Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice
Abstract
1. Introduction
2. Results
2.1. Characterization of TBP
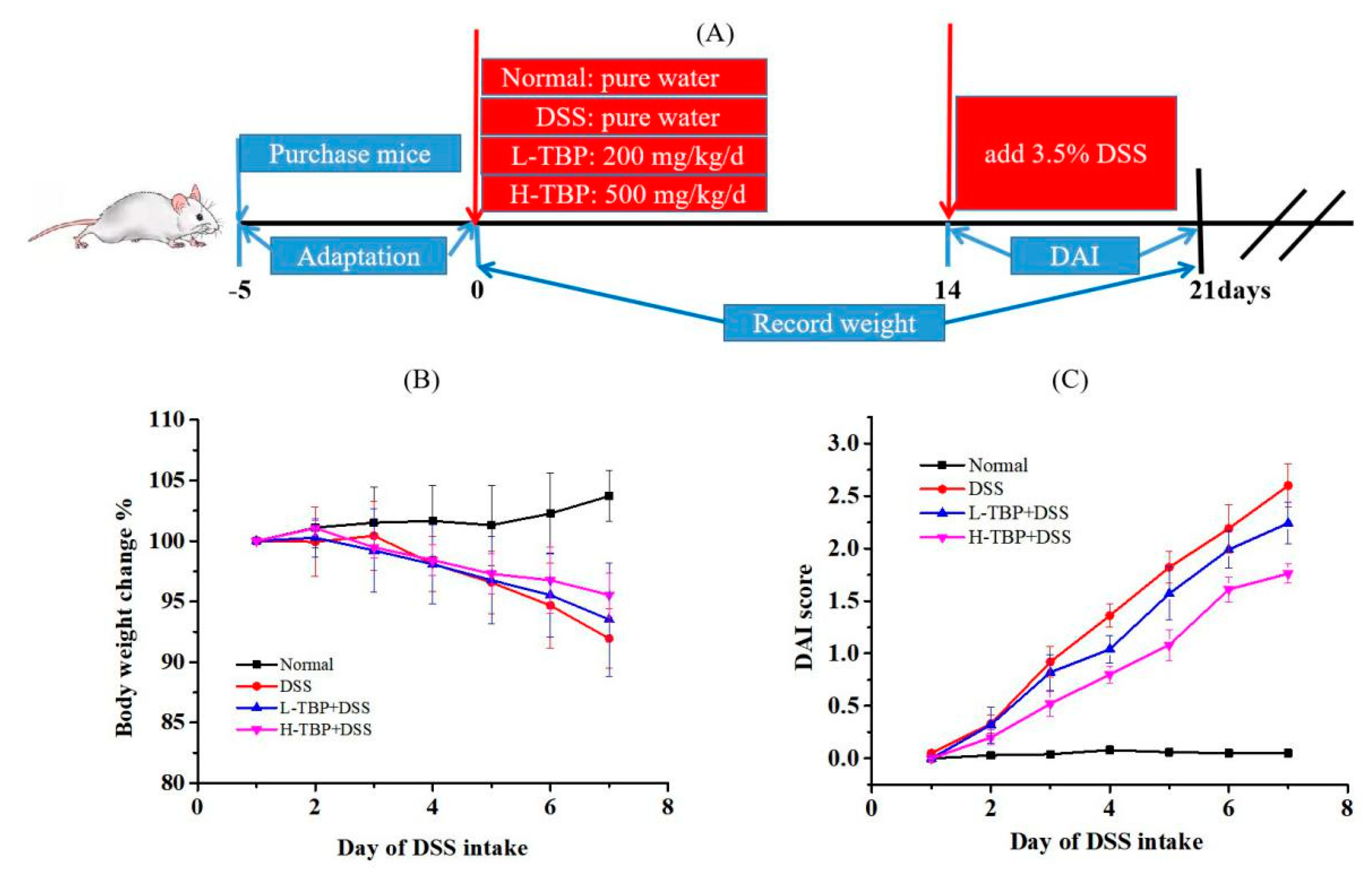
2.2. Effects of TBP on Body Weight and DAI Score of DSS-Induced Colitis Mice
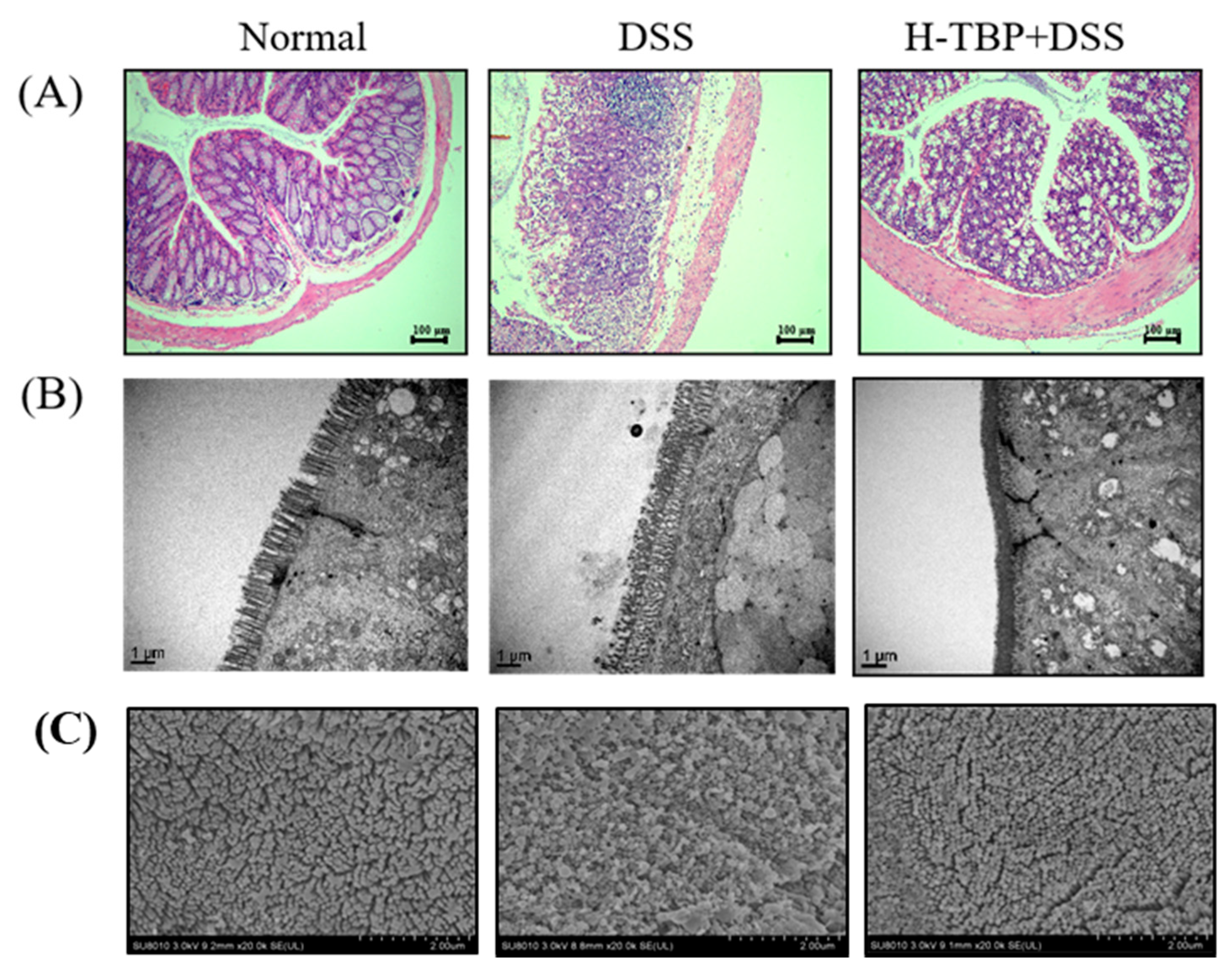
2.3. Effects of TBP on Pathological Changes of Colon Tissues in DSS-Induced Colitis Mice
2.4. Effects of TBP on Cytokines of Colon Tissues in DSS-Induced Colitis Mice
2.5. Effect of TBP on Related Gene in Colon Tissues in DSS-Induced Colitis Mice
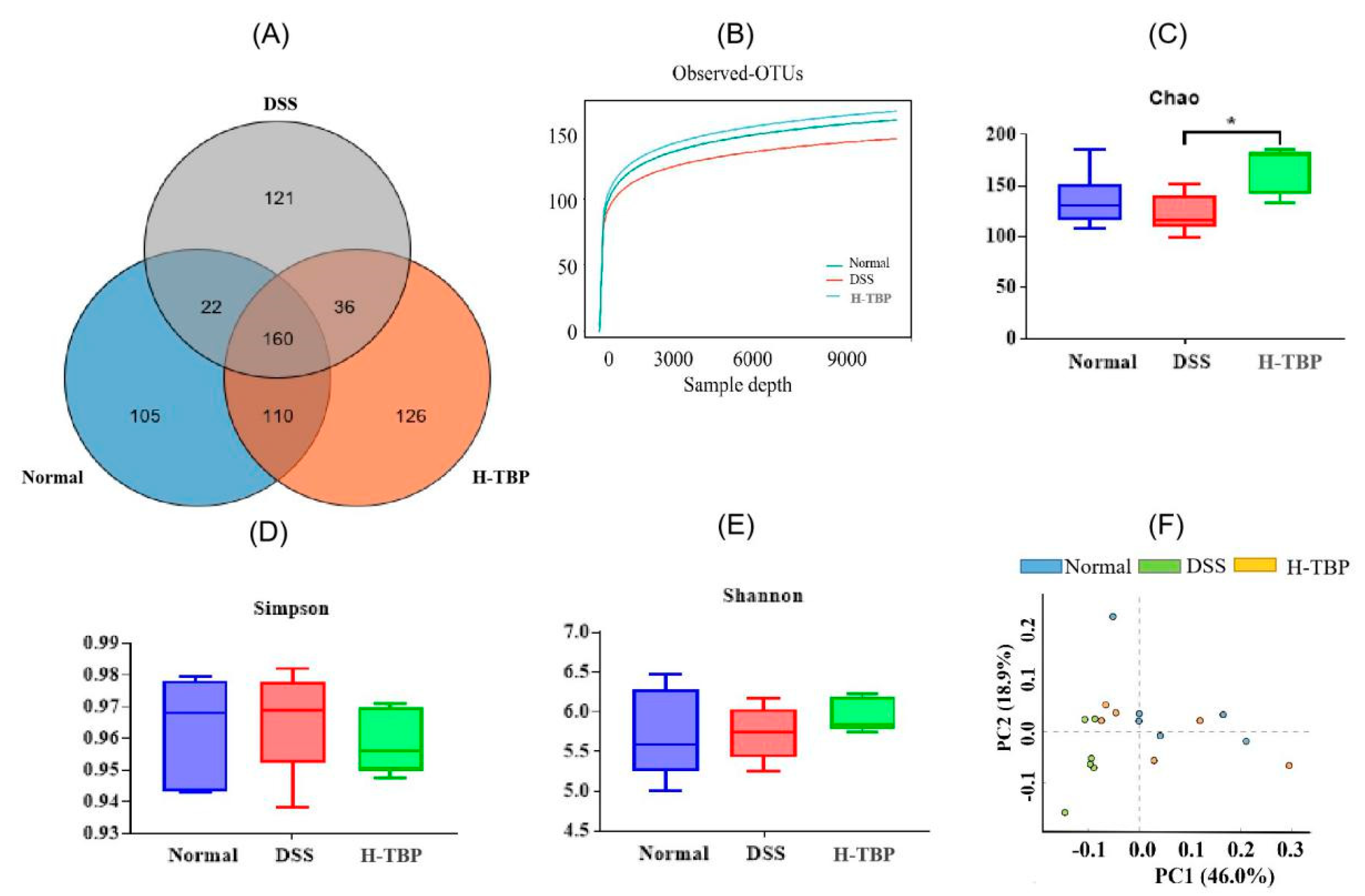
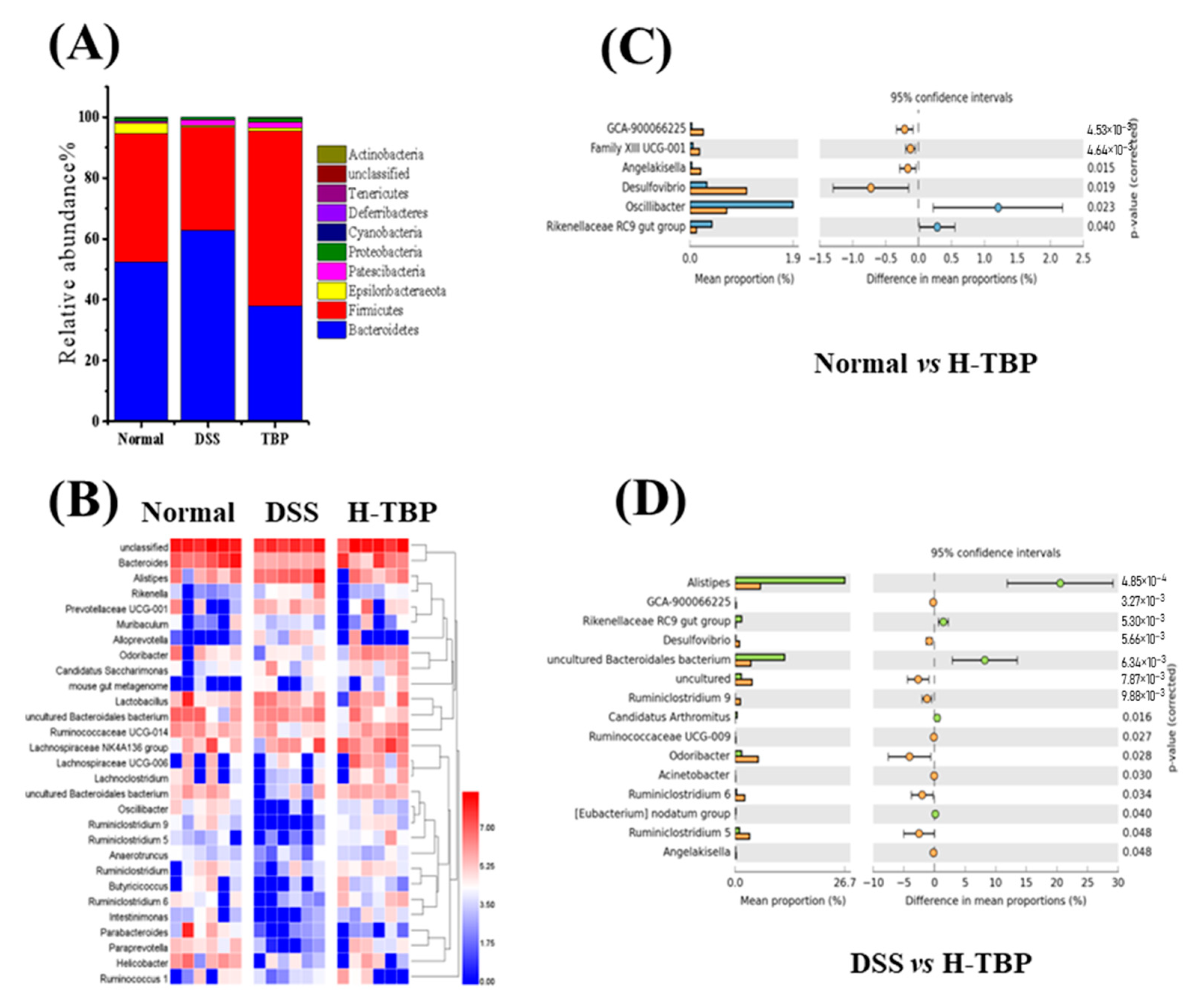
2.6. Effects of TBP on the Gut Microbiota in DSS-Induced Colitis Mice
2.7. Effects of TBP on Short Chain Fatty Acids (SCFAs) in DSS-Induced Colitis Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of TBP
4.3. Animal Experiment Design
4.4. DAI Score Evaluation
4.5. Pathological Evaluation
4.6. Colon Tissues Biochemical Parameter Analysis
4.7. Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Gut Microbiota Analyses
4.9. SCFAs Analyses
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, B.; Lyra, A.; Rocha, R.; Santana, G. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J. Gastroenterol. 2014, 20, 9458–9467. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.P. Traditional chinese medicine combined with western medicine for diagnosis and treatment of ulcerative colitis with anemia. Medicine 2016, 24, 3502–3507. [Google Scholar]
- Porter, R.; Kalla, R.; Ho, G.-T. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F1000Research 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Rana, A.; Ahlawat, A.; Bijjem, K.; Kumar, P. Animal models of inflammatory bowel disease: A review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Evans, R. Microbiology: Wealth management in the gut. Nature 2013, 500, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. J. Biomed Sci. 2018, 25, 79. [Google Scholar] [CrossRef]
- Swidsinski, A.; Weber, J.; Loening-Baucke, V.; Hale, L.; Lochs, H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 2005, 43, 3380–3399. [Google Scholar] [CrossRef]
- Gentile, C.; Weir, T. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Hamley, I. Small bioactive peptides for biomaterials design and therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef]
- Bhat, Z.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, D.; Bouhallab, S. Dietary bioactive peptides: Human studies. Crit. Rev. Food Sci. Nutr. 2015, 57, 335–343. [Google Scholar] [CrossRef]
- Han, S.-H.; Uzawa, Y.; Moriyama, T.; Kawamura, Y. Effect of collagen and collagen peptides from bluefin tuna abdominal skin on cancer cells. Health 2011, 3, 129–134. [Google Scholar] [CrossRef]
- Liu, G.; Yan, W.; Ding, S.; Jiang, H.; Ma, Y.; Wang, H.; Fang, J. Effects of IRW and IQW on oxidative stress and gut microbiota in dextran sodium sulfate-induced colitis. Cell Physiol. Biochem. 2018, 51, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Lee, S.J.; Kim, Y.S.; Kim, E.K.; Ahn, C.B.; Jeon, Y.J.; Moon, S.H.; Jeon, B.T.; Park, P.J. Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysates of Crassostrea gigas. Fish Shellfish Immunol. 2012, 33, 993–999. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Belleville, M.-P.; Amar, R. Production and fractionation of tuna by-product protein hydrolysate by ultrafiltration and nanofiltration: Impact on interesting peptides fractions and nutritional properties. Food Res. Int. 2014, 65, 453–461. [Google Scholar] [CrossRef]
- Oliveira, D.; Bernardi, D.; Drummond, F.; Dieterich, F.; Boscolo, W.; Leivas, C.; Kiatkoski, E.; Waszczynskyj, N. Potential use of tuna (Thunnus albacares) by-product: Production of antioxidant Peptides and recovery of unsaturated fatty acids from tuna head. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Saidi, S.; Saoudi, M.; Amar, R. Valorisation of tuna processing waste biomass: Isolation, purification and characterisation of four novel antioxidant peptides from tuna by-product hydrolysate. Environ. Sci. Pollut. Res. Int. 2018, 25, 17383–17392. [Google Scholar] [CrossRef]
- Parvathy, U.; Binsi, P.K.; Joshy, C.G.; Abubacker, Z.; Ninan, G.; Ravishankar, C. Antioxidant peptides from dark meat of Yellowfin Tuna (Thunnus albacares): Process optimization and characterization. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, E.Y.; Kim, I.H.; Nam, T.J. Peptide derived from desalinated boiled tuna extract inhibits adipogenesis through the downregulation of C/EBP-α and PPAR-γ in 3T3-L1 adipocytes. Int. J. Mol. Med. 2015, 35, 1362–1368. [Google Scholar] [CrossRef]
- Seo, J.; Lee, M.; Go, H.; Park, T.; Park, N. Purification and characterization of YFGAP, a GAPDH-related novel antimicrobial peptide, from the skin of yellowfin tuna, Thunnus albacares. Fish Shellfish Immunol. 2012, 33, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Wang, H.; Hsu, K.; Hwang, J. Anti-inflammatory peptides from enzymatic hydrolysates of tuna cooking juice. Food Agric. Immunol. 2015, 26, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; He, J.; Chen, W.; Jin, W.; Zhu, Y.; Sun, H.; Li, Y.; Shi, Y.; Jing, Y.; et al. Regulation of ROS-NF-κB axis by tuna backbone derived peptide ameliorates inflammation in necrotizing enterocolitis. J. Cell Physiol. 2019, 234, 14330–14338. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Ying, X.-G.; Gao, P.; Wang, C.-L.; Wang, Y.-F.; Yu, X.-W.; Chen, J.; Wang, B.; Luo, H.-Y. Anti-inflammatory activity of a peptide from Skipjack (Katsuwonus pelamis). Mar. Drugs 2019, 17, 582. [Google Scholar] [CrossRef] [PubMed]
- Tsai, B.; Hsieh, D.; Lin, W.-T.; Tamilselvi, S.; Day, C.; Ho, T.-J.; Chang, R.-L.; Padma, V.; Kuo, C.-H.; Huang, C.-Y. Functional potato bioactive peptide intensifies Nrf2-dependent antioxidant defense against renal damage in hypertensive rats. Food Res. Int. 2019, 129, 108862. [Google Scholar] [CrossRef] [PubMed]
- Alemán Pérez, A.; Gimenez, B.; Montero, P.; Gomez-Guillen, M. Antioxidant activity of several marine skin gelatins. LWT Food Sci. Technol. 2011, 44, 407–413. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.; Macedonio, F. Membrane engineering in process intensification—An overview. Fuel Energy Abstr. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Cian, R.; Garzón, A.; Betancur, D.; Guerrero, L.; Drago, S. Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT Food Sci. Technol. 2015, 64, 881–888. [Google Scholar] [CrossRef]
- Neurath, M.; Leppkes, M. Ulcerative colitis. Semin Immunopathol. 2018, 41, 747–756. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 1–15. [Google Scholar] [CrossRef]
- Kim, J.; Shajib, M.S.; Manocha, M.; Khan, W. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012, 60, 3678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Yuan, Z.-W.; Qu, C.; Yu, X.-T.; Huang, T.; Chen, P.; Su, Z.-R.; Dou, Y.-X.; Wu, J.-Z.; Zeng, H.-F.; et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol. Res. 2018, 137, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sui, W.; Gao, C.; Yan, H.; Yin, Y.; Li, H.; Wang, X. L-Glutamate deficiency can trigger proliferation inhibition via down regulation of the mTOR/S6K1 pathway in pig intestinal epithelial cells. J. Anim. Sci. 2016, 94, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yao, W.; He, Q.; Shao, Y.; Zheng, R.; Huang, F. L-leucine stimulates glutamate dehydrogenase activity and glutamate synthesis by regulating mTORC1/SIRT4 pathway in pig liver. Anim. Nutr. 2018, 4, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, A.; Yu, Y.; Guo, S.; Wang, M.; Wang, H. L-Arginine protects ovine intestinal epithelial cells from lipopolysaccharide-induced apoptosis through alleviating oxidative stress. J. Agric. Food Chem. 2019, 67, 1683–1690. [Google Scholar] [CrossRef]
- Sylvia, K.; Demas, G. Acute intraperitoneal lipopolysaccharide influences the immune system in the absence of gut dysbiosis. Physiol. Rep. 2018, 6, e13639. [Google Scholar] [CrossRef]
- Wang, S.; Martins, R.; Sullivan, M.; Friedman, E.; Misic, A.; El-fahmawi, A.; Martinis, E.; O’Brien, K.; Chen, Y.; Bradley, C.; et al. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome 2019, 7, 126. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.; Neubauer, K. Oxidative stress markers in inflammatory bowel diseases: Systematic review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef]
- Carroll, I.; Andrus, J.; Bruno-Barcena, J.; Klaenhammer, T.; Hassan, H.; Threadgill, D. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G729–G738. [Google Scholar] [CrossRef] [PubMed]
- Nurilmala, M.; Hizbullah, H.; Karnia, E.; Kusumaningtyas, E. Ochiai Characterization and antioxidant activity of collagen, gelatin, and the derived peptides from Yellowfin Tuna (Thunnus albacares) skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Winter, M.; Byndloss, M.; Spiga, L.; Duerkop, B.; Hughes, E.; Buettner, L.; Romão, E.; Behrendt, C.; Winter, S. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.; Sirota-Madi, A.; Avila, J.; Fornelos, N.; Haiser, H.; Reinker, S.; Vatanen, T.; Hall, B.; Mallick, H.; McIver, L.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nature Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.; Adams, D.; Fava, F.; Hermes, G.; Hirschfield, G.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2015, 65, 330–339. [Google Scholar] [CrossRef]
- Takiishi, T.; Morales, C.; Câmara, N. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Fábrega, M.-J.; Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badia, J.; Giménez, R.; Galvez, J.; Baldoma, L. Intestinal anti-inflammatory effects of outer membrane vesicles from escherichia coli nissle 1917 in DSS-experimental colitis in mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef]
- Neff, C.; Rhodes, M.E.; Arnolds, K.; Collins, C.; Donnelly, J.; Nusbacher, N.; Jedlicka, P.; Schneider, J.; McCarter, M.; Shaffer, M.; et al. Diverse intestinal bacteria contain putative zwitterionic capsular polysaccharides with anti-inflammatory properties. Cell Host Microbe 2016, 20, 535–547. [Google Scholar] [CrossRef]
- Murugesan, S.; Nirmalkar, K.; Hoyo-Vadillo, C.; Espitia, M.; Ramírez-Sánchez, D.; Garcia-Mena, J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Lv, Y.; Tang, H.; Li, T.; Miao, Y.; Cheng, J.; Wang, G.; Tan, Y.; Zhu, Y.; Xing, X.; et al. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int. J. Biol. Macromol. 2020, 156, 1217–1233. [Google Scholar] [CrossRef]
- Thomson, P.; Medina, D.; Ortúzar, M.; Gotteland, M.; Garrido, D. Anti-inflammatory effect of microbial consortia during the utilization of dietary polysaccharides. Food Res. Int. 2018, 109, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, M.; Schwarzfischer, M.; Hering, L.; Shawki, A.; Sayoc, A.; Santos, A.; Gottier, C.; Lang, S.; Baebler, K.; Geirnaert, A.; et al. Loss of PTPN22 abrogates the beneficial effect of cohousing-mediated fecal microbiota transfer in murine colitis. Mucosal Immunol. 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]




| Molecular Weight Range (Da) | Percentage (%) | Molecular Weight Range (Da) | Percentage (%) |
|---|---|---|---|
| >5000 | 0.16 | 1000–500 | 43.23 |
| 5000–3000 | 0.58 | 500–180 | 48.71 |
| 3000–2000 | 1.41 | < 180 | 4.61 |
| 2000–1000 | 1.30 | - | - |
| Total | 100 | ||
| Amino Acid | Abbreviation | Ratio (g/100 g) |
|---|---|---|
| Glutamic acid # | Glu | 9.16 |
| Aspartic acid # | Asp | 5.68 |
| Leucine * | Leu | 5.35 |
| Lysine * | Lys | 5.14 |
| Alanine # | Ala | 4.27 |
| Arginine # | Arg | 3.67 |
| Valine * | Val | 3.42 |
| Histidine * | His | 3.09 |
| Glycine # | Gly | 3.05 |
| Isoleucine * | Ile | 2.96 |
| Proline # | Pro | 2.59 |
| Threonine * | Thr | 2.58 |
| Serine # | Ser | 2.54 |
| Phenylalanine * | Phe | 2.43 |
| Methionine * | Met | 1.87 |
| Tyrosine # | Tyr | 1.78 |
| Cysteine # | Cys | 0.52 |
| Essential amino acids * | 26.84 | |
| Non-essential amino acids # | 33.26 | |
| Total amino acids | 60.10 | |
| Weight Loss% | Stool Consistency | Occult/Gross Bleeding | DAI Score |
|---|---|---|---|
| 0 | Normal | Normal | 0 |
| 1–5 | Soft stools | Hemoccult positive mildly | 1 |
| 5–10 | - | - | 2 |
| 10–15 | Diarrhea | Visible gross bleeding | 3 |
| >15 | - | - | 4 |
| Gene | Primer | Primer Sequence 5’–3’ | Product Size (bp) |
|---|---|---|---|
| IL-6 | Forward Reverse | CAGGTCTATTTTGGGATCATTGCC TCCCTGATTTCTAAGTGTTGCTGT | 189 |
| TNF-α | Forward Reverse | CACCTCAGACAAAATGCTCTTCAC CTCACACATCTCCTTTCTCATTGC | 100 |
| ZO-1 | Forward Reverse | TACCTCTTGAGCCTTGAACTT CGTGCTGATGTGCCATAATA | 248 |
| Occludin | Forward Reverse | GCCCAGGCTTCTGGATCTATGT GGGGATCAACCACACAGTAGTGA | 124 |
| Claudin-1 | Forward Reverse | GCTGGGTTTCATCCTGGCTTCT CCTGAGCGGTCACGATGTTGTC | 110 |
| β-actin | Forward Reverse | AGTGTGACGTTGACATCCGT GCAGCTCAGTAACAGTCCGC | 298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, X.-W.; Zhou, X.-L.; Wang, R.; Shu, C.-H.; Zhou, Y.-F.; Ying, X.-G.; Zheng, B. Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Mar. Drugs 2021, 19, 127. https://doi.org/10.3390/md19030127
Xiang X-W, Zhou X-L, Wang R, Shu C-H, Zhou Y-F, Ying X-G, Zheng B. Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Marine Drugs. 2021; 19(3):127. https://doi.org/10.3390/md19030127
Chicago/Turabian StyleXiang, Xing-Wei, Xiao-Ling Zhou, Rui Wang, Cong-Han Shu, Yu-Fang Zhou, Xiao-Guo Ying, and Bin Zheng. 2021. "Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice" Marine Drugs 19, no. 3: 127. https://doi.org/10.3390/md19030127
APA StyleXiang, X.-W., Zhou, X.-L., Wang, R., Shu, C.-H., Zhou, Y.-F., Ying, X.-G., & Zheng, B. (2021). Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Marine Drugs, 19(3), 127. https://doi.org/10.3390/md19030127






