Lipophilic Toxins in Wild Bivalves from the Southern Gulf of California, Mexico
Abstract
1. Introduction
2. Results and Discussion
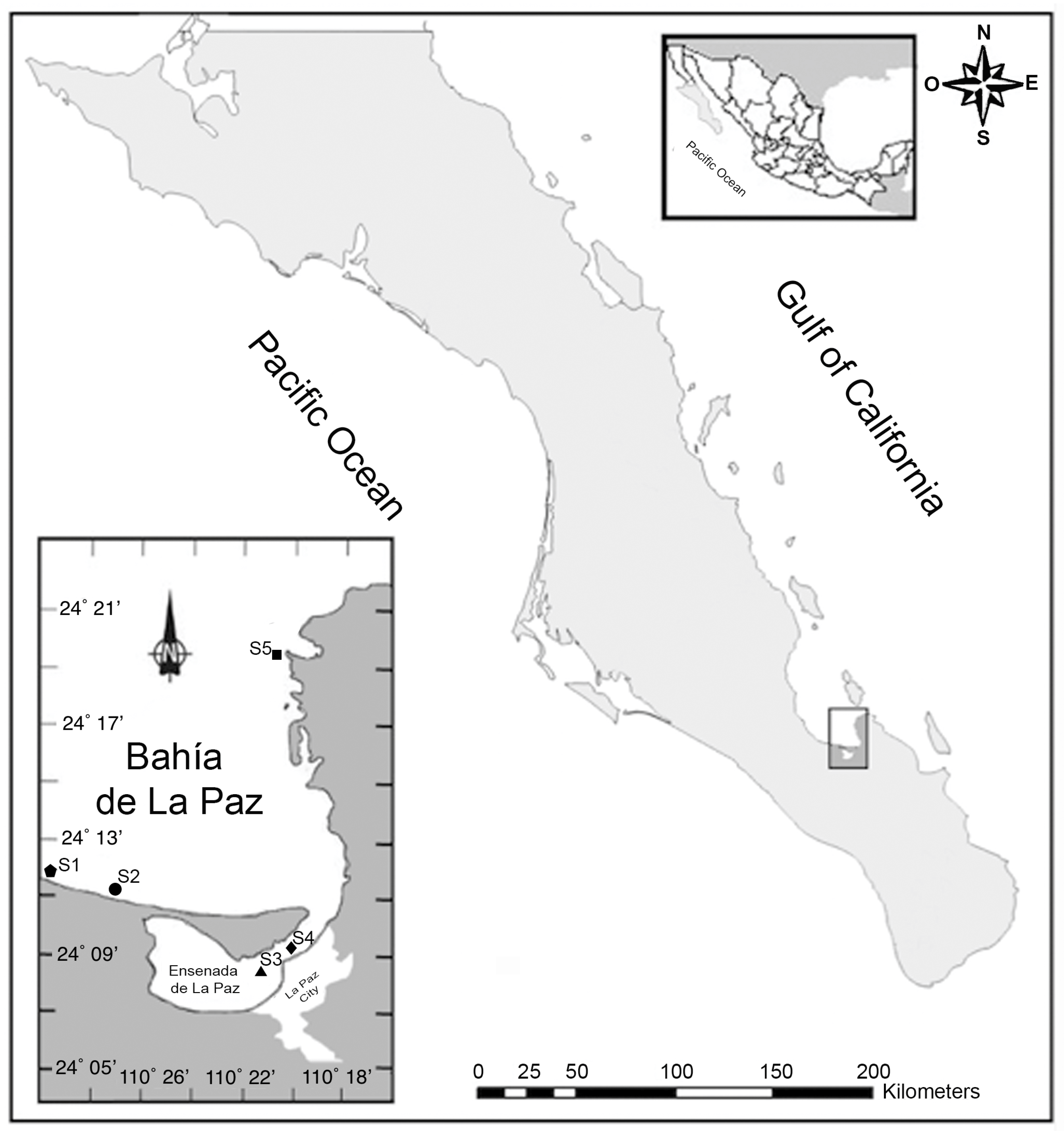
2.1. Study Area and Sampling
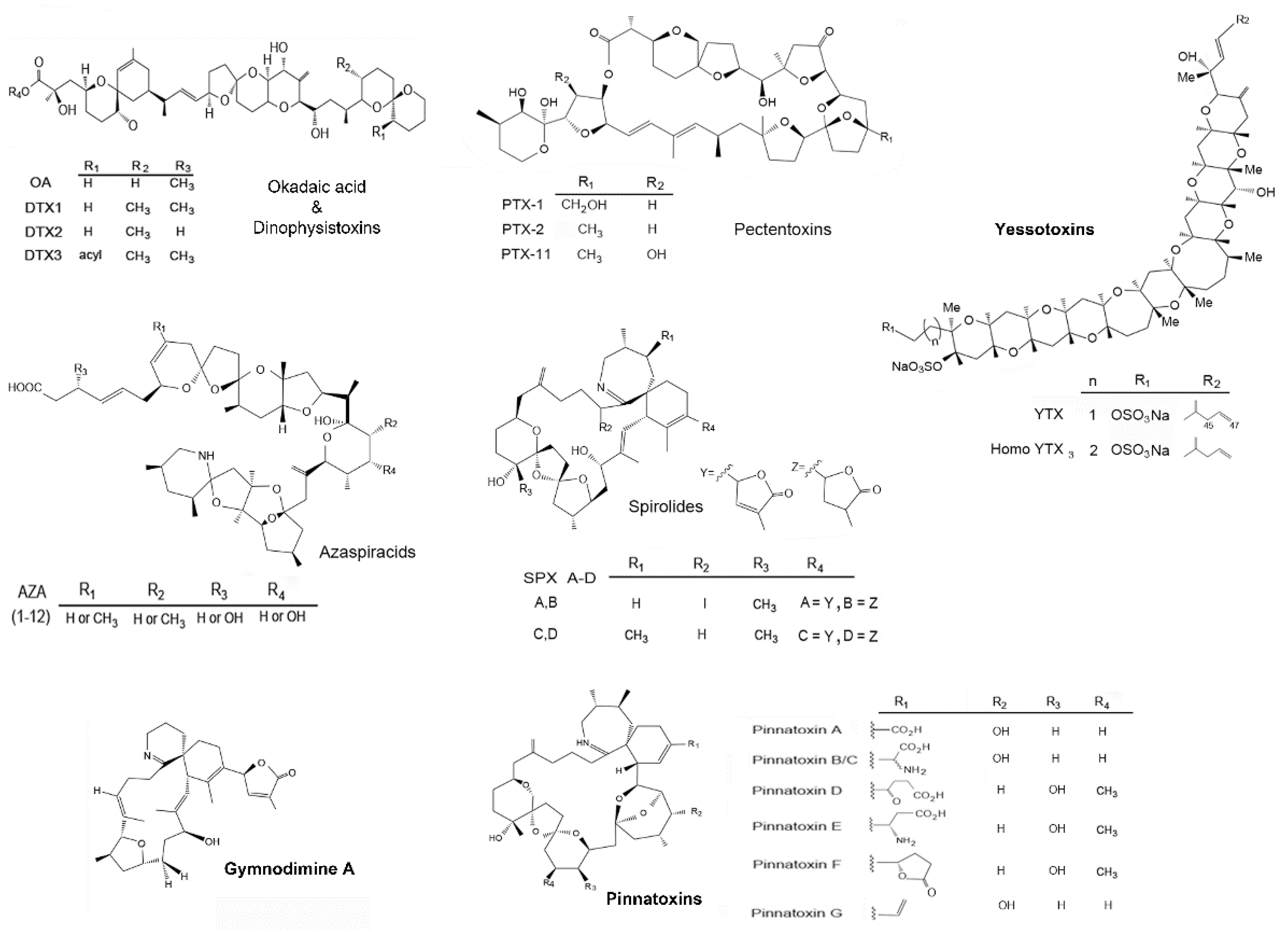
2.2. Content of Lipophilic Toxins in Three Species of Shellfish
2.3. Intoxication Risk According to Shellfish Species
2.4. Toxin Co-Occurrence in Bivalves
3. Materials and Methods
3.1. Study Area and Sample Collection
3.2. Sample Processing
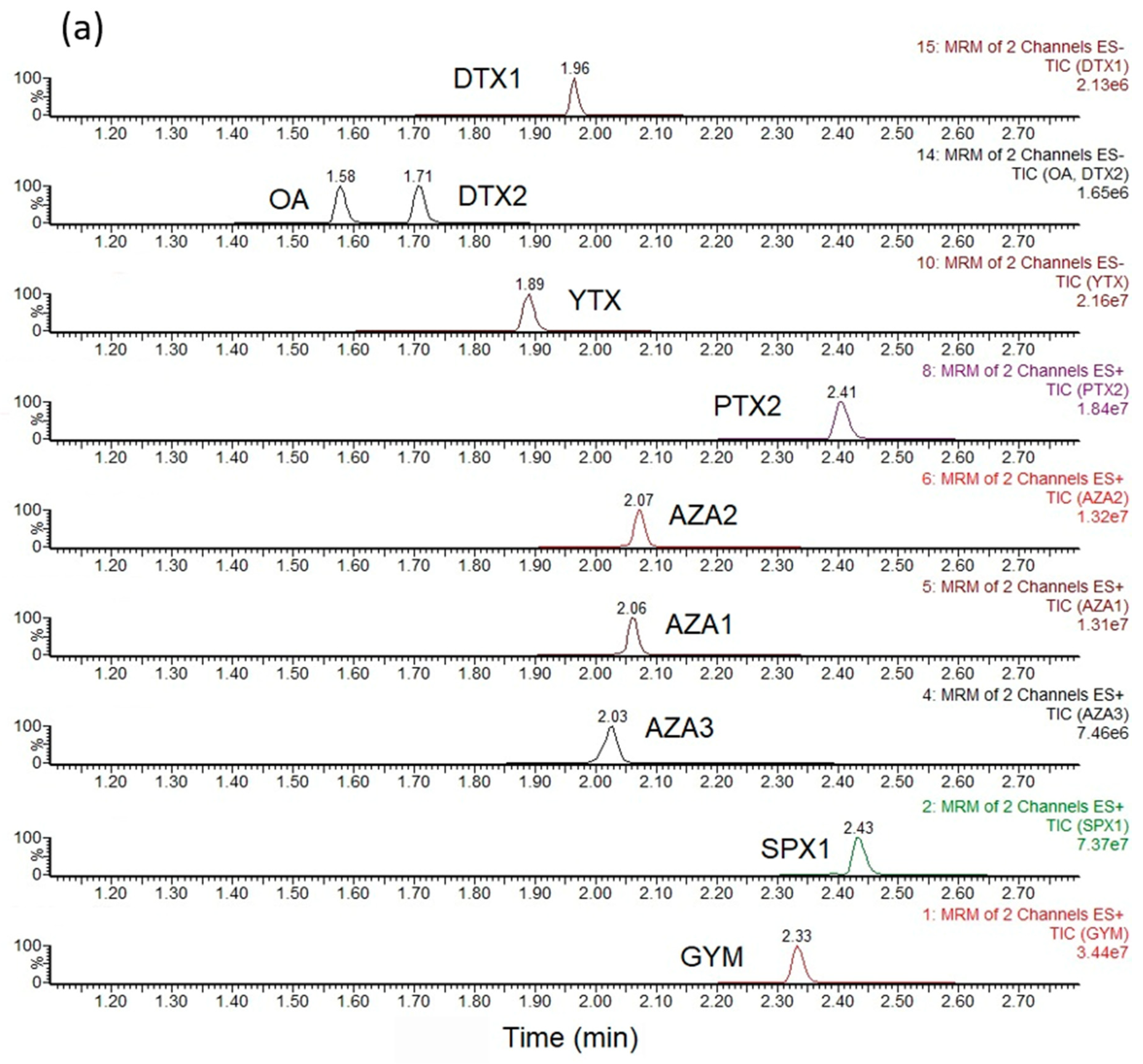
3.3. Sample Analysis—LC-MS/MS
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Brusca, R.; Brusca, R.C. The Gulf of California, Biodiversity and Conservation, 2nd ed.; The Arizona University Press: Tucson, AZ, USA, 2010; pp. 1–6. [Google Scholar]
- Comisión Nacional de Acuacultura y Pesca. Anuario Estadístico de Acuacultura y Pesca 2016, SAGARPA, México. 2016. Available online: https://www.conapesca.gob.mx/work/sites/cona/dgppe/2016/ANUARIO_ESTADISTICO_2016.pdf (accessed on 12 October 2020).
- Cembella, A.; Shumway, S.E.; Lewis, N.I. Anatomical distribution and spatio-temporal variation in paralytic shellfish toxin composition in two bivalve species from the Gulf of Maine. J. Shell. Res. 1993, 12, 389–403. [Google Scholar]
- Bricelj, V.M.; Shumway, S.E. Paralytic shellfish toxins in bivalve mollusks: Occurrence, transfer kinetics, and biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Braña-Magdalena, A.; Lehane, M.; Moroney, C.; Furey, A.; James, K.J. Food safety implications of the distribution of azaspiracids in the tissue compartments of scallops (Pecten maximus). Food Addit. Contam. 2003, 20, 154–160. [Google Scholar] [CrossRef]
- McCarron, P.; Hess, P. Tissue distribution and effects of heat treatments on the content of domoic acid in blue mussels, Mytilus edulis. Toxicon 2006, 47, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Gestal-Otero, J.J. Epidemiology of Marine toxins. In Sea Food and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: Boca Ratón, FL, USA, 2014; pp. 124–167. [Google Scholar]
- Lansberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2010, 10, 113–390. [Google Scholar] [CrossRef]
- Reguera, B.; Rodríguez, F.; Blanco, J. Harmful algae blooms and food safety: Physiological and environmental factors affecting toxin production and their accumulation in Shellfish. In New Trends in Marine and Freshwater Toxins, 2nd ed.; Cabado, A.G., Vieites, J.M., Eds.; Nova Science Publishers: New York, NY, USA, 2013; pp. 53–89. [Google Scholar]
- Holmes, M.J.; Brust, A.; Lewis, R.J. Dinoflagellate toxins: An overview. In Sea Food and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: Boca Ratón, FL, USA, 2014; pp. 3–25. [Google Scholar]
- Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; López-Cortés, D.J.; Gárate-Lizárraga, I.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.E. Ecological and physiological studies of Gymnodinium catenatum in the Mexican Pacific: A review. Mar. Drugs 2010, 8, 1935–1961. [Google Scholar] [CrossRef]
- Graham, H.W. Gymnodinium catenatum, a new dinoflagellate from the Gulf of California. Trans. Am. Microsc. Soc. 1943, 62, 259–261. [Google Scholar] [CrossRef]
- Osorio-Tafall, B.F. El Mar de Cortés y la productividad fitoplanctónica de sus aguas. Anal. Esc. Nal. Cienc. Biol. IPN Mex. 1943, 3, 73–118. [Google Scholar]
- Mee, L.D.; Espinosa, E.; Díaz, G. Paralytic shellfish poisoning with a Gymnodinium catenatum red tide on the Pacific coast of Mexico. Mar. Environm. Res. 1986, 19, 77–92. [Google Scholar] [CrossRef]
- Medina-Elizalde, J.; García-Mendoza, E.; Turner, A.; Sánchez-Bravo, Y.A.; Murillo-Martínez, R. Transformation and depuration of paralytic shellfish toxins in the geoduck clam Panopea globosa from the northern Gulf of California. Front. Mar. Sci. 2018, 5, 335. [Google Scholar] [CrossRef]
- García-Mendoza, E.; Sánchez-Bravo, Y.; Turner, A.; Blanco, J.; O´Neil, A.; Mancera-Flores, J.M.; Pérez-Brunius, P.; Rivas, D.; Almazán-Becerril, A.; Peñá-Manjarrez, J.L. (Lipophilic toxins in cultivated mussels (Mytilus galloprovincialis) from Baja California, Mexico. Toxicon 2014, 90, 111–123. [Google Scholar] [CrossRef]
- Gárate-Lizárraga, I.; Muñetón-Gómez, M.S.; Maldonado-López, V. Florecimiento del dinoflagelado Gonyaulax polygramma frente a la isla Espíritu Santo, Golfo de California, Mexico. Rev. Investig. Mar. 2006, 27, 31–39. [Google Scholar]
- Gárate-Lizárraga, I.; Muñetón-Gómez, M.S.; Pérez-Cruz, B.; Díaz-Ortíz, J.A. Bloom of Gonyaulax spinifera (Dinophyceae: Gonyaulacales) in Ensenada de la Paz Lagoon, Gulf of California. CICIMAR Oceánides 2014, 29, 11–18. [Google Scholar] [CrossRef]
- Durán-Riveroll, L.M.; Cembella, A.D.; Okolodkov, Y.B. A review on the biodiversity and biogeography of toxigenic benthic marine dinoflagellates of the coasts of Latin America. Front. Mar. Sci. 2019, 148, 1–26. [Google Scholar] [CrossRef]
- Heredia-Tapia, A.; Arredondo-Vega, B.O.; Núñez-Vázquez, E.J.; Yasumoto, T.; Yasuda, M.; Ochoa, J.L. Isolation of Prorocentrum lima (Syn. Exuviaella lima) and diarrhetic shellfish poisoning (DSP) risk assessment in the Gulf of California, Mexico. Toxicon 2002, 40, 1121–1127. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A.D.M. First confirmation of human diarrheic poisonings by okadaic acid esters after ingestion of razor clams (Solen margiunatus) and green crabs (Carcinus maenas) in Averio lagoon, Portugal and detection of okadaic acid esters in phytoplankton. Toxicon 2002, 40, 989–996. [Google Scholar] [CrossRef]
- Valdiglesias VPrego-Faraldo, M.V.; Pásaro, E.; Méndez, J.; Laffon, B. Okadaic Acid: More than a diarrheic Toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [PubMed]
- Diario Oficial de la Federación. Norma Oficial Mexicana NOM-242-SSA1-2009. Available online: http://dof.gob.mx/nota_detalle.php?codigo=5177531&fecha=10/02/2011 (accessed on 20 October 2019).
- Guía Técnica del Programa Mexicano de Sanidad de Moluscos Bivalvos. Available online: http://docplayer.es/75122402-Guia-tecnica-del-programa-mexicano-de-sanidad-de-moluscos-bivalvos.html (accessed on 21 October 2019).
- Bialojan, C.; Takai, A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Biochem. J. 1988, 256, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Kumagai, M.; Yasumoto, T. Toxicologic evaluation of yessotoxin. Nat. Toxins 1997, 5, 255–259. [Google Scholar] [CrossRef]
- Munday, R.; Aune, T.; Rossini, G.P. Toxicology of the yessotoxins. In Seafood and Freshwater Toxins, Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; CRC Press: Boca Ratón, FL, USA, 2008; pp. 329–339. [Google Scholar]
- Twiner, M.J.; Rehmann, N.; Hess, P.; Doucette, G.J. Azaspiracid shellfish poisoning: A review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs 2012, 6, 39–72. [Google Scholar] [CrossRef]
- McMahon, T.; Silke, J. Winter toxicity of unknown etiology in mussels. Harmful Algae News 1996, 14, 2. [Google Scholar]
- Satake, M.; Ofuji, K.; Naoki, H.; James, J.K.; Furey, A.; McMahon, T.; Silke, J.; Yasumoto, T. Azaspiracid, a new marine toxin having unique spiro ring assemblies, isolated from Irish mussels, Mytilus Edulis. J. Am. Chem. Soc. 1998, 120, 9967–9968. [Google Scholar] [CrossRef]
- McMahon, T.; Silke, J. Re-occurrence of winter toxicity. Harmful Algae News 1998, 17, 12. [Google Scholar]
- Burgess, V.A. Toxicology Investigations with the Pectenotoxin-2 seco Acids. Ph.D. Thesis, Griffith University, Nathan, Queensland, Australia, 2003. Available online: https://research-repository.griffith.edu.au/handle/10072/365382 (accessed on 7 August 2020).
- Terao, K.; Ito, E.; Yanagi, T.; Yasumoto, T. Histopatological studies on experimental marine toxin poisoning. I. Ultrastructural changes in the small intestine and liber of suckling mice induced by Dinophysistoxin-1 and Pectentoxin-1. Toxicon 1986, 24, 1141–1151. [Google Scholar] [CrossRef]
- Draisci, R.; Lucentini, L.; Giannetti, L.; Boria, P.; Poletti, R. First report of Pectentoxin-2 (PTX-2) in algae (Dinophysis fortii) related to seafood poisoning in Europe. Toxicon 1996, 34, 923–935. [Google Scholar] [CrossRef]
- Torigoe, K.; Murata, M.; Yasumoto, T. Prorocentrolide, a toxic nitrogenous Macrocycle a marine dinoflagellate, Prorocentrum lima. J. Am. Chem. Soc. 1988, 110, 7876–7877. [Google Scholar] [CrossRef]
- Hu, T.; Curtis, J.M.; Oshima, Y.; Quilliam, M.A.; Walter, J.A.; Watson-Wright, W.M.; Wright, J.L. Spirolides B and D, two novel macrocycles isolated from the digestive glands of shellfish. J. Chem. Soc. Chem. Commun. 1995, 2159–2161. [Google Scholar] [CrossRef]
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unpresented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Uemura, D.; Chou, T.; Haino, T.; Nagatsu, A.; Fukusawa, S.; Zheng, S.; Chen, H. Pinnatoxin A: A toxic amphoteric macrocycle from the Okinawan bivalve Pinna muricata. J. Am. Chem. Soc. 1995, 117, 1155–1156. [Google Scholar] [CrossRef]
- Lu, C.K.; Lee, G.H.; Huang, R.; Chou, H.N. Spiro-prorocentrimine, a novel macrocyclic lactone from a benthic Prorocentrum sp. of Taiwan. Tetrahedron Lett. 2001, 42, 1713–1716. [Google Scholar] [CrossRef]
- Selwood, A.I.; Wilkins, A.L.; Munday, R.; Shi, F.; Rhodes, L.L.; Holland, P.T. Portimine: A bioactive metabolite from the benthic dinoflagellate Vulcanodinium rugosum. Tetrahedron Lett. 2013, 54, 4705–4707. [Google Scholar] [CrossRef]
- Fribley, A.M.; Xi, Y.; Makris, C.; Alves-de-Souza, C.; York, R.; Tomas, C.; Wright, J.L.C.; Strangman, W.K. Identification of Portimine B, a new cell permeable spiroimine that induce apoptosis in oral squamous cell carcinoma. Med. Chem. Lett. 2019, 10, 175–179. [Google Scholar] [CrossRef]
- Gill, S.; Murphy, M.; Clausen, J.; Richard, D.; Quilliam, M.; MacKinnon, S.; LaBlanc, P.; Mueller, R.; Pulido, O. Neural injury biomarkers of novel shellfish toxins, spirolides: A pilot study using immunochemical and transcriptional analysis. NeuroToxicology 2003, 24, 593–604. [Google Scholar] [CrossRef]
- Kharrat, R.; Servent, D.; Girard, E.; Ouanounou, G.; Amar, M.; Marrouchi, R.; Benoit, E.; Molgó, J. The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J. Neurochem. 2008, 107, 952–963. [Google Scholar] [CrossRef]
- FAO/IOC/WHO (Food and Agriculture Organization of the United Nations/Intergovernmental Oceanographic Commission of UNESCO/World Health Organization). Report of the Joint FAO/IOC/WHO ad hoc Expert Consultation on Biotoxins in Bivalve Mollusks; FAO/IOC/WHO: Oslo, Norway, 2004; p. 8. [Google Scholar]
- COFEPRIS. Plan de Contingencia Para el Control de Biotoxinas Marinas. 2015. Available online: https://docplayer.es/3456257-Plan-de-contingencia-para-el-control-de-biotoxinas-marinas.html (accessed on 5 November 2019).
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a New Type of Shellfish Poisoning in the Tohoku District. Bull. Jpn. Soc. Sci. Fish. 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Molgó, J.; Araóz, R.; Benoit, E.; Iorga, B.I. Cyclic Imine Toxins: Chemistry, Origin, Metabolism, Pharmacology, Toxicology, and Detection. In Sea Food and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: Boca Ratón, FL, USA, 2014; pp. 951–979. [Google Scholar]
- Hernández-Castro, J.E. Dinoflagelados y Toxinas Lipofílicas en Bancos Naturales de Bivalvos al sur de la Bahía de La Paz, B.C.S., México. Master’s Thesis, Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, La Paz, B.C.S., Mexico, 2017. [Google Scholar]
- Álvarez-Borrego, S.; Lara-Lara, R. The physical environment and primary productivity of the Gulf of California. In The Gulf and Peninsular Province of the Californias, 1st ed.; Dauphin, J.P., Simoneit, B.R., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1991; Volume 47, pp. 555–566. [Google Scholar]
- Selwood, A.I.; Miles, C.O.; Wilkins, A.L.; Ginkel, R.V.; Munday, R.; Rise, F.; McNabb, P. Isolation, structural determination and acute toxicity of Pinnatoxins E, F and G. J. Agric. Food Chem. 2010, 58, 6532–6542. [Google Scholar] [CrossRef]
- Obeso-Nieblas, M.; Shirasago-Germán, B.; Gaviño-Rodríguez, J.; Perez-Lezaman, E.; Obeso-Huerta, H.; Jiménez-Illescas, A. Hydrographic variability in Bahia de La Paz, Gulf of California, Mexico (1995–2005). Rev. Biol. Mar. Ocean. 2008, 43, 559–567. [Google Scholar]
- Gárate-Lizárraga, I.; Bustillos-Guzmán, J.J.; Erler, K.; Muñeton-Gómez, B.; Tripp-Quezada, A. Paralytic shellfish toxins in the chocolata clam, Megapitaria squalida (Bivalvia:Veneridae), in Bahía de La Paz, Gulf of California. Rev. Biol. Trop. 2004, 52, 133–140. [Google Scholar] [PubMed]
- Estrada, N.; Lagos, N.; García, C.; Maeda-Martínez, A.N.; Asencio, F. Effects of the toxic dinoflagellate Gymnodinium catenatum on uptake and fate of paralytic shellfish poisons in the Pacific giant lions-paw scallop Nodipecten subnodosus. Mar. Biol. 2007, 151, 1205–1214. [Google Scholar] [CrossRef]
- Hernández-Sandoval, F.E.; López-Cortés, D.J.; Band-Schmidt, C.J.; Gárate-Lizárraga, I.; Núñez-Vázquez, E.J.; Bustillos-Guzmán, J.J. Toxinas paralizantes en moluscos bivalvos durante una proliferación de Gymnodinium catenatum Graham en la Bahía de La Paz, México. Hidrobiológica 2009, 19, 245–256. [Google Scholar]
- Gárate-Lizarraga, I.; González Armas, R. Occurrence of Pyrodinium bahamense var. compressum along the southern coast of the Baja California Peninsula. Mar. Poll. Bull. 2011, 62, 626–630. [Google Scholar]
- Escobedo-Lozano, A.Y.; Estrada, N.; Asencio, F.; Contreras, G.; Alonso-Rodríguez, R. Accumulation, Biotransformation, Histopathology and Paralysis in the Pacific Calico Scallop Argopecten ventricosus by the Paralyzing Toxins of the Dinoflagellate Gymnodinium catenatum. Mar. Drugs 2012, 10, 1044–1065. [Google Scholar] [CrossRef]
- Dominguez, H.J.; Paz, B.; Daranas, A.H.; Norte, M.; Franco, J.M.; Fernández, J.J. Dinoflagellate polyether within the yessotoxin, pectenotoxin and okadaic acid toxin groups: Characterization, analysis and human health implications. Toxicon 2010, 56, 191–217. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M.G. Harmful Dinophysis species: A review. Harmful Algae. 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U.; Alonso-Rodríguez, R.; Álvarez-Góngora, C.; Barón-Campis, S.A.; Ceballos-Corona, G.; Herrera-Silveira, J.; Meave del Castillo, M.E.; Juárez-Ruíz, N.; Merino-Virgilio, F.; Morales-Blake, A.; et al. Toxic and harmful marine phytoplankton and microalgae (HABs) in Mexican coasts. J. Environ. Sci. Health Part A 2007, 42, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Becerril, D.U.; Barón-Campis, S.A.; Escobar-Morales, S.A. New record of Azadinium spinosum (Dinoflagellata) from the tropical Mexican Pacific. Rev. Biol. Mar. Ocean. 2012, 47, 553–557. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U.; Rodríguez-Palacio, M.C.; Lozano-Ramírez, C. Morphology and life stages of the potentially pinnatoxin producing thecate dinoflagellate Vulcanodinium rugosum from the tropical Mexican Pacific. Bot. Mar. 2013, 56, 535–540. [Google Scholar] [CrossRef]
- Meave del Castillo, M.E.; Zamudio-Resendiz, M.E. Karenia species in the Mexican Pacific. In Proceedings of the 14th International Conference on Harmful Algae, Hersonissos-Crete, Greece, 1–5 November 2010; Pagou, K.A., Hallegraeff, G.M., Eds.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Oostende, Belgium, 2013; pp. 111–113. [Google Scholar]
- Wakeman, K.C.; Yamaguchi, A.; Roy, M.C.; Jenke-Kodama, H. Morphology, phylogeny and novel chemical compounds from Coolia malayensis (Dinophyceae) from Okinawa, Japan. Harmful Algae 2015, 44, 8–19. [Google Scholar] [CrossRef]
- Junqueira de Acevedo-Tibiricá, C.E.; Sibat, M.; Fernandes, L.F.; Bilien, G.; Chomerát, N.; Hess, P.; Mafra, L.L., Jr. Diversity and toxicity of the genus Coolia Meunier in Brazil, and detection of 44-metil Gambierone in Coolia tropicalis. Toxins 2020, 12, 1–24. [Google Scholar]
- Li, X.; Yan, M.; Gu, X.; Lam, V.V.T.; Wai, T.-C.; Baker, D.M.; Thompson, P.D.; Yiu, S.K.F.; Lam, P.K.S.; Leung, P.T.Y. The effect of temperature on physiology, toxicity and toxin content of the benthic dinoflagellate Coolia malayensis from a seasonal tropical región. Water Res. 2020, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, L.; Li, Y.; Zhang, J.; Tan, Z.; Wu, H.; Jiang, T.; Lu, S. Occurrence of marine algal toxins in oyster and phytoplankton samples in Daya Bay, South China Sea. Chemosphere 2017, 183, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Arellano Martínez, M.; Quiñones-Arreola, M.F.; Ceballos-Vázquez, B.P.; Villalejo-Fuerte, M. Reproductive pattern of the squalid callista Megapitaria squalida from northwestern Mexico. J. Shell. Res. 2006, 25, 849–855. [Google Scholar]
- Corona-Fernández, M. Biología Reproductiva de la Almeja Blanca, Dosinia ponderosa (Gray, 1838) en la Bahía de La Paz, B.C.S., México. Master’s Thesis, Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, La Paz, B.C.S., Mexico, December 2016. [Google Scholar]
- Aguilar-Cruz, C.A. Estrategia reproductiva y tejidos de reserva de la almeja blanca Dosinia ponderosa (Gray, 1838) de Puerto Libertad, Sonora, México. Master’s Thesis, Universidad Autónoma de Baja California Sur, Departamento académico de Ciencias Marinas y Costeras, La Paz, B.C.S., Mexico, February 2018. [Google Scholar]
- Blanco, J.; Mariño, C.; Martín, H.; Acosta, C.P. Anatomical distribution of diarrhetic shellfish poisoning (DSP) toxins in the mussel Mytilus galloprovincialis. Toxicon 2007, 50, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Pereira, A.; Braekman, J.C.; Houvenaghel, G. Patterns in Long Term Accumulation of Okadaic Acid and DTX-1 in Blue Mussels, Mytilus Edulis, Experimentally Fed with the DSP-Containing Alga Prorocentrum lima. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Lavoisier, Intercept Ltd.: Paris, France, 1995; pp. 487–492. [Google Scholar]
- Sandoval, F.J.; Gomez-Valdez, J. Tides and currents in Ensenada de La Paz lagoon, Baja California Sur, México. Geofis. Int. 1997, 36, 37–47. [Google Scholar]
- Munday, R.; Towers, N.R.; Mackenzie, L.; Beuzenberg, V.; Holland, P.T.; Miles, C.O. Acute toxicity of gymnodimine to mice. Toxicon 2004, 44, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Marine Biotoxins in Shellfish–Summary on Regulated Marine Biotoxins. Available online: http://www.elika.net/datos/articulos/archivo_en448/marinebiotoxins_en.pdf (accessed on 15 February 2019).
- Aasen, J.A.B.; Espenes, A.; Miles, C.O.; Samdal, I.A.; Hess, P.; Aune, T. Combined oral toxicity of azaspiracid-1 and yessotoxin in female NMRI mice. Toxicon 2011, 57, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.; Espenes, A.; Aasen, J.A.B.; Quilliam, M.A.; Hess, P.; Larsen, S. Study of possible combined toxic effects of azaspiracid-1 and okadaic acid in mice via oral route. Toxicon 2012, 60, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; Ardizzone, M.; Beltramo, D.; Vita, F.; Dell’Ovo, V.; Barreras, A.; Yasumoto, T.; Tubaro, A. Repeated oral co-exposure to yessotoxin and okadaic acid: A short term toxicity study in mice. Toxicon 2013, 76, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.P.; González, V.; Martínez, A.; Paz, B.; Lago, J.; Cordeiro, V.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Occurrence of lipophilic marine toxins in shellfish from Galicia (NW of Spain) and synergies among them. Mar. Drugs 2015, 13, 1666–1687. [Google Scholar] [CrossRef]
- Ferron, P.J.; Dumazeau, K.; Beaulieu, J.F.; Le Hégarat, L.; Fessard, V. Combined effects of lipophilic phycotoxins (okadaic acid, azaspiracid-1 and yessotoxin) on human intestinal cells models. Toxins 2016, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Litaker, L.W.; Berdalet, E. Climate change and harmful benthic microalgae. Harmful Algae 2020, 91, 1–27. [Google Scholar] [CrossRef]
- Reyes-Salinas, A.; Cervantes-Duarte, R.; Morales-Pérez, R.A.; Valdez-Holguín, J.E. Seasonal variability of primary productivity and its relation to the vertical stratification in La Paz bay, B.C.S. Hidrobiológica 2003, 13, 103–110. [Google Scholar]
- Gárate-Lizárraga, I. Proliferation of Amphidinium carterae (Gymnodiniales: Gymnodiniaceae) in Bahía de La Paz, Gulf of California. CICIMAR Oceánides 2012, 27, 37–49. [Google Scholar] [CrossRef]
- European Reference Laboratory for Marine Biotoxins (EURLMB). EU Harmonized Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Mollusks by LC-MS/MS, Version 5 January 2015. Available online: http://www.aecosan.msssi.gob.es/AECOSAN/docs/documentos/laboratorios/LNRBM/ARCHIVO2EU-Harmonised-SOP-LIPO-LCMSMS_Version5.pdf (accessed on 8 August 2020).
- Dhanji-Rapkova, M.; O’Neill, A.; Maskrey, B.H.; Coates, L.; Alves, M.T.; Kelly, R.J.; Hatfield, R.G.; Rowland-Pilgrim, S.; Lewis, A.D.; Algoet, M.; et al. Variability and profiles of lipophilic toxins in bivalves from Great Britain during five and a half years of monitoring: Okadaic acid, dinophysis toxins and pectenotoxins. Harmful Algae 2018, 77, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Dhanji-Rapkova, M.; O’Neill, A.; Maskrey, B.H.; Coates, L.; Swan, S.C.; Teixeira Alves, M.; Kelly, R.J.; Hatfield, R.G.; Rowland-Pilgrim, S.J.; Lewis, A.M.; et al. Variability and profiles of lipophilic toxins in bivalves from Great Britain during five and a half years of monitoring: Azaspiracids and yessotoxins. Harmful Algae 2019, 87, 101629. [Google Scholar] [CrossRef] [PubMed]
- Gerssen, A.; Mulder, P.P.J.; McElhinney, M.A.; de Boer, J. Liquid chromatography-tandem mass spectrometry for the detection of marine lipophilic toxins under alkaline conditions. J. Chrom. A 2009, 1216, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
| Month. | Site | OA | DTX | PTX | YTX | AZA | SPX | GYM | PnTX | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | ||
| J | S3 | 0.5 | * | 0.05 | 3.3 | 0.02 | 0.3 | 0.3 | 5.3 | ||||||||||||||||
| S4 | 0.5 | 0.05 | 0.05 | 5.3 | 0.01 | 0.1 | 0.1 | 6.8 | |||||||||||||||||
| F | S2 | 0.4 | – | * | * | 0.01 | * | 0.30 | * | – | * | 0.18 | * | 1.7 | * | * | 0.1 | ||||||||
| S3 | 0.5 | * | 0.1 | 3.9 | 0.02 | 0.2 | 0.22 | 6.32 | |||||||||||||||||
| S4 | 0.8 | * | 0.02 | 5.7 | 0.01 | 0.1 | 0.07 | 11.1 | |||||||||||||||||
| M | S1 | 2.0 | 3.3 | 0.7 | 0.7 | 2.1 | 3.3 | * | * | * | * | * | 1.2 | * | 5.7 | 7.5 | 6.9 | ||||||||
| S2 | 0.9 | – | * | – | * | – | * | – | 0.9 | – | * | – | * | – | 0.8 | – | |||||||||
| S3 | 1.7 | * | 8.2 | 2.7 | * | 0.7 | 0.6 | 5.1 | |||||||||||||||||
| S4 | 2.6 | * | 0.4 | * | * | * | * | 5.8 | |||||||||||||||||
| A | S1 | 1.7 | 4.2 | * | * | * | * | * | * | * | * | * | * | * | * | 0.9 | – | ||||||||
| S2 | 1.0 | – | * | – | * | – | * | – | * | – | * | – | * | – | 0.8 | – | |||||||||
| S3 | 1.5 | * | 1.5 | * | * | 2.3 | 1.2 | 7.7 | |||||||||||||||||
| S4 | 2.8 | * | * | * | * | * | * | 10.5 | |||||||||||||||||
| M | S1 | 2.6 | 4.3 | * | * | * | * | * | * | * | * | 3.0 | 2.6 | 10.2 | 5.8 | * | * | ||||||||
| S2 | 1.3 | – | * | – | * | – | * | – | * | – | 2.2 | – | 9.9 | – | 0.8 | – | |||||||||
| S3 | 1.9 | * | 0.1 | 7.3 | * | 1.5 | 1.1 | 4.5 | |||||||||||||||||
| S4 | 1.2 | * | 0.1 | 5.2 | * | 0.4 | * | 6.1 | |||||||||||||||||
| J | S1 | 3.5 | 3.4 | 0.9 | – | – | – | – | – | – | – | 3.3 | – | 10.8 | – | * | * | ||||||||
| S2 | 1.1 | – | – | – | – | – | – | – | – | – | 2.9 | – | 11.7 | – | 0.8 | – | |||||||||
| S3 | 4.2 | * | 0.4 | 9.5 | * | 0.9 | 0.8 | 4.5 | |||||||||||||||||
| S4 | 3.5 | * | * | 22.5 | * | 0.6 | 0.5 | 6.1 | |||||||||||||||||
| J | S2 | 0.9 | – | * | – | * | – | 0.5 | – | – | – | 1.7 | – | 24.9 | – | 0.8 | – | ||||||||
| S3 | – | * | * | 5.8 | * | 0.9 | 0.9 | 4.9 | |||||||||||||||||
| S4 | 3.5 | * | * | 7.3 | * | 0.1 | – | 12.6 | |||||||||||||||||
| A | S2 | 1.0 | – | * | – | * | – | * | – | * | – | 1.4 | – | 7.5 | – | 0.8 | – | ||||||||
| S3 | 1.7 | * | * | 13.0 | * | 0.5 | 0.8 | 6.6 | |||||||||||||||||
| S4 | 2.6 | * | * | 4.1 | * | 0.1 | * | 10.7 | |||||||||||||||||
| S | S2 | 1.6 | 1.2 | * | * | * | * | * | * | * | * | 1.7 | 0.8 | 15.5 | 26.2 | 0.8 | 0.8 | ||||||||
| S3 | 5.0 | 0.9 | * | 11.9 | * | 0.5 | 12.6 | 9.4 | |||||||||||||||||
| S4 | 1.9 | * | * | 14.5 | * | 0.1 | 5.6 | 10.4 | |||||||||||||||||
| S5 | – | 1.8 | – | 0.8 | – | * | – | * | – | * | – | 1.3 | * | 23.2 | – | 2.2 | |||||||||
| O | S2 | 3.6 | – | 0.9 | – | * | – | 0.5 | – | – | – | 1.7 | – | 24.9 | – | 1.7 | – | ||||||||
| S3 | 6.3 | * | * | 8.2 | * | 1.1 | 11.3 | 12.3 | |||||||||||||||||
| S4 | * | * | * | 18.9 | 2.4 | * | 5.2 | 13.1 | |||||||||||||||||
| S5 | 1.1 | 1.3 | * | * | * | * | * | * | * | * | 2.0 | 0.8 | 19.0 | 35.4 | 2.3 | 0.9 | |||||||||
| Month. | Site | OA | DTX | PTX | YTX | AZA | SPX | GYM | PnTX | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | ||
| J | S2 | * | – | * | – | * | – | * | – | * | – | 8.9 | – | 33.2 | – | * | – | ||||||||
| S3 | * | * | 0.6 | * | 0.9 | 8.9 | 6.8 | – | |||||||||||||||||
| S4 | * | * | * | * | * | 8.4 | 4.7 | 11.4 | |||||||||||||||||
| S5 | * | – | * | – | * | – | * | – | * | – | 11.0 | – | 38.0 | – | 0.5 | – | |||||||||
| F | S2 | * | – | * | – | * | – | * | – | * | – | 9.5 | – | 29.2 | – | * | – | ||||||||
| S3 | * | * | 0.6 | 11.8 | * | 8.5 | 5.9 | 6.8 | |||||||||||||||||
| S4 | * | * | * | 14.0 | * | 8.4 | 3.9 | * | |||||||||||||||||
| S5 | * | – | * | – | – | * | – | * | – | 1.6 | 11.5 | 8.8 | 30.0 | 5.4 | 0.2 | 0.1 | |||||||||
| M | S2 | * | – | * | – | * | – | * | – | 0.8 | – | 9.9 | – | 20.3 | – | * | – | ||||||||
| S3 | * | * | * | 40.1 | * | 9.1 | 6.1 | 6.8 | |||||||||||||||||
| S4 | * | * | * | 76.5 | * | * | * | * | |||||||||||||||||
| S5 | * | * | * | * | * | * | * | * | 0.9 | 1.2 | 9.9 | 9.8 | 15.2 | 10.5 | 0.2 | 0.1 | |||||||||
| A | S2 | * | – | * | – | * | – | * | – | * | – | 11.4 | – | 17.6 | – | 0.5 | – | ||||||||
| S3 | * | * | * | 29.0 | 0.8 | 8.9 | 6.0 | 8.5 | |||||||||||||||||
| S4 | * | * | * | 88.7 | * | 8.6 | 4.7 | 11.4 | |||||||||||||||||
| S5 | * | – | * | – | * | – | * | – | * | – | 11.3 | 9.2 | 23.9 | 7.8 | 0.2 | 0.1 | |||||||||
| M | S2 | * | – | * | – | * | – | * | – | * | – | 10.0 | – | 12.6 | – | * | – | ||||||||
| S3 | * | * | 1.1 | 32.0 | * | 8.7 | 4.3 | 3.4 | |||||||||||||||||
| S4 | * | * | * | 47.9 | * | 8.2 | 3.5 | 8.1 | |||||||||||||||||
| S5 | * | – | * | – | * | – | * | – | * | 1.0 | 12.1 | 10.0 | 25.9 | 9.6 | 0.5 | 0.5 | |||||||||
| J | S2 | * | – | * | – | * | – | * | – | * | – | 10.9 | – | 21.4 | – | * | – | ||||||||
| S3 | 9.6 | * | * | * | 1.8 | 8.9 | 4.4 | 6.1 | |||||||||||||||||
| S4 | 6.3 | * | * | 28.1 | * | 8.2 | * | 5.2 | |||||||||||||||||
| S5 | * | – | * | 1.1 | * | 1.2 | * | 19.2 | * | – | 11.4 | – | 17.6 | – | * | – | |||||||||
| A | S2 | 1.9 | – | * | – | * | – | * | – | * | – | 5.4 | – | 19.5 | – | * | – | ||||||||
| S3 | 0.8 | * | * | * | * | 0.5 | 0.9 | 12.0 | |||||||||||||||||
| S4 | 1.4 | * | * | 5.8 | * | 0.2 | 0.9 | 7.9 | |||||||||||||||||
| S5 | 0.3 | – | * | – | * | – | * | – | * | – | 1.1 | – | 6.6 | – | * | – | |||||||||
| S | S2 | 0.6 | – | * | – | 0.2 | – | * | – | * | – | 2.4 | – | 15.1 | – | * | – | ||||||||
| S3 | 2.6 | * | * | 3.9 | * | 0.4 | 1.4 | 12.0 | |||||||||||||||||
| S4 | 0.9 | * | * | * | * | * | 0.6 | 7.9 | |||||||||||||||||
| S5 | 0.3 | 0.7 | * | * | * | * | * | * | * | * | 3.4 | 0.8 | 11.7 | 7.4 | * | * | |||||||||
| O | S2 | 0.3 | – | * | – | * | – | * | – | * | – | 1.3 | – | 20.4 | – | * | – | ||||||||
| S3 | 2.0 | * | 0.1 | * | * | 0.9 | 2.9 | 10.3 | |||||||||||||||||
| S4 | 1.3 | * | * | 9.7 | * | * | 1.0 | 13.2 | |||||||||||||||||
| S5 | * | 0.1 | * | 0.4 | * | – | * | – | * | – | 1.4 | 0.3 | 5.4 | 12.1 | * | – | |||||||||
| N | S2 | 1.8 | – | 0.5 | – | * | – | * | – | * | – | 1.3 | – | 26.9 | – | * | – | ||||||||
| S3 | 2.7 | * | * | 8.0 | * | 0.6 | 4.9 | 16.7 | |||||||||||||||||
| S4 | – | – | – | – | – | – | – | – | |||||||||||||||||
| S5 | 0.5 | – | * | – | * | – | * | – | * | – | 2.4 | 0.3 | 16.1 | 9.2 | * | – | |||||||||
| Month | Site | OA | PTX | YTX | SPX | GYM | PnTX | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | Dp | Ms | Am | ||
| F | S2 | * | – | * | – | * | – | * | – | * | – | * | – | ||||||
| S3 | 1.0 | 10.4 | 10.0 | 1.7 | 3.0 | 5.6 | |||||||||||||
| S4 | * | – | – | – | – | – | |||||||||||||
| S5 | 0.4 | – | 0.2 | – | * | – | 2.7 | – | 16.3 | – | * | – | |||||||
| M | S2 | 1.0 | – | 0.4 | – | * | – | 2.1 | – | 30.2 | – | * | – | ||||||
| S3 | 1.3 | 1.5 | * | 1.5 | 4.1 | 8.4 | |||||||||||||
| S4 | 0.9 | 2.1 | 6.2 | 0.5 | 1.9 | 2.6 | |||||||||||||
| S5 | 0.5 | – | 0.1 | – | * | – | 2.9 | – | 12.5 | – | * | – | |||||||
| A | S2 | 0.4 | – | 0.6 | – | * | – | 1.6 | – | 19.6 | – | * | – | ||||||
| S3 | 1.8 | 3.3 | * | 1.6 | 3.7 | 12.8 | |||||||||||||
| S4 | – | – | – | – | – | – | |||||||||||||
| S5 | * | – | * | – | * | – | 1.7 | – | 10.7 | – | * | – | |||||||
| M | S2 | 0.8 | 0.4 | * | 0.1 | * | – | 2.8 | 2.0 | 22.1 | 16.8 | * | – | ||||||
| S3 | 6.4 | 0.4 | * | 1.3 | 1.9 | 12.7 | |||||||||||||
| S4 | 3.8 | 0.6 | 10.2 | 0.7 | 2.4 | 8.0 | |||||||||||||
| S5 | 0.2 | 0.2 | * | 0.1 | * | – | 4.9 | 2.7 | 15.9 | 15.2 | * | – | |||||||
| J | S2 | 0.4 | – | * | – | * | – | 2.0 | – | 14.1 | – | – | – | ||||||
| S3 | – | – | – | – | – | – | |||||||||||||
| S4 | 1.8 | – | 7.2 | * | 0.6 | 8.0 | |||||||||||||
| S5 | * | – | * | – | * | – | 2.1 | 0.6 | 8.6 | 6.1 | * | – | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Valencia, I.; Hernández-Castro, J.E.; Band-Schmidt, C.J.; Turner, A.D.; O’Neill, A.; Núñez-Vázquez, E.J.; López-Cortés, D.J.; Bustillos-Guzmán, J.J.; Hernández-Sandoval, F.E. Lipophilic Toxins in Wild Bivalves from the Southern Gulf of California, Mexico. Mar. Drugs 2021, 19, 99. https://doi.org/10.3390/md19020099
Leyva-Valencia I, Hernández-Castro JE, Band-Schmidt CJ, Turner AD, O’Neill A, Núñez-Vázquez EJ, López-Cortés DJ, Bustillos-Guzmán JJ, Hernández-Sandoval FE. Lipophilic Toxins in Wild Bivalves from the Southern Gulf of California, Mexico. Marine Drugs. 2021; 19(2):99. https://doi.org/10.3390/md19020099
Chicago/Turabian StyleLeyva-Valencia, Ignacio, Jesús Ernestina Hernández-Castro, Christine J. Band-Schmidt, Andrew D. Turner, Alison O’Neill, Erick J. Núñez-Vázquez, David J. López-Cortés, José J. Bustillos-Guzmán, and Francisco E. Hernández-Sandoval. 2021. "Lipophilic Toxins in Wild Bivalves from the Southern Gulf of California, Mexico" Marine Drugs 19, no. 2: 99. https://doi.org/10.3390/md19020099
APA StyleLeyva-Valencia, I., Hernández-Castro, J. E., Band-Schmidt, C. J., Turner, A. D., O’Neill, A., Núñez-Vázquez, E. J., López-Cortés, D. J., Bustillos-Guzmán, J. J., & Hernández-Sandoval, F. E. (2021). Lipophilic Toxins in Wild Bivalves from the Southern Gulf of California, Mexico. Marine Drugs, 19(2), 99. https://doi.org/10.3390/md19020099