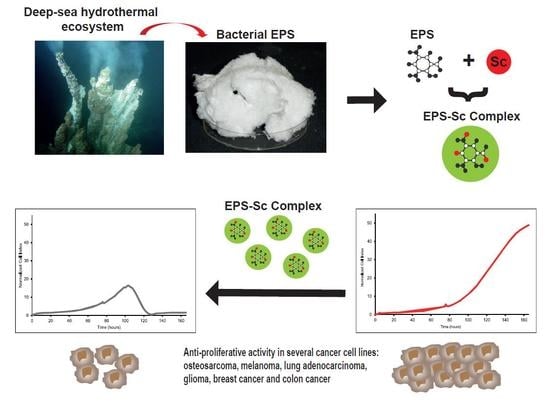
Antiproliferative Properties of Scandium Exopolysaccharide Complexes on Several Cancer Cell Lines
Abstract
1. Introduction
2. Results
2.1. Human MNNG/HOS Osteosarcoma Cell Line
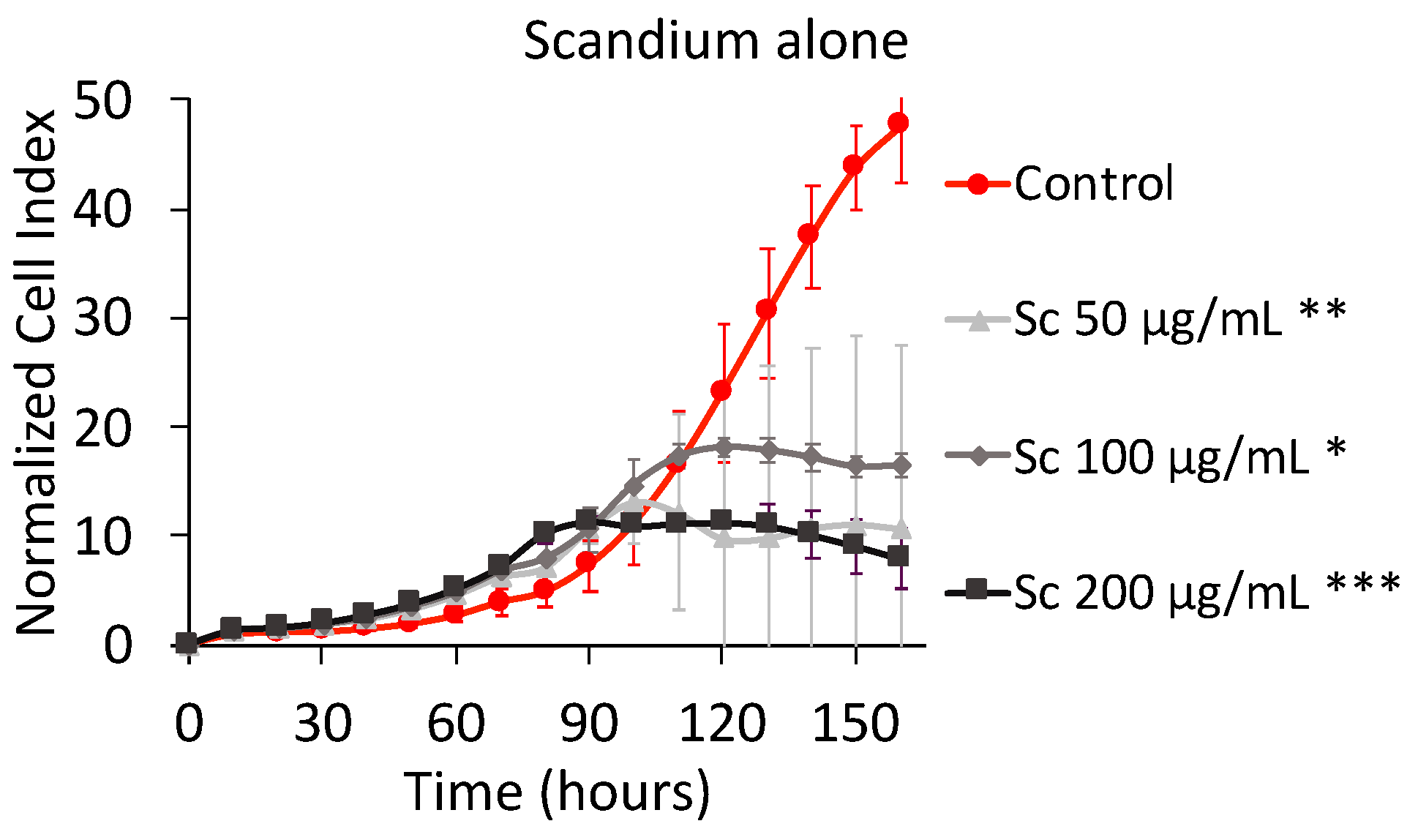
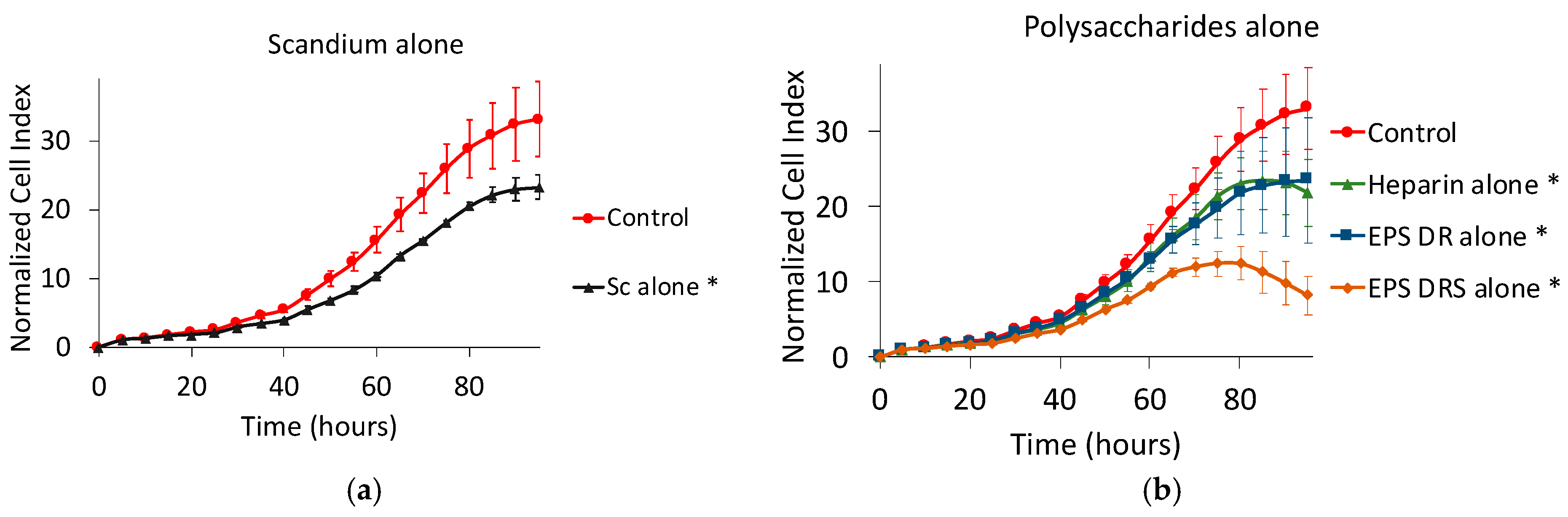
2.1.1. Effect of Scandium Alone on Cell Proliferation and Viability
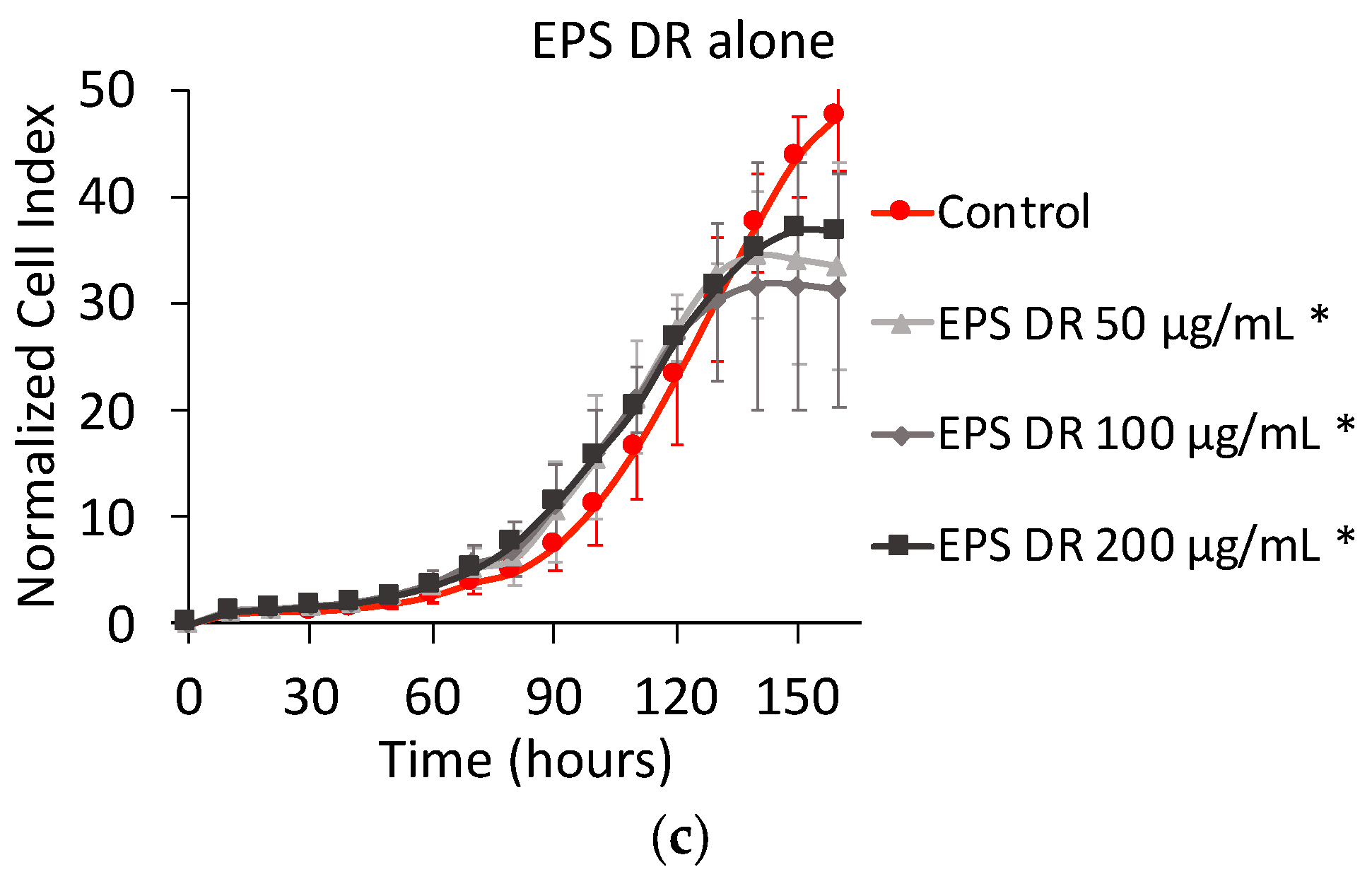
2.1.2. Effect of EPS and Heparin Alone
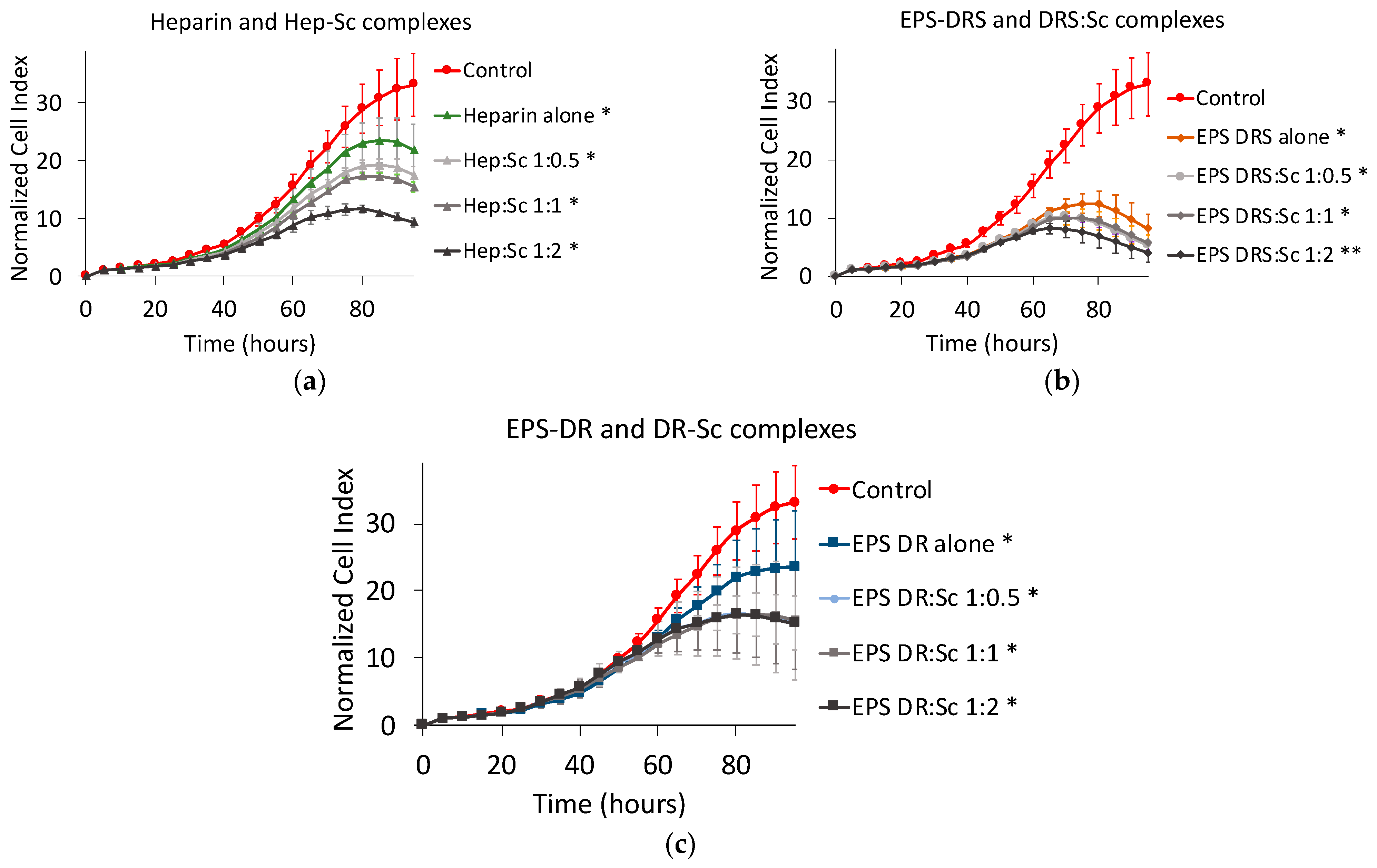
2.1.3. Effect of Polysaccharide:Scandium Complexes
Heparin:Scandium (Hep:Sc) Complex
EPS-DRS:Scandium (EPS-DRS:Sc) Complex
EPS-DR:Scandium (EPS-DR:Sc) Complex
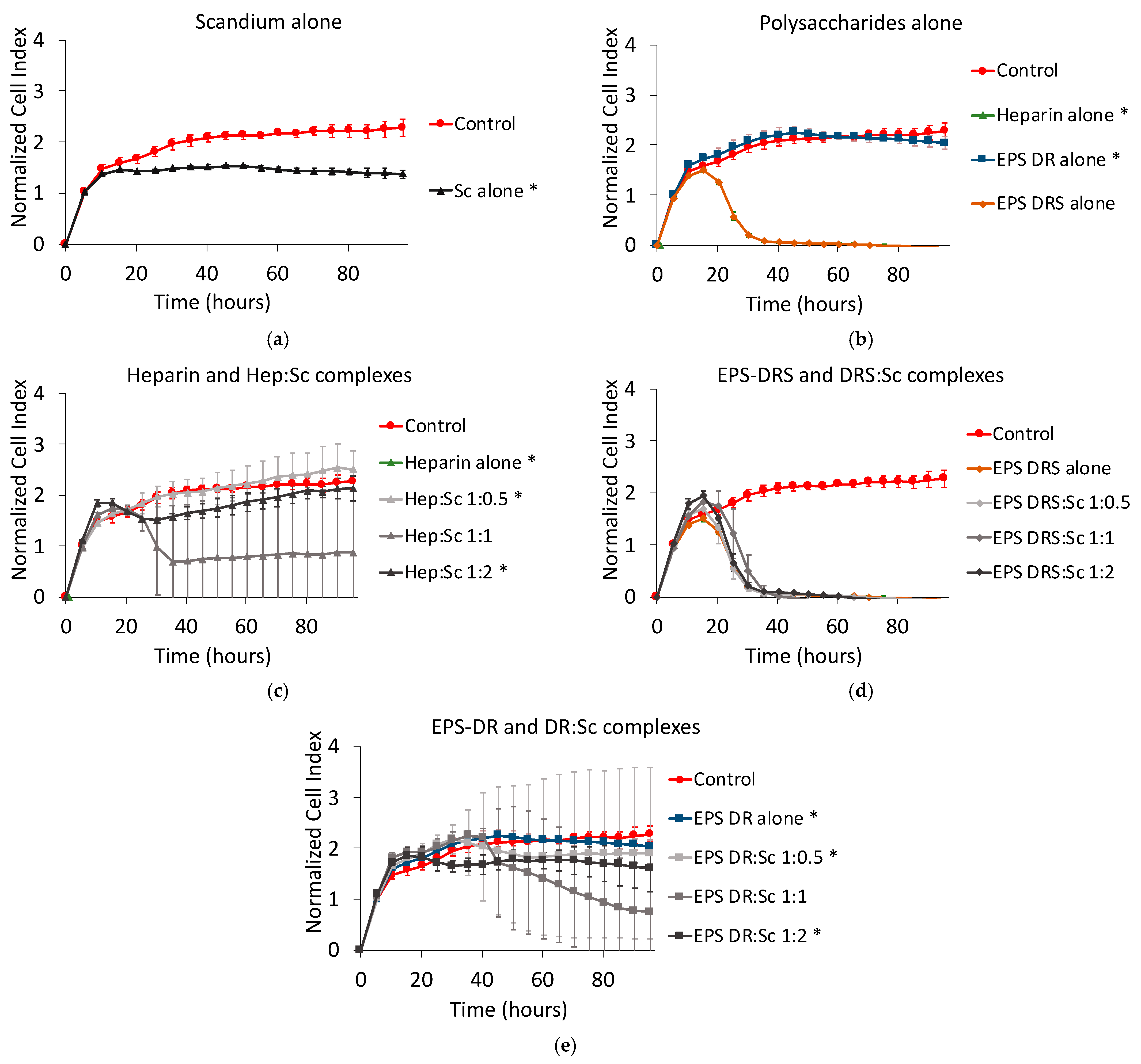
2.2. Human A375 Melanoma Cells
Effect of Scandium and Polysaccharides Alone
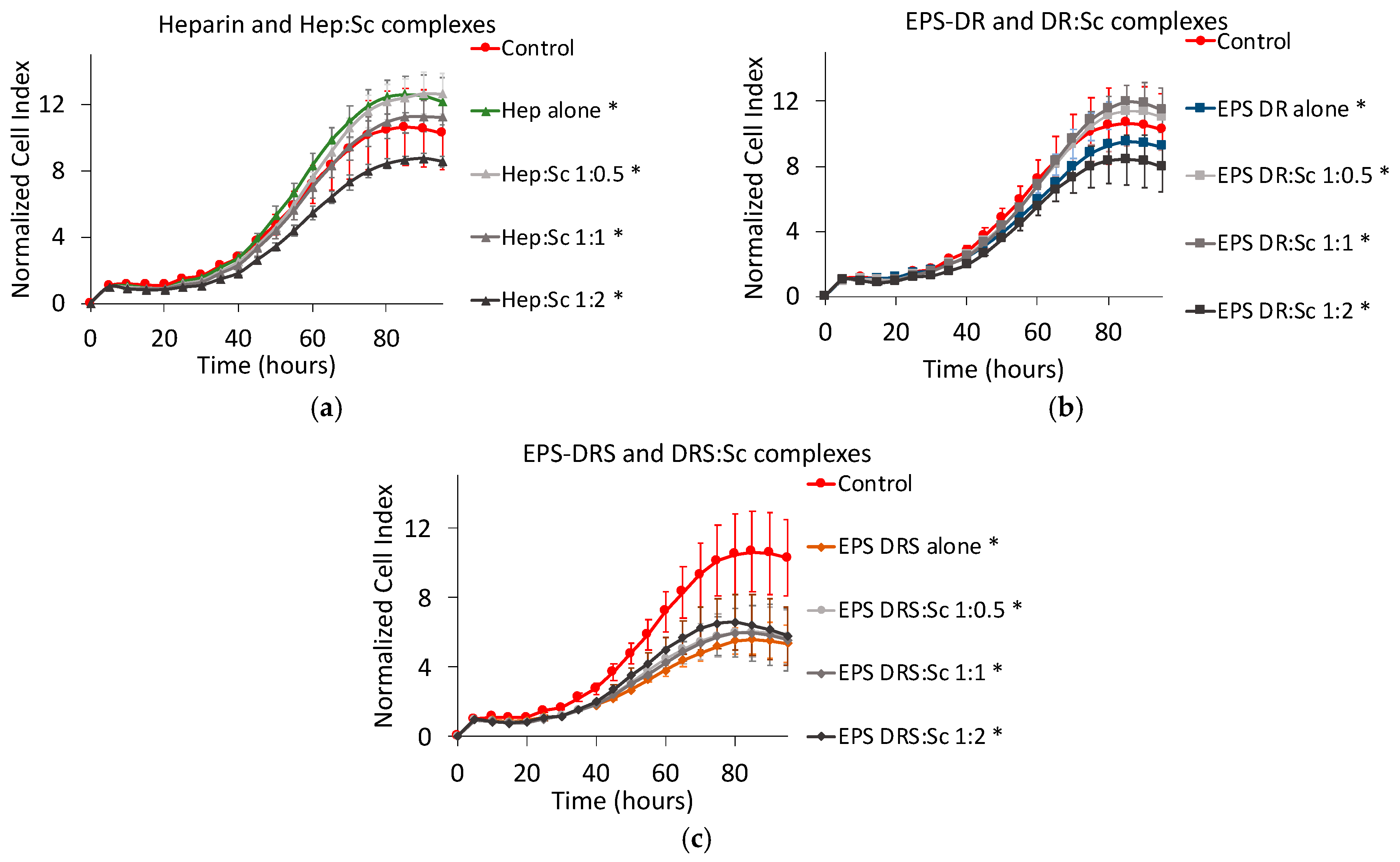
2.3. Effect of Polysaccharide:Scandium Complexes
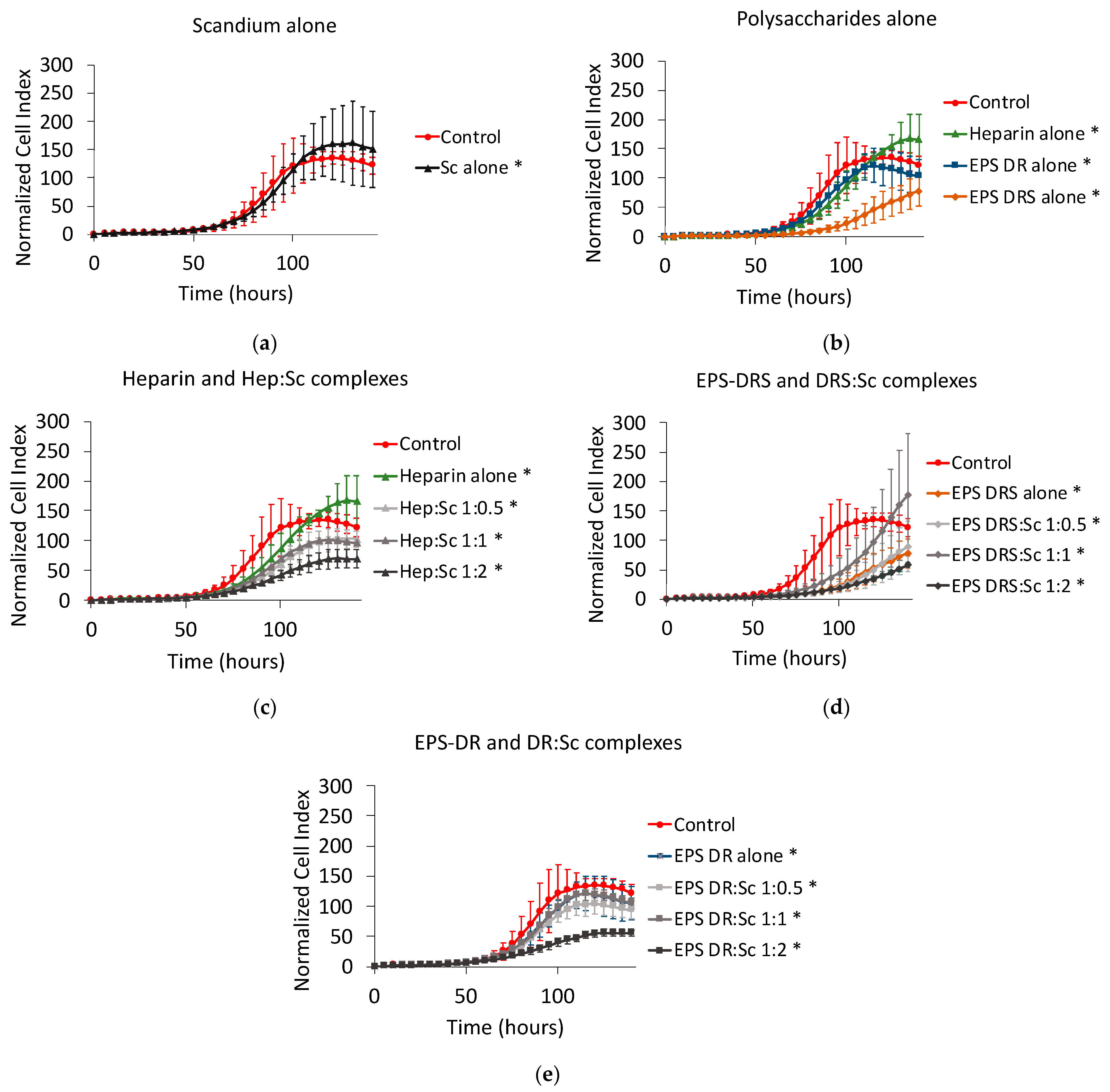
2.4. Human A549 Lung Cancer Cells
2.4.1. Effect of Scandium and Polysaccharides Alone
2.4.2. Effect of Polysaccharide:Scandium Complexes
2.5. Human U251 Glioblastoma Cells
2.6. Human MDA231 Breast Cancer Cells
2.7. Human Caco2 Colon Cancer Cells
3. Discussion
3.1. Effect of Scandium Alone
3.2. Effect of Heparin and EPS Alone
3.3. Synergic Effect of Complexes
3.3.1. Heparin:Sc (Hep:Sc)
3.3.2. EPS-DRS:Sc and EPS-DR:Sc
4. Materials and Methods
4.1. Molecules Assessed
4.2. Proliferation Assay
4.3. Viability Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| CI | cell index |
| EPS | exopolysaccharides |
| GAG | glycosaminoglycanes |
| LMW | low molecular weight |
| LMWH | Low molecular weight heparins |
| NCI | normalized cell index |
| RTCA | Real Cell Time Analysis |
References
- Borsig, L. Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb. Res. 2010, 125, S66–S71. [Google Scholar] [CrossRef]
- Yip, G.W.; Smollich, M.; Götte, M. Therapeutic value of glycosaminoglycans in cancer. Mol. Cancer Ther. 2006, 5, 2139–2148. [Google Scholar] [CrossRef]
- Belting, M. Glycosaminoglycans in cancer treatment. Thromb. Res. 2014, 133, S95–S101. [Google Scholar] [CrossRef]
- Afratis, N.; Gialeli, C.; Nikitovic, D.; Tsegenidis, T.; Karousou, E.; Theocharis, A.D.; Pavão, M.S.; Tzanakakis, G.N.; Karamanos, N.K. Glycosaminoglycans: Key players in cancer cell biology and treatment: GAG Targeting in Cancer Cell Biology. FEBS J. 2012, 279, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H. Thromboprophylaxis with low-molecular-weight heparin in medical patients with cancer. Cancer 2009, 115, 5637–5650. [Google Scholar] [CrossRef]
- Debourdeau, P.; Elalamy, I.; De Raignac, A.; Méria, P.; Gornet, J.M.; Amah, Y.; Korte, W.; Marty, M.; Farge, D. Long-term use of daily subcutaneous low molecular weight heparin in cancer patients with venous thromboembolism: Why hesitate any longer? Support. Care Cancer 2008, 16, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G.; Borsig, L. Cancer Cell Adhesion and Metastasis: Selectins, Integrins, and the Inhibitory Potential of Heparins. Int. J. Cell Biol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review: Medical Application of Glycosaminoglycans. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Jouault, S.C.; Mauray, S.; Theveniaux, J.; Sternberg, C.; Vidal, B.; Fischer, A.M.; Millet, J. Antithrombotic and Anticoagulant Activities of a Low Molecular Weight Fucoidan by the Subcutaneous Route. Thromb. Haemost. 1999, 81, 391–395. [Google Scholar] [CrossRef]
- Guezennec, J. Deep-sea hydrothermal vents: A new source of innovative bacterial exopolysaccharides of biotechnological interest? J. Ind. Microbiol. Biotechnol. 2002, 29, 204–208. [Google Scholar] [CrossRef]
- Colliec-Jouault, S.; Zanchetta, P.; Helley, D.; Ratiskol, J.; Sinquin, C.; Fischer, A.M.; Guezennec, J. Les polysaccharides microbiens d’origine marine et leur potentiel en thérapeutique humaine. Pathol. Biol. 2004, 52, 127–130. [Google Scholar] [CrossRef]
- Roger, O.; Kervarec, N.; Ratiskol, J.; Colliec-Jouault, S.; Chevolot, L. Structural studies of the main exopolysaccharide produced by the deep-sea bacterium Alteromonas infernus. Carbohydr. Res. 2004, 339, 2371–2380. [Google Scholar] [CrossRef]
- Raguénès, G.H.C.; Peres, A.; Ruimy, R.; Pignet, P.; Christen, R.; Loäec, M.; Rougeaux, H.; Barbier, G.; Guezennec, J.G. Alteromonas infernus sp. nov., a new polysaccharide-producing bacterium isolated from a deep-sea hydrothermal vent. J. Appl. Microbiol. 1997, 82, 422–430. [Google Scholar] [CrossRef]
- Ruiz-Velasco, C.; Baud’Huin, M.; Sinquin, C.; Maillasson, M.; Heymann, D.; Colliec-Jouault, S.; Padrines, M. Effects of a sulfated exopolysaccharide produced by Altermonas infernus on bone biology. Glycobiology 2011, 21, 781–795. [Google Scholar] [CrossRef]
- Chopin, N.; Sinquin, C.; Ratiskol, J.; Zykwinska, A.; Weiss, P.; Cérantola, S.; Le Bideau, J.; Colliec-Jouault, S. A Direct Sulfation Process of a Marine Polysaccharide in Ionic Liquid. BioMed Res. Int. 2015, 2015, 50865. [Google Scholar] [CrossRef] [PubMed]
- Jouault, S.C.; Chevolot, L.; Helley, D.; Ratiskol, J.; Bros, A.; Sinquin, C.; Roger, O.; Fischer, A.-M. Characterization, chemical modifications and in vitro anticoagulant properties of an exopolysaccharide produced by Alteromonas infernus. Biochim. Biophys. Acta 2001, 1528, 141–151. [Google Scholar] [CrossRef]
- Bruland, Ø.S.; Pihl, A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur. J. Cancer 1997, 33, 1725–1731. [Google Scholar] [CrossRef]
- Heymann, D.; Ruiz-Velasco, C.; Chesneau, J.; Ratiskol, J.; Sinquin, C.; Colliec-Jouault, S. Anti-Metastatic Properties of a Marine Bacterial Exopolysaccharide-Based Derivative Designed to Mimic Glycosaminoglycans. Molecules 2016, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Cutter, G.R.; Liu, Y. Personalized medicine: The return of the house call? Neurol. Clin. Pract. 2012, 2, 343–351. [Google Scholar] [CrossRef]
- Huclier-Markai, S.; Alliot, C.; Sebti, J.; Brunel, B.; Aupiais, J. A comparative thermodynamic study of the formation of scandium complexes with DTPA and DOTA. RSC Adv. 2015, 5, 99606–99617. [Google Scholar] [CrossRef]
- Mazza, M.; Alliot, C.; Sinquin, C.; Colliec-Jouault, S.; Reiller, P.E.; Huclier-Markai, S. Marine Exopolysaccharide Complexed With Scandium Aimed as Theranostic Agents. Molecules 2021, 26, 1143. [Google Scholar] [CrossRef]
- Zhang, J.D. Introduction to the Data Analysis of the Roche XCELLigence—System with RTCA Package. 11. Available online: www.bioconductor.org › inst › doc (accessed on 22 March 2021).
- Permyakiv, E. Metalloproteomics; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Byrd, B.L.; Watson, E.E.; Cloutier, R.J.; Hayes, R.L. Effect of Stable Scandium on the Long-term Whole Body Retention of Intravenously Administered 46Sc Citrate in the Rat. Health Phys. 1975, 29, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Suzuki, K.T. Exposure, Metabolism, and Toxicity of Rare Earths and Related Compounds. Environ. Health Perspect. 1996, 104, 11. [Google Scholar]
- Rosoff, B.; Siegel, E.; Williams, G.L.; Spencer, H. Distribution and excretion of radioactive rare-earth compounds in mice. Int. J. Appl. Radiat. Isot. 1963, 14, 129–135. [Google Scholar] [CrossRef]
- Moghaddam-Banaem, L.; Jalilian, A.R.; Pourjavid, M.; Bahrami-Samani, A.; Mazidi, M.; Ghannadi-Maragheh, M. Preparation and Quality Control of Scandium-46 Bleomycin as a Possible Therapeutic Agent. Iran. J. Nucl. Med. 2012, 20, 6. [Google Scholar]
- Herath, H.M.T.U.; Silvio, L.D.; Evans, J.R.G. Scandia—A potential biomaterial? J. Mater. Sci. Mater. Electron. 2005, 16, 1061–1065. [Google Scholar] [CrossRef]
- Lima, M.; Rudd, T.; Yates, E. New Applications of Heparin and Other Glycosaminoglycans. Molecules 2017, 22, 749. [Google Scholar] [CrossRef]
- Wright, T.C.; Castellot, J.J.; Petitou, M.; Lormeau, J.C.; Choay, J.; Karnovsky, M.J. Structural Determinants of Heparin’s Growth Inhibitory Activity: Interdependence of oligosaccharide size and charge. J. Biol. Chem. 1989, 264, 1534–1542. [Google Scholar] [CrossRef]
- Volpi, N.; Bolognani, L.; Conte, A.; Petrini, M. Effects of Chondroitin Sulfates with Different Structures on Leukemia Cells: U-937 Cell Proliferation and Differentiation. Leuk. Res. 1993, 17, 789–798. [Google Scholar] [CrossRef]
- Syrokou, A.; Tzanakakis, G.; Tsegenidis, T.; Hjerpe, A.; Karamanos, N.K.; Potten, C.; Darzynkiewicz, Z.; Sasaki, K. Effects of glycosaminoglycans on proliferation of epithelial and fibroblast human malignant mesothelioma cells: A structure-function relationship. Cell Prolif. 1999, 32, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Zafiropoulos, A.; Tzanakakis, G.N.; Karamanos, N.K.; Tsatsakis, A.M. Effects of glycosaminoglycans on cell proliferation of normal osteoblasts and human osteosarcoma cells depend on their type and fine chemical compositions. Anticancer. Res. 2005, 25, 2851–2856. [Google Scholar]
- Hausser, H.-J.; Brenner, R.E. Low doses and high doses of heparin have different effects on osteoblast-like Saos-2 cells in vitro. J. Cell. Biochem. 2004, 91, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.L.; Varki, A.; Borsig, L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb. Res. 2007, 120, S107–S111. [Google Scholar] [CrossRef]
- Abu Arab, W.; Kotb, R.; Sirois, M.; Rousseau, É. The Role of Heparin in Lung Cancer. J. Neoplasms 2017, 1, 14–28. [Google Scholar] [CrossRef]
- Kucukoner, M.; Isikdogan, A.; Kaplan, M.; Inal, A.; Zinciroglu, S.; Cit, M.; Cil, T.; Karadayi, B.; Dirie, A.; Yildiz, I. Can LMWH Improve the Outcome for Patients with Inoperable Stage III Non-Small Cell Lung Cancer? Contemp. Oncol. 2012, 16, 416. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Bereczky, B.; Gilly, R.; Rásó, E.; Vágó, Á.; Tímár, J.; Tóvári, J. Selective antimetastatic effect of heparins in preclinical human melanoma models is based on inhibition of migration and microvascular arrest. Clin. Exp. Metastasis 2005, 22, 69–76. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Kim, B.J.; Kim, H.J.; Jin, J.M.; Yoon, H.J.; Hwang, J.S.; Park, K.-K. Anti-cancer effect of dung beetle glycosaminoglycans on melanoma. BMC Cancer 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Logun, M.T.; Wynens, K.E.; Simchick, G.; Zhao, W.; Mao, L.; Zhao, Q.; Mukherjee, S.; Brat, D.J.; Karumbaiah, L. Surfen-mediated blockade of extratumoral chondroitin sulfate glycosaminoglycans inhibits glioblastoma invasion. FASEB J. 2019, 33, 11973–11992. [Google Scholar] [CrossRef]
- Gomes, A.M.; Stelling, M.P.; Pavão, M.S.G. Heparan Sulfate and Heparanase as Modulators of Breast Cancer Progression. BioMed Res. Int. 2013, 2013, 852093. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaou, G.; Nikitovic, D.; Asimakopoulou, A.; Tsatsakis, A.; Karamanos, N.K.; Tzanakakis, G.N. Heparin—A unique stimulator of human colon cancer cells’ growth. IUBMB Life 2008, 60, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, R.; Maas, S.L.N.; Broekman, M.L.D. Heparin in malignant glioma: Review of preclinical studies and clinical results. J. Neuro Oncol. 2015, 124, 151–156. [Google Scholar] [CrossRef] [PubMed]






| Complex | Ratio | Total Conc. of GAGs | Total Conc. of Sc | Effect on Cell Proliferation | |||||
|---|---|---|---|---|---|---|---|---|---|
| MNNG/HOS | A375 | A549 | U251 | MDA231 | Caco2 | ||||
| M:L | µg mL−1 | µg mL−1 | Osteosarcoma | Melanoma | Lung | Glioblastoma | Breast Cancer | Colon Cancer | |
| Hep:Sc | 1:0.5 | 50 | 0.36 | No effect | NT | ||||
| 100 | 0.71 | + + + | + + | − | − | No effect | + | ||
| 200 | 1.42 | + + + | NT | ||||||
| 1:1 | 50 | 0.71 | + | ||||||
| 100 | 1.42 | + + | + + | No effect | − − | + + | + | ||
| 200 | 2.83 | + + + | NT | ||||||
| 1:2 | 50 | 1.42 | + + + | ||||||
| 100 | 2.83 | + + + | + + + | + | − − | No effect | + + | ||
| 200 | 5.66 | + + + | NT | ||||||
| 1:4 | 50 | 2.83 | + + | ||||||
| 100 | 5.66 | + + | |||||||
| 200 | 11.32 | + + | |||||||
| EPS-DRS:Sc | 1:0.5 | 50 | 0.40 | + + | |||||
| 100 | 0.79 | + | + + + | + + | + | + + + | + | ||
| 200 | 1.58 | + | NT | ||||||
| 1:1 | 50 | 0.79 | + + | ||||||
| 100 | 1.58 | + + | + + + | + + | − − | + + + | − | ||
| 200 | 3.16 | + + + | NT | ||||||
| 1:2 | 50 | 1.58 | + + + | ||||||
| 100 | 3.16 | + + + | +++ | + + | − | + + + | + + | ||
| 200 | 6.31 | + + + | NT | ||||||
| 1:4 | 50 | 3.16 | + + + | ||||||
| 100 | 6.31 | + + | |||||||
| 200 | 12.62 | + + + | |||||||
| EPS-DR:Sc | 1:0.5 | 50 | 0.24 | No effect | |||||
| 100 | 0.48 | No effect | + + | − | − − | No effect | + | ||
| 200 | 0.95 | No effect | NT | ||||||
| 1:1 | 50 | 0.48 | + + + | ||||||
| 100 | 0.95 | + + + | + + | − | − − − | + + | + | ||
| 200 | 1.90 | + + + | NT | ||||||
| 1:2 | 50 | 0.95 | + + | ||||||
| 100 | 1.90 | + + + | + + | + | − − − | + | + + | ||
| 200 | 3.79 | + + + | NT | ||||||
| 1:4 | 50 | 1.90 | No effect | ||||||
| 100 | 3.79 | No effect | |||||||
| 200 | 7.58 | No effect | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Garcia, J.; Mazza, M.; Alliot, C.; Sinquin, C.; Colliec-Jouault, S.; Heymann, D.; Huclier-Markai, S. Antiproliferative Properties of Scandium Exopolysaccharide Complexes on Several Cancer Cell Lines. Mar. Drugs 2021, 19, 174. https://doi.org/10.3390/md19030174
Muñoz-Garcia J, Mazza M, Alliot C, Sinquin C, Colliec-Jouault S, Heymann D, Huclier-Markai S. Antiproliferative Properties of Scandium Exopolysaccharide Complexes on Several Cancer Cell Lines. Marine Drugs. 2021; 19(3):174. https://doi.org/10.3390/md19030174
Chicago/Turabian StyleMuñoz-Garcia, Javier, Mattia Mazza, Cyrille Alliot, Corinne Sinquin, Sylvia Colliec-Jouault, Dominique Heymann, and Sandrine Huclier-Markai. 2021. "Antiproliferative Properties of Scandium Exopolysaccharide Complexes on Several Cancer Cell Lines" Marine Drugs 19, no. 3: 174. https://doi.org/10.3390/md19030174
APA StyleMuñoz-Garcia, J., Mazza, M., Alliot, C., Sinquin, C., Colliec-Jouault, S., Heymann, D., & Huclier-Markai, S. (2021). Antiproliferative Properties of Scandium Exopolysaccharide Complexes on Several Cancer Cell Lines. Marine Drugs, 19(3), 174. https://doi.org/10.3390/md19030174