Dietary Supplementation of Brown Seaweed and/or Nucleotides Improved Shrimp Performance, Health Status and Cold-Tolerant Gene Expression of Juvenile Whiteleg Shrimp during the Winter Season
Abstract
1. Introduction
2. Results
2.1. Water Quality Parameters
2.2. Survival, Growth, and Condition Factor
2.3. Feed Utilization Indices and Whole-Body Proximate Composition
2.4. Nonspecific Immune Responses
2.5. Phagocytic Activity and Phagocytic Index
2.6. Histological Results
2.7. Bioassay of Four Immune-Related Genes in Shrimp P. vannamei
3. Discussion
4. Materials and Methods
4.1. Experimental Site
4.2. Experimental Shrimp and Culture Condition
4.3. Tested Feed Additives (NucleoforceFish™ and Sargassum polycystum)
4.4. Experimental Treatments
- T1: Basal diet; free from tested additives (control).
- T2: Basal diet supplemented with 500 mg/kg nucleotides (as recommended by [79])
- T3: Basal diet supplemented with 500 mg/kg S. polycystum powdered.
- T4: Basal diet supplemented with 500 mg/kg nucleotides and 500 mg/kg S. polycystum powdered.
4.5. Measured Traits
4.5.1. Water Analysis
4.5.2. Growth Performance Parameters
4.5.3. Feed and Nutrients Utilization Parameters
4.5.4. Shrimp and Feed Analytical Methods
4.5.5. Nonspecific Immune Responses
4.5.6. Histological Examination
4.5.7. Hemolymph and Gene Expression Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Wang, Z.; Qu, Y.; Yan, M.; Li, J.; Zou, J.; Fan, L. Physiological responses of pacific white shrimp litopenaeus vannamei to temperature fluctuation in low-salinity water. Front. Physiol. 2019, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Madeira, D.; Mendonça, V.; Dias, M.; Roma, J.; Costa, P.M.; Larguinho, M.; Vinagre, C.; Diniz, M.S. Physiological, cellular and biochemical thermal stress response of intertidal shrimps with different vertical distributions: Palaemon elegans and palaemon serratus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 183, 107–115. [Google Scholar] [CrossRef]
- Wang, X.; Li, E.; Xu, C.; Qin, J.G.; Wang, S.; Chen, X.; Cai, Y.; Chen, K.; Gan, L.; Yu, N. Growth, body composition, ammonia tolerance and hepatopancreas histology of white shrimp l itopenaeus vannamei fed diets containing different carbohydrate sources at low salinity. Aquac. Res. 2016, 47, 1932–1943. [Google Scholar] [CrossRef]
- Han, S.-Y.; Wang, M.-Q.; Liu, M.; Wang, B.-J.; Jiang, K.-Y.; Wang, L. Comparative sensitivity of the hepatopancreas and midgut in the white shrimp litopenaeus vannamei to oxidative stress under cyclic serious/medium hypoxia. Aquaculture 2018, 490, 44–52. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Dong, H.; Li, H.; Liu, Q.; Zhang, J.; Xiong, D. Physiological and immune response in the gills of litopenaeus vannamei exposed to acute sulfide stress. Fish Shellfish Immunol. 2018, 81, 161–167. [Google Scholar] [CrossRef]
- Ghaednia, B.; Mehrabi, M.; Mirbakhsh, M.; Yeganeh, V.; Hoseinkhezri, P.; Garibi, G.; Ghaffar Jabbari, A. Effect of hot-water extract of brown seaweed sargassum glaucescens via immersion route on immune responses of fenneropenaeus indicus. Iran. J. Fish. Sci. 2011, 10, 616–630. [Google Scholar]
- Meenakshi, S.; Saravanan, R.; Balasubramanian, T.; Palavesam, A. In Vivo Administration of Fucoidan from Turbinaria Decurrens Protects Shrimps from White Spot Syndrome Virus. Indian J. Geomarine Sci. 2019, 48, 212–216. [Google Scholar]
- Krüger, D.; van der Werf, M. Benefits of Nucleotide Supplementation in Aquaculture: Shrimps. Available online: https://www.ohly.com/media/4312/applicationinfonucleotidesaqua-shrimp.pdf (accessed on 15 April 2020).
- Lucien-Brun, H.; Vidal, F. Nucleotide Supplementation Improves Survival, Production of White Shrimp, Tilapia in Trials. 2007. Available online: https://www.aquaculturealliance.org/advocate/nucleotide-supplementation-improves-survival-production-of-white-shrimp-tilapia-in-trials/ (accessed on 17 December 2020).
- Devresse, B. Nucleotides—A key nutrient for shrimp immune system. Feed Mix 2000, 8, 20–22. [Google Scholar]
- Do Huu, H. Overview of the application of nucleotide in aquaculture. J. Coast. Life Med. 2016, 4, 816–823. [Google Scholar]
- Thanigaivel, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Antioxidant and antibacterial activity of chaetomorpha antennina against shrimp pathogen vibrio parahaemolyticus. Aquaculture 2014, 433, 467–475. [Google Scholar] [CrossRef]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-value products from macroalgae: The potential uses of the invasive brown seaweed, sargassum muticum. Rev. Environ. Sci. Bio/Technol. 2016, 15, 67–88. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef]
- Schleder, D.D.; Peruch, L.G.B.; Poli, M.A.; Ferreira, T.H.; Silva, C.P.; Andreatta, E.R.; Hayashi, L.; do Nascimento Vieira, F. Effect of brown seaweeds on pacific white shrimp growth performance, gut morphology, digestive enzymes activity and resistance to white spot virus. Aquaculture 2018, 495, 359–365. [Google Scholar] [CrossRef]
- Bolaños, J.M.; Baleta, F.N.; Cairel, J.D. Antimicrobial properties of sargassum spp.(phaeophyceae) against selected aquaculture pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 024–1037. [Google Scholar] [CrossRef]
- Dubrovskaya, Y.V.; Kurilenko, V.; Hang, C.T.T.; Ly, B.M.; Bakunina, I.Y.; Zvyagintseva, T.; Mikhailov, V. The enzymes of a marine bacterial isolate from the brown alga sargassum polycystum agardh, 1821, that catalyzes the transformation of polyanionic oligo-and polysaccharides. Russ. J. Mar. Biol. 2017, 43, 392–399. [Google Scholar] [CrossRef]
- Soliman, M.; Tawfik, E. Identification and assessment of genetic diversity among sargassum species from egypt. Nucleus 2020, 1–6. [Google Scholar] [CrossRef]
- Motshakeri, M.; Ebrahimi, M.; Goh, Y.M.; Othman, H.H.; Hair-Bejo, M.; Mohamed, S. Effects of brown seaweed (sargassum polycystum) extracts on kidney, liver, and pancreas of type 2 diabetic rat model. Evid. Based Complementary Altern. Med. 2014, 2014, 379407. [Google Scholar] [CrossRef]
- Perumal, B.; Chitra, R.; Maruthupandian, A.; Viji, M. Nutritional assessment and bioactive potential of sargassum polycystum c. Agardh (brown seaweed). Indian J. Geo. Mar. Sci. 2019, 48, 492–498. [Google Scholar]
- Ohno, M.; Critchley, A.T. The seaweed resources of Japan. In Seaweed Resources of the World; Critchley, A.T., Ohno, M., Eds.; Japan International Cooperation Agency: Yokosuka, Japan, 1998; pp. 1–14. [Google Scholar]
- Mulyadi, I.N.; Iba, W. Research article efficacy of seaweed (sargassum sp.) extract to prevent vibriosis in white shrimp (litopenaeus vannamei) juvenile. Int. J. Zool. Res. 2020, 16, 1–11. [Google Scholar]
- Mulyadi, N.I.; Iba, W. Phytochemical test of seaweed extract Sargassum sp. J. Fisharies Sci. Innov. 2019, 3, 22–25. [Google Scholar]
- Chotigeat, W.; Tongsupa, S.; Supamataya, K.; Phongdara, A. Effect of fucoidan on disease resistance of black tiger shrimp. Aquaculture 2004, 233, 23–30. [Google Scholar] [CrossRef]
- Gioacchini, G.; Smith, P.; Carnevali, O. Effects of ergosan on the expression of cytokine genes in the liver of juvenile rainbow trout (Oncorhynchus mykiss) exposed to enteric red mouth vaccine. Vet. Immunol. Immunopathol. 2008, 123, 215–222. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesam, A. The effect of fucoidan from brown seaweed, Sargassum wightii on WSSV resistance and immune activity in shrimp penaeus monodon (fab). Fish Shellfish Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef]
- Bagni, M.; Romano, N.; Finoia, M.; Abelli, L.; Scapigliati, G.; Tiscar, P.G.; Sarti, M.; Marino, G. Short-and long-term effects of a dietary yeast β-glucan (macrogard) and alginic acid (ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol. 2005, 18, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Felix, S.; Robins, P.; Rajeev, A. Immune enhancement assessment of dietry incorporated marine alga, Sargassum wightii (phaeophyceae/punctariales) in tiger shrimp penaeus monodon (crustacia/penaeidae) through prophenoloxidase (propo) systems. Indian J. Mar. Sci. 2004, 33, 361–364. [Google Scholar]
- Yeh, S.-T.; Lee, C.-S.; Chen, J.-C. Administration of hot-water extract of brown seaweed sargassum duplicatum via immersion and injection enhances the immune resistance of white shrimp litopenaeus vannamei. Fish Shellfish Immunol. 2006, 20, 332–345. [Google Scholar] [CrossRef]
- Kumlu, M.; Türkmen, S.; Kumlu, M. Thermal tolerance of Litopenaeus vannamei (crustacea: Penaeidae) acclimated to four temperatures. J. Therm. Biol. 2010, 35, 305–308. [Google Scholar] [CrossRef]
- Anaya-Rosas, R.E.; Rivas-Vega, M.E.; Miranda-Baeza, A.; Piña-Valdez, P.; Nieves-Soto, M. Effects of a co-culture of marine algae and shrimp (Litopenaeus vannamei) on the growth, survival and immune response of shrimp infected with vibrio parahaemolyticus and white spot virus (WSSV). Fish Shellfish Immunol. 2019, 87, 136–143. [Google Scholar] [CrossRef]
- Arizo, M.A.; Simeon, E.C.; Layosa, M.J.; Mortel, R.M.; Pineda, C.M.; Lim, J.J.; Maningas, M.B. Crude fucoidan from sargassum polycystum stimulates growth and immune response of macrobrachium rosenbergii against white spot syndrome virus (wssv). Aquac. Aquar. Conserv. Legis. 2015, 8, 535–543. [Google Scholar]
- Do Huu, H.; Tabrett, S.; Hoffmann, K.; Köppel, P.; Lucas, J.S.; Barnes, A.C. Dietary nucleotides are semi-essential nutrients for optimal growth of black tiger shrimp (Penaeus monodon). Aquaculture 2012, 366, 115–121. [Google Scholar] [CrossRef]
- Andrino, K.G.S.; Serrano Jr, A.E.; Corre Jr, V.L. Effects of dietary nucleotides on the immune response and growth of juvenile pacific white shrimp, Litopenaeus vannamei (boone, 1931). Asian Fish. Sci. 2012, 25, 180–192. [Google Scholar]
- Immanuel, G.; Sivagnanavelmurugan, M.; Balasubramanian, V.; Palavesam, A. Effect of hot water extracts of brown seaweeds Sargassum spp. on growth and resistance to white spot syndrome virus in shrimp Penaeus monodon postlarvae. Aquac. Res. 2010, 41, 545–553. [Google Scholar] [CrossRef]
- Immanuel, G.; Vincybai, V.; Sivaram, V.; Palavesam, A.; Marian, M.P. Effect of butanolic extracts from terrestrial herbs and seaweeds on the survival, growth and pathogen (Vibrio parahaemolyticus) load on shrimp Penaeus indicus juveniles. Aquaculture 2004, 236, 53–65. [Google Scholar] [CrossRef]
- Xiong, J.; Jin, M.; Yuan, Y.; Luo, J.X.; Lu, Y.; Zhou, Q.C.; Liang, C.; Tan, Z.L. Dietary nucleotide-rich yeast supplementation improves growth, innate immunity and intestinal morphology of pacific white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2018, 24, 1425–1435. [Google Scholar] [CrossRef]
- Pallaoro, M.F.; do Nascimento Vieira, F.; Hayashi, L. Ulva lactuca (Chlorophyta ulvales) as co-feed for pacific white shrimp. J. Appl. Phycol. 2016, 28, 3659–3665. [Google Scholar] [CrossRef]
- Yang, F.; Xie, S.; Niu, J.; Liu, Y.; Tian, L. Effect of dietary macro-algae in diet of juvenile pacific white shrimp Litopenaeus vannamei. J. Appl. Phycol. 2018, 30, 1335–1344. [Google Scholar] [CrossRef]
- Pholdaeng, K.; Pongsamart, S. Studies on the immunomodulatory effect of polysaccharide gel extracted from Durio zibethinus in Penaeus monodon shrimp against Vibrio harveyi and WSSV. Fish Shellfish Immunol. 2010, 28, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Chithambaran, S.; David, S. Antiviral property and growth promoting potential of punarnava, Boerhaavia diffusa in tiger prawn culture. Indian J. Mar. Sci. 2014, 43, 2236–2243. [Google Scholar]
- Hertrampf, J.W.; Mishra, S.K. Benefits of nucleotides in shrimp farming. Mortality 2006, 46, 6–7. [Google Scholar]
- Biswas, G.; Korenaga, H.; Nagamine, R.; Kono, T.; Shimokawa, H.; Itami, T.; Sakai, M. Immune stimulant effects of a nucleotide-rich baker’s yeast extract in the kuruma shrimp, marsupenaeus japonicus. Aquaculture 2012, 366, 40–45. [Google Scholar] [CrossRef]
- Hertrampf, J. Less stress with nucleotides. Asian Aquac. Mag. 2003, 6, 22–24. [Google Scholar]
- Motshakeri, M.; Ebrahimi, M.; Goh, Y.M.; Matanjun, P.; Mohamed, S. Sargassum polycystum reduces hyperglycaemia, dyslipidaemia and oxidative stress via increasing insulin sensitivity in a rat model of type 2 diabetes. J. Sci. Food Agric. 2013, 93, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Abedian-Kenari, A.; Oujifard, A. Growth, lipid metabolism and intestinal absorbance of white shrimp (Litopenaeus vannamei boone 1931) influenced by dietary nucleotide. Cellulose 2013, 2, 2–25. [Google Scholar]
- Guo, J.; Guo, B.; Zhang, H.; Xu, W.; Zhang, W.; Mai, K. Effects of nucleotides on growth performance, immune response, disease resistance and intestinal morphology in shrimp Litopenaeus vannamei fed with a low fish meal diet. Aquac. Int. 2016, 24, 1007–1023. [Google Scholar] [CrossRef]
- Burrells, C.; Williams, P.; Southgate, P.; Wadsworth, S. Dietary nucleotides: A novel supplement in fish feeds: 2. Effects on vaccination, salt water transfer, growth rates and physiology of atlantic salmon (Salmo salar L.). Aquaculture 2001, 199, 171–184. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Chen, W.-D.; Liu, Y.-J.; Niu, J.; Chen, M.; Tian, L.-X. Effect of different dietary levels of Gracilaria lemaneiformis dry power on growth performance, hematological parameters and intestinal structure of juvenile pacific white shrimp (Litopenaeus vannamei). Aquaculture 2016, 450, 356–362. [Google Scholar] [CrossRef]
- Niu, J.; Chen, X.; Lu, X.; Jiang, S.-G.; Lin, H.-Z.; Liu, Y.-J.; Huang, Z.; Wang, J.; Wang, Y.; Tian, L.-X. Effects of different levels of dietary wakame (Undaria pinnatifida) on growth, immunity and intestinal structure of juvenile Penaeus monodon. Aquaculture 2015, 435, 78–85. [Google Scholar] [CrossRef]
- Wu, C.; Söderhäll, I.; Kim, Y.A.; Liu, H.; Söderhäll, K. Hemocyte-lineage marker proteins in a crustacean, the freshwater crayfish, pacifastacus leniusculus. Proteomics 2008, 8, 4226–4235. [Google Scholar] [CrossRef]
- Ancieta-Probstl, D.; Smullen, R.P.; Barnes, A.C. Enhancing growth performance of shrimp with nucleotide-supplemented diets. Aquac. Asia Pac. 2005, 1, 26–28. [Google Scholar]
- Rahman, M.A.; Islam, M.S. Antioxidant, antibacterial and cytotoxic effects of the phytochemicals of whole leucas aspera extract. Asian Pac. J. Trop. Biomed. 2013, 3, 273–279. [Google Scholar] [CrossRef]
- Schleder, D.D.; Da Rosa, J.R.; Guimarães, A.M.; Ramlov, F.; Maraschin, M.; Seiffert, W.Q.; do Nascimento Vieira, F.; Hayashi, L.; Andreatta, E.R. Brown seaweeds as feed additive for white-leg shrimp: Effects on thermal stress resistance, midgut microbiology, and immunology. J. Appl. Phycol. 2017, 29, 2471–2477. [Google Scholar] [CrossRef]
- Le Moullac, G.; Haffner, P. Environmental factors affecting immune responses in crustacea. Aquaculture 2000, 191, 121–131. [Google Scholar] [CrossRef]
- Sonnenholzner, S.; Rodríguez, J.; Pérez, F.; Betancourt, I.; Echeverría, F.; Calderón, J. Supervivencia y respuesta inmune de camarones juveniles lv desafiados por via oral a wssv a diferentes temperaturas. El Mundo Acuícola 2002, 8, 50–55. Available online: http://www.dspace.espol.edu.ec/handle/123456789/8745 (accessed on 22 March 2021).
- Cheng, W.; Wang, L.-U.; Chen, J.-C. Effect of water temperature on the immune response of white shrimp litopenaeus vannamei to vibrio alginolyticus. Aquaculture 2005, 250, 592–601. [Google Scholar] [CrossRef]
- Wu, W.; Wu, B.; Ye, T.; Huang, H.; Dai, C.; Yuan, J.; Wang, W. Tctp is a critical factor in shrimp immune response to virus infection. PLoS ONE 2013, 8, e74460. [Google Scholar] [CrossRef]
- Galina, J.; Yin, G.; Ardo, L.; Jeney, Z. The use of immunostimulating herbs in fish. An overview of research. Fish Physiol. Biochem. 2009, 35, 669–676. [Google Scholar] [CrossRef]
- Trejo-Flores, J.V.; Luna-González, A.; Álvarez-Ruiz, P.; Escamilla-Montes, R.; Fierro-Coronado, J.A.; Peraza-Gómez, V.; Flores-Miranda, M.d.C.; Diarte-Plata, G.; Rubio-Castro, A. Immune-related gene expression in Penaeus vannamei fed Aloe vera. Lat. Am. J. Aquat. Res. 2018, 46, 756–764. [Google Scholar] [CrossRef]
- Spagnolo, P.; Sato, H.; Marshall, S.E.; Antoniou, K.M.; Ahmad, T.; Wells, A.U.; Ahad, M.A.; Lightman, S.; du Bois, R.M.; Welsh, K.I. Association between heat shock protein 70/hom genetic polymorphisms and uveitis in patients with sarcoidosis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3019–3025. [Google Scholar] [CrossRef]
- Gross, P.; Bartlett, T.; Browdy, C.; Chapman, R.; Warr, G. Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the pacific white shrimp, Litopenaeus vannamei, and the atlantic white shrimp, L. setiferus. Dev. Comp. Immunol. 2001, 25, 565–577. [Google Scholar] [CrossRef]
- Eisenhut, M. Mediators of cellular stress response in bacterial meningitis. Crit. Care Med. 2008, 36, 365–366. [Google Scholar] [CrossRef]
- Liu, C.-H.; Yeh, S.-P.; Kuo, C.-M.; Cheng, W.; Chou, C.-H. The effect of sodium alginate on the immune response of tiger shrimp via dietary administration: Activity and gene transcription. Fish Shellfish Immunol. 2006, 21, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhu, S.; Liu, D.; Guo, X.; Ye, Z. Effects of stocking density of the white shrimp, Litopenaeus vannamei (boone) on immunities, antioxidant status, and resistance against Vibrio harveyi in a biofloc system. Fish Shellfish Immunol. 2017, 67, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, D.; Jang, Y.-H.; Kim, D.-H.; Kang, B.-J.; Heo, M.-S. Dietary effect of Rubus coreanus ethanolic extract on immune gene expression in white leg shrimp, Penaeus vannamei. Fish Shellfish Immunol. 2013, 35, 808–814. [Google Scholar] [CrossRef] [PubMed]
- González-Ruiz, R.; Granillo-Luna, O.N.; Peregrino-Uriarte, A.B.; Gómez-Jiménez, S.; Yepiz-Plascencia, G. Mitochondrial manganese superoxide dismutase from the shrimp Litopenaeus vannamei: Molecular characterization and effect of high temperature, hypoxia and reoxygenation on expression and enzyme activity. J. Therm. Biol. 2020, 88, 102519. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Singh, S.; Ramaiah, N.; Sreepada, R. Identification of upregulated immune-related genes in Vibrio harveyi challenged Penaeus monodon postlarvae. Fish Shellfish Immunol. 2010, 29, 544–549. [Google Scholar] [CrossRef]
- Gueguen, Y.; Garnier, J.; Robert, L.; Lefranc, M.-P.; Mougenot, I.; De Lorgeril, J.; Janech, M.; Gross, P.S.; Warr, G.W.; Cuthbertson, B. Penbase, the shrimp antimicrobial peptide penaeidin database: Sequence-based classification and recommended nomenclature. Dev. Comp. Immunol. 2006, 30, 283–288. [Google Scholar] [CrossRef]
- Destoumieux, D.; Bulet, P.; Strub, J.M.; van Dorsselaer, A.; Bachère, E. Recombinant expression and range of activity of penaeidins, antimicrobial peptides from penaeid shrimp. Eur. J. Biochem. 1999, 266, 335–346. [Google Scholar] [CrossRef]
- Wang, K.H.-C.; Tseng, C.-W.; Lin, H.-Y.; Chen, I.-T.; Chen, Y.-H.; Chen, Y.-M.; Chen, T.-Y.; Yang, H.-L. Rnai knock-down of the Litopenaeus vannamei toll gene (lvtoll) significantly increases mortality and reduces bacterial clearance after challenge with Vibrio harveyi. Dev. Comp. Immunol. 2010, 34, 49–58. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Shanthi, S.; Chen, J.-C.; Espineira, M. Molecular cloning, sequence analysis and expression of fein-penaeidin from the haemocytes of indian white shrimp, Fenneropenaeus indicus. Results Immunol. 2012, 2, 35–43. [Google Scholar] [CrossRef]
- Akev, N.; Can, A.; Sütlüpınar, N.; Çandöken, E.; Özsoy, N.; Özden, T.; Yanardağ, R.; Üzen, E. Twenty years of research on Aloe vera. İstanbul Üniversitesi Eczacılık Fakültesi Derg. 2015, 45, 191–215. [Google Scholar]
- Gnanasekar, M.; Thirugnanam, S.; Zheng, G.; Chen, A.; Ramaswamy, K. Gene silencing of translationally controlled tumor protein (TCTP) by sirna inhibits cell growth and induces apoptosis of human prostate cancer cells. Int. J. Oncol. 2009, 34, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Bangrak, P.; Graidist, P.; Chotigeat, W.; Phongdara, A. Molecular cloning and expression of a mammalian homologue of a translationally controlled tumor protein (TCTP) gene from Penaeus monodon shrimp. J. Biotechnol. 2004, 108, 219–226. [Google Scholar] [CrossRef]
- El-Nokrashy, A.; El-Banna, R.; Edrise, B.; Abdel-Rahim, M.; Jover-Cerdá, M.; Tomás-Vidal, A.; Prince, A.; Davies, S.; El-Haroun, E.; Goda, A.-S. Impact of nucleotide enriched diets on the production of gilthead seabream, Sparus aurata fingerlings by modulation of liver mitochondrial enzyme activitity, antioxidant status, immune gene expression, and gut microbial ecology. Aquaculture 2021, 535, 736398. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, E.G.; Li, Y.; Liu, H.; Vidal-Dorsch, D.E.; Giesy, J.P. Ecological risks posed by ammonia nitrogen (AN) and un-ionized ammonia (NH3) in seven major river systems of China. Chemosphere 2018, 202, 136–144. [Google Scholar] [CrossRef] [PubMed]
- AOAC, (Association of Official Analytical Chemists). Official Methods of Analysis of the Association of Analytical Chemists International, 18th ed.; AOAC, (Association of Official Analytical Chemists): Gathersburg, MD, USA, 2005. [Google Scholar]
- Blaxhall, P.; Daisley, K. Routine haematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Itami, T.; Asano, M.; Tokushige, K.; Kubono, K.; Nakagawa, A.; Takeno, N.; Nishimura, H.; Maeda, M.; Kondo, M.; Takahashi, Y. Enhancement of disease resistance of kuruma shrimp, Penaeus japonicus, after oral administration of peptidoglycan derived from Bifidobacterium thermophilum. Aquaculture 1998, 164, 277–288. [Google Scholar] [CrossRef]
- Engstad, R.E.; Robertsen, B.; Frivold, E. Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in atlantic salmon blood. Fish Shellfish Immunol. 1992, 2, 287–297. [Google Scholar] [CrossRef]
- Supamattaya, K.; Pongmaneerat, J.; Klowklieng, T. The effect of β-glucan (macrogard®) on growth performance, immune response and disease resistance in black tiger shrimp, Penaeus monodon fabricius. Songklanakarin J. Sci. Technol 2000, 22, 677–688. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Walter, K.; Schutt, C. Acid and alkaline phosphatase in serum. Methods Enzym. Anal. 1974, 2, 856–860. [Google Scholar]
- Roberts, R.J.; Smail, D.A. Laboratory methods. In Fish Pathology, 3rd ed.; Roberts, R.J., Saunders, W.B., Eds.; Elsevier Ltd: London, UK, 2004; pp. 380–412. [Google Scholar]
- Xu, Z.; Regenstein, J.M.; Xie, D.; Lu, W.; Ren, X.; Yuan, J.; Mao, L. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish Immunol. 2018, 72, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Rungrassamee, W.; Leelatanawit, R.; Jiravanichpaisal, P.; Klinbunga, S.; Karoonuthaisiri, N. Expression and distribution of three heat shock protein genes under heat shock stress and under exposure to Vibrio harveyi in Penaeus monodon. Dev. Comp. Immunol. 2010, 34, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− δδct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Flores-Miranda, M.d.C.; Luna-González, A.; Cortés-Espinosa, D.; Cortés-Jacinto, E.; Fierro-Coronado, J.; Álvarez-Ruiz, P.; González-Ocampo, H.; Escamilla-Montes, R. Bacterial fermentation of Lemna sp. as a potential substitute of fish meal in shrimp diets. Afr. J. Microbiol. Res. 2014, 8, 1516–1526. [Google Scholar]
- Wang, Y.-C.; Chang, P.-S.; Chen, H.-Y. Differential time-series expression of immune-related genes of pacific white shrimp, Litopenaeus vannamei in response to dietary inclusion of β-1, 3-glucan. Fish Shellfish Immunol. 2008, 24, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.B. Multiple range and multiple f tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
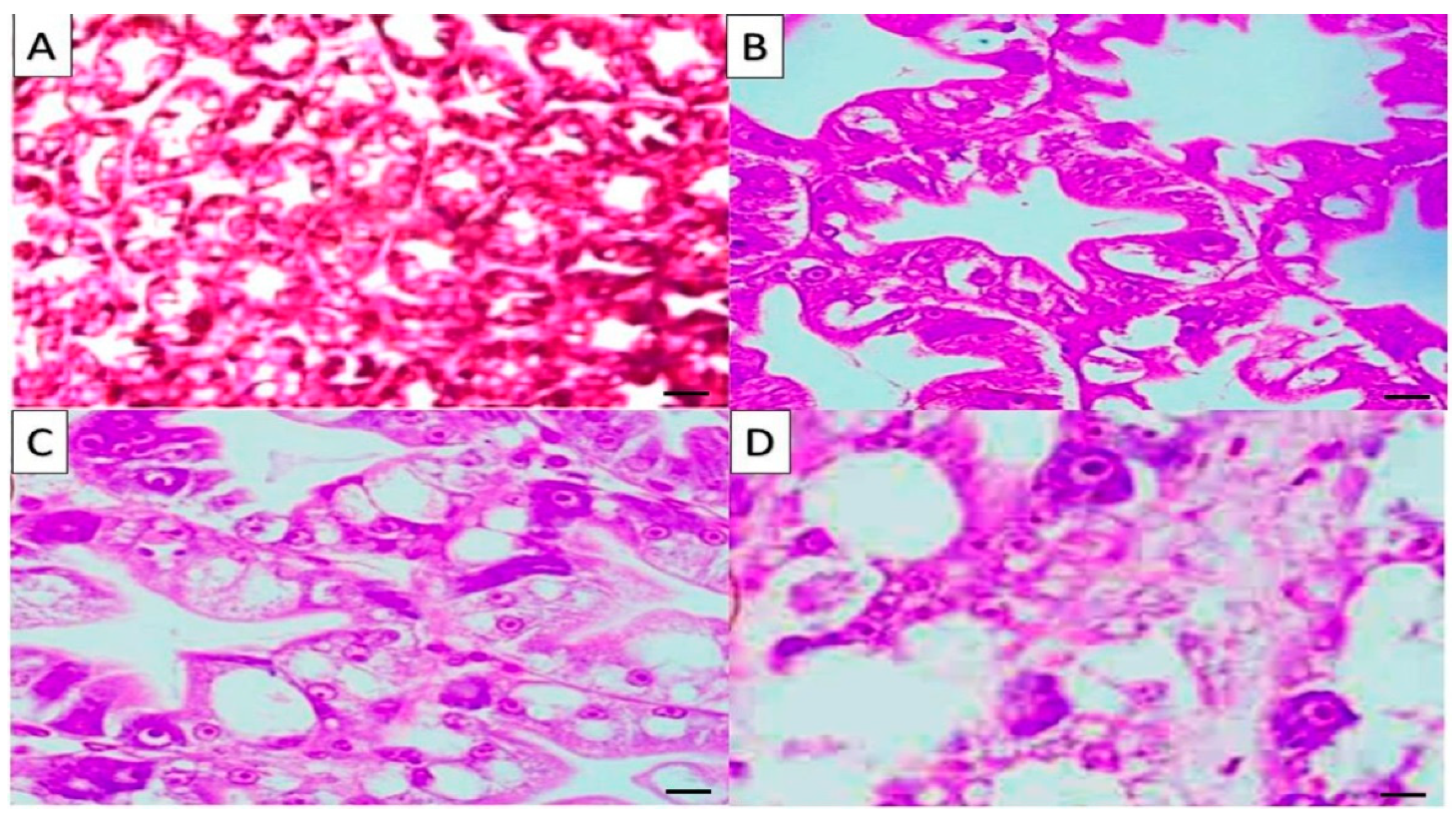

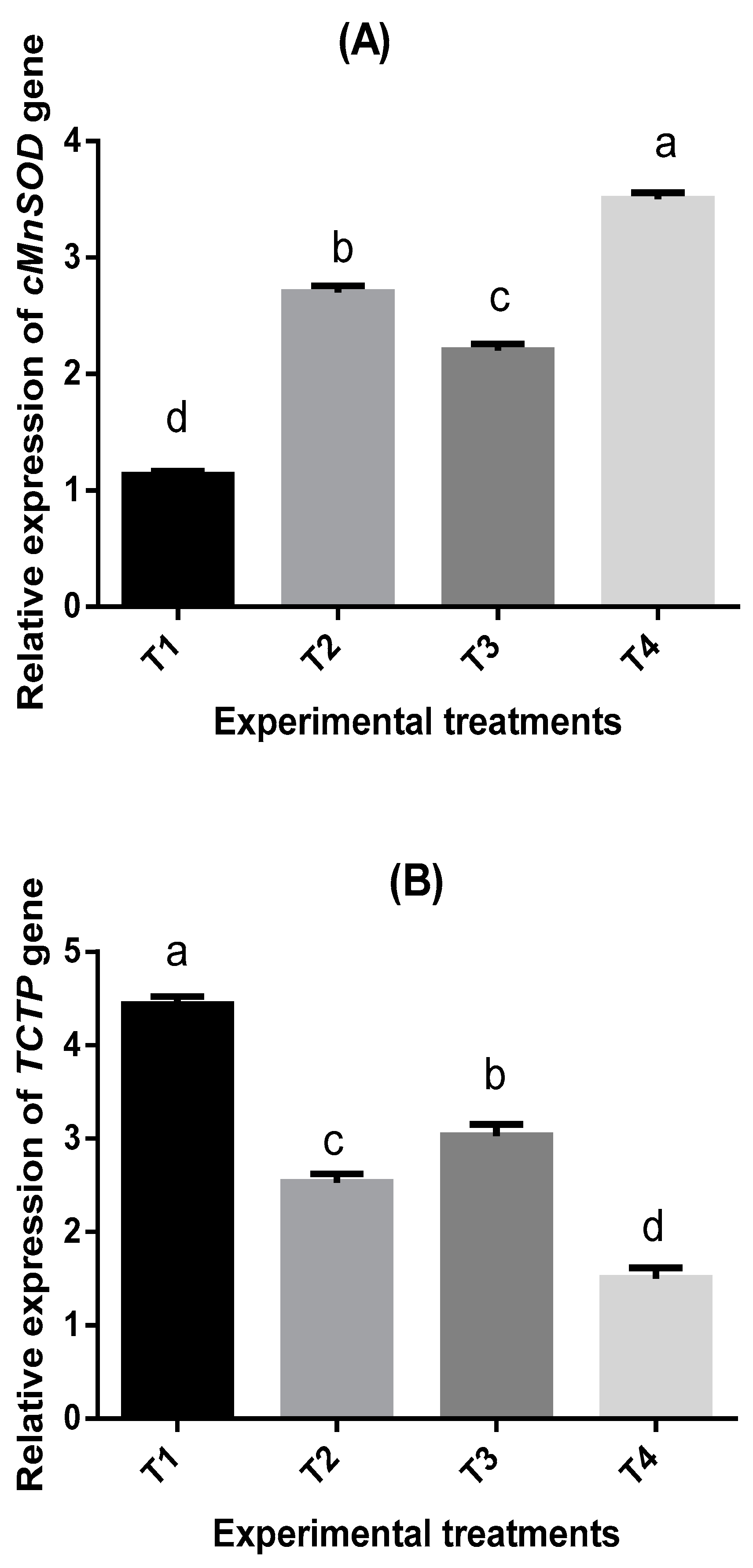
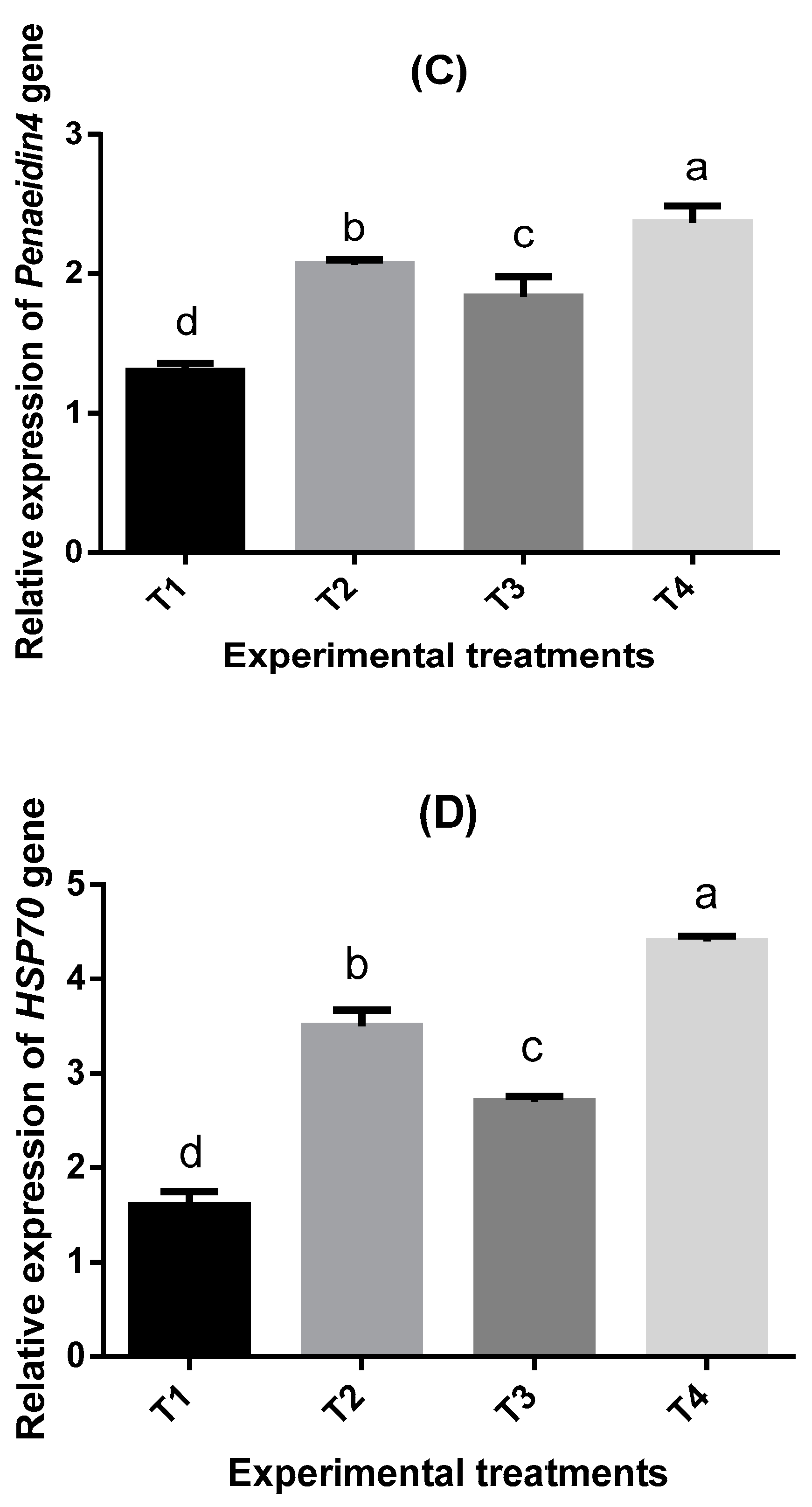
| Parameters | Treatments * | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Water salinity, ppt | 32 ± 0.05 | 32 ± 0.04 | 32 ± 0.08 | 32 ± 0.06 |
| Temperature, °C | 13.33 ± 0.07 | 13.27 ± 0.07 | 13.33 ± 0.03 | 13.43 ± 0.03 |
| pH | 8.20 ± 0.01 a | 8.14 ± 0.05 ab | 8.06 ± 0.04 b | 8.12 ± 0.01 ab |
| DO, ppm | 5.97 ± 0.09 c | 6.37 ± 0.03 b | 6.40 ± 0.06 b | 6.67 ± 0.09 a |
| TAN, ppm | 0.48 ± 0.02 a | 0.39 ± 0.01 b | 0.38 ± 0.01 b | 0.24 ± 0.02 c |
| NH3, ppm | 0.0176 a | 0.0125 b | 0.0102 bc | 0.0075 c |
| Parameters | Treatments * | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Growth performance indices and survival | ||||
| Initial weight (g) | 12.04 ± 0.02 | 12.05 ± 0.01 | 12.06 ± 0.02 | 12.02 ± 0.02 |
| Final weight (g) | 15.28 ± 0.01 d | 15.62 ± 0.04 b | 15.46 ± 0.02 c | 15.82 ± 0.02 a |
| Weight gain (g) | 3.24 ± 0.02 d | 3.57 ± 0.02 b | 3.40 ± 0.04 c | 3.81 ± 0.02 a |
| ADG (g/shrimp/day) | 0.058 ± 0.0 d | 0.064 ± 0.00 b | 0.061 ± 0.00 c | 0.068 ± 0.00 a |
| SGR (%/shrimp/day) | 0.425 ± 0.001 d | 0.463 ± 0.002 b | 0.444 ± 0.005 c | 0.491 ± 0.003 a |
| Condition factor | 0.583 ± 0.003 c | 0.700 ± 0.006 b | 0.690 ± 0.006 b | 0.753 ± 0.003 a |
| Survival rate (%) | 75.00 ± 1.44 b | 82.50 ± 1.44 a | 80.00 ± 1.44 a | 84.17 ± 0.83 a |
| Feed utilization indices | ||||
| FCR | 3.91 ± 0.043 a | 3.14 ± 0.051 c | 3.42 ± 0.035 b | 2.78 ± 0.021d |
| PER | 0.637 ± 0.009 d | 0.793 ± 0.015 b | 0.727 ± 0.009 c | 0.897 ± 0.009 a |
| PPV, % | 46.25 ± 1.37 b | 46.27 ± 2.46 b | 37.80 ± 1.22 c | 64.74 ± 1.07 a |
| Energy utilization (EU %) | 26.77 ± 0.58 b | 25.52 ± 1.48 b | 19.54 ± 0.88 c | 35.14 ± 0.82 a |
| Whole-body proximate composition | ||||
| Dry matter (%) | 20.43 ± 0.02 c | 21.16 ± 0.01 b | 21.79 ± 0.02 a | 21.73 ± 0.08 a |
| Crude protein (%) | 73.45 ± 0.32 a | 70.20 ± 0.59 b | 68.92 ± 0.34 c | 73.35 ± 0.20 a |
| Ether extract (%) | 3.19 ± 0.12 a | 2.58 ± 0.14 b | 2.07 ± 0.11 b | 2.54 ± 0.26 b |
| Ash (%) | 16.05 ± 0.31 a | 14.55 ± 0.37 bc | 13.88 ± 0.35 c | 15.04 ± 0.18 ab |
| Fibre (%) | 4.86 ± 0.03 d | 5.24 ± 0.05 c | 5.46 ± 0.05 b | 5.63 ± 0.04 a |
| Carcass energy (Kcal/100g) | 444.42 ± 0.97 a | 420.26 ± 3.55 b | 408.27 ± 2.83 c | 437.68 ± 2.84 a |
| Parameters | Treatments * | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Total hematocytes count (cells/mm3) | 19.00 ± 0.58 d | 27.00 ± 0.58 b | 22.33 ± 0.88 c | 32.33 ± 1.45a |
| Phagocytosis (%) | 22.20 ± 0.55 d | 29.30 ± 0.61 b | 26.63 ± 0.44 c | 33.37 ± 0.64 a |
| Phagocytic index | 2.57 ± 0.20 d | 4.13 ± 0.27 b | 3.50 ± 0.12 c | 5.13 ± 0.15 a |
| Total protein (mg m L−1) | 5.77 ± 0.43 c | 11.63 ± 0.55 a | 7.97 ± 0.42 b | 12.93 ± 0.43 a |
| Acid phosphatase activity (U L−1) | 15.97 ± 0.72 c | 23.17 ± 0.75 b | 21.40 ± 0.72 b | 25.77 ± 0.47 a |
| Alkaline phosphatase (U L−1) | 6.33 ± 0.72 c | 14.70 ± 1.47 b | 8.80 ± 0.15 c | 20.37 ± 0.50 a |
| Lysozyme activity (U L−1) | 59.57 ± 1.07 d | 78.70 ± 2.57 b | 68.10 ± 1.63 c | 97.17 ± 2.30 a |
| Phenoloxidase activity (U/min/mg) | 15.83 ± 1.28 d | 25.77 ± 0.76 b | 20.30 ± 0.52 c | 29.73 ± 0.50 a |
| Superoxide dismutase activity (U/min/mg) | 0.38 ± 0.02 d | 0.58 ± 0.02 b | 0.48 ± 0.02 c | 0.74 ± 0.04 a |
| Total nitric oxide (μg/mL) | 11.73 ± 0.75 d | 23.13 ± 0.74 b | 19.67 ± 0.88 c | 27.20 ± 0.93 a |
| Parameter | %, DM |
|---|---|
| Protein (%) | 14.99 |
| Ether extract (%) | 7.74 |
| Fiber (%) | 16.98 |
| Carbohydrate (%) | 24.93 |
| Ash content (%) | 28.57 |
| Ingredients (g/kg) | Experimental Diets | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Soybean meal | 340 | 340 | 340 | 340 |
| Wheat meal | 150 | 150 | 150 | 150 |
| Fish meal | 112 | 112 | 112 | 112 |
| Corn gluten, 60% CP | 81 | 80.5 | 80.5 | 80 |
| Rice bran | 80 | 80 | 80 | 80 |
| Corn grain | 60 | 60 | 60 | 60 |
| Shrimp meal | 50 | 50 | 50 | 50 |
| Meat meal | 50 | 50 | 50 | 50 |
| Vitamin and mineral premix | 30 | 30 | 30 | 30 |
| Dicalcium phosphate | 20 | 20 | 20 | 20 |
| Soybean oil | 10 | 10 | 10 | 10 |
| Fish oil | 8 | 8 | 8 | 8 |
| Lecithin | 5 | 5 | 5 | 5 |
| Choline chloride | 2 | 2 | 2 | 2 |
| Cholesterol | 1 | 1 | 1 | 1 |
| Vit. C | 1 | 1 | 1 | 1 |
| Nucleotides | 0 | 0.5 | 0 | 0.5 |
| Sargassum polycystum | 0 | 0 | 0.5 | 0.5 |
| Total | 1000 | 1000 | 1000 | 1000 |
| Proximate composition, %/FM/DM 1 | ||||
| DM, (%) | 90.55/100 | 90.42/100 | 90.45/100 | 90.58/100 |
| CP, (%) | 36.34/40.13 | 36.41/40.27 | 36.17/39.99 | 36.28/40.05 |
| EE, (%) | 4.56/5.04 | 4.51/4.99 | 4.54/5.02 | 4.55/5.03 |
| CF, (%) | 2.36/2.61 | 2.32/2.57 | 2.37/2.62 | 2.34/2.58 |
| Ash, (%) | 11.22/12.39 | 11.18/12.36 | 11.30/12.49 | 11.26/12.43 |
| NFE, (%) 1 | 45.52 | 45.58 | 45.62 | 45.57 |
| Gross energy (MJ/kg diet) 2 | 18.25 | 18.26 | 18.22 | 18.24 |
| Gross energy (kcal/kg) 3 | 4350.84 | 4352.77 | 4343.66 | 4348.68 |
| Energy/protein (kcal/g protein) | 119.73 | 119.55 | 120.09 | 119.86 |
| Ca, (%) | 2.15 | 2.11 | 2.19 | 2.17 |
| Total P, (%) | 1.53 | 1.52 | 1.56 | 1.54 |
| Available P, (%) | 1.25 | 1.23 | 1.26 | 1.25 |
| Genes | Primers | Sequence (5’–3’) | References |
|---|---|---|---|
| SOD | SOD-F | ATCCACCACACAAAGCATCA | [74] |
| SOD-R | AGCTCTCGTCAATGGCTTGT | ||
| TCTP | TCTP-F | CAATGGACCCTGATGGC | [61] |
| TCTP-R | GCTTCTCCTCTGTTAGACCGTAT | ||
| Penaeidin4 | Pen4-F | GCCCGTTACCCAAACCATC | [74] |
| Pen4-R | CCGTATCTGAAGCAGCAAAGTC | ||
| HSP70 | HSP70 F | GGCAAGGAGCTGAACAAGTC | [93] |
| HSP70 R | TCTCGATACCCAGGGACAAG | ||
| β-actin | β-actin-F | CCACGAGACCACCTACAAC | [94] |
| β-actin-R | AGCGAGGGCAGTGATTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Rahim, M.; Bahattab, O.; Nossir, F.; Al-Awthan, Y.; Khalil, R.H.; Mohamed, R. Dietary Supplementation of Brown Seaweed and/or Nucleotides Improved Shrimp Performance, Health Status and Cold-Tolerant Gene Expression of Juvenile Whiteleg Shrimp during the Winter Season. Mar. Drugs 2021, 19, 175. https://doi.org/10.3390/md19030175
Abdel-Rahim M, Bahattab O, Nossir F, Al-Awthan Y, Khalil RH, Mohamed R. Dietary Supplementation of Brown Seaweed and/or Nucleotides Improved Shrimp Performance, Health Status and Cold-Tolerant Gene Expression of Juvenile Whiteleg Shrimp during the Winter Season. Marine Drugs. 2021; 19(3):175. https://doi.org/10.3390/md19030175
Chicago/Turabian StyleAbdel-Rahim, Mohamed, Omar Bahattab, Fatma Nossir, Yahya Al-Awthan, Riad H. Khalil, and Radi Mohamed. 2021. "Dietary Supplementation of Brown Seaweed and/or Nucleotides Improved Shrimp Performance, Health Status and Cold-Tolerant Gene Expression of Juvenile Whiteleg Shrimp during the Winter Season" Marine Drugs 19, no. 3: 175. https://doi.org/10.3390/md19030175
APA StyleAbdel-Rahim, M., Bahattab, O., Nossir, F., Al-Awthan, Y., Khalil, R. H., & Mohamed, R. (2021). Dietary Supplementation of Brown Seaweed and/or Nucleotides Improved Shrimp Performance, Health Status and Cold-Tolerant Gene Expression of Juvenile Whiteleg Shrimp during the Winter Season. Marine Drugs, 19(3), 175. https://doi.org/10.3390/md19030175








