, A.; Jiménez, A.; Fiszman, S.M. Yogurt enrichment with functional asparagus fibre. Effect of fibre extraction method on rheological properties, colour, and sensory acceptance. Eur. Food Res. Technol. 2008, 227, 1515–1521. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; de Carvalho, F.A.L.; Domínguez, R.; Trindade, M.A.; Pateiro, M.; Lorenzo, J.M. Healthy beef burgers: Effect of animal fat replacement by algal and wheat germ oil emulsions. Meat Sci. 2021, 173, 108396. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.S.; Pateiro, M.; Campagnol, P.C.B.; Domínguez, R. Healthy Spanish salchichón enriched with encapsulated n − 3 long chain fatty acids in konjac glucomannan matrix. Food Res. Int. 2016, 89, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Horita, C.N.; Baptista, R.C.; Caturla, M.Y.R.; Lorenzo, J.M.; Barba, F.J.; Sant’Ana, A.S. Combining reformulation, active packaging and non-thermal post-packaging decontamination technologies to increase the microbiological quality and safety of cooked ready-to-eat meat products. Trends Food Sci. Technol. 2018, 72, 45–61. [Google Scholar] [CrossRef]
- Georgantelis, D.; Blekas, G.; Katikou, P.; Ambrosiadis, I.; Fletouris, D.J. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Sci. 2007, 75, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martín, E.; Pérez-Palacios, T.; Carrascal, J.R.; Rojas, T.A. Enrichment of chicken nuggets with microencapsulated omega-3 fish oil: Effect of frozen storage time on oxidative stability and sensory quality. Food Bioprocess Technol. 2016, 9, 285–297. [Google Scholar] [CrossRef]
- Aquilani, C.; Pérez-Palacios, T.; Sirtori, F.; Jiménez-Martín, E.; Antequera, T.; Franci, O.; Acciaioli, A.; Bozzi, R.; Pugliese, C. Enrichment of Cinta Senese burgers with omega-3 fatty acids. Effect of type of addition and storage conditions on quality characteristics. Grasas Y Aceites 2018, 69, e235. [Google Scholar] [CrossRef]
- Henna Lu, F.S.; Norziah, M.H. Contribution of microencapsulated n-3 PUFA powder toward sensory and oxidative stability of bread. J. Food Process. Preserv. 2011, 35, 596–604. [Google Scholar] [CrossRef]
- De Conto, L.C.; Porto Oliveira, R.S.; Pereira Martin, L.G.; Chang, Y.K.; Steel, C.J. Effects of the addition of microencapsulated omega-3 and rosemary extract on the technological and sensory quality of white pan bread. LWT Food Sci. Technol. 2012, 45, 103–109. [Google Scholar] [CrossRef]
- Keenan, D.F.; Resconi, V.C.; Smyth, T.J.; Botinestean, C.; Lefranc, C.; Kerry, J.P.; Hamill, R.M. The effect of partial-fat substitutions with encapsulated and unencapsulated fish oils on the technological and eating quality of beef burgers over storage. Meat Sci. 2015, 107, 75–85. [Google Scholar] [CrossRef]
- Domínguez, R.; Bohrer, B.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Recent discoveries in the field of lipid bio-based ingredients for meat processing. Molecules 2021, 26, 190. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Munekata, P.E.; Pateiro, M.; López-Fernández, O.; Lorenzo, J.M. Immobilization of oils using hydrogels as strategy to replace animal fats and improve the healthiness of meat products. Curr. Opin. Food Sci. 2021, 37, 135–144. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Campagnol, P.C.B.; Lorenzo, J.M.; Sichetti Munekata, P.E.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Influence of partial pork backfat replacement by fish oil on nutritional and technological properties of liver pâté. Eur. J. Lipid Sci. Technol. 2017, 119, 1600178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pateiro, M.; Domínguez, R.; Varzakas, T.; Munekata, P.E.S.; Movilla Fierro, E.; Lorenzo, J.M. Omega-3-Rich Oils from Marine Side Streams and Their Potential Application in Food. Mar. Drugs 2021, 19, 233. https://doi.org/10.3390/md19050233
Pateiro M, Domínguez R, Varzakas T, Munekata PES, Movilla Fierro E, Lorenzo JM. Omega-3-Rich Oils from Marine Side Streams and Their Potential Application in Food. Marine Drugs. 2021; 19(5):233. https://doi.org/10.3390/md19050233
Chicago/Turabian StylePateiro, Mirian, Rubén Domínguez, Theodoros Varzakas, Paulo E. S. Munekata, Elena Movilla Fierro, and José M. Lorenzo. 2021. "Omega-3-Rich Oils from Marine Side Streams and Their Potential Application in Food" Marine Drugs 19, no. 5: 233. https://doi.org/10.3390/md19050233
APA StylePateiro, M., Domínguez, R., Varzakas, T., Munekata, P. E. S., Movilla Fierro, E., & Lorenzo, J. M. (2021). Omega-3-Rich Oils from Marine Side Streams and Their Potential Application in Food. Marine Drugs, 19(5), 233. https://doi.org/10.3390/md19050233











