Abstract
Edible marine algae are rich in bioactive compounds and are, therefore, a source of bioavailable proteins, long chain polysaccharides that behave as low-calorie soluble fibers, metabolically necessary minerals, vitamins, polyunsaturated fatty acids, and antioxidants. Marine algae were used primarily as gelling agents and thickeners (phycocolloids) in food and pharmaceutical industries in the last century, but recent research has revealed their potential as a source of useful compounds for the pharmaceutical, medical, and cosmetic industries. The green, red, and brown algae have been shown to have useful therapeutic properties in the prevention and treatment of neurodegenerative diseases: Parkinson, Alzheimer’s, and Multiple Sclerosis, and other chronic diseases. In this review are listed and described the main components of a suitable diet for patients with these diseases. In addition, compounds derived from macroalgae and their neurophysiological activities are described.
1. Introduction
Neurodegenerative diseases are pathologies characterized by the irreversible destruction of certain neurons, which leads to the progressive and disabling loss of certain functions of the nervous system. Some of them are now considered the biggest causes of dementia in the world []. It is estimated that 9.9 million European citizens and 35.6 million worldwide suffer from some form of dementia. These figures are expected to double in 2030, and triple in 2050. People living with dementia have little access to adequate health care, even in most high-income countries, where only about 50% of people living with dementia are properly diagnosed and followed up. In low- and middle-income countries, less than 10% of cases are diagnosed. As the population ages, due to the increase in life expectancy, the number of people with dementia is increasing. Although the neurodegenerative diseases of Alzheimer’s (AD) and Parkinson’s (PD) are forms of dementia, there are other syndromes that have similar symptoms―such as depression, hallucinations, and memory loss―syndromes that include dementia of Lewy bodies, vascular dementia, frontotemporal dementia, etc. [].
For this reason, much attention has been paid by scientists to safe and efficient neuroprotective agents. Several categories of natural and synthetic neuroprotective agents have been described. However, it is believed that synthetic neuroprotective agents may have side effects, such as tiredness, drowsiness, numbness in the upper and lower limbs, balance difficulties, nervousness, or anxiety, etc. []. Thus, currently, researchers are interested in evaluating natural bioactive compounds that can act as neuroprotective agents. Adaptogens comprise a category of medicinal and nutritional products based on plants that promote adaptability, resilience, and survival of living organisms under stress. Common adaptogenic plants used in various traditional medical systems (TMS) and conventional medicine provide a modern justification for their use in the treatment of stress-induced and age-related diseases []. In this sense, seaweed can be a potential source of neuroprotective agents [].
The development of neuroprotective agents from seaweed still faces several challenges. The justification for the treatment of the neuroprotective effects of marine algae on the central nervous system (CNS) has been based on observations and experiments established in vitro or only on animal models. So far, few of the neuroprotective effects of seaweed have been studied directly in humans []. Thus, more clinical studies and other large-scale controlled studies are needed [].
Another major challenge in the development of studies on marine algae to obtain neuroprotective agents is that many of the drugs currently available are unable to provide effective neuroprotection. Possible reasons for this failure include the inappropriate use of specific medication for a particular disease, or the disease’s progression stage was too advanced, or suboptimal doses were used. Thus, future studies need to focus on the synergistic benefits of consuming different species of seaweed (or their extracts), recommended doses, ingestion times, and preparation methods for bioactive seaweed compounds to maximize the desired protective effect in preventing neurodegenerative diseases [,].
Currently, several lines of study attempt to provide information on the biological activities and neuroprotective effects of seaweed, including antioxidants, anti-neuroinflammatory agents, cholinesterase inhibitory activity, and inhibition of neuronal death []. Recent studies have shown that microglia activation and the resulting production of pro-inflammatory and neurotoxic factors are sufficient to induce neurodegeneration in animal models. In addition, microglia activation and excessive amounts of proinflammatory mediator release by microglia were observed during the pathogenesis of AD, PD, MS, dementia complex, as well as neuronal post-death in strokes and traumatic brain injuries (Figure 1). Therefore, a mechanism to regulate the release of the inflammatory response by microglia may have important therapeutic potential for the treatment of neurodegenerative diseases. Several published works point out that seaweed constitutes a relevant source of neuroprotective agents, with particular interest for preventive therapeutics (Table 1) [].
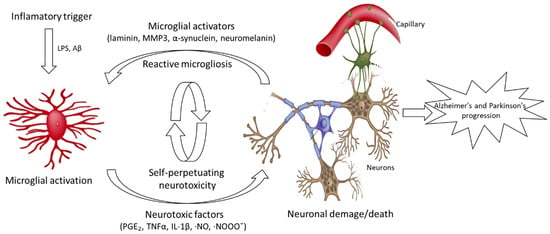
Figure 1.
Microglia-mediated neurotoxicity in Alzheimer’s and Parkinson’s diseases.
2. Alzheimer’s and Parkinson’s Diseases
Typically, afflicting adults in mid-life, neurodegenerative diseases are characterized by motor or cognitive changes that get progressively worse with age, and that usually reduce life expectancy. Human neurodegenerative disease results from the influence of several environmental and genetic causes []. Among the series of identified disease conditions that relate to extensive loss of function and quantity of neurons, Parkinson’s and Alzheimer’s are well-defined neurodegenerative diseases. However, the etiology of those diseases has not yet been identified clearly, even though some of them were reported centuries before [].
Currently, with increasing life expectancy and demographic changes in the population, neurodegenerative diseases, such as AD and PD, are becoming frighteningly common [,]. AD is the most common form of dementia, accounting for about 50 to 70% of all cases.
AD is a type of dementia that causes a global, progressive, and irreversible deterioration of various cognitive functions (memory, attention, concentration, language, thinking, among others). This deterioration results in changes in behavior, personality, and functional capacity of the person, making it difficult to perform their daily activities. The name of this disease is due to Alois Alzheimer, a German doctor who in 1907 first described the disease [].
In AD, dysregulation of the level of beta-amyloid (Aβ) leads to the appearance of senile plaques that contain depositions of Aβ. Aβ is a complex biological molecule that interacts with many types of receptors and/or forms insoluble assemblies and, eventually, its non-physiological depositions alternate with normal neuronal conditions [].
In neuropathological terms, AD is characterized by neuronal death in certain parts of the brain, with some causes yet to be determined. The appearance of fibrillary braids and senile plaques make communication between nerve cells impossible, which causes changes in the overall functioning of the person. In the early stages, the symptoms of AD can be very subtle. However, they often begin with lapses of memory and difficulty in finding the right words for everyday objects. These symptoms worsen as brain cells die and communication between them is altered [].
Decreased levels of the neuro-mediators acetylcholine (ACh) and butyrylcholine (BCh) have been observed in the brains of patients with AD. For this reason, inhibition of Acetylcholinesterase enzyme (AChE) and Butyrylcholinesterase enzyme (BuChE), responsible for the hydrolysis of ACh and BCh, has become a treatment option for AD [].
Involvement of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), has been suggested in diseases such as AD, schizophrenia, amyotrophic lateral sclerosis, PD, and other degenerative diseases of the basal ganglia, systemic atrophy, and multiple and progressive supranuclear degeneration are theorized by having the activity of free radicals as mediators. The neurotoxicity of H2O2 is mainly exerted by the formation of the highly reactive hydroxyl (OH•) radical, although the depletion of glutathione (GSH) levels and the secondary rupture of calcium homeostasis may also contribute to the toxic effects of H2O2 [,,]. Fallarero et al. [] showed that Halimeda incrassata (Chlorophyta) is an effective ROS scavenger in mouse hypothalamic (GT1–7) cells.
PD is a degenerative and slowly progressive disease of specific areas of the central nervous system (brain and spinal cord). It is characterized by tremors when the muscles are at rest (resting tremor), increased muscle tone (stiffness), slow movement of the muscles, and difficulty maintaining balance (postural instability). In many people, thinking becomes compromised. In PD, nerve cells and part of the basal ganglia (called black substance) degenerate. Basal ganglia are structures related to the movement, although they do not send connections directly to the spinal cord or cranial nerves. Functionally, the following structures are part of the basal ganglia: The caudate nucleus, the putamen, the pale globe, the subthalamic nuclei, and the substantia nigra. Like all nerve cells, those of the basal ganglia release chemical messengers, the neurotransmitters, which stimulate the next nerve cell (neuron) and allow it to send a nerve impulse. Dopamine is the major neurotransmitter used in the basal ganglia. Its general effect is to intensify the nerve impulses so that they reach the muscles. Thus, the fundamental functions of the base ganglia are related to the cognitive part of the movement, such as planning and performing complex motor acts. Its dysfunction determines changes in the reciprocal control of muscles, causing stiffness, tremors, and akinesia [,].
3. Seaweeds and Their Neurophysiological Activities
Macroalgae-derived compounds with neuroprotective activity may provide some important nutrients for the prevention and treatment of neurodegenerative diseases such as AD, PD, and other neurodegenerative diseases. Much of these bioactive compounds are derived from Phaeophyceae, brown algae (57.6%), followed by Rhodophyta, red algae (28.3%) and Chlorophyta, green algae (14.1%) [] (Table 1).
Among the various components, carbohydrates are the most abundant constituents of seaweed. In addition, polysaccharides are generally the main component of red, green, and brown algae [], and monosaccharides and oligosaccharides are also present. The reserve polysaccharides are laminarin in brown algae, floridean starch (more branched than amylopectin) in red algae, and starch in green algae. The algal cell walls are characterized by the presence of unusual polysaccharides that can be sulfated, acetylated, etc. Thus, seaweed carbohydrates are promising compounds in several fields, such as food, pharmaceutical, and biomedical [,,]. The therapeutic applications of these notable polysaccharides are, among others, antiviral, antibacterial, and antitumor activity, antioxidant, antidiabetic, antilipidemic properties, anti-inflammatory, and immunomodulatory characteristics [,]. Studies related to the absorption of bioactive compounds extracted from algae, namely polysaccharides, provide vital information on the most appropriate administration routes. If a drug formulation has a high absorption rate, it can be used for the delayed/controlled release of an active ingredient. Understanding the pharmacokinetics of polysaccharides derived from seaweeds (alginates, laminarins, fucoidans, etc.), may lead to their extensive use not only as drugs, but also to improve the bioavailability of certain poorly soluble compounds in pharmaceutical formulations (Shikov et al., 2020) []. Laminarin, a polysaccharide composed of (1,3)-β-d-glucan with some β (1,6) branching, particularly abundant in species of the genus Laminaria (Figure 2a) [], has been shown to have antibacterial and chemo-preventive activities, along with pre-biotic, important in the modulation of the intestinal microbiota, which in turn can regulate neuro-inflammation [].

Figure 2.
Some seaweeds with neurophysiological activities: (a)—Laminaria hyperborea (B); (b)—Fucus vesiculosus (B); (c)—Caulerpa racemosa (G); (d)—Hypnea musciformis (R); (e)—Chondrus crispus (G); (f)—Kappaphycus alvarezii (R); (g)—Bifurcaria bifurcata (B); (h)—Lithothamnion corallioides (R); (i)—Palmaria palmata (R); (j)—Ulva rigida (G); (k)—Neopyropia leucosticta (R); (l)—Porphyra umbilicalis (R); (m)—Gracilaria gracilis (R); (n)—Ulva lactuca (G); (o)—Saccharina latissima (B); (p)—Saccorhiza polyschides. B: Brown Algae; G: Green Algae; R: Red Algae.
PD is generally characterized, as we have seen previously, by the loss of dopaminergic neurons, and the presence of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can induce PD []. The administration of this substance may result in motor dysfunction, such as occurs in PD, which makes it a suitable experimental model for this disease [].
A compound (fucoidan) has been found to attenuate the neurotoxicity of MPTP activity. This sulfated polysaccharide, derived from Saccharina japonica (Phaeophyceae), has been demonstrated in mice models to be effective at a dosage of 25 mg kg−1 in protecting the cells from MPTP-induced neurotoxicity by reducing the behavioral deficits and cell death and increasing the level of dopamine []. Tau is a microtubule-associated protein (MAP) found in axons, and this protein is responsible for regulating the stability of microtubules [,,]. Hyper-phosphorylation of tau results in its dissociation from microtubules and aggregation in the form of neurofibrillary tangles []. Hyper-phosphorylated tau protein is a major component in neurofibrillary tangles, which is a hallmark of AD, and dysregulation of kinases and phosphatases has been found to increase tau hyper-phosphorylation levels [,]. Only three compounds (Spiralisone A, B, and Chromone 6) from seaweed have kinase inhibitory activity, and these compounds have been isolated from brown algae (Phaeophyceae). Besides, these compounds were isolated from a single species of brown algae, the Zonaria spiralis harvested in Australia, and all of them are phloroglucinols. The most active compound is spiralisone B, inhibiting the kinases-cyclin-dependent kinase 5 (CDK5/p25), casein kinase 1 (CK1δ), and glycogen synthase kinase 3β (GSK3β), with IC50 values of 3, 5, and 5.4 μM, respectively [].
Polysaccharide extracts from seaweed have very significant neuroprotective and repairing activities. These polysaccharides could be the next great advance in the treatment of neurodegenerative diseases. Fucoidan, ulvan, and their derivatives are potential agents to treat Alzheimer’s disease, according to a recent review made by Bauer et al. [].
A study carried out by Jhamandas et al. [] successfully showed that fucoidan isolated from Fucus vesiculosus (Figure 2b) (Phaeophyceae), could protect cholinergic neuronal death in rats induced by the beta amyloid protein (Aβ1). Fucoidan pretreatment blocked the activation of caspase-9 and caspase-3. Caspase-9 and caspase-3 have been suggested to mediate the terminal stages of neuronal apoptosis []. Therefore, fucoidan’s ability to block the activation of caspase-9 and caspase-3 suggests that the inhibition of neuronal death, promoted by fucoidan, occurs mainly through apoptotic inhibition. In neurodegenerative diseases, apoptosis may be pathogenic, and targeting this process may mitigate neurodegenerative diseases [].
Fucoidans extracted from the brown algae Laminaria hyperborea and Saccharina latissima have recently been suggested as potential therapeutics in age-related macular degeneration (AMD), and in other pathological pathways that include lipid dysregulation, inflammation, oxidative stress, and pro-angiogenic signaling []. However, knowing the pharmacokinetic profile of these bioactive molecules, including their molecular weight, is essential to implement drug development processes [].
Eight compounds have been found from macroalgae (Phaeophyceae and Chlorophyta) with neuroprotective activity against beta amyloid protein (Aβ), and five compounds of them are extracted from the green alga Caulerpa racemosa (Figure 2c) [].
In the study made by Najam et al. [], the administration of methanolic extract of Hypnea musciformis (Figure 2d) (Rhodophyta) significantly increased the level of dopamine on rats and mice. The possible effect of H. musciformis on dopamine and other biogenic amines in the brain indicates that H. musciformis probably has a psychotropic and anxiolytic profile. Increasing the level of dopamine may also be beneficial in view of the etiology of PD. In this study, the serotonin level was decreased after the administration of H. musciformis. The regular use of algae as a diet alleviates the symptoms of anxiety because the known anxiolytics also manifest their effect by reducing the concentration of serotonin [].
Mohibbullah et al. [] collected 23 edible seaweeds from Korean and Indonesian coasts to screen for marine seaweeds with potent neuroprotective activity. Hippocampal neurons of rats (DIV 9) were cultured in the presence of ethanol extracts and the cultures were treated with three different concentrations of seaweed extract: 5, 15, and 30 µg mL−1. About 1/3 of the tested seaweeds exhibited neuroprotective activity. Cell viability and cell cytotoxicity testing revealed that the ethanol Gracilariopsis chorda (Rhodophyta) extract afforded the most neuroprotection at a concentration of 15 µg mL−1, at which G. chorda significantly increased cell viability to 3.2–119.0%, and decreased cell death to 10.3–80.5%. Undaria pinnatifida (brown alga) had almost the same level of neuroprotection as G. chorda, and others like Sargassum fulvellum, Sargassum nigrifolium (brown algae), Neopyropia yezoensis (as Porphyra yezoensis), Gracilaria coronopifolia, Agarophyton tenuistipitatum (formerly Gracilaria tenuistipitata) (red algae), Ecklonia bicyclis (as Eisenia bicyclis) (brown alga), and Grateloupia cornea (formerly Carpopeltis cornea) (red alga) also exhibited moderate neuroprotective effects [].
Liu et al. [] demonstrated that dietary supplementation of the worms with an extract from the cultivated red seaweed Chondrus crispus (Figure 2e) (Rhodophyta) decreased the accumulation of α-synuclein and protected the worms from the neuronal toxin-induced 6-hydroxy-dopamine (6-OHDA model), and the brought dopaminergic neurodegeneration. These effects were associated with a corrected slowness of movement. The authors also showed that increased tolerance to oxidative stress and a positive regulation of the stress response genes, sod-3 and skn-1, may have served as a molecular mechanism for the protection mediated by C. crispus extract against pathology’s of PD. In all, in addition to its potential as a functional food, the tested red algae, C. crispus, may find promising pharmaceutical applications for the creation of novel anti-neurodegenerative drugs [].
According to Tirtawijaya et al. [], ethanolic extract of Kappaphycus alvarezii (Figure 2f) (Rhodophyta) significantly increased numbers of axodendritic intersections, branching points and branching tips, in cultures of fetal rat hippocampal neurons. Thus, K. alvarezii may be useful as a diet supplement or pharmaceuticals for people who are prone to neurological disorders.
In the works of Silva and collaborators [], the neuroprotective effects of the Bifurcaria bifurcata (Figure 2g) (Phaeophyceae) extracts were evaluated in a neurotoxic model induced by 6-hydroxydopamine (6-OHDA) in a human neuroblastoma cell line (SH-SY5Y), while the mechanisms associated to neuroprotection were investigated by the determination of mitochondrial membrane potential, H2O2 production, Caspase-3 activity, and by observation of Deoxyribonucleic Acid (DNA) fragmentation. The extracted diterpenes eleganolone and eleganonal exhibited antioxidant potential, being interesting candidates for further neuroprotective studies [].
A food supplement approved by the Food and Drug Administration (FDA), named Aquamin, is a natural multi-mineral derived from the marine edible red macroalgae Lithothamnion corallioides (Figure 2h). Aquamin was evaluated for its anti-neuroinflammatory potential, and in cortical glial-enriched cells was able to suppress the release of lipopolysaccharides (LPS)-induced tumor necrosis factor (TNF)-α and interleukin (IL)-1β. Several authors suggested that anti-inflammatory and antioxidative agents could prevent the deposition of Aβ and the subsequent brain damage [,].
According to Olasehinde et al. [], aqueous ethanol extracts rich in phlorotannins, phenolic acids and flavonoids from Gracilaria gracilis (Rhodophyta) (Figure 2n) and Ulva lactuca (Chlorophyta) (Figure 2o) exhibited AChE and BChE inhibitory activities. In addition, the sulfated polysaccharides obtained from Ulva rigida, as well as the algae species mentioned above, also showed potent inhibitory effects on BChE and AChE in vitro.

Table 1.
Bioactive properties of some compounds extracted from seaweeds.
Table 1.
Bioactive properties of some compounds extracted from seaweeds.
| Species | Extraction Methods | Compounds of Interest and Fractions | Activity | References | |
|---|---|---|---|---|---|
| Phaeophyceae (brown seaweeds) | |||||
| Agarum clathratum subsp. yakishiriense | Ethanol extract at 60 °C for 2 h | The ethanol extract was suspended in distilled water and subjected to a series of partitioning with n-hexane, dichloromethane, ethyl acetate and n-butanol; the mass of crude extract (95% EtOH) and fractions was 840.46 mg | In vivo (animal models) neuronal protection from ischemic injury | [] | |
| Alaria esculenta | Extracted with methanol/water (1:1) at 50 °C with stirring for 2 h at room temperature | Fractions | The fraction below 5 kDa decreased the melting point of α-synuclein, whereas the fraction above 10 kDa raised the melting point. Both of these fractions were found to inhibit the formation of amyloid aggregates by α-synuclein, in vitro | [] | |
| Cystoseira humilis | Methanolic extract | Fraction | In vitro AChE inhibitory capacity: 50% 10 mg mL−1 | [] | |
| Gongolaria nodicaulis (as C. nodicaulis) | Methanolic extract | Fraction | In vitro AChE inhibitory capacity: 64.4% In vitro BuChE inhibitory capacity: 110% 10 mg mL−1 | [] | |
| Ericaria selaginoides (as Cystoseira tamariscifolia) | Methanolic extract | Fraction | In vitro AChE inhibitory capacity: 85% In vitro BuChE inhibitory capacity: 86% 10 mg mL−1 | [] | |
| Gongolaria usneoides (as Cystoseira usneoides) | Methanolic extract | Fraction | In vitro AChE inhibitory capacity: 47% 10 mg mL-1 | [] | |
| Dictyopteris undulata | Zonarol was prepared as a 10 mM stock solution in dimethyl sulfoxide (DMSO) | Sesquiterpene: Zonarol | Activates the Nrf2/ARE pathway, induces phase-2 enzymes, and protects neuronal cells from oxidative stress, in vitro | [] | |
| Dictyota humifusa | Methanolic extract | Extract | Inhibiting AChE IC50 = 4.8 mg mL−1, in vitro | [] | |
| Ecklonia bicyclis and E. bicyclis (as Eisenia bicyclis) | MeOH extract | Phlorotannins | Suppression of BACE-1 enzyme activity IC50 = 5.35 μM, in vitro | [] | |
| Ethyl acetate extraction | Phlorotannins | Decreased Aβ-induced cell death IC50 = 800 µM, in vivo | [] | ||
| Ethanolic extract | Phlorotannins | Protection from retinal neuronal death, in vivo | [] | ||
| Ethanolic extract | Phlorotannins | In vitro inhibitory properties against AChE, BChE, and total ROS with inhibition percentages (%) of 68.01, 95.72, and 73.20 at concentrations of 25 μg mL−1, respectively | [] | ||
| Ecklonia cava | The seaweed (1 kg) was extracted with 95% ethanol (10 L) for 2 h in a water bath at 50 °C | Phlorotannins: Dieckol and phlorofucofuroeckol | Improvement of memory and possible involvement of the AChE inhibition, in vivo | [] | |
| Ethanolic extract | Phlorotannin: Triphlorethol-A | Anti-oxidative activity: Scavenging activity against ROS and DPPH via activation of ERK protein, in vivo | [] | ||
| Methanolic extract | Phlorotnnins | In vitro scavenging activity against hydroxyl, superoxide, and peroxyl radicals IC50 = 392.5, 115.2 and 128.9 µM, respectively | [] | ||
| Enzymatic extract | Phlorotannins | The phlorotannin-rich fraction significantly potentiated the pentobarbital-induced sleep at >50 mg kg−1, in vivo | [] | ||
| 80% MeOHextract | Phlorotannins | Neuroprotective effects against H2O2-induced oxidative stress in murine hippocampal HT22 cells IC50 = 50 µM, in vivo | [] | ||
| Ethanolic extract | Phloroglucinol | Reduce the toxicity ROS induced by hydrogen peroxide IC50 = 10 µg mL−1, in vivo | [] | ||
| Ethanolic extract | Phlorotannin: 8,8’-Bieckol | Phlorotannin: 8,8’-Bieckol reduce COX-2, NO, and prostaglandin E2 (PGE2) IC50 = 100 μM, in vivo | [] | ||
| Ethanolic extract | Extract | Extracts have potential analgesic effects in the case of postoperative pain and neuropathic pain, in vivo | [] | ||
| Ethanolic extract | Phlorotannin (eckol) | Inhibiting BuChE IC50 = 29 μM, in vitro and in vivo model studies | [] | ||
| Ethanolic extract | Phlorotannin (7-phloroeckol) | Inhibiting BuChE IC50 = 0.95 μM, in vitro and in vivo model studies | [] | ||
| E. kurome | Provided by Marine Drug and FoodInstitute, Ocean University of China China | Acidic oligosaccharide sugar chain (AOSC) | Blocking the fibril formation of Aβ IC50 = 100 µg mL−1, in vitro | [] | |
| E. maxima | Crude extract was sequentially extracted with n-hexane, dichloromethane, ethyl acetate, and finally n-butanol | Phlorotannins | IC50 values for the solvent fractions ranged from 62.61 to 150.8 μg mL−1, with the ethyl acetate fraction having the best inhibitory activity, in vitro | [] | |
| E. stolonifera | Ethanolic extract | Phlorotannin (dieckol) | Inhibiting AChE 17.11 μM, in vitro | [] | |
| Ethanolic extract | Phlorotannin (eckstolonol) | Inhibiting AChE and BuChE IC50 = 42.66 and 230.27 μM, in vitro | [] | ||
| Ethanolic extract | Phlorotannin (eckol) | Inhibiting AChE IC50 = 20.56 μM, in vitro | [] | ||
| Ethanolic extract | Phlorotannin (2-phloroeckol) | Inhibiting AChE IC50 = 38.13 μM, in vitro | [] | ||
| Ethanolic extract | Phlorotannin (7-phloroeckol) | Inhibiting AChE and BuChE IC50 = 4.89 and 136.71 μM, in vitro | [] | ||
| Ethanolic extract | Phlorotannin (phlorofucofuroeckol A) | Inhibiting AChE and BuChE IC50 = 4.89 and 136.71 μM, in vitro | [] | ||
| Ethanolic extract | Sterol (fucosterol) | Inhibiting AChE IC50 = 421.72 μM, in vitro | [] | ||
| Methanolic extract | Phlorotannins | Inhibiting AChE IC50 = 108.11 mg mL−1, in vitro | [] | ||
| Fucus vesiculosus | Fucoidan (Sigma) | Fucoidan | Fucoidan completely blocks microglial uptake of fDNA at only 40 ng mL−1, in vivo | [] | |
| Fucoidan (Sigma) | Fucoidan | In vitro anti-oxidative activity: Inhibit superoxide radicals, hydroxyl radicals, and lipid peroxidation IC50 = 0.058, 0.157 and 1.250 mg mL−1, respectively | [] | ||
| Fucoidan (Sigma) | Fucoidan | Fucoidan has protective effect via inducible nitric oxide synthase (iNOS), in vivo | [] | ||
| Fucoidan (Sigma) | Fucoidan | Fucoidan inhibits TNF-alpha- and IFN-gamma-stimulated NO production via p38 MAPK, AP-1, JAK/STAT, and IRF-1, in vivo | [] | ||
| Fucoidan (Sigma) | Fucoidan | Fucoidan inhibits beta amyloid induced microglial clustering at 10 µM, in vivo | [] | ||
| Extracted using 70% acetone | Phlorotnnins | Suppressing the overproduction of intracellular ROS induced by hydrogen peroxide IC50 = 0.068 mg mL−1, in vivo | [] | ||
| Ishige okamurae | Methanolic extraction | Phlorotannin: 6,6ʹ-Bieckol | Inhibiting AChE IC50 = 46.42 μM, in vitro | [] | |
| Methanolic extraction | Phlorotannin: Diphlorethohydroxycarmalol (DPHC) | In vivo neuroprotection against hydrogen peroxide (H2O2)-induced oxidative stress in murine hippocampal neuronal cells IC50 = 50 µM | [] | ||
| Marginariella boryana | Sequential extractions with H2SO4 and HCl | Sulfated fucans | Prevents the accumulation of Aβ | [] | |
| Padina australis | Dichloromethane extract | Extracts | Inhibiting AChE IC50 = 0.149 mg mL−1, in vitro | [] | |
| P. gymnospora | Methanolic extract | Bioassay-guided fractionation of the active n-hexane and ethyl acetate (EtOAc) soluble fractions | Inhibiting AChE IC50 = 3.5 mg mL−1, in vitro | [] | |
| Methanolic extract | Extracts | Inhibiting AChE IC50 = 3.5 mg mL−1, in vitro | [] | ||
| Acetone extracts | Extrats | IC50 value <10 μg mL−1 for both AChE and BuChE, in vitro | [] | ||
| P. tetrastromatica | Acetone extract | Fucoxanthin | Anti-oxidative activity: Reduce lipid peroxidation in rats IC50 = 0.83 μM, in vivo | [] | |
| Chloroform and ethanol extracts | Extract | Chloroform extract at 600 mg Kg−1 showed significant anticonvulsant activity, in vivo | [] | ||
| Papenfussiella lutea | Sequential extractions with H2SO4 and HCl | Sesquiterpenes | Inhibiting AChE IC50 = 48–65 μM, in vivo | [] | |
| Saccharina japonica | Fucoidan | Fucoidan | Protective effect in MPTP-induced neurotoxicity. In addition, reduce behavioural deficits and cell death and increase dopamine IC50 = 25 mg kg−1, once per day in mice, in vivo and in vitro | [] | |
| Extracted from seaweeds commercially cultured in Qingdao, China | Fucoidan | Inhibiting microglia which inhibits LPS-induced NO production via suppression of p38 and ERK phosphorylation IC50 = 125 μg mL−1, in vivo | [] | ||
| Fucoidan (CY110115) was obtained from Ci Yuan Biotechnology Co., Ltd., Xi’an, China. The purity of the chemical was more than 98.0%. | Fucoidan | Anti-oxidative activity: Reduce the toxicity of H2O2 in PC12 cells via activation of PI3K/Akt pathway IC50 = 60 µg mL−1, in vivo | [] | ||
| Ethanolic extract | Extracts | Promoted neurite outgrowth in a dose-dependent manner with optimal concentrations of 15 μg mL−1, in vitro | [,] | ||
| Extracted from seaweeds commercially cultured in Qingdao, China | Fucoidan | Reduced 6-hydroxydopamine (6-OHDA) and reduced the loss of dopaminergic in neurons IC50 = 20 mg kg−1 in rats, in vivo | [] | ||
| Sargassum fulvellum | MeOH-extract | Pigment: Pheophytin A | Produce neurite outgrowth (from 20 to 100% in the present of 10 ng mL−1 of NGF) and activate IC50 = 3.9 μg mL−1 in PC12 cells, in vivo | [] | |
| S. macrocarpum | Extracted with chloroform at room temperature | Carotenoids | Promote neurite outgrowth activity to 0.4 in PC12 cells IC50 = 6.25 μg mL−1, in vivo | [] | |
| Methanol extract | Meroterpenoid: Sargaquinoic acid | Signalling pathway of TrkA-MAP kinase pathway IC50 = 3 μg mL−1, in vivo | [] | ||
| Methanol extract | Meroterpene: Sargachromenol | Promote survival of PC-12 cells and neurite outgrowth through activation of cAMP and MAP kinase pathways IC50 = 9 μM, in vivo | [] | ||
| S. micracanthum | Methanol extract | Plastoquinones | Anti-oxidative activity: Lipid peroxidation IC50 = 0.95–44.3 μg mL−1 DPPH IC50 = 3–52.6 μg mL-1, in vivo | [] | |
| S. fulvellum | Ethanolic extract | Extracts | Promoted neurite outgrowth in a dose-dependent manner with optimal concentrations of 5 μg mL−1, in vivo | [] | |
| S. fusiforme (as Hijikia fusiformis) | Methanol extract | Fucoxanthin | Anti-oxidative activity: DPPH radical scavenging, in vitro | [] | |
| Alcohol extract | Fucoidan | Ameliorating learning and memory deficiencies, and otential ingredient on treatment of Alzheimer’s disease, in vivo | [] | ||
| S. horneri | Ethanol extract | Total sterols and β-sitosterol | Antidepressant effect, in vivo | [] | |
| S. polycystum | Hexane, dichloromethane, and methanol extracts | Extracts | Inhibiting AChE IC50 = 0.115, 0.180 and 0.162 mg mL−1, respectively, in vitro | [] | |
| S. sagamianum | MeOH extract | Sesquiterpenes | Inhibiting AChE IC50 = 48–65 μM, in vitro | [] | |
| MeOH extract | Plastoquinones: Sargaquinoic acid and sargachromenol | Inhibiting AChE IC50 = 23.2 and 32.7 μM, respectively Inhibiting BuChE IC50 = 26 μM (for sargaquinoic), in vitro | [] | ||
| S. siliquastrum | Extracted with 80% aqueous MeOH | Fucoxanthin | Anti-oxidative activity: Inhibit hydrogen peroxide in Vero cells IC50 = 100 uM, in vivo | [] | |
| Extracted with CH2Cl2 and MeOH | Meroditerpenoids | These compounds exhibited moderate to significant radical-scavenging activity as well as weak inhibitory activities against sortase A and isocitrate lyase, in vitro | [] | ||
| Sargassum sp. | Methanolic extract | Extract | Inhibiting AChE IC50 = 1 mg mL−1, in vitro | [] | |
| S. swartzii (as Sargassum wightii) | Extracted with CH2Cl2 and MeOH | Alginic acid | Polysaccharide inhibition activities to COX-2, lipoxygenase (5-LOX), xanthine oxidase (XO) and myeloperoxidase (MPO) in type II collagen induced arthritic rats IC50 = 100 mg kg−1, in vivo | [] | |
| Petroleum ether, hexane, benzene, and dichloromethane extracts | Extracts | Inhibiting AChE IC50 = 19.33, 46.81, 27.24, 50.56 µg mL−1, respectively Inhibiting BuChE IC50 = 17.91, 32.75, 12.98, 36.16 µg mL−1, respectively, in vivo | [] | ||
| S. vulgare | Methanolic extract | Extracts | Inhibiting AChE IC50 = 3.5 mg mL−1, in vitro | [] | |
| Scytothamnus australis | 6 h with 1% (w/v) H2SO4 at 20 °C, 6 h with 0.2 M HCl at 20 °C, 6 h with 2% CaCl2 at 75 °C | Sulfated fucans | Prevents the accumulation of Aβ, in vivo | [] | |
| Splachnidium rugosum | 6 h with 1% (w/v) H2SO4 at 20 °C, 6 h with 0.2 M HCl at 20 °C, 6 h with 2% CaCl2 at 75 °C | Sulfated fucans | Prevents the accumulation of Aβ, in vivo | [] | |
| Turbinaria decurrens | Dried seaweed powder was depig-mented with acetone followed by hot water extraction at 90–95 °C for 3–4 h | Fucoidan | Potential neuroprotective effect in Parkinson’s deasese, in vivo | [] | |
| Undaria pinnatifida | Ethanolic extract | Extract | Promoted neurite outgrowth in a dose-dependent manner with optimal concentrations of 5 μg mL−1, in vitro | [,] | |
| Ethanolic extract | Extract | Neurogenesis, neuroprotection, anti-inflammatory and anti-Alzheimer’s, in vivo | [] | ||
| Glycoprotein | Glycoprotein | Neurogenesis, neuroprotection, anti-inflammatory and anti-Alzheimer’s Showed predominantly AChE, BChE, and BACE1 inhibitory activities with IC50 values of 63.56, 99.03 and 73.35 μg mL−1, respectively, in vitro and in vivo | [] | ||
| Ethanolic extract | Sulfated fucans | Prevents the accumulation of Aβ, in vivo | [] | ||
| Zonaria spiralis | Ethanolic extract | Phloroglucinol: Spiralisone A and Chromone 6 | Kinases inhibitory to CDK5/p25, CK1δ and GSK3β IC50 = 10.0, <10 and <10 μM, respectively, in vitro | [] | |
| Rhodophyta (red seaweeds) | |||||
| Amphiroa beauvoisii | 50% Aqueous methanol extract | Phenolic, flavonoid extracts | Inhibiting AChE IC50 = 0.12 mg mL−1, in vitro | [] | |
| A. bowerbankii | Methanolic extract | Extracts | Inhibiting AChE IC50 = 5.3 mg mL−1, in vitro | [] | |
| A. ephedraea | Methanolic extract | Extracts | Inhibiting AChE IC50 = 5.1 mg mL−1, in vitro | [] | |
| Asparagopsis armata | Methanolic extract | Extracts | AChE inhibitory capacity: 58.4% BuChE inhibitory capacity: 81.4% 10 mg mL−1, in vitro | [] | |
| Bryothamnion triquetrum | Water extract | Fractions | Protect GT1–7 cells death produced by severe (180 min.) chemical hypoxia/aglycemia insult, in vitro | [,] | |
| Chondracanthus acicularis | Alcaline extraction | Lambda-carrageenan | Anti-oxidative activity: Inhibit superoxide radicals, hydroxyl radicals and lipid peroxidation IC50 = 0.046, 0.357 and 2.267 mg mL−1, respectively, in vitro | [] | |
| Chondrophycus undulatus (as Laurencia undulata) | Glycerol glycosides: Floridoside | Glycerol glycosides: Floridoside | Suppress pro-inflammatory responses in microglia by markedly inhibiting the production of nitric oxide (NO) and reactive oxygen species (ROS) IC50 = 10 μM | [] | |
| Chondrus crispus | Methanolic extract | Floridoside and d-Isofloridoside | Extract-mediated protection against Parkinson’s disease pathology, in vitro and in vivo | [] | |
| Eucheuma denticulatum | Alcaline extraction | Iota-carrageenan | Anti-oxidative activity: Inhibit superoxide radicals, hydroxyl radicals and lipid peroxidation IC50 = 0.332, 0.281 and 0.830 mg mL−1, respectively, in vitro | [] | |
| Gelidiella acerosa | Petroleum ether, hexane, benzene, dichloromethane, chloroform, ethyl acetate, acetone, methanol, and water extracts | Extracts | Inhibiting AChE Benzene extract, IC50 = 434.61 μg mL−1 Ethyl acetate, IC50 = 444.44 μg mL−1 Inhibiting BuChE Benzene extract, IC50 = 163.01 μg mL−1 Chloroform extract, IC50 = 375 μg mL−1, in vitro | [] | |
| Petroleum ether and successively extracted with benzene | Phytol | In vitro and in vivo antioxidant activities (25–125 μg mL−1) with an IC50 value of 95.27 μg mL−1 and cholinesterase inhibitory potential (5–25 μg mL−1) with IC50 values of 2.704 and 5.798 μg mL−1 for AChE and BuChE, respectively, in vitro | [] | ||
| Gelidium amansii | Ethanol extract | Extract | Neurogenesis (synaptogenesis promotion), in vitro and in vivo | [,] | |
| G. foliaceum | 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.16 mg mL−1, in vitro | [] | |
| Gloiopeltis furcata | 2-(3-Hydroxy-5-oxotetrahydrofuran-3-yl) acetic acid, glutaric acid, succinic acid, nicotinic acid, (E)-4-hydroxyhex-2-enoic acid, cholesterol, 7-hydroxycholesterol, uridine, glycerol, phlorotannin, fatty acids | Inhibiting AChE 1.4–12.50 μg mL−1 Inhibiting BuChE 6.56–75.25 μg mL−1, in vitro | [] | ||
| Hydropuntia edulis (as Gracilaria edulis) | Methanolic extract | Extract | Inhibiting AChE IC50 = 3 mg mL−1, in vitro | [] | |
| Methanolic extract | Extract | Inhibiting AChE IC50 = 3 mg mL−1, in vitro | [] | ||
| Gracilaria gracilis | Methanolic extract | Extract | Inhibiting AChE IC50 = 1.5 mg mL−1, in vitro | [] | |
| Gracilariopsis chorda | Ethanolic extract | Extract | Neuronal cell viability and cell cytotoxicity testing revealed that the ethanol extract afforded the most neuroprotection at a concentration of 15 µmL−1, at which G. chorda extract significantly increased cell viability to 119.0–3.2%, and decreased cell death to 80.5–10.3%, in vivo | [] | |
| G. chorda | Ethanolic extract | Extract | Extract concentration-dependently increased neurite outgrowth, with an optimal concentration of 30 mu g mL−1, in vivo | [] | |
| Hypnea valentine | Methanolic extract | Extract | Inhibiting AChE IC50 = 2.6 mg mL−1, in vitro | [] | |
| Methanolic extract | Extracts | Inhibiting AChE IC50 = 2.6 mg mL−1, in vitro | [] | ||
| Kappaphycus alvarezii | Alcaline extraction | Kappa-carrageenan | Anti-oxidative activity: Inhibit superoxide radicals, hydroxyl radicals and lipid peroxidation IC50 = 0.112, 0.335 and 0.323 mg mL−1, respectively, in vitro | [] | |
| Ethanolic extract | Extracts | Promotes neurite outgrowth in hippocampal neurons, in vivo | [] | ||
| Ochtodes secundiramea | Dichloromethane/methanol extract | Halogenated monoterpenes | Extract showed 48% AChE inhibition at 400 µg mL−1, in vitro | [] | |
| Porphyra/Pyropia sp. (Korean purple laver) | In vitro digestion | Phycoerythrobilin | Antioxidant activity IC50 = 0.048 mmol g−1, in vitro | [] | |
| Neopyropia yezoensis (as Porphyra yezoensis) | Ethanolic extract | Extract | Increased neurite outgrowth at an optimal concentration of 15 µg mL−1, in vivo | [] | |
| Rhodomela confervoides | Ethyl acetate extract | Bromophenols | Antioxidant activity IC50 = 5.22–23.60 µM, in vitro | [] | |
| Rhodomelopsis africana | 50% aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.12 mg mL−1, in vitro | [] | |
| Chlorophyta (green seaweeds) | |||||
| Caulerpa racemosa | Methanolic extract | Extract | Inhibiting AChE IC50 = 5.5 mg mL−1, in vitro | [] | |
| Extracted with MeOH and partitioned between H2O and hexane, chloroform, ethyl acetate and n-butanol. | Alkaloid: Caulerpin | Inhibition of nociception 100 μM kg−1 in Swiss albino mice, in vivo | [] | ||
| Ethanol extract | Bisindole alkaloid (racemosin A) | Increase 5.5% of cell viability in SH-SY5Y cells (neuroblast from neural tissue) IC50 = 10 µM, in vivo | [] | ||
| Ethanol extract | Bisindole alkaloid (racemosin B) | Increase 14.6% of cell viability in SH-SY5Y cells (neuroblast from neural tissue) IC50 = 10 µM, in vivo | [] | ||
| Hexane, dichloromethane and methanol extracts | Extracts | Inhibiting AChE IC50 = 0.086, 0.089 and 0.095 mg mL−1, respectively Inhibiting BuChE IC50 = 0.156, >0.2 and 0.118 mg mL−1, respectively, in vitro | [] | ||
| Ethanol extract | Terpenoid (α-tocospirone) | 13.85% increases in cell viability in SH-SY5Y cells IC50 = 10 μM, in vivo | [] | ||
| Ethanol extract | Sterol (23E)-3β-hydroxystigmasta-5,23-dien28-one | 11.31% increases in cell viability in SH-SY5Y cells IC50 = 10 μM, in vivo | [] | ||
| Ethanol extract | Sterol (22E)-3β-hydroxycholesta-5,22-dien24-one | 15.98% increases in cell viability in SH-SY5Y cells IC50 = 10 μM, in vivo | [] | ||
| Cladophora vagabunda (as Cladophora fascicularis) | Methanolic extract | Extract | Inhibiting AChE IC50 = 2 mg mL−1, in vitro | [] | |
| Codium capitatum | Methanolic extract | Extract | Inhibiting AChE IC50 = 7.8 mg mL−1, in vitro | [] | |
| 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.11 mg mL−1, in vitro | [] | ||
| C. duthieae | 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.14 mg mL−1, in vitro | [] | |
| C. fragile | 80% aqueous methanol extract | Sterol: Clerosterol | Exhibit reducing activity to COX-2, iNOS and TNF-α IC50 = 3 μg mL−1, in vitro and in vivo | [] | |
| Halimeda incrassata | Water extracts | Extracts | Neuroprotective and antioxidant properties, in vitro and in vivo | [] | |
| H. cuneata | Methanolic extract | Extracts | Inhibiting AChE IC50 = 5.7 mg mL−1, in vitro | [] | |
| 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.07 mg mL-1, in vitro | [] | ||
| Ulva australis (as Ulva pertusa) | Water at 125 °C for 4 h; polysaccharides were precipitated by the addition of 4000 mL of 95% (v/v) ethanol | Sulfated polysaccharide (ulvan) | Scavenging activity for superoxide radicals, in vitro | [] | |
| U. fasciata | Methanolic extract | Extracts | Inhibiting AChE IC50 = 4.8 mg mL−1, in vitro | [] | |
| 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.13 mg mL−1, in vitro | [] | ||
| U. prolifera (as Enteromorpha prolifera) | 95% ethanol extract | Pheophorbide A | Antioxidant activity IC50 = 71.9 µM, in vitro | [] | |
| U. reticulata | Methanolic extract | Extract | Inhibiting AChE IC50 = 10 mg mL−1, in vitro | [] | |
| Methanolic extract | Extract | Inhibiting AChE IC50 = 10 mg mL−1, in vitro | [] | ||
4. Multiple Sclerosis, Other Chronic Diseases, and the Seaweed Diet
Multiple Sclerosis (MS) is the most common of the chronic, inflammatory, neurological demyelination diseases of the CNS, of autoimmune origin. Although the cause of the disease is still unknown, MS has been the focus of many studies worldwide, which has made possible a constant and significant evolution in patients’ quality of life. Patients are usually young, especially women in their 20s and 40s [,]. MS cannot be cured and can be manifested by various symptoms such as: Severe fatigue, depression, muscle weakness, impaired balance of motor coordination, joint pain, bowel and bladder dysfunction, and changes in visual acuity [].
Both modifiable lifestyle factors and the quality of the diet can affect the course of the disease, dietary guidelines for people with MS have the potential to reduce symptoms related to the disease. Potential mechanisms by which the quality of the diet can influence the course of the disease in patients with MS include epigenetic changes in gene expression and changes in the composition of the intestinal microbiome, which can result in reduced inflammation. The quality of the diet can also influence the sufficiency of nutrients needed for neuronal structure [].
There is currently no formal recommendation for the application of a specific dietary protocol for MS, although evidence has accumulated favoring the implementation of low-fat diets. The first of these diets was developed by Roy Swank in the 1950s, characterized by a low amount of saturated fats, not exceeding 15 g, and by supplementation with cod liver oil []. A modified version of this diet, the McDougall diet, has been studied and has recently shown to be useful in the fight against MS-related fatigue [].
The synthesis of poly-unsaturated fatty acids (PUFAs), omega 3 (ω-3 fat acids), and 6 (ω-6 fat acids) is made by vegetables. In marine algae, despite their low lipid content, these fatty acids may form a significant part of the lipid profile of algae. Thus, PUFAs are important components of cell membranes and precursors of eicosanoids, essential bio-regulators of various cellular processes. PUFAs, effectively, reduce the risk of cancer, diabetes, hypercholesterolemia, cardiovascular diseases, osteoporosis, and MS progression. As there is historical and recurrent use of seaweed in Asia, in addition to increasing food use in other parts of the world, there is great potential for an increase in the presence of ω-3 PUFAs, especially in the western diets [,,,].
The major commercial sources of ω-3 PUFAs are fish, but their wide usage as food additives is limited because of the typical “fishy smell”, unpleasant taste, and lack of stability (since they are powerful antioxidants) []. The term “a balanced diet” of a heterotrophic organism implies the intake of essential nutrients for growth and reproduction. Some of the essential nutrients may be used for reconstruction, decomposed, and/or used in the production of new metabolites essential to the primary metabolism. However, there are others which cannot be produced, requiring them to be obtained externally through ingestion. Among these are poly-unsaturated fats, of which the most familiar are the fatty acids of omega type, as we have seen previously. These fatty acids control the cholesterol that binds to lipoproteins (carriers of these fats in blood plasma), that is, the balance between HDL-C (High Density Lipoprotein Cholesterol), or good cholesterol, and LDL-C (Low Density Lipoprotein Cholesterol), the bad cholesterol. The first should be kept at a high rate, the second at a low rate. HDL-C carries excess cholesterol into the bloodstream to the liver, where it is catabolized, while LDL-C does reverse transport, thus promoting its accumulation in tissues and organs []. EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are carboxylic acids of omega-3 type, which are considered the most important polyunsaturated fatty acids for human health (220 mg daily), because the human body is unable to produce them, and only obtains them through the intake of foods that contain them. Amongst the main sources of this type of unsaturated fats are, in addition to algae, the fish from the cold deep waters (sardines, salmon, trout, tuna, and mackerels, etc.). There is also another family of polyunsaturated fatty acids, the omega-6 group, whose primary sources are, in addition to the algae, vegetable oils (soy, corn, sunflower, etc.), fats, and eggs. Curiously, in the human body, both groups of these polyunsaturated fatty acids are reported to interact, i.e., so that omega-3 can act effectively give rise to all its potential benefits, there needs to be a balance between the consumption of omega-3 and omega-6 in the diet [].
Omega-3 is the only fatty acid which can lower triglycerides (i.e., decreases hepatic synthesis of these fats) and hence the conversion of LDL-C in the liver—which is thus available to be transported to various tissues, which can then be deposited, thereby increasing the likelihood of some diseases. Accordingly, high levels of EPA may play an important role in the prevention of thrombotic strokes, and may also lower the risk of atherosclerosis, ischemic heart disease or angina pectoris, and the risk of myocardial stroke [,].
For example, the red seaweed Dulse (Palmaria palmata) (Figure 2i), and the brown seaweeds Kombu (Saccharina latissima) (Figure 2p) and Saccorhiza polyschides (Figure 2q), beyond the polyphenols, vitamins A, B12, and C (see Table 2), high levels of fiber, protein, minerals, and arginine, has low concentration of saturated fatty acids, and various polyunsaturated fatty acids—such as linoleic and arachidonic of omega-6 family, or EPA omega-3 family. It is therefore a good seaweed for restorative in anemia states, asthenia (weakness), and for postoperative processes. It also strengthens the vision (has high levels of vitamin A) and is recommended for the treatment of gastric and intestinal disorders, and for the regeneration of mucosa (respiratory, gastric, and vaginal). Due to the high content of vitamin B12, this red seaweed is suggested to provide a protection against cardiovascular diseases [,]; and because this vitamin also reduces the homocysteine levels in blood, when high amounts are deposited in blood vessels [,].

Table 2.
Vitamin content of some edible seaweeds (mg/100 g edible portion).
Phlorotannins (brown algae, such as Fucus vesiculosus and Ascophyllum nodosum, polyphenols) are structural analogues of condensed tannins from terrestrial plants and are found exclusively in algae and macrophytes. Phlorotannins are polymers made up of phloroglucinol (1,3,5-trihydroxybenzene) and can make up 25% of the dry algae biomass. They exhibit anti-inflammatory, antioxidant, antibacterial, antiallergic, and anti-tumor properties, and inhibit antiplasmins, matrix metalloproteinases, etc. [].
According to Valado et al. [], the daily intake of vegetable jelly for 60 days showed a reduction in serum total cholesterol (TC) and LDL-C levels in women, leading to the conclusion that carrageenan has bioactive potential in reducing TC concentration.
The results obtained in the work of Nunes et al. [] strongly reinforce the possibility of using Ulva lactuca (Figure 2o) and Zonaria tournefortii (Phaeophyceae) lipids to improve the nutraceutical characteristics of food products and develop supplements based on macroalgae to improve or maintain health. Considering the above, including U. lactuca and Z. tournefortii in human nutrition can be a means of reducing the ω6/ω3 fat acids ratio.
In addition to controlling the amount of saturated fats in the diet, nutritional conduits like non-MS subjects are recommended, according to the patient’s biochemical individuality and current recommendations. Food quality should be prioritized in order to maintain or restore patients’ nutritional status and avoid chronic diseases such as obesity, diabetes mellitus type 2, systemic arterial hypertension, dyslipidemias, and cardiovascular diseases [,].
Although poorly understood and studied, dietary habits and the nutritional status of individuals with MS suggest that they may suffer from different nutritional imbalances. Among these, we can highlight obesity (more common), cachexia, low weight, and vitamin deficiencies. If they occur, vitamin deficiencies may interfere with the proper functioning of the immune system and the symptoms of the disease, the most important being vitamins A and D (modulators of the immune system), C, E, and B12 (important for myelin synthesis) (see Table 2) [].
Once this relationship was established, studies in animal models have confirmed the neuroprotective properties of vitamin D, opening precedents for studies in humans []. One of the most important studies in the field [] showed that the supplementation of 400 IU daily of vitamin D was able to reduce the number of relapsing, seeming to reduce the severity of the disease.
Vitamin D (Calciferol), another fat-soluble vitamin, whose functions are maintaining phosphorus and calcium concentrations in the blood, regulate bone metabolism and promote calcium fixation in bones and teeth; in children, it is key to bone growth [,].
Seaweeds, when included in a varied and balanced diet, can contribute effectively to provide adequate intake of a wide variety of vitamins (Table 2). Vitamins can be divided into those that are soluble in water, and those that are liposoluble. Water-soluble vitamins include B-complex vitamins and vitamin C. The B-complex vitamins are the largest group, and perform activities associated with muscle tone, metabolism, cell growth, and nervous system. For example, Sea Lettuce (Ulva spp., Figure 2j) (green algae) and Nori (Neopyropia/Pyropia/Porphyra spp., Figure 2k,l) (red algae) are excellent sources of vitamin B12 (Cobalamin), which is essential in DNA synthesis. Vitamin C (Ascorbic acid) is a water-soluble vitamin very important for oral health (gums), iron absorption, and resistance of the body to infections [,].
Vitamins A, D, E, and K are all fat-soluble. The liver is the major organ responsible for the storage, metabolism, and distribution of Vitamin A (Retinol) to peripheral tissues []. It is also suggested that the liver, besides functioning as a deposit site for vitamin A, can use retinol for its normal functioning, such as the proliferation and differentiation of its cells []. Vitamin A plays a key role in maintaining the integrity of visual processes, as an inadequate condition is the leading cause of preventable blindness in childhood. Another important nutritional role of vitamin A is its involvement in the immune system [].
Vitamins E (Tocopherol) and K also have several biological functions, including antioxidant activity and blood clotting. In addition to its biochemical functions and antioxidant activity, vitamins derived from seaweed have other health benefits, such as: Preventing cardiovascular diseases; reducing hypertension; and reducing the risk of cancer [,,].
The genus and species to which the macroalgae belong is a critical factor that can affect the vitamin composition. For example, the level of Niacin (vitamin B3) in some Phaeophyceae (Laminaria spp., Figure 2a) is approximately one tenth of the level found in Rhodophyta, Neopyropia tenera (as Porphyra tenera). Other factors that may influence vitamin content include geographic location, water temperature, salinity, and harvest time. Vitamin content may also be affected by processing, as both the dehydration and the temperature at which macroalgae are subjected may affect the levels of vitamin present [,].
5. Conclusions
The stress of our day-to-day lives and lack of time often lead us to opt for fast food, rich in calories and saturated fats, which leads to a lack of essential nutrients, obesity, and the appearance of diseases related to an excessive intake of sugars and fats, such as diabetes and arteriosclerosis, among others.
Algae are a natural food that provides us with a high nutritional value, but low in calories. With very low values in fats, marine algae have polysaccharides that behave, for the most part, as non-caloric fibers. Algae provide a rich and healthy diet (in trace elements and vitamins), offering a multitude of flavors, aromas, and textures, becoming an alternative food that easily awakens the curiosity of the most curious palates. Because they are rich in minerals, vitamins, and fiber, but low in lipids, they are undoubtedly an excellent choice for weight loss regimens and can even facilitate intestinal transit, lower blood cholesterol levels, and reduce certain affections such as colon cancer, and helps to delay, or at least minimize, the effects of neurodegenerative diseases, such as AD, PD, MS, among others.
Several in vitro studies have proven the efficacy of nutraceuticals and food products fortified from bioactive compounds from seaweed, however, the most challenging factor in the food industry is to develop new products that can attract consumers where strange or unfamiliar products are approaching them.
Author Contributions
L.P. and A.V. proceeded to the literature search, reading, and interpretation of the cited articles and books, and both participated in the writing of this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work had the support of “Fundação para a Ciência e Tecnologia (FCT)”, through the strategic projects (No. UID/MAR/04292/2020) granted to MARE.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Farlow, M. Alzheimer disease (2010). In Textbook of Geriatric Medicine and Gerontology–Brocklehurst’s, 8th ed.; Fillit, H.M., Rockwood, K., Woodhouse, K., Eds.; Saunders/Elservier: Philadelphia, PA, USA, 2017; 1168p. [Google Scholar]
- FCG—Fundação Calouste Gulbenkian. O Cérebro e as Doenças Neurodegenerativas; Dossier Ciência em Cena, Gulbenkian Descobrir, Maratona da Saúde: Lisboa, Portugal, 2015; 30p, Available online: https://content.gulbenkian.pt/wp-content/uploads/sites/16/2018/04/24100926/Dossie_2015_Neurodegenerativas.pdf (accessed on 12 January 2021).
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.-A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Neurological activities of seaweeds and their extracts (Chapter 11), In Therapeutic and Nutritional Uses of Algae, 1st ed.; Pereira, L., Ed.; Science Publishers’ (SP), An Imprint of CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 485–502. [Google Scholar] [CrossRef]
- Catanesi, M.; Caioni, G.; Castelli, V.; Benedetti, E.; d’Angelo, M.; Cimini, A. Benefits under the Sea: The role of marine compounds in neurodegenerative disorders. Mar. Drugs 2021, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Déléris, P.; Nazih, H.; Bard, J.M. Seaweeds in human health (Chapter 10). In Seaweed in Health and Disease Prevention, 1st ed.; Fleurence, J., Levine, I., Eds.; Academic Press: London, UK, 2016; pp. 319–367. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Lessing, D.; Bonini, N.M. Maintaining the brain: Insight into human neurodegeneration from Drosophila mutants. Nat. Rev. Genet. 2009, 10, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Wood-Kaczmar, A.; Gandhi, S.; Wood, N. Understanding the molecular causes of Parkinson’s disease. Trends Mol. Med. 2006, 12, 521–528. [Google Scholar] [CrossRef]
- Skovronsky, D.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 151–170. [Google Scholar] [CrossRef]
- García-Ayllón, M.-S.; Cauli, O.; Silveyra, M.-X.; Rodrigo, R.; Candela, A.; Compañ, A.; Jover, R.; Pérez-Mateo, M.; Martínez, S.; Felipo, V. Brain cholinergic impairment in liver failure. Brain 2008, 131, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J. 2017, 150, 118–129. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.; Zeisel, J.M.; Bennett, K. World Alzheimer Report 2020: Design Dignity Dementia: Dementia-Related Design and the Built Environment, 1st ed.; Alzheimer’s Disease International: London, UK, 2020; Volume 1, 248p, Available online: https://www.alzint.org/u/WorldAlzheimerReport2020Vol1.pdf (accessed on 12 January 2021).
- Cavdar, H.; Senturk, M.; Guney, M.; Durdagi, S.; Kayik, G.; Supuran, C.T.; Ekinci, D. Inhibition of acetylcholinesterase and butyrylcholinesterase with uracil derivatives: Kinetic and computational studies. J. Enzym. Inhib. Med. Chem. 2019, 34, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.L. Mechanisms of cell injury by activated oxygen species. Environ. Health Perspect. 1994, 102, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rimpler, M.M.; Rauen, U.; Schmidt, T.; Möröy, T.; De Groot, H. Protection against hydrogen peroxide cytotoxicity in Rat-1 fibroblasts provided by the oncoprotein BCL-2: Maintenance of calcium homoeostasis is secondary to the effect of BCL-2 on cellular glutathione. Biochem. J. 1999, 340, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Pôrto, W.G. Radicais livres e neurodegeneração. Entendimento fisiológico: Base para nova terapia? Rev. Neurociências 2001, 9, 70–76. [Google Scholar] [CrossRef]
- Fallarero, A.; Loikkanen, J.J.; Männistö, P.T.; Castañeda, O.; Vidal, A. Effects of aqueous extracts of Halimeda incrassata (Ellis) Lamouroux and Bryothamnion triquetrum (S.G. Gmelim) Howe on hydrogen peroxide and methyl mercury-induced oxidative stress in GT1–7 mouse hypothalamic immortalized cells. Phytomedicine 2003, 10, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Usigli, H.A. Parkinson Disease—Professional Version. MSD Manual; Global Medical Knowledge: Kenilworth, NJ, USA, 2019; Available online: https://www.msdmanuals.com/en-pt/professional/neurologic-disorders/movement-and-cerebellar-disorders/parkinson-disease (accessed on 12 January 2021).
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Pereira, L. Nutritional composition of the main edible algae (Chapter 2). In Therapeutic and Nutritional Uses of Algae, 1st ed.; Pereira, L., Ed.; Science Publishers’ (SP), An Imprint of CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 65–127. ISBN 9781498755382. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef]
- Gaspar, R.; Fonseca, R.; Pereira, L. Illustrated Guide to the Macroalgae of Buarcos Bay, Figueira da Foz, Portugal; MARE UC, DCV, FCT: Coimbra, Portugal, 2020; 128p. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.Y.; Bi, D.C.; Fang, W.S.; Wei, G.B.; Xu, X. Alginate-derived oligosaccharide inhibits neuroinflammation and promotes microglial phagocytosis of β-amyloid. Mar. Drugs 2015, 13, 5828–5846. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of marine-derived drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Langston, J.; Forno, L.; Tetrud, J.; Reeves, S.; Kaplan, J.; Karluk, D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 1999, 46, 598–605. [Google Scholar] [CrossRef]
- Gerlach, M.; Maetzler, W.; Broich, K.; Hampel, H.; Rems, L.; Reum, T.; Riederer, P.; Stöffler, A.; Streffer, J.; Berg, D. Biomarker candidates of neurodegeneration in Parkinson’s disease for the evaluation of disease-modifying therapeutics. J. Neural Transm. 2012, 119, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Wischik, C.; Crowther, R.; Walker, J.; Klug, A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: Identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. USA 1988, 85, 4051–4055. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, D.N.; Hyman, A.; Cobb, N.H.; Kirschner, M. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 1992, 3, 1141–1154. [Google Scholar] [CrossRef]
- Hirokawa, N.; Funakoshi, T.; Sato-Harada, R.; Kanai, Y. Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J. Cell Biol. 1996, 132, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Santa-Maria, I.; Perez, M.; Hernandez, F.; Moreno, F. Tau phosphorylation, aggregation, and cell toxicity. J. Biomed. Biotechnol 2006, 2006, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genotypes, phenotypes, and treatments. Science 1997, 275, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Seereeram, A.; Noble, W. Mediators of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Expert Rev. Neurother. 2009, 9, 1647–1666. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, X.; Conte, M.M.; Khalil, Z.; Capon, R.J. Spiralisones A–D: Acylphloroglucinol hemiketals from an Australian marine brown alga, Zonaria spiralis. Org. Biomol. Chem. 2012, 10, 9671–9676. [Google Scholar] [CrossRef]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [CrossRef]
- Cowan, C.M.; Thai, J.; Krajewski, S.; JReed, J.C.; Nicholson, D.W.; Kaufmann, S.H.; Roskams, A.J. Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J. Neurosci. 2001, 21, 7099–7109. [Google Scholar] [CrossRef]
- Vila, M.; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 365–375. [Google Scholar] [CrossRef]
- Dörschmann, P.; Klettner, A. Fucoidans as potential therapeutics for age-related macular degeneration—current evidence from in vitro research. Int. J. Mol. Sci. 2020, 21, 9272. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and tissue distribution of fucoidan from Fucus vesiculosus after oral administration to rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef]
- Najam, R.; Ahmed, S.P.; Azhar, I. Pharmacological activities of Hypnea musciformis. Afr. J. Biomed. Res. 2010, 13, 69–74. [Google Scholar]
- Mohibbullah, M.; Hannan, M.A.; Choi, J.Y.; Bhuiyan, M.M.H.; Hong, Y.K.; Choi, J.S.; Choi, I.S.; Moon, I.S. The edible marine alga Gracilariopsis chorda alleviates hypoxia/reoxygenation-induced oxidative stress in cultured hippocampal neurons. J. Med. Food 2015, 18, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Banskota, A.H.; Critchley, A.T.; Hafting, J.; Prithiviraj, B. Neuroprotective effects of the cultivated Chondrus crispus in a C. elegans model of Parkinson’s disease. Mar. Drugs 2015, 13, 2250–2266. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.; Moon, I.S.; Hong, Y.K. The tropical carrageenophyte Kappaphycus alvarezii extract promotes axodendritic maturation of hippocampal neurons in primary culture. J. Appl. Phycol. 2018, 30, 3233–3241. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and neuroprotective potential of the brown seaweed Bifurcaria bifurcata in an in vitro Parkinson’s disease model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; O’Gorman, D.M.; Nolan, Y.M. Evidence that the marine-derived multi-mineral Aquamin has anti-inflammatory effects on cortical glial-enriched cultures. Phytother. Res. 2011, 25, 765–767. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of Alzheimer’s disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Yoo, K.Y.; Park, J.H.; Yan, B.C.; Ahn, J.H.; Lee, J.C.; Kwon, H.M.; Kim, J.D.; Kim, Y.M.; You, S.G.; et al. Comparison of neuroprotective effects of extract and fractions from Agarum clathratum against experimentally induced transient cerebral ischemic damage. Pharm. Biol. 2014, 52, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Giffin, J.C.; Richards, R.C.; Craft, C.; Jahan, N.; Leggiadro, C.; Chopin, T.; Szemerda, M.; MacKinnon, S.L.; Ewart, K.V. An extract of the marine alga Alaria esculenta modulates α-synuclein folding and amyloid formation. Neurosci. Lett. 2017, 644, 87–93. [Google Scholar] [CrossRef]
- Custódio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.J.; Vizetto-Duarte, C.; Pereira, H.; Barreira, L.; Varela, J. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a Human dopaminergic cell line from hydrogen peroxide-induced cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef]
- Stirk, W.A.; Reinecke, D.L.; Staden, J.V. Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J. Appl. Phycol. 2007, 19, 271–276. [Google Scholar] [CrossRef]
- Jung, H.A.; Oh, S.H.; Choi, J.S. Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3211–3215. [Google Scholar] [CrossRef]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, S.M.; Kang, S.W.; Jeon, S.I.; Um, B.H.; Jung, S.H. Edible seaweed, Eisenia bicyclis, protects retinal ganglion cells death caused by oxidative stress. Mar. Biotechnol. 2012, 14, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Haulader, S.; Karki, S.; Jung, H.J.; Kim, H.R.; Jung, H.A. Acetyl- and butyryl-cholinesterase inhibitory activities of the edible brown alga Eisenia bicyclis. Arch. Pharm. Res. 2015, 38, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Myung, C.S.; Shin, H.C.; Bao, H.Y.; Yeo, S.J.; Lee, B.H.; Kang, J.S. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res. 2005, 39, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Z.-J.; Ryu, B.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Cho, S.; Han, D.; Kim, S.B.; Yoon, M.; Yang, H.; Jin, Y.H.; Jo, J.; Yong, H.; Lee, S.H.; Jeon, Y.J.; et al. Depressive effects on the central nervous system and underlying mechanism of the enzymatic extract and its phlorotannin-rich fraction from Ecklonia cava edible brown seaweed. Biosci. Biotechnol. Biochem. 2012, 76, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Cha, S.H.; Ko, J.Y.; Kang, M.C.; Kim, D.; Heo, S.J.; Kim, J.S.; Heu, M.S.; Kim, Y.T.; Jung, W.K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.; Kang, K.A.; Lee, N.H.; Hyun, J.W.; Kim, H.-S. Phloroglucinol exerts protective effects against oxidative stress-induced cell damage in SH-SY5Y cells. J. Pharmacol. Sci. 2012, 119, 186–192. [Google Scholar] [CrossRef]
- Yang, Y.-I.; Jung, S.-H.; Lee, K.-T.; Choi, J.-H. 8,8’-Bieckol, isolated from edible brown algae, exerts its anti-inflammatory effects through inhibition of NF-kB signaling and ROS production in LPS-stimulated macrophages. Int. Immunopharmacol. 2014, 23, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Lim, D.W.; Cho, S.; Han, D.; Kim, Y.T. The edible brown seaweed Ecklonia cava reduces hypersensitivity in postoperative and neuropathic pain models in rats. Molecules 2014, 19, 7669–7678. [Google Scholar] [CrossRef]
- Choi, B.W.; Lee, H.S.; Shin, H.C.; Lee, B.H. Multifunctional activity of polyphenolic compounds associated with a potential for Alzheimer’s disease therapy from Ecklonia cava. Phytother. Res. 2015, 29, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Geng, M.; Li, J.; Xin, X.; Wang, J.; Tang, M.; Zhang, J.; Zhang, X.; Ding, J. Acidic oligosaccharide sugar chain, a marine-derived acidic oligosaccharide, inhibits the cytotoxicity and aggregation of amyloid beta protein. J. Pharm. Sci. 2004, 95, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; Staden, J.V. Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.E. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Lee, S.-H.; Kim, S.-K. Phlorotannins from Ishige okamurae and their acetyl- and butyrylcholinesterase inhibitory effects. J. Funct. Foods 2009, 1, 331–335. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Liu, D.; Woodward, S.; Barger, S.W.; Mrak, R.E.; Griffin, W.S. Microglial activation by uptake of fDNA via a scavenger receptor. J. Neuroimmunol. 2004, 147, 50–55. [Google Scholar] [CrossRef]
- De Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- Lee, H.-R.; Do, H.; Lee, S.-R.; Sohn, E.-R.; Pyo, S.; Son, E. Effects of fucoidan on neuronal cell proliferation: Association with NO production through the iNOS pathway. J. Food Nutr. Sci. 2007, 12, 74–78. [Google Scholar] [CrossRef]
- Do, H.; Pyo, S.; Sohn, E.H. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J. Nutr. Biochem. 2009, 21, 671–679. [Google Scholar] [CrossRef]
- Huang, W.C.; Yen, F.C.; Shiao, Y.J.; Shie, F.S.; Chan, J.L.; Yang, C.N.; Sung, Y.L.; Huang, F.L.; Tsay, H.J. Enlargement of Abeta aggregates through chemokine-dependent microglial clustering. Neurosci. Res. 2009, 63, 280–287. [Google Scholar] [CrossRef]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef]
- Heo, S.J.; Cha, S.H.; Kim, K.N.; Lee, S.H.; Ahn, G.; Kang, D.H.; Oh, C.; Choi, Y.U.; Affan, A.; Kim, D.; et al. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef]
- Wozniak, M.; Bell, T.; Dénes, Á.; Falshaw, R.; Itzhaki, R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540. [Google Scholar] [CrossRef]
- Gany, S.A.; Tan, S.C.; Gan, S.Y. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014, 8, 1251–1257. [Google Scholar] [CrossRef]
- Natarajan, S.; Shanmugiahthevar, K.P.; Kasi, P.D. Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: Seaweeds inhabiting South Indian coastal areas (Hare Island, Gulf of Mannar). Nat. Prod. Res. 2009, 23, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, B.; Malar, D.S.; Sathya, S.; Devi, K.P. Antiaggregation potential of Padina gymnospora against the toxic Alzheimer’s beta-amyloid peptide 25–35 and cholinesterase inhibitory property of its bioactive compounds. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.; Bhaskar, N.; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.R.; Harle, U.N.; Ittadwar, A.M. Insignificant anticonvulsant activity of Padina tetrastromatica (brown macroalgae) in mice. J. Pharm. Negat. Results 2016, 7, 33–36. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Zhang, L.J.; Zhang, T.; Luo, D.Z.; Jia, Y.J.; Guo, Z.X.; Zhang, Q.B.; Wang, X.; Wang, X.M. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide activated primary microglia. Clin. Exp. Pharmacol. Physiol. 2010, 37, 422–428. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, C.; Yin, J.; Shen, J.; Tian, J.; Li, C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell. Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar] [CrossRef]
- Hannan, M.A.; Mohibbullah, M.; Hong, Y.-K.; Nam, J.H.; Moon, I.S. Gelidium amansii promotes dendritic spine morphology and synaptogenesis, and modulates NMDA receptor-mediated postsynaptic current. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Mohibbullah, M.; Hwang, S.Y.; Lee, K.; Kim, Y.C.; Hong, Y.K.; Moon, I.S. Differential neuritogenic activities of two edible brown macroalgae, Undaria pinnatifida and Saccharina japonica. Am. J. Chin. Med. 2014, 42, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; He, Y.; Zheng, Y.; Zhang, W.J.; Wang, Q.; Jia, Y.J.; Song, H.L.; An, H.T.; Zhang, H.B.; Qian, Y.J. Therapeutic effects of fucoidan in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease: Role of NADPH oxidase-1. CNS Neurosci. Ther. 2014, 20, 1036–1044. [Google Scholar] [CrossRef]
- Ina, A.; Hayashi, K.; Nozaki, H.; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef]
- Tsang, C.; Sagara, A.; Kamei, Y. Structure-activity relationship of a neurite outgrowth-promoting substance purified from the brown alga, Sargassum macrocarpum, and its analogues on PC12D cells. J. Appl. Phycol. 2001, 13, 349–357. [Google Scholar] [CrossRef]
- Kamei, Y.; Tsang, C.K. Sargaquinoic acid promotes neurite outgrowth via protein kinase A and MAP kinases-mediated signaling pathways in PC12D cells. Int. J. Dev. Neurosci. 2003, 21, 255–262. [Google Scholar] [CrossRef]
- Tsang, C.; Ina, A.; Goto, T.; Kamei, Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience 2005, 132, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.; Iwashima, M.; Wakasugi, H.; Saito, H.; Matsunaga, T.; Ogasawara, M.; Takahashi, S.; Suzuki, H.; Hayashi, T. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem. Pharm. Bull. 2005, 53, 1159–1163. [Google Scholar] [CrossRef]
- Hannan, M.A.; Kang, J.Y.; Hong, Y.K.; Lee, H.S.; Chowdhury, M.T.H.; Choi, J.-S.; Choi, I.S.; Moon, I.S. A brown alga Sargassum fulvellum facilitates neuronal maturation and synaptogenesis. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 535–544. [Google Scholar] [CrossRef]
- Yan, X.J.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, Z.; Chen, M.; Sun, Z.; Ling, Y.; Jiang, J.; Huang, C. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zheng, L.; Qi, L.; Wang, S.; Guan, L.; Xia, Y.; Cai, J. Structural features and potent antidepressant effects of total sterols and β-sitosterol extracted from Sargassum horneri. Mar. Drugs 2016, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Park, S.H.; Kim, E.S.; Choi, B.W.; Ryu, S.Y.; Lee, B.H. Cholinesterase inhibitory activity of two farnesylacetone derivatives from the brown alga Sargassum sagamianum. Arch. Pharm. Res. 2003, 26, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Rhu, G.; Park, S.H.; Kim, E.S.; Shin, J.; Roh, S.; Shin, H.C.; Lee, B.H. Anticholinesterase activity of plastoquinones from Sargassum sagamianum, lead compounds for Alzheimer’s disease therapy. Phytother. Res. 2007, 21, 423–426. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Jung, M.; Jang, K.H.; Kim, B.; Lee, B.H.; Choi, B.W.; Oh, K.-B.; Shin, J. Meroditerpenoids from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2008, 71, 1714–1719. [Google Scholar] [CrossRef]
- Sarithakumari, C.H.; Renju, G.L.; Kurup, G.M. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology 2013, 21, 261–268. [Google Scholar] [CrossRef]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Antioxidant and anti-cholinesterase activity of Sargassum wightii. Pharm. Biol. 2013, 51, 1401–1410. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, R.; Balasubramanian, T. Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int. J. Biol. Macromol. 2016, 86, 425–433. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.H.; Mohibbullah, M.; Hannan, M.A.; Hong, Y.-K.; Choi, J.-S.; Choi, I.S.; Moon, S. Undaria pinnatifida promotes spinogenesis and synaptogenesis and potentiates functional presynaptic plasticity in hippocampal neurons. Am. J. Chin. Med. 2015, 43, 529–542. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Kim, E.Y.; Lee, J.M.; Mohibbullah, M.; Alam, M.B.; Moon, I.S.; Kim, J.M.; Kong, I.S. Anti-Alzheimers and antiinflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Res. Int. 2015, 77, 118–124. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Amoo, S.O.; Aremu, A.O.; Stirk, W.A.; Gruz, J.; Subrtova, M.; Dolezal, K.; Van Staden, J. Phenolic profiles, antioxidant capacity, and acetylcholinesterase inhibitory activity of eight South African seaweeds. J. Appl. Phycol. 2015, 27, 1599–1605. [Google Scholar] [CrossRef]
- Fallarero, A.; Peltoketo, A.; Loikkanen, J.; Tammela, P.; Vidal, A.; Vuorela, P. Effects of the aqueous extract of Bryothamnion triquetrum on chemical hypoxia and aglycemia-induced damage in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine 2006, 13, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Li, Y.X.; Dewapriya, P.; Ryu, B.M.; Kim, S.K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Assessment of anticholinesterase activity of Gelidiella acerosa: Implications for its therapeutic potential against Alzheimer’s disease. Evid. Based Complement. Alternat. Med. 2012, 497242, 8. [Google Scholar] [CrossRef]
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef]
- Hannan, M.A.; Kang, J.-K.; Hong, Y.-K.; Lee, H.S.; Choi, J.-S.; Choi, I.S.; Moon, I.S. The Marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytother. Res. 2013, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Jeong, S.Y.; Jung, H.A.; Choi, J.S.; Min, B.S.; Woo, M.H. Anticholinesterase and antioxidant constituents from Gloiopeltis furcata. Chem. Pharm. Bull. 2010, 58, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Mohibbullah, M.; Hannan, M.A.; Park, I.S.; Moon, I.S.; Hong, Y.K. The edible red seaweed Gracilariopsis chorda promotes axodendritic architectural complexity in hippocampal neurons. J. Med. Food 2016, 19, 638–644. [Google Scholar] [CrossRef]
- Suganthy, N.; Pandian, S.K.; Devi, K.P. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar marine biosphere reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 2010, 468, 216–219. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.N.; Moon, I.S.; Hong, Y.K. The ethanol extract of the rhodophyte Kappaphycus alvarezii promotes neurite outgrowth in hippocampal neurons. J. Appl. Phycol. 2016, 28, 2515–2522. [Google Scholar] [CrossRef]
- Machado, L.P.; Carvalho, L.R.; Young, M.C.M.; Cardoso-Lopes, E.M.; Centeno, D.C.; Zambotti-Villela, L.; Colepicolo, P.; Yokoya, N.S. Evaluation of acetylcholinesterase inhibitory activity of Brazilian red macroalgae organic extracts. Revista Brasileira de Farmacognosia 2015, 25, 657–662. [Google Scholar] [CrossRef]
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Antioxidant activity of the phycoerythrobilin compound formed from a dried Korean purple laver (Porphyra sp.) during in vitro digestion. Food Sci. Technol. 2010, 16, 347–352. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Bhuiyan, M.M.H.; Hannan, M.A.; Getachew, P.; Hong, Y.K.; Choi, J.S.; Choi, I.S. The edible red alga Porphyra yezoensis promotes neuronal survival and cytoarchitecture in primary hippocampal neurons. Cell. Mol. Neurobiol. 2016, 36, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.-M.; Gloer, J.B.; Wang, B.-G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef]
- De Souza, E.T.; de Lira, D.P.; de Queiroz, A.C.; da Silva, D.J.C.; de Aquino, A.B.; Mella, E.A.C.; Lorenzo, V.P.; de Miranda, V.P.; de Araújo-Júnior, J.X.; Chaves, M.C.O.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef]
- Liu, D.-Q.; Mao, S.-C.; Zhang, H.-Y.; Yu, X.-Q.; Feng, M.-T.; Wang, B.; Feng, L.-H.; Guo, Y.-W. Racemosins A and B, two novel bisindole alkaloids from the green alga Caulerpa racemosa. Fitoterapia 2013, 91, 15–20. [Google Scholar] [CrossRef]
- Yang, P.; Liu, D.-Q.; Liang, T.-J.; Li, J.; Zhang, H.-Y.; Liu, A.-H.; Guo, Y.-W.; Mao, S.C. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg. Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Ahn, E.M.; Kim, B.; Park, C.-I.; Jang, J.H. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013, 86, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X. Antioxidant activity of different sulphate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2015, 37, 195–199. [Google Scholar] [CrossRef]
- Cho, M.; Lee, K.-S.; Kang, I.-J.; Won, M.-H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef]
- Grisante, A.I.; Stanich, P. Esclerose múltipla: Aspectos nutricionais e o papel dos nutrientes específicos. ConScientiaeSaúde 2006, 5, 67–74. [Google Scholar] [CrossRef][Green Version]
- Valado, A.; Leitao, M.J.; Martinho, A.; Pascoal, R.; Cerqueira, J.; Correia, I.; Batista, S.; Sousa, L.; Baldeiras, I. Multiple Sclerosis: Association of gelatinase B/matrix metalloproteinase-9 with risk and clinical course the disease. Mult. Scler. Relat. Disord. 2017, 11, 71–76. [Google Scholar] [CrossRef][Green Version]
- Wahls, T.L.; Chenard, C.A.; Snetselaar, L.G. Review of two popular eating plans within the Multiple Sclerosis community: Low saturated fat and modified paleolithic. Nutrients 2019, 11, 352. [Google Scholar] [CrossRef]
- Swank, R.L. Treatment of Multiple Sclerosis with low-fat diet. AMA Arch. Neurol. Psychiatry 1953, 69, 91–103. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; Celis de la Rosa, A.J. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in Multiple Sclerosis treated with interferon beta-1b. Oxid. Med. Cell Longev. 2013, 709493. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses, 1st ed.; Pomin, V.H., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 15–47. ISBN -13. [Google Scholar]
- Pereira, L. Edible Seaweeds of the World, 1st ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016; 463p, ISBN 9781498730471. [Google Scholar]
- Pereira, L. Biodiversity and description of the main algae with bioactive properties. In Therapeutic and Nutritional Uses of Algae, 1st ed.; Chapter 1; Pereira, L., Ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 1–64. ISBN 9781498755382. [Google Scholar] [CrossRef]
- Mišurcová, L.; Ambrožová, J.; Samek, D. Seaweed lipids as nutraceuticals. In Advances in Food and Nutrition Research, 1st ed.; Chapter 27; Se-Kwon, K., Ed.; Academic Press: Burlington, NJ, USA, 2011; Volume 64, pp. 339–355. [Google Scholar] [CrossRef]
- Pereira, L.; Correia, F. Algas Marinhas da Costa Portuguesa—Ecologia, Biodiversidade e Utilizações, 1st ed.; Nota de Rodapé Edições: Paris, France, 2015; pp. 196–201. ISBN 978-989-20-5754-5. [Google Scholar]
- Aragão, M.J. Plantas e Algas Medicinais, 1st ed.; Livros Horizonte: Lisboa, Portugal, 2009; pp. 153–174. ISBN 9789722416580. [Google Scholar]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Fernandes, F.; Pereira, D.M.; Azevedo, I.C.; Sousa-Pinto, I.; Andrade, P.B.; Valentão, P. Fatty acid patterns of the kelps Saccharina latissima, Saccorhiza polyschides and Laminaria ochroleuca: Influence of changing environmental conditions. Arab. J. Chem. 2020, 13, 45–58. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural deep eutectic solvents as alternatives for extracting phlorotannins from brown algae. Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Valado, A.; Pereira, M.; Caseiro, A.; Figueiredo, J.P.; Loureiro, H.; Almeida, C.; Cotas, J.; Pereira, L. Effect of carrageenans on vegetable jelly in humans with hypercholesterolemia. Mar. Drugs 2020, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.; Rosa, G.P.; Ferraz, S.; Barreto, M.C.; Pinheiro de Carvalho, M.A.A. Fatty acid composition, TLC screening, ATR-FTIR analysis, anti-cholinesterase activity, and in vitro cytotoxicity to A549 tumor cell line of extracts of 3 macroalgae collected in Madeira. J. Appl. Phycol. 2020, 32, 759–771. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Tolerable Upper Intake Levels for Vitamins and Minerals, 1st ed; EFSA: Parma, Italy, 2006; 480p, Available online: https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 12 January 2021).
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernan, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of Multiple Sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo-Moyano, B.; Benito-Leon, J.; Mitchell, A.J.; Hernandez-Gallego, J. A systematic review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in Multiple Sclerosis. Neuroepidemiology 2013, 40, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.D.; Yamamoto, Y.; Zolfaghari, R.; Rosales, F.J.; Dietz, J.; Shimada, T.; Li, N.; Ross, A.C. Regulation of hepatic vitamin A storage in a rat model of controlled vitamin A status during aging. J. Nutr. 2000, 130, 1280–1286. [Google Scholar] [CrossRef]
- Roenigk, H.H. Liver toxicity of retinoid therapy. J. Am. Acad. Dermatol. 1989, 19, 199–208. [Google Scholar] [CrossRef]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A deficiency and the lung. Nutrients 2008, 10, 1–29. [Google Scholar] [CrossRef] [PubMed]
- LTS. Health and Food Technology. Home Economics, Learning and Teaching, Resource Management; Learning & Teaching Scotland: Scotland, UK, 2005; 170p, Available online: http://www.sqa.org.uk/files/nq/HE_Health_and_Food_Technology_Higher.pdf (accessed on 12 January 2021)ISBN -13978-1843990888.
- Škrovánková, S. Seaweed vitamins as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 357–369. [Google Scholar] [CrossRef] [PubMed]
- OHT. Seaweed: A Rich Source of Vitamins and Bioactive Compounds; Ocean Harvest Technology: Galway, Ireland, 2013; Available online: https://oceanharvesttechnology.wordpress.com/2013/02/25/seaweed-a-rich-source-of-vitamins-and-bioactive-compounds/ (accessed on 12 January 2021).
- García, I.; Castroviejo, R.; Neira, C. Las Algas en Galicia: Alimentación y Otros Usos, 1st ed.; Consellería de Pesca, Marisqueo e Acuicultura—Xunta de Galícia: La Coruña, Spain, 1993; 231p, ISBN 84-453-0718-5. [Google Scholar]
- Morrissey, J.; Kraan, S.; Guiry, M.D. A Guide to Commercially Important Seaweeds on the Irish Coast, 1st ed; Bord Iascaigh Mhara/Irish Fisheries Board: Dublin, Ireland, 2001; pp. 5–40. [Google Scholar]
- Taboada, C.; Millán, R.; Míguez, I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2010, 90, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lloréns, J.L.P.; Carero, I.H.; Oñate, J.J.V.; Murillo, F.G.B.; León, A. Las Algas se Comen: Un Periplo por La Biologia, La Historia, Las Curiosidades y La Gastronomia, 1st ed.; Editorial UCA: Universidade de Cádiz, Cádiz, Spain, 2016; 336p, ISBN 978-84-9828-567-3. [Google Scholar]
- Sáa, C.F. Atlantic Sea Vegetables—Nutrition and Health: Properties, Recipes, Description, 1st ed.; Algamar: Redondela-Pontevedra, Spain, 2002; 300p. [Google Scholar]
- Quirós, A.R.B.; Ron, C.; López-Hernández, J.; Lage-Yusty, M.A. Determination of folates in seaweeds by high-performance liquid chromatography. J. Liq. Chromatogr. A 2004, 1032, 135–139. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, S.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweed. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Thennarasan, S. Murugesan Biochecmial composition of marine brown alga Lobophora variegata from Mandapam in the south east coast of Tamil Nadu. Int. J. Phytopharm. 2015, 5, 25–29. [Google Scholar] [CrossRef]
- Kolb, N.; Vallorani, L.; Milanovi, N.; Stocchi, V. Evaluation of marine algae Wakame (Undaria pinnatifida) and Kombu (Laminaria digitata, L. japonica) as food supplements. Food Technol. Biotechnol. 2004, 42, 57–61. [Google Scholar]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Tsuchiya, Y. Physiological studies on the vitamin C content of marine algae. Tohoku J. Agric. Res. 1950, 1, 97–102. [Google Scholar]
- Noda, H. Health benefits and nutritional properties of Nori. J. Appl. Phycol. 1993, 5, 255–258. [Google Scholar] [CrossRef]
- Watanabe, F.; Takenaka, S.; Katsura, H.; Miyamoto, E.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Characterization of a vitamin B12 compound in the edible purple laver, Porphyra yezoensis. Biosci. Biotechnol. Biochem. 2000, 64, 2712–2715. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).