Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum nigrum M13 and Alternaria alternata 13A
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of the Endophytic Fungal Strains
2.2. Fermentation, Isolation, and Structure Elucidation
2.3. Biological Activity
2.3.1. Antimicrobial Activity
2.3.2. Biofilm Inhibitory Activity
3. Material and Methods
3.1. General Experimental Procedures
3.2. Fungal Strains
3.3. Cultivation and Fermentation of Endophytic Fungi
3.4. Extraction and Isolation
3.5. Advanced Marfey’s Analysis
3.6. Mosher Ester Analysis
3.7. Bioactivity Studies
3.7.1. Antimicrobial Assay
3.7.2. Biofilm Inhibitory Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nogawa, T.; Kawatani, M.; Okano, A.; Futamura, Y.; Aono, H.; Shimizu, T.; Kato, N.; Kikuchi, H.; Osada, H. Structure and biological activity of Metarhizin C, a stereoisomer of BR-050 from Tolypocladium album RK17-F0007. J. Antibiot. 2019, 72, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Bernan, V.S.; Greenstein, M.; Maiese, W.M. Marine microorganisms as a source of new natural products. Adv. Appl. Microbiol. 1997, 43, 57–90. [Google Scholar] [PubMed]
- El-Hady, F.K.A.; Shaker, K.H.; Souleman, A.M.; Fayad, W.; Abdel-Aziz, M.S.; Hamed, A.A.; Iodice, C.; Tommonaro, G. Comparative correlation between chemical composition and cytotoxic potential of the coral-associated fungus Aspergillus sp. 2C1-EGY against human colon cancer cells. Curr. Microbiol. 2017, 74, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- El-Neekety, A.A.; Abdel-Aziz, M.S.; Hathout, A.S.; Hamed, A.A.; Sabry, B.A.; Ghareeb, M.A.; Aly, S.E.; Abdel-Wahhab, M.A. Molecular identification of newly isolated non-toxigenic fungal strains having antiaflatoxigenic, antimicrobial and antioxidant activities. Der Pharm. Chem. 2016, 8, 121–134. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Imhoff, J. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Schol–Schwarz, M.B. The genus Epicoccum Link. Trans. Brit. Mycol. Soc. 1959, 42, 149–173. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterisation, and toxicological potential of Alternaria-mycotoxins (TeA, AOH and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Baute, M.A.; Deffieux, G.; Baute, R.; Neveu, A. New antibiotics from the fungus Epicoccum nigrum. J. Antibiot. 1978, 31, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- de Lima Favaro, L.C.; de Souza Sebastianes, F.L.; Araújo, W.L. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS ONE 2012, 7, e36826. [Google Scholar]
- Bamford, P.C.; Norris, G.L.F.; Ward, G. Flavipin production by Epicoccum spp. Trans. Brit. Mycol. Soc. 1961, 44, 354–356. [Google Scholar] [CrossRef]
- Brown, A.E.; Finlay, R.; Ward, J.S. Antifungal compounds produced by Epicoccum purpurascens against soil born plant pathogenic fungi. Soil. Biol. Biochem. 1987, 19, 657–664. [Google Scholar] [CrossRef]
- Guo, H.; Sun, B.; Gao, H.; Chen, X.; Liu, S.; Yao, X.; Liu, X.; Che, Y. Diketopiperazines from the Cordyceps– colonising fungus Epicoccum nigrum. J. Nat. Prod. 2009, 72, 2115–2119. [Google Scholar] [CrossRef]
- Wang, J.-M.; Ding, G.-Z.; Fang, L.; Dai, J.-G.; Yu, S.-S.; Wang, Y.-H.; Chen, X.-G.; Ma, S.-G.; Qu, J.; Xu, S.; et al. Thiodiketopiperazines produced by endophytic fungus Epicoccum nigrum. J. Nat. Prod. 2010, 73, 1240–1249. [Google Scholar] [CrossRef]
- Sun, H.-H.; Mao, W.-J.; Jiao, J.-Y.; Xu, J.-C.; Li, H.-Y.; Chen, Y.; Qi, X.-H.; Chen, Y.-L.; Xu, J.; Zhao, C.-Q.; et al. Structural characterisation of extracellular polysaccharides produced by the marine fungus Epicoccum nigrum JJY–40 and their antioxidant activities. Mar. Biotechnol. 2011, 13, 1048–1055. [Google Scholar] [CrossRef]
- Hamed, A.A.; Soldatou, S.; Qader, M.M.; Arjunan, S.; Miranda, K.J.; Casolari, F.; Pavesi, C.; Diyaolu, O.A.; Thissera, B.; Eshelli, M.; et al. Screening fungal endophytes derived from under-explored Egyptian marine habitats for antimicrobial and antioxidant properties in factionalised textiles. Microorganisms 2020, 8, 1617. [Google Scholar] [CrossRef]
- Fujii, K.; Ikai, Y.; Oka, H.; Suzuki, M.; Harada, K. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: combination of Marfey’s method with mass spectrometry and its practical application. Anal. Chem. 1997, 69, 5146–5151. [Google Scholar] [CrossRef]
- He, R.; Wang, B.; Wakimoto, T.; Wang, M.; Zhu, L.; Abe, I. Cyclodipeptides from metagenomic library of a Japanese marine sponge. J. Braz. Chem. Soc. 2013, 24, 1926–1932. [Google Scholar] [CrossRef]
- Cimmino, A.; Puopolo, G.; Perazzolli, M.; Andolfi, A.; Melck, D.; Pertot, I.; Evidente, A. Cyclo(L–Pro–L–Tyr), The fungicide isolated from lysobacter capsici az78: A structure–activity relationship study. Chem. Heterocycl. Comp. 2014, 50, 290–295. [Google Scholar] [CrossRef]
- Daoud, N.N.; Foster, H.A. Antifungal activity of Myxococcus species 1 production, physiochemical and biological properties of antibiotics from Myxococcus flavus S110 (Myxobacterales). Microbios 1993, 73, 173–184. [Google Scholar]
- Hoye, T.R.; Jeffrey, C.S.; Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2007, 2, 2451–2458. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Yancheva, D.; Cherneva, E.; Petronijevic, Z.; Lamshoeft, M.; Herebian, D. Identification and synthesis of three cyclodidepsipeptides as potential precursor of enniatin B in Fusarium sporotrichioides. J. Mol. Structure 2011, 985, 397–402. [Google Scholar] [CrossRef]
- Sun, J.; Awakawa, T.; Noguchi, H.; Abe, I. Induced production of mycotoxins is an endophytic fungus from the medicinal plant Datura stramonium L. Bioorg. Med. Chem. Lett. 2012, 22, 6397–6400. [Google Scholar] [CrossRef]
- Hu, L.; Chen, N.; Hu, Q.; Yang, C.; Yang, Q.; Qang, F.-F. An unusual piceatannol dimer from Rheum austral D. Don with antioxidant activity. Molecules 2014, 19, 11453–11464. [Google Scholar] [CrossRef]
- Kamisuki, S.; Takahashi, S.; Mizushina, Y.; Hanashima, S.; Kuramochi, K.; Kobayashi, S.; Sakaguchi, K.; Nakata, T.; Sugawara, F. Total synthesis of dehydroaltenusin. Tetrahedron 2004, 60, 5695–5700. [Google Scholar] [CrossRef]
- Mousa, W.K.; Schwan, A.; Davidson, J.; Strange, P.; Liu, H.; Zhou, T.; Auzanneau, F.I.; Raizada, M.N. An endophytic fungus isolated from finger millet (Eleusine coracana) produces anti–fungal natural products. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Bashyal, B.P.; Wellensiek, B.P.; Ramakrishnan, R.; Faeth, S.H.; Ahmed, N.; Gunatilaka, A.A.L. Aflatoxins with potent anti–HIV activity from Alternaria tenuissima QUE1Se, a fungal endophyte of Quercus emoryi. Bioorg. Med. Chem. 2014, 22, 6112–6116. [Google Scholar] [CrossRef]
- Okuno, T.; Natsume, I.; Sawai, K.; Sawamura, K.; Furusaki, A.; Matsumoto, T. Structure of antifungal and phytotoxic pigments produced by Alternaria sp. Tetrahedron Lett. 1983, 24, 5653–5656. [Google Scholar] [CrossRef]
- O’Meara, S. Antimicrobial resistance. Nature 2020, 586, S49. [Google Scholar] [CrossRef]
- Fdhila, F.; Vazquez, V.; Sanchez, J.L.; Riguera, R. DD-diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef]
- Houston, D.R.; Synstad, B.; Eijsink, V.G.; Stark, M.J.; Eggleston, I.M.; van Aalten, D.M. Structure-based exploration of cyclic dipeptide chitinase inhibitors. J. Med. Chem. 2004, 47, 5713–5720. [Google Scholar] [CrossRef]
- Li, J.; Wangb, W.; Xua, S.X.; Magarveyb, N.A.; McCormicka, J.K. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. USA 2011, 108, 3360–3365. [Google Scholar] [CrossRef]
- Cimmino, A.; Bejarano, A.; Masi, M.; Puopolo, G.; Evidente, A. Isolation of 2,5-diketopiperazines from Lysobacter capsici AZ78 with activity against Rhodococcus fascians. Nat. Prod. Res. 2020, 30. [Google Scholar] [CrossRef]
- Mangamuri, U.K.; Muvva, V.; Poda, S.; Manavathi, B.; Bhujangarao, C.; Yenamandra, V. Chemical characterization and bioactivity of diketopiperazine derivatives from the mangrove derived Pseudocardia endophytica. Egypt. J. Aquat. Res. 2016, 42, 169–175. [Google Scholar] [CrossRef]
- Rhee, K.-H. Cyclic dipeptides exhibit synergistic, broad spectrum antimicrobial effects and have anti-mutagenic properties. Int. J. Antimicrob. Agents 2004, 24, 423–427. [Google Scholar] [CrossRef]
- Gallardo, G.L.; Peña, N.I.; Chacana, P.; Terzolo, H.R.; Cabrera, G.M. L-Tenuazonic acid, a new inhibitor of Paenibacillus larva. World J. Microbiol. Biotechnol. 2004, 20, 609–612. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, S.; Wei, J.; Fang, N.; Yin, L.; Sun, J. Porric acid D from marine-derived fungus Alternaria sp. isolated from Bohai sea. Chem. Nat. Compd. 2012, 47, 893–895. [Google Scholar] [CrossRef]
- Kjer, J.; Wray, V.; Edrada-Ebel, R.A.; Ebel, R.; Pretsch, A.; Lin, W.H.; Proksch, P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2009, 72, 2053–2057. [Google Scholar] [CrossRef]
- Gu, W. Bioactive metabolites from Alternaria brassicicola ML–P08, an endophytic fungus residing in Malus halliana. World J. Microbiol. Biotechnol. 2009, 25, 1677–1683. [Google Scholar] [CrossRef]
- Pletzer, D.; Hancock, R.E.W. Antibiofilm peptides: Potential as broad-spectrum agents. J. Bacteriol. 2016, 198, 2572–2578. [Google Scholar] [CrossRef]
- Antunes, A.L.; Trentin, D.S.; Bonfanti, J.W.; Pinto, C.C.; Perez, L.R.; Macedo, A.J.; Barth, A.L. Application of a feasible method for determination of biofilm antimicrobial susceptibility in staphylococci. APMIS 2010, 118, 873–877. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Sun, S.; Chang, A.; Dai, X.; Li, H.; Wang, Y.; and Hu Zhu, H. A cyclic dipeptide from marine fungus Penicillium chrysogenum DXY-1 exhibits anti-quorum sensing activity. ACS Omega 2021, 6, 7693–7700. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Bérubé, C.; Voyer, N.; Grenier, D. Anti-biofilm and anti-adherence properties of novel cyclic dipeptides against oral pathogens. Bioorg. Med. Chem. 2019, 27, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef]

 and HMBC
and HMBC  correlations of compounds 1, 7, and 8; (B) molecular mechanics simulations of compound 7.
correlations of compounds 1, 7, and 8; (B) molecular mechanics simulations of compound 7.
 and HMBC
and HMBC  correlations of compounds 1, 7, and 8; (B) molecular mechanics simulations of compound 7.
correlations of compounds 1, 7, and 8; (B) molecular mechanics simulations of compound 7.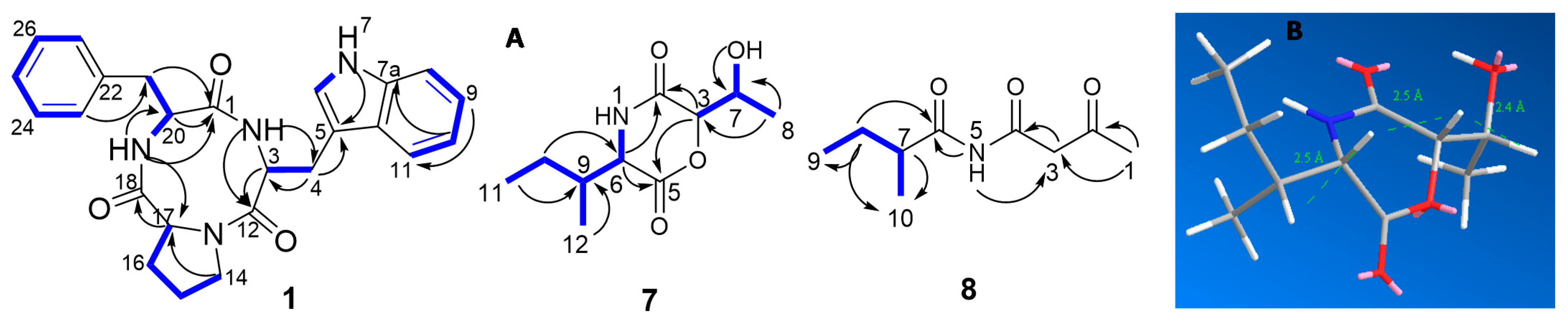

| Position | 1H (Mult., J in Hz) | 13C, Mult. | HMBC |
|---|---|---|---|
| Tryptophan moiety | |||
| 1-CO | 165.1, C | ||
| 2-NH | 7.73 (br s) | C-3, C-4, C-12, C-17, C-18 | |
| 3 | 4.30 (t, 5.4) | 55.2, CH | C-4, C-5, C-12, C-18 |
| 4 | 3.24–3.07 (m) | 25.8, CH2 | C-3, C-5, C-6, C-12, C-11a |
| 5 | 109.4, C | ||
| 6 | 7.17 (m) | 124.3, CH | C-5, C-7a, C-11a |
| 7-NH | 10.85 (br s) | C-5, C-6, C-7a, C-11a | |
| 7a | 136.0, C | ||
| 8 | 7.32 (d, 7.9) | 111.2, CH | C-10, C-11a |
| 9 | 7.05 (t, 7.6) | 120.8, CH | C-11, C-7a |
| 10 | 6.96 (t, 7.6) | 118.2, CH | C-8, C-11a |
| 11 | 7.56 (d, 7.8) | 118.6, CH | C-5, C-9, C-7a, C-11a |
| 11a | 127.4, C | ||
| Proline moiety | |||
| 12-CO | 165.5, C | ||
| 14 | 3.38–3.26 (m) | 44.5, CH2 | C-15, C-16, C-17 |
| 15 | 1.71–1.61 (m) | 21.8, CH2 | C-14, C-16, C-17 |
| 16 | 1.98–1.39 (m) | 27.7, CH2 | C-14, C-15, C-17, C-18 |
| 17 | 4.08 (m) | 58.4, CH | C-16, C-18 |
| Phenylalanine moiety | |||
| 18-CO | 168.9, C | ||
| 19-NH | 7.97 (br s) | C-1, C-17, C-18, C-20, C-21 | |
| 20 | 4.34 (t, 5.4) | 55.7, CH | C-1, C-18, C-21, C-22 |
| 21 | 3.03 (dd, 14.6, 4.9) | 35.4, CH2 | C-1, C-20, C-22, C-23/27 |
| 22 | 137.3, C | ||
| 23/27 | 7.26 (d, 6.8) | 129.8, CH | C-21, C-25 |
| 24/26 | 7.25 (t, 6.8) | 127.9, CH | C-22 |
| 25 | 7.19 (m) | 126.3, CH | C-23/C-27 |
| Position | Phragamide A (7) | Phragamide B (8) | ||
|---|---|---|---|---|
| δH (Mult., J in Hz) | δC, Mult. | δH (Mult., J in Hz) | δC, Mult. | |
| 1 | 2.15 (s) | 30.0, CH3 | ||
| 2 | 168.1, C | 201.9, C | ||
| 3 | 4.65 (s) | 81.8, CH | 3.76 (br s) | 52.9, CH2 |
| 4 | 169.1, C | |||
| 5 | 166.0, C | |||
| 6 | 4.10 (br s) | 57.5, CH | 176.7, C | |
| 7 | 4.05 (m) | 68.4, CH | 2.50 (m) | 41.8, CH |
| 8 | 1.14 (d, 6.2) | 18.6, CH3 | 1.51–1.33 (m) | 26.3, CH2 |
| 9 | 1.93 (m) | 38.3, CH | 0.81 (t, 7.6) | 10.9, CH3 |
| 10 | 1.41–1.28 (m) | 24.5, CH2 | 0.98 (d, 6.9) | 16.2, CH3 |
| 11 | 0.87 (t, 7.2) | 11.8, CH3 | ||
| 12 | 0.92 (d, 7.5) | 14.7, CH3 | ||
| 1-NH | 8.36 (br s) | |||
| 5-NH | 10.76 (br s) | |||
| 7-OH | 5.45 (brs) | |||
| Compound | Minimum Inhibitory Concentration (MIC, µg/mL) * | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | K. pneumonia | P. vulgaris | P. aeruginosa | C. albicans | |
| 1 | 2.5 | 2.5 | 10 | 20 | 10 | 30 | 30 |
| 2 | 50 | 40 | - | - | - | - | - |
| 3 | 20 | 20 | - | - | - | - | - |
| 4 | 10 | 10 | 30 | 30 | 30 | - | - |
| 5 | 10 | 10 | 30 | 30 | 30 | - | - |
| 6 | 40 | 40 | - | - | - | - | 40 |
| 7 | 5 | 5 | 20 | - | - | 10 | 20 |
| 8 | 30 | 40 | 30 | 40 | 40 | - | 50 |
| 9 | 10 | 10 | - | - | - | - | - |
| 10 | - | - | - | - | - | - | - |
| 11 | 20 | 20 | - | - | - | 30 | 50 |
| 12 | 20 | 30 | - | - | - | 40 | 50 |
| 13 | 30 | 30 | - | - | - | - | 40 |
| 14 | 30 | 30 | - | - | - | - | 40 |
| 15 | 30 | 20 | - | - | - | - | 40 |
| 16 | 30 | 20 | - | - | - | - | 30 |
| Cip | 0.62 | 0.31 | 1.25 | 1.25 | 1.25 | 2.5 | - |
| Nys | - | - | - | - | - | - | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qader, M.M.; Hamed, A.A.; Soldatou, S.; Abdelraof, M.; Elawady, M.E.; Hassane, A.S.I.; Belbahri, L.; Ebel, R.; Rateb, M.E. Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Mar. Drugs 2021, 19, 232. https://doi.org/10.3390/md19040232
Qader MM, Hamed AA, Soldatou S, Abdelraof M, Elawady ME, Hassane ASI, Belbahri L, Ebel R, Rateb ME. Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Marine Drugs. 2021; 19(4):232. https://doi.org/10.3390/md19040232
Chicago/Turabian StyleQader, M. Mallique, Ahmed A. Hamed, Sylvia Soldatou, Mohamed Abdelraof, Mohamed E. Elawady, Ahmed S. I. Hassane, Lassaad Belbahri, Rainer Ebel, and Mostafa E. Rateb. 2021. "Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum nigrum M13 and Alternaria alternata 13A" Marine Drugs 19, no. 4: 232. https://doi.org/10.3390/md19040232
APA StyleQader, M. M., Hamed, A. A., Soldatou, S., Abdelraof, M., Elawady, M. E., Hassane, A. S. I., Belbahri, L., Ebel, R., & Rateb, M. E. (2021). Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Marine Drugs, 19(4), 232. https://doi.org/10.3390/md19040232








