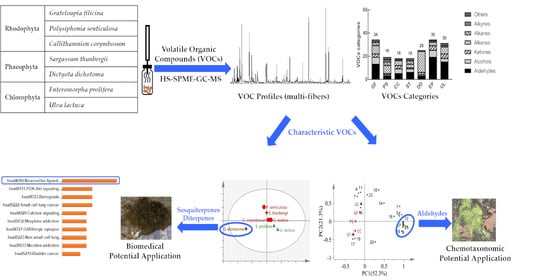
Characteristic Volatile Composition of Seven Seaweeds from the Yellow Sea of China
Abstract
1. Introduction
2. Results and Discussion
2.1. Headspace VOC Composition of Seaweeds
2.1.1. Headspace VOC Composition of Rhodophyta
2.1.2. Headspace VOC Composition of Phaeophyta
2.1.3. Headspace VOC Composition of Chlorophyta
2.2. Characteristic VOC Molecules and Potential Application in Chemotaxonomy
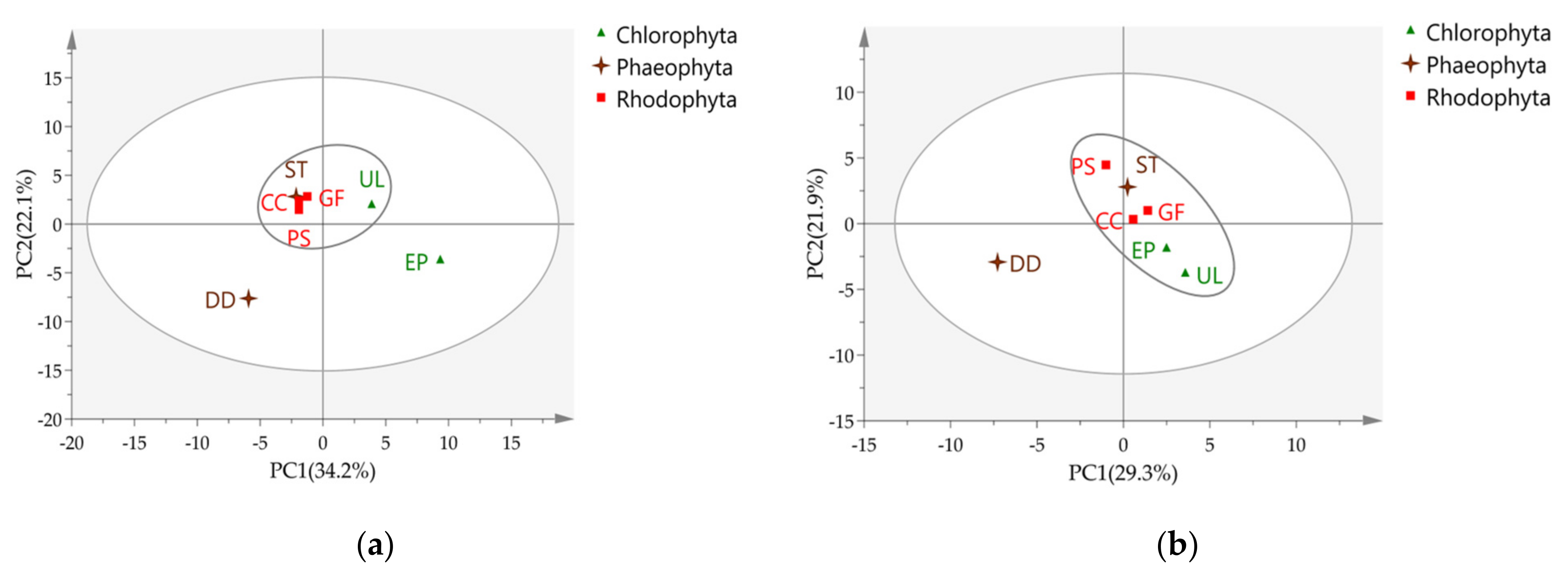
2.2.1. PCA on Total VOCs Variables
2.2.2. PCA on Aldehyde Variables
2.3. Network Pharmacology and Potential Biomedical Application of D. Dichotoma
3. Materials and Methods
3.1. Sample Collection
3.2. Headspace Solid Phase Microextraction (HS-SPME)
3.3. Gas Chromatography–Mass Spectrometry (GC–MS) Analyses
3.4. Chemometrics and Network Pharmacology Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Penuelas, J.; Llusia, J. Plant VOC emissions: Making use of the unavoidable. Trends Ecol. Evol. 2004, 19, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Owen, S.M.; Boissard, C.; Hewitt, C.N. Volatile organic compounds (VOCs) emitted from 40 Mediterranean plant species: VOC speciation and extrapolation to habitat scale. Atmos. Environ. 2001, 35, 5393–5409. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Guenther, A.; Hoffmann, T.; Piedade, M.T.; Warnke, J. Natural volatile organic compound emissions from plants and their roles in oxidant balance and particle formation. In Amazonia and Global Change; American Geophysical Union: Washington, DC, USA, 2009; Volume 186, pp. 183–206. [Google Scholar] [CrossRef]
- Rinnan, R.; Steinke, M.; McGenity, T.; Loreto, F. Plant volatiles in extreme terrestrial and marine environments. Plant Cell Environ. 2014, 37, 1776–1789. [Google Scholar] [CrossRef]
- Jerkovic, I.; Kranjac, M.; Marijanovic, Z.; Roje, M.; Jokic, S. Chemical diversity of headspace and volatile oil composition of two brown algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef]
- De Alencar, D.B.; Diniz, J.C.; Rocha, S.A.S.; Dos Santos Pires-Cavalcante, K.M.; Freitas, J.O.; Nagano, C.S.; Sampaio, A.H.; Saker-Sampaio, S. Chemical composition of volatile compounds in two red seaweeds, Pterocladiella capillacea and Osmundaria obtusiloba, using static headspace gas chromatography mass spectrometry. J. Appl. Phycol. 2017, 29, 1571–1576. [Google Scholar] [CrossRef]
- Yamamoto, M.; Baldermann, S.; Yoshikawa, K.; Fujita, A.; Mase, N.; Watanabe, N. Determination of volatile compounds in four commercial samples of Japanese green algae using solid phase microextraction gas chromatography mass spectrometry. Sci. World J. 2014, 2014, 289780:1–289790:8. [Google Scholar] [CrossRef]
- Zuo, Z. Why algae release volatile organic compounds—The emission and roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef]
- Rocha, F.; Homem, V.; Castro-Jimenez, J.; Ratola, N. Marine vegetation analysis for the determination of volatile methylsiloxanes in coastal areas. Sci. Total Environ. 2019, 650, 2364–2373. [Google Scholar] [CrossRef]
- Akakabe, Y.; Kajiwara, T. Bioactive volatile compounds from marine algae: Feeding attractants. J. Appl. Phycol. 2008, 20, 661–664. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat. Prod. Rep. 2002, 19, 108–122. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Houser, L.T. Dimethylsulfide release during macroinvertebrate grazing and its role as an activated chemical defense. Mar. Ecol. Prog. Ser. 2003, 250, 175–181. [Google Scholar] [CrossRef]
- Schnitzler, I.; Pohnert, G.; Hay, M.; Boland, W. Chemical defense of brown algae (Dictyopteris spp.) against the herbivorous amphipod Ampithoe longimana. Oecologia 2001, 126, 515–521. [Google Scholar] [CrossRef]
- Wiesemeier, T.; Hay, M.; Pohnert, G. The potential role of wound-activated volatile release in the chemical defence of the brown alga Dictyota dichotoma: Blend recognition by marine herbivores. Aquat. Sci. 2007, 69, 403–412. [Google Scholar] [CrossRef]
- Kajiwara, T.; Matsui, K.; Akakabe, Y.; Murakawa, T.; Arai, C. Antimicrobial browning-inhibitory effect of flavor compounds in seaweeds. J. Appl. Phycol. 2006, 18, 413–422. [Google Scholar] [CrossRef]
- Wang, X.J.; Xu, J.L.; Yan, X.J. Analysis of the semivolatile organic compounds of two seaweeds. Haiyang Kexue 2010, 34, 25–28. (In Chinese) [Google Scholar]
- Zhang, M.; Li, R.X.; Hu, C.M.; Yang, L.E.; Tang, J.; Lu, Q.Q.; Zhang, T.; Shen, Z.G.; Shen, S.D.; Xu, P.; et al. The metabolism of 8-heptadecene in Pyropia (Bangiaceae, Rhodophyta). J. Appl. Phycol. 2014, 26, 1181–1187. [Google Scholar] [CrossRef]
- Lu, S.J.; Yosemoto, S.; Satomi, D.; Handa, H.; Akakabe, Y. Two types of volatile polyenes in the brown alga Sargassum thunbergii. J. Oleo Sci. 2018, 67, 1463–1471. [Google Scholar] [CrossRef]
- Jerkovic, I.; Marijanovic, Z.; Roje, M.; Kus, P.M.; Jokic, S.; Coz-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Li, H.; Zhao, Z.S.; Xia, X.; Li, B.; Zhang, J.R.; Yan, X.J. Diterpenes from the marine algae of the genus Dictyota. Mar. Drugs 2018, 16, 159. [Google Scholar] [CrossRef]
- Roberts, D.L.; Rowland, R.L. Macrocyclic diterpenes. α- and β-4,8,13-Duvatriene-1,3-diols from Tobacco. J. Org. Chem. 1962, 27, 3989–3995. [Google Scholar] [CrossRef]
- Silk, P.J.; Mayo, P.D.; LeClair, G.; Brophy, M.; Pawlowski, S.; MacKay, C.; Hillier, N.K.; Hughes, C.; Sweeney, J.D. Semiochemical attractants for the beech leaf-mining weevil, Orchestes fagi. Entomol. Exp. Appl. 2017, 164, 102–112. [Google Scholar] [CrossRef]
- Miao, F.F.; Ding, Y.; Lin, J.L.; He, H.P.; Zhu, W.C.; Su, X.R. Analysis of volatile compounds in Enteromorpha prolifera harvested during different seasons by electronic nose and HS-SPME-GC-MS. Mod. Food Sci. Technol. 2014, 30, 258–263. (In Chinese) [Google Scholar] [CrossRef]
- Reese, K.L.; Fisher, C.L.; Lane, P.D.; Jaryenneh, J.D.; Moorman, M.W.; Jones, A.D.; Frank, M.; Lane, T.W. Chemical profiling of volatile organic compounds in the headspace of algal cultures as early biomarkers of algal pond crashes. Sci. Rep. 2019, 9, 13866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.J.; Lin, T.F.; Zhang, T.Q.; Li, C.; Gao, N.Y. Characterization of typical taste and odor compounds formed by Microcystis aeruginosa. J. Environ. Sci. 2013, 25, 1539–1548. [Google Scholar] [CrossRef]
- Jones, S.; Fernandes, N.V.; Yeganehjoo, H.; Katuru, R.; Qu, H.B.; Yu, Z.L.; Mo, H.B. β-Ionone induces cell cycle arrest and apoptosis in human prostate tumor cells. Nutr. Cancer 2013, 65, 600–610. [Google Scholar] [CrossRef]
- Teixeira, V.L.; Kelecom, A. A chemotaxonomic study of diterpenes from marine brown algae of the genus Dictyota. Sci. Total Environ. 1988, 75, 271–283. [Google Scholar] [CrossRef]
- Goulitquer, S.; Ritter, A.; Thomas, F.; Ferec, C.; Salauen, J.P.; Potin, P. Release of volatile aldehydes by the brown algal kelp Laminaria digitata in response to both biotic and abiotic stress. ChemBioChem 2009, 10, 977–982. [Google Scholar] [CrossRef]
- Kamenarska, Z.; Ivanova, A.; Stancheva, R.; Stoyneva, M.; Stefanov, K.; Dimitrova-Konaklieva, S.; Popov, S. Volatile compounds from some Black Sea red algae and their chemotaxonomic application. Bot. Mar. 2006, 49, 47–56. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Nagashima, F.; Gradstein, R.S.; Asakawa, Y. Volatile components from selected Mexican, Ecuadorian, Greek, German and Japanese liverworts. Nat. Prod. Commun. 2008, 3, 133–140. [Google Scholar] [CrossRef]
- Ji, X.M.; Bossé, Y.; Landi, M.T.; Gui, J.; Xiao, X.J.; Qian, D.; Joubert, P.; Lamontagne, M.; Li, Y.F.; Gorlov, I.; et al. Identification of susceptibility pathways for the role of chromosome 15q25.1 in modifying lung cancer risk. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- El-Shaibany, A.; Al-Habori, M.; Al-Maqtari, T.; Al-Mahbashi, H. The yemeni brown algae Dictyota dichotoma exhibit high in vitro anticancer activity independent of its antioxidant capability. Biomed Res. Int. 2020, 2425693:1–2425693:9. [Google Scholar] [CrossRef]
- Adkins, D.E.; Khachane, A.N.; McClay, J.L.; Åberg, K.; Bukszár, J.; Sullivan, P.F.; van den Oord, E.J.C.G. SNP-based analysis of neuroactive ligand–receptor interaction pathways implicates PGE2 as a novel mediator of antipsychotic treatment response: Data from the CATIE study. Schizophr. Res. 2012, 135, 200–201. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 1 June 2020).
- Lab of System Pharmacology. Available online: https://tcmspw.com/tcmsp.php (accessed on 21 December 2020).
- Unitprot. Available online: https://www.uniprot.org/ (accessed on 21 December 2020).
- STRING. Available online: https://string-db.org/ (accessed on 21 December 2020).
- DAVID Bioinformatics Resources 6.8. Available online: https://david.ncifcrf.gov/ (accessed on 21 December 2020).




| NO. | Compound | Molecular Formula | Compound Class | RI a | Area Percentage (%) | Identification | ||
|---|---|---|---|---|---|---|---|---|
| GF b | PS b | CC b | ||||||
| 1 | (E,E,E)-2,4,6-Octatriene T | C8H12 | Alkene | <800 | 1.03 * | 0.00 | 0.00 | MS |
| 2 | 3,5,5-Trimethyl-1-hexene T | C9H18 | Alkene | <800 | 8.24 * | 1.74 * | 2.74 * | MS |
| 3 | 3,5-Dimethyl-1-hexene T | C8H16 | Alkene | <800 | 0.36 * | 0.00 | 0.00 | MS |
| 4 | 1-Hexen-3-ol T | C6H12O | Alcohol | <800 | 19.58 * | 0.00 | 4.44 * | MS |
| 5 | 2-Propyl-furan T | C7H10O | Furan derivative | <800 | 0.30 * | 1.02 * | 0.00 | MS |
| 6 | 3-Ethyl-1,4-hexadiene T | C8H14 | Alkene | 846 | 1.16 * | 1.25 * | 0.00 | MS |
| 7 | 2-Methylpropylidene-Cyclopentane | C9H16 | Alkene | 908 | 1.48 * | 0.00 | 0.00 | MS, RI |
| 8 | (Z)-2-Octen-1-ol T | C8H16O | Unsaturated alcohol | 927 | 8.5 # | 0.00 | 0.00 | MS |
| 9 | Isocumene | C9H12 | Others | 931 | 0.00 | 1.67 * | 0.00 | MS, RI |
| 10 | 3-Cyclohexene-1-ethanol | C8H14O | Alcohol | 935 | 2.90 # | 0.00 | 0.00 | MS.RI |
| 11 | (E)-2-Heptenal | C7H12O | Aldehyde | 960 | 4.78 * | 1.51 * | 0.00 | MS, RI |
| 12 | 1-Octen-3-ol S | C8H16O | Alcohol | 980 | 18.72 * | 0.00 | 2.15 * | MS, RI |
| 13 | 3,7-Dimethyl-1-octene T | C10H20 | Alkene | 985 | 0.00 | 0.00 | 7.81 * | MS |
| 14 | 2,7-Octadien-1-ol T | C8H14O | Unsaturated alcohol | 986 | 8.39 * | 0.00 | 0.00 | MS |
| 15 | 4-Methyl-2-propyl-1-pentanol T | C9H20O | Alcohol | 990 | 0.00 | 0.00 | 3.59 # | MS |
| 16 | 5-Methyl-1-undecene T | C12H24 | Alkene | 1025 | 0.00 | 6.91 * | 12.70 * | MS |
| 17 | (E)-2-Undecen-1-ol T | C11H22O | Unsaturated alcohol | 1039 | 0.25 * | 1.28 * | 0.00 | MS |
| 18 | (9Z)-1,9-Dodecadiene T | C12H22 | Alkene | 1092 | 0.00 | 0.00 | 1.77 # | MS |
| 19 | Ectocarpene T | C11H16 | Alkene | 1105 | 0.00 | 21.83 *, 14.71 # | 0.00 | MS |
| 20 | 1-Undecyne | C11H20 | Alkyne | 1108 | 0.00 | 2.46 * | 8.86 * | MS, RI |
| 21 | Dictyopterene D’ T | C11H18 | Alkene | 1112 | 0.00 | 1.74 *, 0.95 # | 0.00 | MS |
| 22 | (Z)-6-Nonenal | C9H16O | Aldehyde | 1113 | 3.25 * | 0.00 | 0.00 | MS, RI |
| 23 | Decanal | C10H20O | Aldehyde | 1173 | 0.07 * | 0.00 | 0.00 | MS, RI |
| 24 | β-Cyclocitral | C10H16O | C10-Norisoprenoid | 1192 | 0.79 *, 0.69 # | 1.55 * | 0.95 * | MS, RI |
| 25 | β-Cyclohomocitral | C11H18O | Aldehyde | 1236 | 0.07 * | 0.00 | 0.00 | MS, RI |
| 26 | 2,4-Decadienal | C10H16O | Unsaturated aldehyde | 1275 | 0.33 * | 0.00 | 0.00 | MS, RI |
| 27 | Undecanal | C11H22O | Aldehyde | 1290 | 0.47 *, 0.52 # | 0.00 | 0.00 | MS, RI |
| 28 | (E,E)-2,4-Decadienal | C10H16O | Unsaturated aldehyde | 1298 | 0.98 *, 0.66 # | 0.69 * | 0.00 | MS, RI |
| 29 | Dodecanal | C12H24O | Aldehyde | 1400 | 0.37 *, 0.95 # | 0.00 | 0.00 | MS, RI |
| 30 | α-Ionone | C13H20O | C13-Norisoprenoid | 1420 | 1.50 *, 2.01 # | 0.00 | 1.16 *, 1.60 # | MS, RI |
| 31 | Dihydropseudoionone | C13H22O | C13-Norisoprenoid | 1447 | 0.15 *, 0.40 # | 0.00 | 0.00 | MS, RI |
| 32 | (E)-β-Ionone S | C13H20O | C13-Norisoprenoid | 1481 | 2.65 *, 5.68 # | 1.37 * | 2.39 *, 4.01 # | MS, RI |
| 33 | 1-Pentadecene | C15H30 | Alkene | 1484 | 3.84 *, 2.33 # | 1.33 # | 0.00 | MS, RI |
| 34 | Pentadecane S | C15H32 | Alkane | 1492 | 0.57 *, 0.88 # | 1.39 *, 11.19# | 0.00 | MS.RI |
| 35 | Tridecanal S | C13H26O | Aldehyde | 1503 | 5.55 *, 19.20 # | 0.00 | 2.28 *, 5.60 # | MS, RI |
| 36 | Cyclopentadecane T | C15H30 | Alkane | 1508 | 0.31 * | 1.16 # | 0.00 | MS |
| 37 | Dihydroactinidiolide | C11H16O2 | C11-Norisoprenoid | 1525 | 0.06 * | 0.00 | 0.00 | MS, RI |
| 38 | TetradecanalS | C14H28O | Aldehyde | 1608 | 1.75 # | 0.00 | 1.16 # | MS, RI |
| 39 | 8-Heptadecene | C17H34 | Alkene | 1681 | 0.10 * | 2.85# | 0.00 | MS, RI |
| 40 | Z-11-Pentadecenal T | C15H28O | Aldehyde | 1689 | 0.26 *, 1.78 # | 0.00 | 1.09 # | MS |
| 41 | Heptadecane S | C17H36 | Alkane | 1693 | 1.73 *, 5.76 # | 3.19 *, 15.93 # | 2.04 *, 3.15 # | MS, RI |
| 42 | Pentadecanal | C15H30O | Aldehyde | 1711 | 3.23 *, 20.11 # | 0.00 | 11.64 *, 35.43 # | MS, RI |
| 43 | Perhydrofarnesyl acetone | C18H36O | Ketone | 1840 | 0.00 | 0.00 | 0.91 *, 3.14 # | MS, RI |
| 44 | 1,11-Dodecadiyne T | C12H18 | Alkyne | 1845 | 0.00 | 0.81 # | 0.00 | MS |
| 45 | (Z,Z)-6,9-Pentadecadien-1-ol T | C15H28O | Unsaturated alcohol | 1889 | 0.00 | 0.00 | 0.46 *, 1.92 # | MS |
| Total Identified (%) | 90.59 *, 74.10 # | 49.61 *, 48.92 # | 60.53 *,62.46 # | |||||
| NO. | Compound | Molecular Formula | Compound Class | RI a | Area Percentage (%) | Identification | |
|---|---|---|---|---|---|---|---|
| ST b | DD b | ||||||
| 1 | 2-Propyl-furan T | C7H10O | Furan derivative | <800 | 1.37 * | 0.00 | MS |
| 2 | (E)-2-Hepten-1-ol T | C7H14O | Alcohol | 830 | 0.00 | 4.62 * | MS |
| 3 | Sulcatone T | C8H14O | C13-Norisoprenoid | 852 | 0.00 | 5.21 * | MS |
| 4 | 2-Propyl-1-pentanol T | C8H18O | Alcohol | 896 | 0.82 * | 0.00 | MS |
| 5 | Isocumene | C9H12 | Others | 931 | 1.14 * | 0.00 | MS, RI |
| 6 | 2,7-Dimethyl-1-octanol T | C10H22O | Alcohol | 1023 | 0.94 * | 0.00 | MS |
| 7 | (E)-2-Undecen-1-ol T | C11H22O | Alcohol | 1039 | 1.48 * | 0.00 | MS |
| 8 | Ectocarpene T | C11H16 | Alkene | 1105 | 3.40 * | 0.00 | MS |
| 9 | β-Cyclocitral | C10H16O | C10-Norisoprenoid | 1192 | 1.05 * | 0.00 | MS, RI |
| 10 | 2,4-Decadienal | C10H16O | Unsaturated aldehyde | 1275 | 0.00 | 0.72 * | MS, RI |
| 11 | α-Cubebene | C15H24 | Sesquiterpene | 1338 | 0.00 | 0.81 * | MS, RI |
| 12 | β-Bourbonene | C15H24 | Sesquiterpene | 1376 | 0.00 | 1.07 *, 1.39 # | MS, RI |
| 13 | Cedrene | C15H24 | Sesquiterpene | 1418 | 0.00 | 0.28 # | MS, RI |
| 14 | β-Copaene | C15H24 | Sesquiterpene | 1423 | 0.00 | 0.57 * | MS, RI |
| 15 | cis-Muurola-3,5-diene | C15H24 | Sesquiterpene | 1441 | 0.00 | 0.48 * | MS, RI |
| 16 | β-Gurjunene | C15H24 | Sesquiterpene | 1458 | 0.00 | 1.72 * | MS, RI |
| 17 | γ-Muurolene | C15H24 | Sesquiterpene | 1468 | 0.00 | 2.82 *, 0.62 # | MS, RI |
| 18 | Germacrene D S | C15H24 | Sesquiterpene | 1477 | 0.00 | 34.83 *, 62.00 # | MS, RI |
| 19 | (E)-β-Ionone S | C13H20O | C13-Norisoprenoid | 1481 | 1.22 *, 0.93 # | 0.52 *, 0.44 # | MS, RI |
| 20 | 1-Pentadecene | C15H30 | Alkene | 1484 | 4.31 *, 5.46 # | 0.00 | MS, RI |
| 21 | α-Selinene | C15H24 | Sesquiterpene | 1488 | 0.00 | 0.38 # | MS, RI |
| 22 | Pentadecane S | C15H32 | Alkane | 1492 | 9.24 *, 13.87 # | 5.94 *, 7.98 # | MS.RI |
| 23 | Tridecanal S | C13H26O | Aldehyde | 1503 | 11.46 *, 11.02 # | 2.51 *, 1.74 # | MS, RI |
| 24 | Cyclopentadecane T | C15H30 | Alkane | 1508 | 2.94 *, 1.51 # | 0.00 | MS |
| 25 | Muurola-4,9-diene | C15H24 | Sesquiterpene | 1510 | 0.00 | 3.37 * | MS, RI |
| 26 | β-Cadinene | C15H24 | Sesquiterpene | 1518 | 0.00 | 0.52 # | MS, RI |
| 27 | δ-Cadinene | C15H24 | Sesquiterpene | 1520 | 0.00 | 5.28 * | MS, RI |
| 28 | 1,4-Cadinadiene | C15H24 | Sesquiterpene | 1529 | 0.00 | 0.44 * | MS, RI |
| 29 | α-Muurolene T | C15H24 | Sesquiterpene | 1535 | 0.00 | 0.82 * | MS |
| 30 | Tetradecanal S | C14H28O | Aldehyde | 1608 | 0.62 *, 0.48 # | 0.00 | MS, RI |
| 31 | Cubenol | C15H26O | Sesquiterpene | 1637 | 0.00 | 3.69 *, 4.70 # | MS, RI |
| 32 | 8-Heptadecene | C17H34 | Alkene | 1681 | 5.97 *, 14.82 # | 0.00 | MS, RI |
| 33 | Heptadecane S | C17H36 | Alkane | 1693 | 1.63 *, 1.59 # | 0.00 | MS, RI |
| 34 | (Z)-11-Pentadecenal T | C15H28O | Aldehyde | 1689 | 5.49 *, 5.85 # | 0.00 | MS |
| 35 | Pentadecanal | C15H30O | Aldehyde | 1711 | 6.86 *, 11.33 # | 0.46 # | MS, RI |
| 36 | (Z,Z,Z)-7,10,13-Hexadecatrienal T | C16H26O | Unsaturated aldehyde | 1890 | 0.91 # | 0.00 | MS |
| 37 | 1,5,9-Trimethyl-12-(1-methylethyl)-4,8,13-Cyclotetradecatriene-1,3-diol T | C20H34O2 | Diterpene | 1989 | 0.00 | 5.70 # | MS |
| 38 | Thunbergol | C20H34O2 | Diterpene | 2089 | 0.00 | 3.30 # | MS, RI |
| 39 | Geranyl-α-terpinene T | C20H32 | Diterpene | >2100 | 0.00 | 1.04 *, 0.99 # | MS |
| Total Identified (%) | 59.94 *, 67.77 # | 76.46 *, 90.51 # | |||||
| NO. | Compound | Molecular Formula | Compound Class | RI a | Area Percentage (%) | Identification | |
|---|---|---|---|---|---|---|---|
| EP b | UL b | ||||||
| 1 | 3,5,5-Trimethyl-1-hexene T | C9H18 | Alkene | <800 | 0.00 | 1.34 * | MS |
| 2 | 3,5-Dimethyl-1-hexene T | C8H16 | Alkene | <800 | 3.75 * | 1.27 * | MS |
| 3 | 2-Propyl-furan T | C7H10O | Furan derivatives | <800 | 4.61 * | 4.74 *, 3.82 # | MS |
| 4 | 2,4-Octadiene | C8H14 | Alkene | 805 | 0.00 | 3.06 * | MS, RI |
| 5 | 3-Ethyl-1,4-hexadiene T | C8H14 | Alkene | 846 | 3.59 * | 10.17 * | MS |
| 6 | (E)-2-Heptenal | C7H12O | Aldehyde | 960 | 9.64 * | 4.60 * | MS, RI |
| 7 | 1,2-Dimethyl-cycloheptene T | C9H16 | Alkene | 988 | 3.32 * | 3.40 * | MS |
| 8 | 4-Heptenal T | C7H12O | Aldehyde | 1001 | 2.94 * | 0.00 | MS |
| 9 | (4E)-2-Methyl-4-hexen-3-ol T | C7H14O | Alcohol | 1025 | 3.08 * | 0.00 | MS |
| 10 | (E)-2-Undecen-1-ol T | C11H22O | Unsaturated alcohol | 1039 | 3.15 * | 1.27 * | MS |
| 11 | 2,4-Dimethyl-Cyclohexanol | C8H16O | Alcohol | 1045 | 0.80 * | 1.90 * | MS, RI |
| 12 | 3-Cyclohexene-1-carboxaldehyde T | C8H12O | Aldehyde | 1056 | 0.50 * | 0.00 | MS |
| 13 | (Z)-6-Nonenal | C9H16O | Aldehyde | 1113 | 0.00 | 1.94 * | MS, RI |
| 14 | 2,4-Nonadienal | C9H14O | Unsaturated aldehyde | 1180 | 0.95 * | 0.52 * | MS, RI |
| 15 | Decanal | C10H20O | Aldehyde | 1173 | 1.36 * | 0.58 * | MS, RI |
| 16 | 9-Oxabicyclo [6.1.0]nonan-4-ol T | C8H14O2 | Alcohol | 1186 | 0.70 * | 0.00 | MS |
| 17 | β-Cyclocitral | C10H16O | C10-Norisoprenoid | 1192 | 0.89 *, 1.05 # | 3.05 *, 3.17 # | MS, RI |
| 18 | (Z)-2-Decenal | C10H18O | Aldehyde | 1243 | 0.70 * | 0.00 | MS, RI |
| 19 | Citral | C10H16O | C10-Norisoprenoid | 1253 | 0.32 * | 0.00 | MS, RI |
| 20 | 1-Butenylidene-cyclohexane T | C10H16 | Alkane | 1260 | 0.81 * | 1.80 *, 1.80 # | MS |
| 21 | Undecanal | C11H22O | Aldehyde | 1290 | 0.31 * | 0.15 * | MS, RI |
| 22 | 2,4-Decadienal | C10H16O | Unsaturated aldehyde | 1275 | 0.00 | 0.96 *, 0.48 # | MS, RI |
| 23 | (E,E)-2,4-Decadienal | C10H16O | Unsaturated aldehyde | 1298 | 0.77 *, 1.61 # | 3.02 *, 2.59 # | MS, RI |
| 24 | 4,4,6-Trimethyl-cyclohex-2-en-1-ol | C9H16O | Alcohol | 1330 | 0.28 * | 0.00 | MS, RI |
| 25 | 2-Undecenal | C11H20O | Aldehyde | 1353 | 0.65 * | 0.00 | MS, RI |
| 26 | (E)-4,5-Epoxydec-2-enal | C10H16O2 | Unsaturated Aldehyde | 1368 | 1.70 *, 0.85 # | 0.28 * | MS, RI |
| 27 | 6,10-Dimethyl-2-undecanone | C13H26O | Ketone | 1395 | 0.56 * | 0.19 * | MS, RI |
| 28 | Dodecanal | C12H24O | Aldehyde | 1400 | 0.43 * | 0.00 | MS, RI |
| 29 | α-Ionone | C13H20O | C13-Norisoprenoid | 1420 | 0.21 *, 0.45 # | 2.59 *, 4.24 # | MS, RI |
| 30 | (E)-Geranylacetone | C13H22O | C13-Norisoprenoid | 1447 | 0.93 *, 1.74 # | 0.29 *, 0.48 # | MS, RI |
| 31 | (E)-β-Ionone S | C13H20O | C13-Norisoprenoid | 1481 | 3.53 *, 5.97 # | 8.69 *, 18.13 # | MS, RI |
| 32 | Tridecanal S | C13H26O | Aldehyde | 1503 | 1.31 *, 2.37 # | 0.37 *, 0.65 # | MS, RI |
| 33 | Dihydroactinidiolide | C11H16O2 | C11-Norisoprenoid | 1525 | 0.00 | 0.29 * | MS, RI |
| 34 | Tetradecanal S | C14H28O | Aldehyde | 1608 | 3.34 *, 6.32 # | 0.79 # | MS, RI |
| 35 | 8-Heptadecene | C17H34 | Alkene | 1681 | 2.72 # | 5.32 *, 19.70 # | MS, RI |
| 36 | (Z)-11-Pentadecenal T | C15H28O | Aldehyde | 1689 | 0.40 *, 0.81 # | 0.77 * | MS |
| 37 | Pentadecanal | C15H30O | Aldehyde | 1711 | 8.49 *, 24.40 # | 3.83 *, 7.36 # | MS, RI |
| 38 | (Z)-11-Hexadecenal | C16H30O | Aldehyde | 1793 | 0.59 *, 1.18 # | 0.00 | MS, RI |
| 39 | (Z,Z)-6,9-Pentadecadien-1-ol T | C15H28O | Unsaturated alcohol | 1889 | 0.83 # | 0.54 *, 0.93 # | MS |
| 40 | (Z,Z,Z)-7,10,13-Hexadecatrienal T | C16H26O | Unsaturated aldehyde | 1890 | 0.46 *, 6.13 # | 2.83 *, 4.96 # | MS |
| Total Identified (%) | 65.07 *, 56.43 # | 69.79 *, 69.10 # | |||||
| No. | Aldehydes | Molecular Formula | Related Fiber | Algae |
|---|---|---|---|---|
| 2 | 4-Heptenal | C7H12O | DVB/CAR/PDMS | EP |
| 3 | 3-Cyclohexene-1-carboxaldehyde | C8H12O | DVB/CAR/PDMS | EP |
| 7 | β-Cyclocitral | C10H16O | PDMS | EP, UL Δ, GF, PS, CC, ST |
| 8 | (Z)-2-Decenal | C10H18O | DVB/CAR/PDMS | EP |
| 9 | Citral | C10H16O | DVB/CAR/PDMS | EP |
| 11 | 2,4-Decadienal | C10H16O | PDMS | UL Δ, GF, DD |
| 12 | (E)-4,5-Epoxydec-2-enal | C10H16O2 | DVB/CAR/PDMS, PDMS | EP Δ, UL |
| 15 | 2-Undecenal | C11H20O | DVB/CAR/PDMS | EP |
| 18 | Tetradecanal | C14H28O | DVB/CAR/PDMS, PDMS | EP Δ, UL, GF, CC, ST |
| 21 | (Z)-11-Hexadecenal | C16H30O | DVB/CAR/PDMS, PDMS | EP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Chen, J.; Chen, L.; Shi, L.; Liu, H. Characteristic Volatile Composition of Seven Seaweeds from the Yellow Sea of China. Mar. Drugs 2021, 19, 192. https://doi.org/10.3390/md19040192
Wang P, Chen J, Chen L, Shi L, Liu H. Characteristic Volatile Composition of Seven Seaweeds from the Yellow Sea of China. Marine Drugs. 2021; 19(4):192. https://doi.org/10.3390/md19040192
Chicago/Turabian StyleWang, Pengrui, Jiapeng Chen, Lujing Chen, Li Shi, and Hongbing Liu. 2021. "Characteristic Volatile Composition of Seven Seaweeds from the Yellow Sea of China" Marine Drugs 19, no. 4: 192. https://doi.org/10.3390/md19040192
APA StyleWang, P., Chen, J., Chen, L., Shi, L., & Liu, H. (2021). Characteristic Volatile Composition of Seven Seaweeds from the Yellow Sea of China. Marine Drugs, 19(4), 192. https://doi.org/10.3390/md19040192