Abstract
Sustainable agricultural practices increasingly demand novel, environmentally friendly compounds which induce plant immunity against pathogens. Stimulating plant immunity using seaweed extracts is a highly viable strategy, as these formulations contain many bio-elicitors (phyco-elicitors) which can significantly boost natural plant immunity. Certain bioactive elicitors present in a multitude of extracts of seaweeds (both commercially available and bench-scale laboratory formulations) activate pathogen-associated molecular patterns (PAMPs) due to their structural similarity (i.e., analogous structure) with pathogen-derived molecules. This is achieved via the priming and/or elicitation of the defense responses of the induced systemic resistance (ISR) and systemic acquired resistance (SAR) pathways. Knowledge accumulated over the past few decades is reviewed here, aiming to explain why certain seaweed-derived bioactives have such tremendous potential to elicit plant defense responses with considerable economic significance, particularly with increasing biotic stress impacts due to climate change and the concomitant move to sustainable agriculture and away from synthetic chemistry and environmental damage. Various extracts of seaweeds display remarkably different modes of action(s) which can manipulate the plant defense responses when applied. This review focuses on both the similarities and differences amongst the modes of actions of several different seaweed extracts, as well as their individual components. Novel biotechnological approaches for the development of new commercial products for crop protection, in a sustainable manner, are also suggested.
1. Introduction
Changing environmental and climatic conditions have the potential to increase the susceptibility of crops to numerous pathogens, i.e., biotic stress [1,2]. The proliferation of plant diseases greatly reduces crop yield and quality. Many pathogens produce toxins and, in so doing contaminate the produce after harvest, which can cause severe economic loss to crop production worldwide [3,4]. Whilst not all of the foregoing problems occur with all crops, all at the same time, nor is their occurrence entirely predictable, it is current agricultural practice to take an “insurance approach”. This relies on the use (sometimes over-usage) of numerous synthetic chemical pesticides to control plant pathogens; however, the extensive input of pesticides is harmful to the farmers, consumers as well as ecosystems [5].
Through the advent of techniques in molecular biology, transgenic crops which carry various disease-resistance genes have been obtained from distant, usually wild, relatives. However, the successful implementation of the limited number of crops within which disease resistance has been achieved, through genetic modification/insertion (i.e., GMO crops) will require regulatory clearance from government authorities. In general, global public opinion is not favorable to this approach [6]. Alternatively, sustainable strategies of induced biotic resistance in important cultivated plants can also be achieved by the application of natural elicitors which can be derived from various seaweeds [7]. Over time, plants have developed an inherent immune system in order to fight pathogen aggression [6]. Plants respond to pathogen infestations either by Systemic Acquired Resistance (SAR) or Induced Systemic Resistance (ISR). SAR determines hypersensitive responses against pathogen infestations and is mediated by salicylic acid and pathogenesis-related (PR) proteins [8].
Plants activate their resistance mechanisms against pathogens by recognizing pathogen-associated molecular patterns (PAMPs) [9]. Various PAMPs can bind to the pattern recognition receptors on the plant cell membrane including flagellin (the major protein of the bacterial flagellum) and complex polysaccharides, such as chitin or different glucans. The binding of PAMPs to the appropriate pattern recognition receptor on the plant cell membrane leads to the activation of downstream signalling events, which, ultimately, will trigger a defense response [10]. Elicitors are those compounds recognized as PAMPS and these trigger the induction of the expression of genes involved in defense responses. During evolution, various seaweeds developed their own efficient defense mechanisms, hence there is a paucity of epidemics of infectious disease in natural seaweed populations [11,12]. Seaweeds are integral inhabitants of global coastal ecosystems and provide important ecosystem services which sustain the biodiversity of the in-shore marine environment [12]. From ancient times, seaweeds were used as natural soil conditioners [13,14]. In more recent decades, the uses of many seaweeds have been expanded immensely, and are evolving as a new trend, with increasing prospects their usage, i.e., food and bioactive compounds within functional foods and feed ingredients, phycocolloid production, plant biostimulants, biofuels and bioremediation [15,16,17]. Polyphenols, such as phlorotannins, and carbohydrates such as carrageenan, laminarins, fucoidans and ulvans, may act as elicitors which, when applied, can induce immunity against various plant pathogens [12,18,19,20]. The presence of these bioactive compounds has drawn the interest of various agrochemical companies for the production of commercial biostimulants using the biomass of a selected number of seaweeds [20,21,22].
The various modes of actions of seaweed-derived biostimulants largely depends on the raw materials and the applied methods of extraction [20]. In this review, we discuss similarities and differences among the various modes of actions of selected extracts derived from seaweeds.
2. Bioactive Compounds Present in Different Seaweeds and Their Mode of Action
Marine macroalgae are one of the most abundant sources of various bioactive compounds and have been widely used in agriculture to induce plant defense against various pathogens [23]. Table 1 lists the reports published on bioactive compounds from seaweeds and their role in eliciting responses from plant pathogens. The polymers highlighted are as reported in the literature. It may be that these are not always purified and may well represent polymer-rich fractions. There could well be cross-contamination with other co-extracted, seaweed-derived polymers. This is an avenue for future research that we suggest needs consolidated attention, whereby the bioactivities of purified fractions from complex seaweed extracts should be examined rigorously in order to determine modes of action and cause and effect responses.

Table 1.
Seaweed-derived elicitors (phyco-elicitors) and their roles in the defense mechanism of plants.
2.1. Alginates
Alginates are linear polysaccharides composed of alternating units of β-d-mannuronate and α-l-guluronate residues linked by 1,4 glycosidic linkages and are important constituents of the cell wall of marine brown algae [63]. Alginates are widely researched for their commercial uses in food products, agriculture, materials, medicine, and biological science [63,64]. The egg-box interaction of alginates with calcium ions is reported to increase their biological activity [65,66]. Alginates, as derived from seaweeds, are made up of various ratios of mannuronic (M) and guluronic (G) acids. These ratios differ (M:G) with the part of the algal thallus from where they are isolated and also determine the rheological properties of the commercial alginate extracts. However, the individual effects of either M and G or the M:G ratios to bioefficacy in plant responses are not known. Clearly, further work is called for. Alginates have been shown to exhibit bio-stimulatory activities and mitigate stress tolerance in plants [67,68,69,70]. They also act as PAMPs and their application induces the innate immune system of plants. Bouissil et al. [25] showed that sodium alginate, as isolated from Bifurcaria bifurcata and Fucus spiralis, induced natural defense responses in the roots of date palm (Phoenix dactylifera) by regulating PAL and polyphenol metabolism. The activity of PAL was significantly higher in roots of the date palm treated with alginate-based elicitors [25]. Dey et al. [24] showed that an application of sodium alginate to tomatoes reduced the progression of early blight disease, caused by Alternaria solani. The foliar application of alginates induced SAR by stimulating the accumulation of H2O2 in response to pathogen infection and by reducing the activity of catalase (CAT) involved in the scavenging of H2O2. In addition to this, the activity of superoxide dismutase (SOD) was found to be increased upon the application of sodium alginate in tomatoes [24]. In the same study, the transcript expression of the non-expressor of pathogenesis related protein 1 (NPR1), β-1,3-glucanase (PR2), lipoxygenase D (LOXD), and ACC oxidase 1 genes involved in the defense signalling pathways was found to be increased in the alginate-pretreated seedlings. Similarly, sodium alginate induced the biosynthesis of stilbenes and flavonoids, which are known to play major roles in defense responses, in the cell suspension of Vitis vinifera, and which also induced the expression of genes involved in their biosynthesis, including that of PAL, cinnamate 4-hydroxylase, and 4-coumarate: CoA ligase, stilbene synthase and chalcone synthase [71]. The alginate-treated V. vinifera cell suspension also showed a higher activity of pathogenesis-related proteins (PR), such as chitinase and β-1,3-glucanase [71]. Oligoguluronates obtained after the acid hydrolysis of sodium alginate isolated from Laminaria hyperborea elicited defense responses against pathogenic bacteria inhabiting the surface of the thallus of L. hyperborea and an epiphyte Laminariocolax tomentosoides [72]. These oligoguluronates induced oxidative stress in L. digitata and, in response, high levels of iodide were released to scavenge ROS [72]. Alginate oligosaccharides (AOS), prepared by the enzymatic treatment of the alginates isolated from brown algae, induced the biosynthesis of phytoalexins in response to the Magnaporthe grisea infection in rice [73]. A foliar spray of 1 mg/mL of AOS reduced the incidence of rice blast disease from 17.7 to 10.8% by regulating the activities of defense responsive enzymes such as peroxidase (POD), CAT and PAL. To decipher the molecular action of AOS in plant defense, Zhang et al. [27] used Arabidopsis thaliana to evaluate AOS-induced resistance to Pseudomonas syringae pv. tomato DC3000. A pre-treatment with 25 mg/L of AOS reduced the disease symptoms by 35.6%, as compared to control. The authors proposed that the phenotype was due to the early induction of signalling molecules, such as ROS and nitric oxide (NO) in AOS-treated leaves [27]. The elicitation properties of AOS in inducing resistance was lessened in the sid2 mutant plants impaired in the SA biosynthetic pathway. In addition to this, the transcript of SA-dependent PR1 was found to be significantly higher in AOS-treated plants [27]. These results suggest that AOS-pre-treatment elicited resistance in A. thaliana by activating the SA-dependent defense-signalling pathway and stimulated natural, endogenous systems by regulating defense-responsive signalling pathways.
2.2. Carrageenans
Carrageenans are linear, partially hydrophilic sulphated polygalactans composed mainly of alternating units of d-galactose and 3,6-anhydro-galactose, linked by α-1,3 and β-1,4-glycosidic linkages [12,74]. Based on the degree and position of the sulphate groups, carrageenans are classified into six main types: iota (ι)-, kappa (κ)-, lambda (λ)-, mu (μ)-, nu (ν)- and theta (θ)-carrageenans [12,75]. Kappa (κ)-carrageenans are mainly isolated from the red alga Kappaphycus alvarezii through the hot extraction process and consist of d-galactose sulphated at the C4 position, linked to anhydro-galactose [76]. Structurally, beta (β)-carrageenans are identical to the kappa (κ)-carrageenans but lack sulphate on the C4 of the 1,3-linked units [12]. λ-carrageenans are more hydrophilic than κ-carrageenans and consist of d-galactose, having the sulphate group at the C2 position linked to a d-galactose sulphated at the C2 and C6 positions. λ-carrageenans are extracted from red algae such as Gigartina and Chondrus using an alcohol precipitation process [12]. ι-carrageenans are composed of d-galactose having the sulphate group at the C4 position, linked to an anhydro galactose sulphated at the C2 positions, and are commercially extracted from the red alga Eucheuma denticulatum [76]. Variation in sulphate content (i.e., 20% κ-carrageenan, 33% ι-carrageenan, 41% λ-carrageenan) leads to differential biological activities. Carrageenans are widely used in a large number of commercial applications in food and dairy industries, drug delivery, and the pharmaceutical industry, including antiviral, antitumor, immunomodulatory, antihyperlipidemic and anticoagulant properties [12,75,77,78,79]. Carrageenans and their pre-cursor of hydrolytic cleavage products, the oligo-carrageenans (OCs), also elicit natural plant defense responses against pests and pathogens by modulating the activity of different defense pathways, including salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) signalling pathways [12]. The level of sulphation of the types of carrageenans is suggested to influence their specific activities and, therefore, their targeted applications for plant defenses [12]. It should be stated that further research is required in order to determine their absolute specificities. Clearly this work is of commercial importance and should be viewed as urgently required.
Diseases caused by viroids cause important commercial losses in agriculture [12,80]. Viroids replicate in the nucleus or chloroplast and spread by moving from cell to cell via plasmodesmata. Sangha et al. [40] showed that λ-carrageenan changed the biochemical status of tomato plants, inducing the defense mechanisms against Tomato Chlorotic Dwarf Viroid (TCDVd) and thereby controlling viroid replication. λ-carrageenan-treated plants showed changes in the regulation of several genes, including up-regulation of genes such as lipoxygenase (LOX), allene oxide synthase and PR1, suggesting a JA-mediated response in treated plants against TCDVd [40].
Carrageenans are reported to protect plants and animals from viral infections by limiting their binding to receptors and internalization of viral particles into the host cells [76,81,82,83]. Sulphated polysaccharide 4 (SPS4) extracted from the red alga Hypnea musciformis contains 98% κ-carrageenan [47]. The infiltration of tobacco leaves with 200 μM SPS4 significantly reduced the number of lesions caused by tobacco mosaic virus (TMV) infection by inducing the accumulation of secondary metabolites, such as sesquiterpenoid and scopoletin. SPS4 activated plant defense mechanisms against viral infection by controlling the expression of the genes involved in SA- and JA/ET-dependent signalling pathways. Gene expression analysis showed that PR1a, PR2, PR5, PR3 and Def1.2 (defensin protein) were upregulated in leaves infiltrated with SPS4, in response to TMV infection [47]. Nagorskaya et al. [84,85] reported on the antiviral activity of κ/β-carrageenan isolated from the red alga Tichocarpus crinitus against TMV and potato virus X particles. The detached leaf assay showed that κ/β-carrageenan from T. crinitus induced lytic processes in Datura stramonium by controlling the intracellular accumulation and translocation of potato virus X particles [84]. A foliar spray of κ-, λ-, or ι-oligo-carrageenans, at the concentrations of 0.5, 1 or 5 mg/mL, induced defense responses in tobacco plants, resulting in enhanced protection against TMV, Pectobacterium carotovorum and Botrytis cinerea by inducing PAL biosynthesis—an important enzyme involved in the regulation of secondary metabolism and defense-responsive signalling pathways [48]. This study showed that OCs induced a long-term protection against TMV, which was dependent on dose, time, and number of treatments, mimicking a vaccine-like action, particularly when λ-carrageenan was used.
Carrageenans and oligo-carrageenans can also significantly reduce the progression of fungal and bacterial diseases [12]. κ-OCs prepared by the enzymatic hydrolysis of κ-carrageenan were found to elicit the activity of laminarinase involved in plant defense in the cells of Rubus fruticosus [86]. Mani and Nagarathnam [44] showed that κ-carrageenan isolated from K. alvarezii had antifungal properties against Colletotrichum gloeosporioides which causes anthracnose disease in Capsicum annuum. An in vitro assay revealed that κ-carrageenan inhibited the mycelial growth of C. gloeosporioides by increasing its plasma membrane permeability. A foliar spray of C. annuum with κ-carrageenan elicited defense responses by regulating the enzymatic activity of POD and the expression of SA-/JA-dependent genes. Detailed proteomic analyses revealed the induction of proteins involved in nitric oxide synthesis, pathogenesis-related protein production and phytoalexin synthesis, whilst gene expression analyses indicated that cyclin-dependent protein kinase (CDPK), PR1 and NHO1 were also up-regulated [46]. Amongst other carrageenans, λ-carrageenan was found to be the most potent elicitor because of its high sulphur content, inducing systemic resistance against P. parasitica var. nicotianae in tobacco cells [44]. The induced resistance found in the tobacco cells was due to the higher expression of sesquiterpene cyclase, involved in the synthesis of the phytoalexin capsidiol, a functional chitinase coded by PR3 genes. In addition to this, the cellular SA, and the transcripts of lipoxygenase (LOX) and ACC oxidase (ACO), involved in JA- and ET-biosynthesis, were also found to be upregulated in λ-carrageenan-treated cells [44]. Pettongkhao et al. [43] showed that the leaves of the rubber tree (Hevea brasiliensis) sprayed with 0.5 mg/mL of λ-carrageenan, as isolated from the red alga Acanthophora spicifera stimulated immunity against Phytophthora palmivora by inducing the expression of SA-dependent defense responsive genes, which was further substantiated by the higher accumulation of SA and scopoletin. The activity of catalase, involved in ROS-scavenging, was suppressed in λ-carrageenan-treated rubber tree leaves, whilst POD activity was induced in treated leaves. The plausible explanation to these expression patterns is that the higher SA content in treated rubber leaves might have inhibited catalase activity [87], while the induction of peroxidase stimulated plant defense by regulating the process of lignification [88,89]. Le Mire et al. [45] showed that a foliar spray of λ-carrageenan reduced the progression of Septoria tritici blotch (STB) disease in wheat, caused by the fungal pathogen Zymoseptoria tritici, by inducing both SA- and JA-dependent signalling pathways. In another report, λ-carrageenan was reported to elicit a JA-dependent defense response in A. thaliana against Sclerotinia sclerotiorum by inducing the expression of the JA-induced, defense-related genes, such as AOS, PDF1.2 and PR3 [42]. S. sclerotiorum infected the plant by producing oxalic acid, which reduced local defense responses by the treated plants against the pathogen. Sangha et al. [42] reported that λ-carrageenan suppressed the S. sclerotiorum-mediated accumulation of oxalic acid by inducing in planta oxalate oxidase activity. The complementation assay using an A. thaliana mutant showed that the defense-eliciting activity of λ-carrageenan was observed in the salicylic acid-deficient mutant ics1 but did not rescue the susceptibility of jar1 plants from S. sclerotiorum infection. However, ι-carrageenan-treated plants showed an increased susceptibility towards S. sclerotiorum infection [42].
Taken together, these results indicate that carrageenans and their oligomers, as derived from a variety of red seaweeds, are a very important source of bioactive compounds, eliciting the natural defense system and conferring resistance against a wide range of broad-spectrum pathogens in treated plants.
2.3. Laminarins
Laminarins (alt. spelling laminarans) are important constituents of the cell walls of brown seaweeds and provide flexibility to those algal thalli to withstand the pressure exerted by hydrodynamic forces [90,91,92,93]. Laminarins are generally low molecular weight (e.g., 5 kDa) storage β-glucans comprising (1,3)-β-d-glucans, having (1,3)-β-d-glucopyranose residues with some 6-O-branching in the main chain and some β-(1,6)-intrachain links [90]. Various published reports have found laminarins to possess biostimulant properties, and they are used extensively as immunostimulants, antitumour agents, anticoagulants, and as wound-healing agents in pharmaceutical, and cosmetic industries [92,93]. Laminarins also possess antioxidant and antimicrobial activities [90,94,95]. Currently, Iodus and Vacciplant (Arysta LifeScience, Cary, NC, USA) are products derived from laminarins that have been commercialized in various countries in order to control powdery mildew in strawberry and cereals, bacterial fire blight on apple trees and the grey mould in grapevine [39,96,97]. Foliar sprays of laminarins extracted from Laminaria digitata reduced the dependence of fungicides for the control of grey mould and powdery mildew in strawberry [29]. Similarly, Pugliese et al. [34] showed that the foliar application of laminarins reduced the natural incidence of powdery mildew in strawberry caused by Erysiphe necator. Laminarins extracted from L. digitata elicited the defense response in tobacco cells in a dose-dependent manner [31]. A treatment with 200 μg mL−1 laminarin determined a strong alkalinization of the extracellular medium followed by NADPH oxidase-dependent release of H2O2 triggering the subsequent induction of defense-related enzymes such as PAL, caffeic acid O-methyltransferase (CaOMT), and lipoxygenase. These enzymes are involved in phenylpropanoid, lignin and fatty acid biosynthesis, suggesting that laminarins mobilize the metabolic machinery of tobacco cells to elicit defense responses. The infiltration of laminarin restricted the progression of soft-rot disease caused by P. carotovorum in tobacco plants by inducing plant natural defense mechanisms [31]. Similarly, Aziz et al. [30] showed the elicitation of the early signalling events by laminarins in grape cells. In addition to the increased alkalization and higher release of H2O2 in the growth medium, laminarins also induced Ca2+ influx in grapevine cells. Cells grown in the medium supplemented with laminarins showed the activation of two mitogen-activated protein kinases, of defense-related genes and enzymes such as chitinase and β-1,3-glucanases, and increased biosynthesis of phytoalexins [30]. The foliar application of laminarins to the grapevine plants, conferred resistance against B. cinerea and Plasmopara viticola. However, laminarins did not induce hypersensitive response or cell death, but elicited the plants’ own innate immune system in both grapevine and tobacco [30,31]. Xin et al. [36] showed that laminarins from L. digitata protected tea plants (Camellia sinensis) against the tea green leafhopper (TLH), Empoasca (Matsumurasca) onukii. Laminarins were found to trigger immediate defense responses in plants by activating early defense-response genes, such as MAPKs and WRKY, and elevate the biosynthesis of SA and ABA, whilst also inducing oxidative bursts [36]. The induction of the MAPKs and WRKYs by laminarins regulates the activation of transcription factors, which subsequently modulate the activation of defense-responsive genes, such as NPR1, PDF1.2 [36]. The leaves of the tea plants sprayed with laminarins showed a higher activity of PAL, polyphenol peroxidase (PPO), chitinase, flavonol synthase and increased callose deposition. In addition to this, laminarin treatment increased tea volatile emissions involved in defense response. All these metabolic and defense responses helped laminarin-treated plants to reduce the TLH infestations [36]. All these results together suggest that laminarins from various seaweed extracts have the potential to induce early defense responses against different plant pathogens, including bacteria, fungi, and insects (Figure 1).
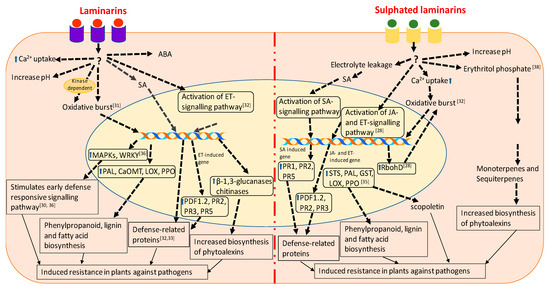
Figure 1.
Schematic representation of the cellular functions and signalling pathways involved in defense mechanisms elicited in laminarin and sulphated laminarin. The drawing represents a synthesis of the work described by [28,31,32,33,34,35,38].
Menard et al. [32] showed that the sulphated form of β-1,3 glucan, β-1,3 glucan sulphate, is a more effective elicitor of plant defense responses than β-1,3 glucan. The comparative structural analyses of laminarins and sulphated laminarin (PS3) revealed that chain length was very important for the biological activity of both the compounds, and that the sulphation of the laminarin increased its bioactivity, which cannot be achieved by using other anionic groups [32]. In tobacco cells, the oxidative bursts induced by PS3 were Ca2+ dependent, but partially kinase independent. In contrast, laminarin induced oxidative bursts in a kinase-dependent manner only. The treatment of tobacco cells with PS3 determined electrolyte leakage, which caused the accumulation of SA and scopoletin, whereas such responses were not observed in the presence of laminarins [32,96]. Complementation assays in A. thaliana revealed that PS3 elicited the expression of ethylene- and SA-dependent PR proteins, while laminarins from L. digitata induced the expression of ethylene-dependent PR proteins only [32]. Laminarins are considered as a standard substrate for β-1,3 glucanases, whereas its chemical sulphation makes it resistant to degradation by β-1,3 glucanases [32]. The infiltration of PS3 into the leaves of transgenic PR1–β-glucuronidase (GUS) tobacco plants induced the expression of GUS, providing further evidence for the activation of the SA-dependent signalling pathway [33]. PS3 treatment of tobacco cells did not induce the expression of acidic PR1 proteins, which is considered a clear sign of SAR activation in plant cells [33,98]. The infiltration of tobacco leaves with 200 μg mL−1 of PS3 produced a 100% reduction in lesions upon TMV infection, while the same concentration of laminarins caused only a 60% reduction in lesions [33]. These results suggested that PS3 had stronger antiviral effects, as compared to laminarins, while the synergistic application of PS3 and laminarins together induced stronger oxidative bursts, as compared to laminarin and PS3 alone, but no synergy was observed in the expression of PR proteins [32,33]. These results provide an insight that the combination of the various forms of the bioelicitors can be further used for the development of potential novel biostimulant for plant disease management.
Unlike laminarins, PS3 did not elicit the early defense response, but induced resistance by enhancing prolonged plasma membrane depolarization in grapevine [28]. Microarray analysis revealed that PS3 induced 132 genes in grapevine, while laminarins from L. digitata induced only 94 genes. Interestingly, 94% of the induced genes were expressed in both the PS3 and laminarin treatments. Among the 33 genes expressed specifically in the PS3-primed grapevine leaves, most of the genes were involved in signalling pathways, such as phospholipase C, calmodulin, CBL-interacting protein kinase (CIPK 14), serine/threonine-protein kinase (AFC2) and the transcription regulators NAC78, AP2/ERF, jumonji, squamosa-binding protein and ankyrin repeat 2 (AKR2). PS3- and laminarin-treated plants showed the expression of the genes that are common in the SA- and JA- dependent pathways [28]. However, Trouvelot et al. [35] demonstrated that a foliar spray of PS3 induced a JA-dependent defense response in Vitis vinifera cv. Marselan against downy mildew caused by P. viticola. In addition to this, PS3 elicited NADPH oxidase-dependent oxidative burst and the expression of stilbene synthase (STS), PAL, LOX, glutathione-S-transferase (GST), protease inhibitor (PIN), and basic class I chitinase (CHIT1b). The treatment of PS3-primed leaves with 2-deoxy-d-glucose (i.e., an inhibitor of callose synthase) and 5, 8, 11, 14-eicosatetraynoic acid (i.e., an inhibitor of 9-lipoxygenase) reverted the PS3-induced resistance. This suggested that PS3 elicited the defense response by callose deposition and induced hypersensitive responses, such as cell death [35]. These results were further confirmed by Gauthier et al. [28] who demonstrated that PS3-priming induced the expression of the respiratory burst homolog gene (RbohD) which is involved in oxidative bursts and also HSR203J which is involved in HR responses in those grapevines leaves infected with P. viticola [28]. Adrian et al. [38] studied the effects of PS3 on the global metabolism of grapevine leaves infected with P. viticola. Erythritol phosphate, which is involved in the production of monoterpenes and sesquiterpenes, was found to be significantly induced in the leaves of grapevines treated with PS3, in response to P. viticola infection [38]. Figure 1 represents comparative molecular mechanisms involved in the induction of plant immunity by laminarins and sulphated laminarins. These results suggest that the laminarins, laminarin-derived products, and particularly sulphated laminarins, induced defense responses in treated plants by triggering various metabolic, physiological, and biochemical processes.
2.4. Ulvans
Ulvans are sulphated polysaccharides, extracted from the cell wall of green seaweeds, in particular from various species of the genus Ulva, which generally accounts for 9–36% of their dry biomass [99,100,101]. Ulvans are extracted from the algal biomass either by acid or enzyme-based extraction procedures, and are mainly composed of sulphated rhamnose, uronic acids (glucuronic acid and iduronic acid) and xylose [100,102,103]. The structural backbone of ulvans consists of monosaccharides (e.g., rhamnose, xylose, glucuronic acid and iduronic acid) joined by α- and β-(1,4)-linkages with characteristic repeating disaccharide units (i.e., ulvano-biuronic acid, ulvano-bioses) [100,101]. Commercially, ulvans have been widely used as ingredients in food, pharmaceuticals, and biomedical applications. They possess immune-modulatory, anti-inflammatory, antioxidant, antibacterial, antiviral, anticoagulant, and antihyperlipidemic activities [100,101,104]. In recent years, ulvans have been used widely for the modulation of active defense mechanisms in plants against a broad range of pathogens [49,96,105]. Foliar spray applications of ulvans from Ulva fasciata on the leaves of bean plants significantly reduced the colonisation of epicotyl xylem vessels by Fusarium oxysporum and thereby reduced the development of Fusarium wilt [51]. Similarly, in apple leaves, the spraying of ulvans from U. fasciata was found to reduce the severity of Glomerella leaf spot (caused by C. gloeosporioides) by 50%, by inhibiting the formation of appressoria [105]. Likewise, ulvans from U. lactuca and oligo-ulvans, prepared by enzymatic degradation, triggered a rapid and transient oxidative burst, and elicited the antioxidant activity of related defense-responsive enzymes, thereby inducing resistance in the apple fruits against Penicillium expansum and B. cinerea infections [106]. A commercial biostimulant based on ulvans (from hydrolysis of Ulva armoricana) (Elicityl Ltd., Crolles, France) was found to confer resistance against C. gloeosporioides, causing anthracnose disease in papaya. The induced resistance was attributed to an increased activity of defense-related enzymes involved in antioxidant metabolism [55]. Delgado et al. [54] demonstrated that the foliar application of ulvans induced resistance in bean plants against rust, caused by Uromyces appendiculatus and angular leaf spot, caused by Pseudocercospora griseola.
Unlike laminarins, ulvans induced resistance against Alternaria brassicicola and Colletotrichum higginsianum, independent of their degree of sulphation [58]. Briand et al. [105] showed that a foliar spray of ulvans, prepared from U. armoricana, induced the expression of genes involved mainly in the defense response in Medicago truncatula. These applications conferred significant protection for M. truncatula, pea, and pepper against C. trifolii, Mycosphaerella pinodes and Phytophthora capsicum, respectively [105]. Similarly, a foliar spray of ulvans on the leaves of the common bean (Phaseolus vulgaris) conferred resistance against anthracnose disease, caused by Colletotrichum lindemuthianum by inducing the activity of defense-related enzymes [57]. In a different study, Ben Salah et al. [47] showed that a twig of olive (Olea europaea) dipped in a solution of ulvans (2 g/L) stimulated phenolic metabolism, and thereby increased its resistance to the Verticillium wilt of olive (VWO), caused by V. dahliae. The priming of rice and barley by ulvans elicited defense responses in the whole plants and reduced the incidence of powdery mildew caused by Blumeria graminis, by 45% in wheat and by 80% in barley, respectively [52]. It was found that wheat and rice cells primed with ulvans did not induce oxidative bursts independently, but rather induced oxidative bursts in the cells which already had been primed with chitin and chitosan [52]. de Freitas and Stadnik [58] showed that a foliar spray application of ulvans from U. fasciata reduced the severity of colonisation by A. brassicicola of wild-type (WT) A. thaliana, but did not protect AtrbohD plants impaired in NADPH oxidase, which is required for the production of ROS during oxidative burst. Furthermore, ulvan priming reduced electrolyte loss by 130% in WT, but failed to control it in the AtrbohD mutant. The infiltration of diphenyleneiodonium into the leaves of WT and AtrbohD impaired NADPH oxidase activity and hydrogen peroxide accumulation, thus reverting the ulvan-induced resistance in A. thaliana against A. brassicicola [58]. Taken together, ulvans induced disease resistance in A. thaliana by stimulating NADPH oxidase-dependent ROS accumulation but did not activate the hypersensitive response. Ulvans failed to induce the defense response in AtrbohD, as the treatment lacked the respiratory burst oxidase homologues, required for triggering of cell-to-cell signalling cascades which result in ROS production [58]. Ulvans and methyl jasmonate (MeJA) were found to induce similar types of responses in M. truncatula [54]. Microarray analysis revealed a 40% identical expression in the plants treated with MeJA and ulvans, and these genes were related to JA-dependent defense responses, including lipoxygenase, hydroxyproline-rich glycoproteins, proline-rich proteins, cysteine-rich antifungal proteins (i.e., defensin) and wound-induced proteins [54]. Treatments with ulvans modulated the expression of genes involved in phytohormone metabolism. Gibberellic Acid Insensitive (GAI), Repressor of GAI (RAG) and Scarecrow (SCR) (GRAS) transcription factors (TF) are expressed in response to gibberellins and bacterial and fungal elicitors. Ulvans induced the expression of GRAS TFs, suggesting that ulvan treatments were able to regulate the crosstalk occurring between elicitors and gibberellin responses [54,107,108]. Jaulneau et al. [54] demonstrated that a foliar spray of ulvans on the leaves of A. thaliana induced the expression of PDF1.2, which was significantly reduced in ulvan-sprayed jasmonic acid resistant, jar1.1 and the abscisic acid deficient, aba3.1 mutants. On the contrary, the SA-dependent genes PR1a and PR5 were not induced by the treatment of ulvans extracted from U. armoricana, suggesting that the activated JA-dependent defense-responsive pathway acted synergistically with the ABA pathway. Collectively, these results suggested that, in general, ulvans induced resistance in a JA-dependent fashion, thereby regulating cross-talk amongst various defense-responsive processes.
In addition to these bioactive polysaccharides, oligofucans isolated from Pelvetia canaliculata elicited a defense response in tobacco suspension culture by inducing alkalization and oxidative bursts in treated cells [59]. The tobacco cells treated with oligofucans exhibited the strong induction of PAL, LOX, PR proteins, and phytoalexin [59]. The infiltration of oligofucans in the leaves of tobacco, systemically induce the SA-dependent defense response against TMV [59]. The foliar spray of tomato seedlings with oligoulvans and oligoglucuronans from U. lactuca showed a strong induction of PAL activity, followed by the accumulation of SA and phenolic compounds [60]. The oligoulvan- and oligoglucuronan-treated seedlings showed reduced susceptibility towards wilt disease caused by F. oxysporum f. sp. lycopersici.
2.5. Phenolics
Polyphenols represent a class of secondary metabolites that are widely distributed in various seaweeds [109]. Polyphenolic compounds derived from marine sources are widely used as antibacterial and antiviral agents in pharmaceuticals [110,111,112]. Phlorotannins are unique phenols (tannins) found in brown algae. Structurally, phlorotannins consist of monomers of phloroglucinol (1,3,5-trihydroxybenzene) joined by either ether (C−O) or aryl−aryl (C−C) linkages [112,113]. These unique polyphenols are known to play the role of inducing chemical defenses against herbivory [114]. The bioactive phenolic compounds extracted from Sargassum muticum and Ascophyllum nodosum posses radical scavenging activities [115,116]. Jormalaninen et al. [117] showed that the accumulation of phlorotannins was induced in Fucus vesiculosus in the presence of two species of snails, i.e., Theodoxus fluviatilis and Physa fontinalis, which are known to feed on brown algae. Arnold et al. [118], proposed that phlorotannins were synthesized as reactive, polyphenolic secondary metabolites in specialised cells called physodes (i.e., intracellular vesicular inclusions) of brown algae, and then converted to unreactive forms as cell wall components. Thus, an increased accumulation rate of phlorotannins, upon attack by herbivores, is not necessarily the result of chemical defense mechanisms; they are likely required for the reconstruction of the damaged cell walls. Polyphenolics and phlorotannins extracted from F. vesiculosus by ultrasound-assisted extraction exhibited antioxidant properties [119]. Amongst the brown algae, A. nodosum is a rich source of phlorotannins, and an extraction of these was found to reduce the occurrence of food-borne pathogens in pigs, without impacting the normal physiology of the intestine [112,120]. Eckol, a phlorotannin obtained from Ecklonia maxima, was shown to stimulate plant growth and seed germination [121]. Foliar applications of eckol induced myrosinase activity in treated cabbage and stimulated natural defense responses related to aphid infestation by regulating the glucosinolate-myrosinase system [62]. The use of phenolics as constituents of biostimulant formulations for plant disease management needs to be further explored in much more detail. Taking into consideration their antioxidant and antiviral properties, demonstrated in both plants and in other experimental models (i.e., animals) [112,119], these bioactive compounds derived from seaweeds are likely to be an important resource for improving natural defense mechanisms in these forms of life, leading to the development of novel commercial products of benefit to plants, animals, and microbes.
3. Seaweeds: Sources of Extracts Used as Biostimulants
The presence of bioactive compounds has fuelled the increasing interest in utilising the seaweeds as a sustainable raw material [119]. The biostimulants prepared from the various seaweeds elicits plant defense by following a unique mode of action (Table 2).

Table 2.
Roles of different extracts from various seaweeds in inducing disease resistance in different plants.
3.1. Ascophyllum Nodosum
The phaeophycean rockweed, Ascophyllum nodosum, is a widely distributed intertidal species that can form dense, mono-specific, sub-marine forests around the periphery of the North Atlantic Ocean [91]. Rockweed is commonly found on the north-western coast of Europe, east Greenland, and the North East coast of North America. Similar to other intertidal species, A. nodosum is able to survive extreme climatic challenges [13], and the brown alga is a rich source of secondary metabolites [122,123]. A. nodosum is also a significant source of sterols, phlorotannins, fucoidans, ascophyllan, mannitol, alginic acid and laminarin [20,123,124,125,126,127,128]. These unique characteristics make A. nodosum one of the most important sources of raw materials for the commercial production of bioactive compounds [13,20,129]. The elicitors present in the different extracts and formulations were shown to reduce disease severity and incidence, i.e., ameliorate biotic stresses in plants such as tomato, strawberry, carrot, cucumber and the model plant A. thaliana (Table 2) [20,130,131,132,133,134].
A liquid extract prepared from A. nodosum was found to elicit D-glycanases activities [135]. Betaines present in an alkaline extract of A. nodosum reduced the replication of root-knot nematode, Meloidogyne javanica, in mono-xenic cultures of A. thaliana [136]. Alternate applications of Stimplex® (Acadian Seaplants Ltd., Dartmouth, NS, Canada), an Environmental Protection Agency (EPA)-registered pesticide, in combination with the fungicide chlorothalonil (2 g L−1) reduced the progression of Alternaria cucumerina, B. cinerea, Didymella applanata and F. oxysporum in cucumber plants grown under greenhouse conditions [137]. Similarly, the integrated use of an alkaline extract prepared from A. nodosum (ANE) with fungicides (i.e., chlorothalonil and cupraneb) reduced the severity of an infection caused by A. solani and Xanthomonas campestris pv. vesicatoria in tomato and sweet pepper, under greenhouse and field conditions [130,138]. The increased induced resistance to the pathogens observed was likely due to the enhanced activity of PAL, PPO, peroxidase (PO), and β-1,3 glucanases [130].
Tomato and sweet pepper plants treated with ANE showed a significantly higher transcript expression of PIN II (proteinase inhibitor) and ETR-1 (ethylene receptor) genes, which are involved in JA and ET-mediated defense signalling pathways. Likewise, no significant changes were observed in the expression of the PR-1a in both crops [138]. In addition to the defense-responsive genes, ANE-treated tomato and sweet pepper plants showed a higher expression of isopentenyltransferases (IPT), Indole acetic acid (IAA) and Ga2Ox, genes that are known to be involved in hormonal biosynthesis [138]. Taken together, these results suggest that the A. nodosum extract induced innate immunity by regulating crosstalk between defense-responsive signalling pathways and hormonal biosynthesis.
A foliar spray of 0.2% ANE improved resistance against Podosphaera aphanis, which is the causative agent of powdery mildew in strawberries by enhancing the biosynthesis of secondary metabolites and defense-related enzymes [131]. Similarly, a foliar spray of 0.2% of Stimplex® controlled the progression of fungal pathogens, i.e., Alternaria radicina and B. cinerea in carrot by inducing the activity of several defense-related enzymes, such as PO, PPO, PAL, chitinase and β-1,3-glucanase [133]. The treated carrot plants showed a higher expression of PR-1, chitinase, lipid transfer protein (LTP), PAL, CHS, NPR-1 and PR-5 in response to the pathogen [133].
Marmarine® (International Ferti Techology Corporation, Amman, Jordan), induced defense responses against Phytophthora melonis in cucumber [139]. The combination of foliar and root drench of Marmarine® was found more effective in inducing disease resistance, as compared to foliar and root drench alone. Marmarine® induced systemic resistance in cucumber by increasing the expression of cucumber pathogen-induced 4 (Cupi4), lipoxygenase (LOX), PAL, and galactinol synthase (GolS) genes involved in defense responses [139].
A. nodosum extracts prepared by Acadian Seaplants Limited, induced systemic defense responses against Pseudomonas syringae and S. sclerotiorum by inducing the expression of the genes involved in the JA-dependent pathway [145]. The pre-treatment of Stella Maris® (Acadian Seaplants), a commercial extract prepared from A. nodosum, inhibited the growth of several bacterial pathogens in A. thaliana by inducing a strong oxidative burst of reactive oxygen species (ROS) [132]. In addition to this, Stella Maris®, also induced the expression of cytochrome P450 family polypeptide (CYP71A12), an antimicrobial phytoalexin known to damage the cell wall and disrupt the metabolism of bacterial pathogens [132]. The expression of other defense-related genes, such as WRKY30 and PR1, was also find higher in A. thaliana in response to a P. syringae infection [132].
The combination of liquid seaweed extract prepared from A. nodosum (Acadian Seaplants) and chitosan reduces the severity of Fusarium-head-blight (FHB) caused by Fusarium graminearum by eliciting the expression of pathogenesis-related genes (TaPR1.1, TaPR2, TaPR3, TaGlu2) and defense-related enzymes [150]. F. graminearum infection results in the loss of yield and accumulation of mycotoxin in the infected grain, causing reduced quality of grains for animal and human consumption [171]. The levels of mycotoxins, deoxynivalenol and sambucinol, were found to be lower in those wheat grains harvested from the plants treated with a combination of liquid seaweed extract and chitosan [150]. Similarly, the combination of liquid seaweed extract prepared from A. nodosum and chitosan inhibited the growth of Erisyphe pisi causing powdery mildew in pea [172]. Seasol Commercial® (Seasol International, Bayswater, Australia), is an alkaline hydrolysis product prepared from Duvillaea potatorum and A. nodosum and was found to reduce the progression of Plasmodiophora brassicae, causing club-root in broccoli [144]. The treatment of the broccoli with the seaweed extract reduced the number of the plasmodia formed in the roots [144]. Islam et al. [134] studied the protective effects of Seasol®, ANE and an alkaline extract of Duvillaea potatorum on A. thaliana, before and after inoculation with the root pathogen Phytophthora cinnamomi, using a transcriptomics approach. Global transcriptomics analysis revealed that Seasol Commercial® reduced the progression of P. cinnamomi by regulating proteolytic pathways, respiratory burst, and various defense-related responses. Overall, each seaweed extract acted differently, but all were successful in inducing plant defense-related genes [134]. The root drench treatment of 0.5% Dalgin® (Sustainable Agro Solutions, Lleida, Spain), is another commercial extract prepared from A. nodosum that can significantly reduce the severity of a P. capsici infection of tomatoes by inducing the expression of several defense-related genes and also the activity of many oxidative enzymes [142]. Another report published by Somai-Jemmali et al. [141] showed that Dalgin Active®, consisting of 22.6% A. nodosum extract, 0.135 % vitamins, 1.43% nitrogen and 6.78% free amino acid, induced defense responses in both bread and durum wheat against the fungal pathogen Z. tritici. A foliar spray of this extract induced the plant defense response by eliciting expression of multiple genes involved in defense responses, antioxidant metabolism and both the phenylpropanoid and octadecanoid pathways [141].
The extensive cultivation of the seaweed Kappaphycus alvarezii (Rhodophyta), a commercially important carrageenophyte, is hampered by infestations by the endo-epiphytic red alga Neosiphonia apiculata [147]. An extract with the abbreviated name AMPEP (Ascophyllum Marine Plant Extract Powder) [173,174], prepared from the soluble powder extract of A. nodosum, exhibited a “vaccine-like” effect on K. alvarezii, as tested in Brazil, and induced the natural defense in this alga by reducing the effects of surface level oxidative bursts. AMPEP also increased the daily growth rate and carrageenan yield and quality of the treated crops [147]. AMPEP, as a biostimulant solution for promoting the growth of K. alvarezii propagules was found to reduce the percentage occurrence of Neosiphonia [148], and improved carrageenan quality [149] in studies conducted in SE Asia.
3.2. Ecklonia Maxima
Ecklonia maxima, commonly known as kelp or “sea bamboo”, is a brown seaweed (Phaeophyceae) distributed mainly in the southern hemisphere, on the southern Atlantic coast of Africa [175]. E. maxima is known to be a rich source of polyamines, phlorotannins, and of ACC (1-amino-cyclopropane-1-carboxylic acid) [176,177,178]. The biomass of this brown seaweed has been largely used as an integral constituent of animal feed, nutritional supplements, and soil conditioner [177]. Kelpak® is an extract of E. maxima (Kelp Products International, Simon’s Town, South Africa) which is prepared by using a cold, cell-burst technique [179]. Kelpak® contains various growth-promoting compounds and has been reported to improve plant productivity under stress conditions [176,180,181,182]. An application of the liquid extract reduced occurrence of Verticillium wilt in green pepper caused by Verticillium dahliae [151]. A soil drench treatment of tomato with Kelpak® significantly reduced the infestation of the root-knot-causing nematode Meloidogyne incognita, whilst foliar applications of Kelpak® were not found to be effective in controlling M. incognita infection [152]. The commercial extract prepared from E. maxima adversely affected the hatching and sensory perception of root-nematodes in vitro. This suggested beneficial applications in controlling the infection of tomato roots by M. chitwoodi and M. hapla [153].
3.3. Sargassum spp.
Sargassum is one of the largest genera of brown algae. Due to its abundance, it is an integral part of several marine ecosystems; however, some species are invasive in nature [183]. For example, Sargassum muticum, commonly known as “Japanese wireweed”, a native to Japan, has invaded wide areas of the Atlantic coasts of Europe since its introduction in the region, and now it is one of the most abundant Sargassaceae species used on the European markets [184]. S. muticum offer an auspicious source of bioactive compounds as they develop chemical defense mechanisms which helps the invasive seaweed to establish in new, highly dispersed geographical environments [184]. A laboratory-scale, aqueous extract prepared from S. muticum represents a natural source of bioactive molecules, such as phlorotannins, laminarins, alginic acid, phenolic compounds, antioxidants, carotenoids, and anticancer compounds such as fucoxanthin [185]. Sargassum fusiforme¸ also known as jade grass, is a perennial, warm-temperature alga, mainly found in south coastal areas of China, Japan, North Korea and South Korea [186]. The bioactive compounds present in an aqueous extract of S. fusiforme induced resistance in Solanum lycopersicum against several pathogens [167]. Foliar applications of S. fusiforme extract on tomato plants reduced disease severity caused by powdery mildew by 37%, while the severity of late blight, caused by Phytopthtora infestans, and of gray mold, caused by B. cinerea, was reduced by 36 and 80%, respectively. The extract did not exhibit any direct antifungal activity, but rather induced systemic defense responses in tomato against pathogen infestations [167]. On the contrary, the organic solvent and aqueous extracts from S. vulgare inhibited the mycelial growth of F. oxysporum f. sp. tuberosi and controlled the progression of Fusarium dry rot on those potato tubers treated with the extracts [165,187]. Similarly, Ammar et al. [164] demonstrated that an aqueous extract from S. vulgare-controlled Pythium leek disease caused by P. aphanidermatum. Ali et al. [168] showed that the foliar application of an extract prepared from S. vulgare resulted in a significant reduction in the progression of disease caused by X. campestris and A. solani in tomato and sweet peppers under both, greenhouse and field conditions. S. vulgare extract induced resistance in treated plants by regulating expression of genes involved in defense-signalling pathways (i.e., PR-1a, PINII, and ETR-1) and phytohormone biosynthesis [168]. The foliar application of an extract prepared by the alkali treatment of Sargassum polycystum was reported to reduce leaf fall disease caused by Phytophthora palmivora by inducing systemic acquired resistance. The application of the extract induced enzymatic activities of catalase, endo-1,3-d-glucanase, and peroxidase in response to P. palmivora [166].
In a recent study, the foliar treatment of laboratory-scale extracts (i.e., hot and cold water and alkali) from Sargassum tenerrimum, at both vegetative and reproductive stages, reduced the progression of charcoal-rot caused by Macrophomina phaseolina [163]. Endogenous phytohormone analysis of the treated plants revealed higher salicylic acid (SA) accumulation in the treated vs. control groups, suggesting that induction of SA-dependent, defense pathways against M. phaseolina was responsible for the observed, reduced infection [163]. The cross talk of endogenously produced phytohormones, in response to different stresses, is an energy-efficient strategy of plants [188]. A higher accumulation of abscisic acid (ABA) was reported to increase the susceptibility of plants to pathogen infection [189]. The extract prepared from S. tenerrimum significantly reduced ABA accumulation in tomato plants infected with M. phaseolina. In addition, this Sargassum extract also induced endogenous phytohormones such as indoleacetic acid (IAA) and kinetin in M. phaseolina-infected tomato plants, during the vegetative and reproductive stages of pathogen development [163]. Taken together, these results suggest that bioactive formulations prepared from various Sargassum spp. may elicit the innate defense responses of plants by regulating the crosstalk between endogenous phytohormones and other biological processes.
3.4. Kappaphycus Alvarezii
Elkhorn sea moss, Kappaphycus alvarezii (Rhodophyta), is an industrially important red seaweed, primarily cultivated for the extraction of the phycocolloid carrageenan and some quaternary ammonium compounds [190,191]. K-sap is a commercial name given to a product obtained after mechanical crushing of the fresh seaweed. It contains phytohormones, such as IAA, kinetin, trans-zeatin and gibberellic acid (GA3) [154,192,193]. The foliar application of 5% of K-sap induced systemic defense responses in tomato plants against M. phaseolina [154]. The expression of the defense-response gene was found to be higher in the K-sap-treated tomato plants. The application of K-sap to M. phaseolina-inoculated plants induced the expression of SA-dependent PR-1, PR-3 coding chitinase and PR-5 coding osmotins by 2-fold, 4.5-fold, and 1194-fold, respectively. The trend in gene expression was supported by higher levels of SA accumulation [154]. K-sap was found to modulate endogenous plant phytohormone biosynthesis in response to M. phaseolina infections, thereby mediating crosstalk between different signalling pathways [154]. The potential for bioactive compounds from K. alvarezii needs to be explored further in priming plant defense against plant pathogens.
3.5. Gracilaria spp.
The genus Gracilaria belongs to the Rhodophyta and its members are distributed throughout the world in tropical and temperate waters [194,195]. Most of the species are commercially exploited primarily for their use as raw materials in agar production [194]. However, there have been a few studies focusing on the use of Gracilaria extracts as plant biostimulants [196,197,198,199,200]. Gracilaria extracts are rich sources of fatty acids, florisides, sterols, polyols, terpenoids and hydrocolloid polysachhrides [200]. Palmitic acid and the agarans isolated from the aqueous extract of Gracilaria caudata and G. domingensis promoted the early growth of lettuce [200]. Soliman et al. [156] showed that organic fractions of aqueous extract obtained from G. confervoides had antifungal activities against the plant pathogens Rhizoctonia solani, Fusarium solani and M. phaseolina. The maximum reduction in the radial growth of the pathogen on potato dextrose agar was observed when the medium was amended with a chloroform fraction of the aqueous extract of G. confervoides. The incidence of the disease caused by these pathogens was reduced when cucumber plants were grown in the soil amended with the powder of G. confervoides [156]. Silver nanoparticles synthesized using G. cortica exhibit antifungal activity against Candida albicans and C. glabrata [201].
3.6. Ulva spp.
The genus Ulva, commonly known as “sea lettuce”, is one of the most abundant green macroalgae (Chlorophyta) throughout the world [102]. Ulva spp. are perhaps the best researched members of the Ulvophyceae and indeed some species are used as experimental models in order to study macroalgal development, growth, and morphogenesis. Ulva biomass is used in the restoration of degraded environments [202,203]. Sea lettuce is a rich source of unique, sulphated polysaccharides called ulvans. These contain rhamnose, sulphate, xylose, iduronic acid, galactose, and glucose [54,204], and biomass may also used as a source of biofuel [205]. Cluzet et al. [160] demonstrated that the elicitors present in an extract of Ulva sp. prepared by hot water extraction induced immunity in Medicago truncatula against Phytophthora parasitica var. nicotianae by triggering the induction of PR-10, the hallmark of a plant defense response against pathogens. In addition to this, the foliar spray of an extract from Ulva sp. also protected M. truncatula from the fungal pathogen Colletotrichum trifolii, which is the causative agent of anthracnose. The differential expression analysis of expressed sequence tags (ESTs), obtained by a microarray analysis, revealed the induction of 152 genes in M. truncatula sprayed with the extract from Ulva sp. These ESTs were mainly involved in the biosynthesis of phytoalexins, pathogenesis-related proteins and cell wall proteins [160]. The bioactive compounds present in the ethyl acetate fraction derived from an aqueous extract of U. lactuca reduced the post-harvest losses in citrus by controlling the occurrence of green mould caused by Pencillium digitatum [159]. The elicitors isolated from U. lactuca stimulated the natural defense in the treated tomato seedlings against F. oxysporum f. sp. lycopersici [60]. A polysaccharide-enriched extract from U. lactuca showed a significant reduction in the progression of A. solani infection in tomato seedlings, as compared to extracts obtained from Caulerpa sertularioides, Padina gymnospora and Sargassum liebmanni [157]. A foliar spray of 0.1 mg mL−1 of the U. lactuca extract on tomato seedlings showed highest induction of activities of defense-related proteins, such as PPO, POD, and trypsin inhibitor [157]. The application of U. lactuca extract induced the expression of genes involved in wound response, JA biosynthesis, and defense-response. Interestingly, PAL was downregulated in those tomato plants treated with U. lactuca [157]. Heliosol® (Action-Pin, France), a commercial extract derived from the extract of Ulva armoricana, induced natural defenses in bean, grapevine and cucumber against powdery mildews caused by Erysiphe polygoni, E. necator and Sphareotheca fuliginea, respectively [154]. In the same study, Jaulneau et al. [154,155] showed that the infiltration of the extract of U. armoricana into the leaves of tobacco, expressing the reporter construct P1Lox: GUS, was a determinant for a higher β-glucuronidase activity. The aqueous and methanolic extracts from U. fasciata reduced both mycelial growth and the conidial germination of Colletotrichum lindemuthianum. Foliar applications of these extracts on to the leaves of Phaseolus vulgaris induced defense mechanisms against the anthracnose disease caused by C. lindemuthianum [53]. In another study, Rajesh et al. [206] synthesized silver nanoparticles impregnated with an ethyl acetate fraction of U. fasciata, they reported a reduced growth of X. campestris. These results indicate that Ulva spp. have an immense potential for being used in various disease management programs; however, current research lacks more in-depth knowledge of the bioactive compounds present in Ulva spp. and their modes of action.
In addition to the well-characterised seaweeds, others have also been reported for being used as a source of bioactive compounds for the management of plant diseases. The mixture of the alcoholic and aqueous extract from the tropical brown alga, Turbinaria conoides, demonstrated an antifungal property against the root rot pathogen F. oxysporum [169]. Esserti et al. [170] showed that methanolic extracts from the brown seaweeds, Cystoseira myriophylloides, L. digitata, and F. spiralis, controlled the progression of disease caused by V. dahliae and Agrobacterium tumefaciens in tomato plants. The treated plants showed reduced symptoms of disease, not by inhibiting pathogen growth, but by inducing plant defense responses, which included the enhanced synthesis of defense-responsive enzymes, such as polyphenol oxidase and POD [170]. An extract from A. spicifera conferred resistance against X. campestris and A. solani in tomato and sweet pepper by modulating the expression of genes involved in defense signalling and phytohormone biosynthesis [168]. Similarly, Ramkissoon et al. [158] determined the elicitor activity of tropical seaweeds, U. lactuca, S. filipendula and Gelidium serrulatum, luxuriantly found along the coast of Trinidad and the southern Caribbean, against X. campestris and A. solani infection in tomato. The alkaline extract prepared from U. lactuca and S. filipendula conferred resistance against X. campestris and A. solani infection in tomato plants by inducing the JA-dependent signalling system, while the alkaline extract from G. serrulatum induced both SA- and JA-dependent signalling pathways against both pathogens.
4. Conclusions
Climate change and urbanisation have undoubtedly increased the pressure on the agricultural sector to improve the productivity and quality of all staple crops. Rapid changes in environmental conditions have detrimental effects on plant immunity and their ability to fight against pathogens, thus augmenting the pressure on the agricultural sector to increase the productivity of crops [207]. The expanded, continuous use of synthetic chemicals to reduce the growth of pathogens is no longer a viable option because of their deleterious effects on human health and the environment, thus affecting short- and long-term sustainability.
To the rescue? Seaweed-based bio-elicitors offer a sustainable alternative for enhancing plant immunity against pathogens. Particularly during the last decade, the booming biostimulant industry has been characterized by the increasing utilisation of various seaweed-based extracts, in particular for relieving abiotic stresses. There is growing consensus that their applications have contributed to the reduction in synthetic chemical inputs to the environment [20]. Although the increasing number of reports on the use of seaweed extracts and their various, constituent bioactive compounds is encouraging, it is important to have a better understanding of their holistic modes of action and biotic responses, particularly in improving plant immunity (Figure 2). The biostimulant activity of various formulations seems to be strongly influenced by the extraction procedure, and so too the provenance/origin of the raw materials, seasonality (particularly in those originating in temperate areas), and ultimately in the potential synergistic/antagonistic activities of the numerous constituents of the extracts.
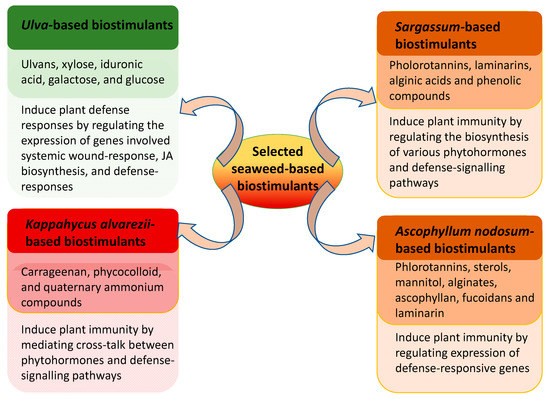
Figure 2.
Bioactive components present in various seaweed extracts and plausible defense mechanism elicited by these extracts that induce plant immunity against pathogens.
In the current scenario, there is a requirement for a thorough re-evaluation of many biostimulants that have been revealed as promising in laboratory settings, and the development of products which are useful and commercially viable in real-world conditions.
The information summarized in this review aimed at providing a platform to researchers, emphasizing the necessity to evaluate thoroughly modes of actions in seaweed extract products in inducing plant immunity; it was also intended to generate a plethora of research questions that need to be answered, such as: will the combination of different seaweed extracts have synergistic effects and increased beneficial bioactivity? Is the usage of various bioactive compounds isolated from crude extracts commercially viable? Should the research focus more on the usage of seaweeds as enhancers of plant growth/yield, or as potential pest control agents? Do the modes of applications (i.e., drench or spray) and frequency of applications have different metabolic responses? Clearly, further research aiming at the identification, isolation and characterization of the bioactive compounds present in the extracts of various seaweeds will elucidate some of these important aspects.
Author Contributions
Conceptualization, P.S.S., T.B., B.P. and A.T.C.; writing—original draft preparation, P.S.S.; writing—review and editing, P.S.S., T.B., B.P. and A.T.C.; supervision, B.P.; funding acquisition, B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by Accelerate Cluster grant (IT08347) from Mitacs (Canada), awarded to B.P. The funder provided support in the form of salary for P.S.S.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of atmospheric and climatic change on plant-pathogen interactions. Plant. Pathol. 2011, 60, 54–69. [Google Scholar] [CrossRef]
- Luck, J.; Spackman, M.; Freeman, A.; TreBicki, P.; Griffiths, W.; Finlay, K.; Chakraborty, S. Climate change and diseases of food crops. Plant. Pathol. 2011, 60, 113–121. [Google Scholar] [CrossRef]
- MacKenzie, D.R.; Shane, W.W. Crop losses due to plant pathogens. CRC. Crit. Rev. Plant. Sci. 1984, 2, 21–47. [Google Scholar]
- Strange, R.N.; Scott, P.R. Plant Disease: A Threat to Global Food Security. Annu. Rev. Phytopathol. 2005, 43. [Google Scholar] [CrossRef] [PubMed]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant. Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. (Amsterdam) 2015, 196, 39–48. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic Acid and its Function in Plant Immunity. J. Integr. Plant. Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Göhre, V.; Robatzek, S. Breaking the Barriers: Microbial Effector Molecules Subvert Plant Immunity. Annu. Rev. Phytopathol. 2008, 46, 189–215. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant. Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Potin, P.; Bouarab, K.; Küpper, F.; Kloareg, B. Oligosaccharide recognition signals and defence reactions in marine plant-microbe interactions. Curr. Opin. Microbiol. 1999, 2, 276–283. [Google Scholar] [CrossRef]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from Red Seaweeds as Promoters of Growth and Elicitors of Defense Response in Plants. Front. Mar. Sci. 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Marine Nutraceuticals: Prospects and Perspectives; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781466513525. [Google Scholar]
- Nabti, E. Biotechnological Applications of Seaweeds; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2017; ISBN 9781536109832. [Google Scholar]
- Duan, D.; Critchley, A.T.; Fu, X.; Pereira, L. Preface: Bioactive substances of various seaweeds and their applications and utilization. J. Oceanol. Limnol. 2019, 37, 779–782. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; González, A.; Barrientos, H.; Matsuhiro, B.; Arce, P.; Zuñiga, G.; Moenne, A. Long-term protection against tobacco mosaic virus induced by the marine alga oligo-sulphated-galactan Poly-Ga in tobacco plants. Mol. Plant. Pathol. 2011, 12, 437–447. [Google Scholar] [CrossRef]
- Stadnik, M.J. Ulvan effect on conidial germination and appressoria formation of Colletotrichum gloeosporioides. Phytoparasitica 2014, 42, 631–640. [Google Scholar]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant. Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant. Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Ramanujam, R.; Venkatesan, G.; Nagarathnam, R. Sodium alginate potentiates antioxidant defense and PR proteins against early blight disease caused by Alternaria solani in Solanum lycopersicum Linn. PLoS ONE 2019, 14, e0223216. [Google Scholar] [CrossRef] [PubMed]
- Bouissil, S.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of alginate extracted from Moroccan brown algae to stimulate natural defense in date palm roots. Molecules 2020, 25, 720. [Google Scholar] [CrossRef] [PubMed]
- An, Q.D.; Zhang, G.L.; Wu, H.T.; Zhang, Z.C.; Zheng, G.S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2009, 106, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Howlader, P.; Liu, T.; Sun, X.; Jia, X.; Zhao, X.; Shen, P.; Qin, Y.; Wang, W.; Yin, H. Alginate Oligosaccharide (AOS) induced resistance to Pst DC3000 via salicylic acid-mediated signaling pathway in Arabidopsis thaliana. Carbohydr. Polym. 2019, 225, 115221. [Google Scholar] [CrossRef]
- Gauthier, A.; Trouvelot, S.; Kelloniemi, J.; Frettinger, P.; Wendehenne, D.; Daire, X.; Joubert, J.M.; Ferrarini, A.; Delledonne, M.; Flors, V.; et al. The sulfated laminarin triggers a stress transcriptome before priming the SA- And ROS-dependent defenses during Grapevine’s induced resistance against Plasmopara viticola. PLoS ONE 2014, 9, e88145. [Google Scholar] [CrossRef]
- Meszka, B.; Bielenin, A. Activity of laminarin in control of strawberry diseases. Phytopathologia 2011, 62, 15–23. [Google Scholar]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin Elicits Defense Responses in Grapevine and Induces Protection Against Botrytis cinerea and Plasmopara viticola. Mol. Plant. Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef]
- Klarzynski, O.; Plesse, B.; Joubert, J.M.; Yvin, J.C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant. Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef]
- Ménard, R.; Alban, S.; De Ruffray, P.; Jamois, F.; Franz, G.; Fritig, B.; Yvin, J.C.; Kauffmann, S. β-1,3 glucan sulfate, but not β-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant. Cell 2004, 16, 3020–3032. [Google Scholar] [CrossRef]
- Ménard, R.; De Ruffray, P.; Fritig, B.; Yvin, J.C.; Kauffmann, S. Defense and resistance-inducing activities in tobacco of the sulfated β-1,3 glucan PS3 and its synergistic activities with the unsulfated molecule. Plant. Cell Physiol. 2005, 46, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Monchiero, M.; Gullino, M.L.; Garibaldi, A. Application of laminarin and calcium oxide for the control of grape powdery mildew on Vitis vinifera cv. Moscato. J. Plant. Dis. Prot. 2018, 125, 477–482. [Google Scholar] [CrossRef]
- Trouvelot, S.; Varnier, A.L.; Allègre, M.; Mercier, L.; Baillieul, F.; Arnould, C.; Gianinazzi-Pearson, V.; Klarzynski, O.; Joubert, J.M.; Pugin, A.; et al. A β-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol. Plant. Microbe Interact. 2008, 21, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Cai, X.; Chen, S.; Luo, Z.; Bian, L.; Li, Z.; Ge, L.; Chen, Z. A Disease Resistance Elicitor Laminarin Enhances Tea Defense against a Piercing Herbivore Empoasca (Matsumurasca) onukii Matsuda. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Krzyzaniak, Y.; Gauvrit, C.; Jamois, F.; Domergue, F.; Joubès, J.; Ferrières, V.; Adrian, M.; Legentil, L.; Daire, X.; et al. An ethoxylated surfactant enhances the penetration of the sulfated laminarin through leaf cuticle and stomata, leading to increased induced resistance against grapevine downy mildew. Physiol. Plant. 2016, 156, 338–350. [Google Scholar] [CrossRef]
- Adrian, M.; Lucio, M.; Roullier-Gall, C.; Héloir, M.C.; Trouvelot, S.; Daire, X.; Kanawati, B.; Lemaître-Guillier, C.; Poinssot, B.; Gougeon, R.; et al. Metabolic fingerprint of PS3-induced resistance of grapevine leaves against Plasmopara viticola revealed differences in elicitor-triggered defenses. Front. Plant. Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Renard-Merlier, D.; Randoux, B.; Nowak, E.; Farcy, F.; Durand, R.; Reignault, P. Iodus 40, salicylic acid, heptanoyl salicylic acid and trehalose exhibit different efficacies and defence targets during a wheat/powdery mildew interaction. Phytochemistry 2007, 68, 1156–1164. [Google Scholar] [CrossRef]
- Sangha, J.; Kandasamy, S.; Khan, W.; Bahia, N.; Singh, R.; Critchley, A.; Prithiviraj, B. λ-Carrageenan Suppresses Tomato Chlorotic Dwarf Viroid (TCDVd) Replication and Symptom Expression in Tomatoes. Mar. Drugs 2015, 13, 2875–2889. [Google Scholar] [CrossRef]
- Sangha, J.S.; Khan, W.; Ji, X.; Zhang, J.; Mills, A.A.S.; Critchley, A.T.; Prithiviraj, B. Carrageenans, sulphated polysaccharides of red seaweeds, differentially affect Arabidopsis thaliana resistance to Trichoplusia ni (Cabbage Looper). PLoS ONE 2011, 6, e26834. [Google Scholar] [CrossRef]
- Sangha, J.S.; Ravichandran, S.; Prithiviraj, K.; Critchley, A.T.; Prithiviraj, B. Sulfated macroalgal polysaccharides λ-carrageenan and ι-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotinia sclerotiorum. Physiol. Mol. Plant. Pathol. 2010, 75, 38–45. [Google Scholar] [CrossRef]
- Pettongkhao, S.; Bilanglod, A.; Khompatara, K.; Churngchow, N. Sulphated polysaccharide from Acanthophora spicifera induced Hevea brasiliensis defense responses against Phytophthora palmivora infection. Plants 2019, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.T.; Fournier, J. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]
- Le Mire, G.; Siah, A.; Marolleau, B.; Gaucher, M.; Maumené, C.; Brostaux, Y.; Massart, S.; Brisset, M.N.; Haissam Jijakli, M. Evaluation of l-carrageenan, CpG-ODN, glycine betaine, spirulina platensis, and ergosterol as elicitors for control of Zymoseptoria tritici in wheat. Phytopathology 2019, 109, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.D.; Nagarathnam, R. Sulfated polysaccharide from Kappaphycus alvarezii (Doty) Doty ex P.C. Silva primes defense responses against anthracnose disease of Capsicum annuum Linn. Algal Res. 2018, 32, 121–130. [Google Scholar] [CrossRef]
- Ghannam, A.; Abbas, A.; Alek, H.; Al-Waari, Z.; Al-Ktaifani, M. Enhancement of local plant immunity against tobacco mosaic virus infection after treatment with sulphated-carrageenan from red alga (Hypnea musciformis). Physiol. Mol. Plant. Pathol. 2013, 84, 19–27. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Contreras, R.A.; González, A.; Moenne, A. Oligo-carrageenans induce a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiol. Mol. Plant. Pathol. 2012, 79, 31–39. [Google Scholar] [CrossRef]
- Ben Salah, I.; Aghrouss, S.; Douira, A.; Aissam, S.; El Alaoui-Talibi, Z.; Filali-Maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against Verticillium wilt of olive. J. Plant. Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- De Freitas, M.B.; Ferreira, L.G.; Hawerroth, C.; Duarte, M.E.R.; Noseda, M.D.; Stadnik, M.J. Ulvans induce resistance against plant pathogenic fungi independently of their sulfation degree. Carbohydr. Polym. 2015, 133, 384–390. [Google Scholar] [CrossRef]
- De Borba, M.C.; de Freitas, M.B.; Stadnik, M.J. Ulvan enhances seedling emergence and reduces Fusarium wilt severity in common bean (Phaseolus vulgaris L.). Crop. Prot. 2019, 118, 66–71. [Google Scholar] [CrossRef]
- Paulert, R.; Ebbinghaus, D.; Urlass, C.; Moerschbacher, B.M. Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant. Pathol. 2010, 59, 634–642. [Google Scholar] [CrossRef]
- Paulert, R.; Talamini, V.; Cassolato, J.E.F.; Duarte, M.E.R.; Noseda, M.D.; Paulert, R.; Stadnik, M.J. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant. Dis. Prot. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.T.; Dumas, B. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signalling pathway. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Chiquito-Contreras, R.G.; Murillo-Amador, B.; Carmona-Hernandez, S.; Chiquito-Contreras, C.J.; Hernandez-Montiel, L.G. Effect of marine bacteria and ulvan on the activity of antioxidant defense enzymes and the bio-protection of papaya fruit against Colletotrichum gloeosporioides. Antioxidants 2019, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.Z.; de Freitas, M.B.; Stadnik, M.J. Effectiveness of saccharin and ulvan as resistance inducers against rust and angular leaf spot in bean plants (Phaseolus vulgaris). Crop. Prot. 2013, 47, 67–73. [Google Scholar] [CrossRef]
- De Freitas, M.B.; Stadnik, M.J. Race-specific and ulvan-induced defense responses in bean (Phaseolus vulgaris) against Colletotrichum lindemuthianum. Physiol. Mol. Plant. Pathol. 2012, 78, 8–13. [Google Scholar] [CrossRef]
- De Freitas, M.B.; Stadnik, M.J. Ulvan-induced resistance in Arabidopsis thaliana against Alternaria brassicicola requires reactive oxygen species derived from NADPH oxidase. Physiol. Mol. Plant. Pathol. 2015, 90, 49–56. [Google Scholar] [CrossRef]
- Klarzynski, O.; Descamps, V.; Plesse, B.; Yvin, J.-C.; Kloareg, B.; Fritig, B. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant. Microbe Interact. 2003, 16, 115–122. [Google Scholar] [CrossRef]
- El Modafar, C.; Elgadda, M.; El Boutachfaiti, R.; Abouraicha, E.; Zehhar, N.; Petit, E.; El Alaoui-Talibi, Z.; Courtois, B.; Courtois, J. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hortic. (Amsterdam) 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Abouraïcha, E.F.; El Alaoui-Talibi, Z.; Tadlaoui-Ouafi, A.; El Boutachfaiti, R.; Petit, E.; Douira, A.; Courtois, B.; Courtois, J.; El Modafar, C. Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. J. Appl. Phycol. 2017, 29, 471–480. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Pendota, S.C.; Van Staden, J. Enhancing growth, phytochemical constituents and aphid resistance capacity in cabbage with foliar application of eckol—A biologically active phenolic molecule from brown seaweed. New Biotechnol. 2016, 33, 273–279. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Zhao, X.; Wang, H.; Yin, H. Preparation of alginate oligosaccharides and their biological activities in plants: A review. Carbohydr. Res. 2020, 494, 108056. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Braccini, I.; Grasso, R.P.; Pérez, S. Conformational and configurational features of acidic polysaccharides and their interactions with calcium ions: A molecular modeling investigation. Carbohydr. Res. 1999, 317, 119–130. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Kailemia, M.J.; Ruhaak, L.R.; Lebrilla, C.B.; Amster, I.J. Oligosaccharide analysis by mass spectrometry: A review of recent developments. Anal. Chem. 2014, 86, 196–212. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Chen, S.; He, J.; Huang, Y. Characterization of calcium alginate/deacetylated konjac glucomannan blend films prepared by Ca2+ crosslinking and deacetylation. Food Hydrocoll. 2018, 82, 363–369. [Google Scholar] [CrossRef]
- Liu, L.; Wu, F.; Ju, X.J.; Xie, R.; Wang, W.; Niu, C.H.; Chu, L.Y. Preparation of monodisperse calcium alginate microcapsules via internal gelation in microfluidic-generated double emulsions. J. Colloid Interface Sci. 2013, 404, 85–90. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zhong, L.; Wu, G. Preparation of high-stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat. Phys. Chem. 2009, 78, 251–255. [Google Scholar] [CrossRef]
- Xu, A.; Zhan, J.C.; Huang, W.D. Oligochitosan and sodium alginate enhance stilbene production and induce defense responses in Vitis vinifera cell suspension cultures. Acta Physiol. Plant. 2015, 37, 1–13. [Google Scholar] [CrossRef]
- Küpper, F.C.; Müller, D.G.; Peters, A.F.; Kloareg, B.; Potin, P. Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of sporophytes of Laminariales. J. Chem. Ecol. 2002, 28, 2057–2081. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, W.; Jiang, L.; Hou, Y.; Yang, F.; Chen, W.; Li, X. Elicitor activity of algino-oligosaccharide and its potential application in protection of rice plant (Oryza saliva L.) against Magnaporthe grisea. Biotechnol. Biotechnol. Equip. 2015, 29, 646–652. [Google Scholar] [CrossRef]
- De Ruiter, G.A.; Rudolph, B. Carrageenan biotechnology. Trends Food Sci. Technol. 1997, 8, 389–395. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Singh, R.P.; Dilworth, A.D. Tomato chlorotic dwarf viroid in the ornamental plant Vinca minor and its transmission through tomato seed. Eur. J. Plant. Pathol. 2009, 123, 111–116. [Google Scholar] [CrossRef]
- González, M.E.; Alarcón, B.; Carrasco, L. Polysaccharides as antiviral agents: Antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987, 31, 1388–1393. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Nagorskaya, V.P.; Reunov, A.V.; Lapshina, L.A.; Ermak, I.M.; Barabanova, A.O. Inhibitory effect of κ/β-carrageenan from red alga Tichocarpus crinitus on the development of a potato virus X infection in leaves of Datura stramonium L. Biol. Bull. 2010, 37, 653–658. [Google Scholar] [CrossRef]
- Nagorskaia, V.P.; Reunov, A.V.; Lapshina, L.A.; Ermak, I.M.; Barabanova, A.O. Influence of kappa/beta-carrageenan from red alga Tichocarpus crinitus on development of local infection induced by Tobacco Mosaic Virus in Xanthi-nc tobacco leaves. Izv. Akad. Nauk Ser. Biol. 2008, 35, 360–364. [Google Scholar]
- Patier, P.; Potin, P.; Rochas, C.; Kloareg, B.; Yvin, J.; Licnart, Y. Free or silica-bound oligokappa-carrageenans elicit laminarinase activity in Rubus cells and protoplasts. Plant. Sci. 1995, 9452, 27–35. [Google Scholar] [CrossRef]
- Chen, Z.; Silva, H.; Klessig, D.F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant. Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Raimundo, S.C.; Pattathil, S.; Eberhard, S.; Hahn, M.G.; Popper, Z.A. β-1,3-Glucans are components of brown seaweed (Phaeophyceae) cell walls. Protoplasma 2017, 254, 997–1016. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Li, M.; Jin, M. Influence of Laminarin polysaccahrides on oxidative damage. Int. J. Biol. Macromol. 2011, 48, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, M.J.; Freitas, M.B. De Algal polysaccharides as source of plant resistance inducers. Trop. Plant. Pathol. 2014, 39, 111–118. [Google Scholar] [CrossRef]
- Elmer, P.A.G.; Reglinski, T. Biosuppression of Botrytis cinerea in grapes. Plant. Pathol. 2006, 55, 155–177. [Google Scholar] [CrossRef]
- Brederode, F.T.; Linthorst, H.J.M.; Bol, J.F. Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant. Mol. Biol. 1991, 17, 1117–1125. [Google Scholar] [CrossRef]
- Mantri, V.A.; Kazi, M.A.; Balar, N.B.; Gupta, V.; Gajaria, T. Concise review of green algal genus Ulva Linnaeus. J. Appl. Phycol. 2020, 32, 2725–2741. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Sankaranarayanan, S.; Gajaria, T.K.; Li, G.; Kujawski, W.; Kujawa, J.; Navia, R. A short review on the valorization of green seaweeds and ulvan: Feedstock for chemicals and biomaterials. Biomolecules 2020, 10, 991. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- McKinnell, J.P.; Percival, E. 606. Structural investigations on the water-soluble polysaccharide of the green seaweed Enteromorpha compressa. J. Chem. Soc. (Resumed) 1962, 3141–3148. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact. Carbohydrates Diet. Fibre 2019, 18, 100182. [Google Scholar] [CrossRef]
- Briand, X.; Cluzet, S.; Dumas, B.; Esquerre-Tugaye, M.; Salamagne, S. Ulvans as Activators of Plant Defense and Resistance Reactions Against Biotic or Abiotic Stresses. U.S. Patent 7,820,176, 26 October 2010. [Google Scholar]
- Abouraïcha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hortic. (Amsterdam) 2015, 181, 121–128. [Google Scholar] [CrossRef]
- Day, R.B.; Tanabe, S.; Koshioka, M.; Mitsui, T.; Itoh, H.; Ueguchi-Tanaka, M.; Matsuoka, M.; Kaku, H.; Shibuya, N.; Minami, E. Two rice GRAS family genes responsive to N-acetylchitooligosaccharide elicitor are induced by phytoactive gibberellins: Evidence for cross-talk between elicitor and gibberellin signaling in rice cells. Plant. Mol. Biol. 2004, 54, 261–272. [Google Scholar] [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D.G. DELLAs Control Plant Immune Responses by Modulating the Balance of Jasmonic Acid and Salicylic Acid Signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; O’hara, C.; Magee, P.J.; Mcsorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Rivas, A.; Martínez, A.; Rodrigo, D. Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem. 2017, 235, 34–44. [Google Scholar] [CrossRef]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Kubanek, J.; Lester, S.E.; Fenical, W.; Hay, M.E. Ambiguous role of phlorotannins as chemical defenses in the brown alga Fucus vesiculosus. Mar. Ecol. Prog. Ser. 2004, 277, 79–93. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Anaëlle, T.; Serrano Leon, E.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jormalainen, V.; Honkanen, T.; Koivikko, R.; Eränen, J. Induction of phlorotannin production in a brown alga: Defense or resource dynamics? Oikos 2003, 103, 640–650. [Google Scholar] [CrossRef]
- Arnold, T.M.; Targett, N.M. To grow and defend: Lack of tradeoffs for brown algal phlorotannins. Oikos 2003, 100, 406–408. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Allwood, J.W.; Evans, H.; Austin, C.; McDougall, G.J. Extraction, Enrichment, and LC-MSn-Based Characterization of Phlorotannins and Related Phenolics from the Brown Seaweed, Ascophyllum nodosum. Mar. Drugs 2020, 18, 448. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Eckol—A new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2015, 27, 581–587. [Google Scholar] [CrossRef]
- Toth, G.B.; Pavia, H. Water-borne cues induce chemical defense in a marine alga (Ascophyllum nodosum). Proc. Natl. Acad. Sci. USA 2000, 97, 14418–14420. [Google Scholar] [CrossRef]
- Pavia, H.; Brock, E. Extrinsic factors influencing phlorotannin production in the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 2000, 193, 285–294. [Google Scholar] [CrossRef]
- Knights, B.A. Sterols in Ascophyllum nodosum. Phytochemistry 1970, 9, 903–905. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.B. Inducible Chemical Resistance to Herbivory in the Brown Seaweed Ascophyllum nodosum. Ecology 2000, 81, 3212–3225. [Google Scholar] [CrossRef]
- Marais, M.F.; Joseleau, J.P. A fucoidan fraction from Ascophyllum nodosum. Carbohydr. Res. 2001, 336, 155–159. [Google Scholar] [CrossRef]
- Nakayasu, S.; Soegima, R.; Yamaguchi, K.; Oda, T. Biological activities of fucose-containing polysaccharide ascophyllan isolated from the brown alga Ascophyllum nodosum. Biosci. Biotechnol. Biochem. 2009, 73, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Okimura, T.; Yamaguchi, K.; Oda, T. The potent activity of sulfated polysaccharide, ascophyllan, isolated from Ascophyllum nodosum to induce nitric oxide and cytokine production from mouse macrophage RAW264.7 cells: Comparison between ascophyllan and fucoidan. Nitric Oxide Biol. Chem. 2011, 25, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Hiltz, D.; Norrie, J.; Prithiviraj, B. Ascophyllum nodosum extract mitigates salinity stress in Arabidopsis thaliana by modulating the expression of miRNA involved in stress tolerance and nutrient acquisition. PLoS ONE 2018, 13, e0206221. [Google Scholar] [CrossRef]
- Ali, N.; Ramkissoon, A.; Ramsubhag, A.; Jayaraj, J. Ascophyllum extract application causes reduction of disease levels in field tomatoes grown in a tropical environment. Crop. Prot. 2016, 83, 67–75. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant. Pathol. J. 2019, 35, 406. [Google Scholar]
- Cook, J.; Zhang, J.; Norrie, J.; Blal, B.; Cheng, Z. Seaweed Extract (Stella Maris®) Activates Innate Immune Responses in Arabidopsis thaliana and Protects Host against Bacterial Pathogens. Mar. Drugs 2018, 16, 221. [Google Scholar] [CrossRef]
- Jayaraj, J.; Wan, A.; Rahman, M.; Punja, Z.K. Seaweed extract reduces foliar fungal diseases on carrot. Crop. Prot. 2008, 27, 1360–1366. [Google Scholar] [CrossRef]
- Islam, M.T.; Gan, H.M.; Ziemann, M.; Hussain, H.I.; Arioli, T.; Cahill, D. Phaeophyceaean (Brown Algal) Extracts Activate Plant Defense Systems in Arabidopsis thaliana Challenged with Phytophthora cinnamomi. Front. Plant. Sci. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Patier, P.; Yvin, J.C.; Kloareg, B.; Liénart, Y.; Rochas, C. Seaweed liquid fertilizer from Ascophyllum nodosum contains elicitors of plant d-glycanases. J. Appl. Phycol. 1993, 5, 343–349. [Google Scholar] [CrossRef]
- Wu, Y.; Jenkins, T.; Blunden, G.; von Mende, N.; Hankins, S.D. Suppression of fecundity of the root-knot nematode, Meloidogyne javanica, in monoxenic cultures of Arabidopsis thaliana treated with an alkaline extract of Ascophyllum nodosum. J. Appl. Phycol. 1998, 10, 91–94. [Google Scholar] [CrossRef]
- Jayaraman, J.; Norrie, J.; Punja, Z.K. Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 2011, 23, 353–361. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef]
- Abkhoo, J.; Sabbagh, S.K. Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J. Appl. Phycol. 2016, 28, 1333–1342. [Google Scholar] [CrossRef]
- Viencz, T.; Oliari, I.C.R.; Ayub, R.A.; Faria, C.M.D.R.; Botelho, R.V. Postharvest quality and brown rot incidence in plums treated with Ascophyllum nodosum extract. Semin. Agrar. 2020, 41, 753–766. [Google Scholar] [CrossRef]
- Somai-Jemmali, L.; Siah, A.; Randoux, B.; Magnin-Robert, M.; Halama, P.; Hamada, W.; Reignault, P. Brown alga Ascophyllum nodosum extract-based product, Dalgin Active®, triggers defense mechanisms and confers protection in both bread and durum wheat against Zymoseptoria tritici. J. Appl. Phycol. 2020, 32, 3387–3399. [Google Scholar] [CrossRef]
- Panjehkeh, N.; Abkhoo, J. Influence of Marine Brown Alga Extract (Dalgin) on Damping-off Tolerance of Tomato. J. Mater. Environ. Sci. 2016, 7, 2369–2374. [Google Scholar]
- Hankins, S.D.; Hockey, H.P. The effect of a liquid seaweed extract from Ascophyllum nodosum (Fucales, Phaeophyta) on the two-spotted red spider mite Tetranychus urticae. Hydrobiologia 1990, 204–205, 555–559. [Google Scholar] [CrossRef]
- Wite, D.; Mattner, S.W.; Porter, I.J.; Arioli, T. The suppressive effect of a commercial extract from Durvillaea potatorum and Ascophyllum nodosum on infection of broccoli by Plasmodiophora brassicae. J. Appl. Phycol. 2015, 27, 2157–2161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Subramanian, S.; Sangha, J.S.; Gray, B.A.; Singh, R.P.; Hiltz, D.; Critchley, A.T.; Prithiviraj, B. Extracts of the marine brown macroalga, Ascophyllum nodosum, induce jasmonic acid dependent systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato DC3000 and Sclerotinia sclerotiorum. Eur. J. Plant. Pathol. 2011, 131, 237–248. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Reis, R.P.; Critchley, A.T. In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J. Appl. Phycol. 2010, 22, 101–104. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Reis, R.P.; Berrogain, F.D.; Critchley, A.T. Extract powder from the brown alga Ascophyllum nodosum (Linnaeus) Le Jolis (AMPEP): A “vaccine-like” effect on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva. J. Appl. Phycol. 2012, 24, 427–432. [Google Scholar] [CrossRef]
- Borlongan, I.A.G.; Tibubos, K.R.; Yunque, D.A.T.; Hurtado, A.Q.; Critchley, A.T. Impact of AMPEP on the growth and occurrence of epiphytic Neosiphonia infestation on two varieties of commercially cultivated Kappaphycus alvarezii grown at different depths in the Philippines. J. Appl. Phycol. 2011, 23, 615–621. [Google Scholar] [CrossRef]
- Ali, M.K.M.; Yasir, S.M.; Critchley, A.T.; Hurtado, A.Q. Impacts of Ascophyllum marine plant extract powder (AMPEP) on the growth, incidence of the endophyte Neosiphonia apiculata and associated carrageenan quality of three commercial cultivars of Kappaphycus. J. Appl. Phycol. 2018, 30, 1185–1195. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Patel, J.S.; Sumarah, M.W.; Renaud, J.B.; Mantin, E.G.; Prithiviraj, B. A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduce Fusarium head blight and mycotoxin contamination in wheat. PLoS ONE 2019, 14, e0220562. [Google Scholar] [CrossRef]
- Rekanovic, E.; Potocnik, I.; Milijasevic-Marcic, S.; Stepanovic, M.; Todorovic, B.; Mihajlovic, M. Efficacy of seaweed concentrate from Ecklonia maxima (Osbeck) and conventional fungicides in the control of Verticillium wilt of pepper. Pestic. Fitomed. 2010, 25, 319–324. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Effect of seaweed concentrate from Ecklonia maxima (Osbeck) Papenfuss on Meloidogyne incognita infestation on tomato. J. Appl. Phycol. 1993, 5, 37–43. [Google Scholar] [CrossRef]
- Ngala, B.M.; Valdes, Y.; dos Santos, G.; Perry, R.N.; Wesemael, W.M.L. Seaweed-based products from Ecklonia maxima and Ascophyllum nodosum as control agents for the root-knot nematodes Meloidogyne chitwoodi and Meloidogyne hapla on tomato plants. J. Appl. Phycol. 2016, 28, 2073–2082. [Google Scholar] [CrossRef]
- Agarwal, P.; Patel, K.; Das, A.K.; Ghosh, A.; Agarwal, P.K. Insights into the role of seaweed Kappaphycus alvarezii sap towards phytohormone signalling and regulating defence responsive genes in Lycopersicon esculentum. J. Appl. Phycol. 2016, 28, 2529–2537. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.T.; Dumas, B. An Ulva armoricana extract protects plants against three powdery mildew pathogens. Eur. J. Plant. Pathol. 2011, 131, 393. [Google Scholar] [CrossRef]
- Soliman, A.S.; Ahmed, A.Y.; Abdel-ghafour, S.E.; El-sheekh, M.M.; Sobhy, H.M. Antifungal bio-efficacy of the red algae Gracilaria confervoides extracts against three pathogenic fungi of cucumber plant. Middle East. J. Appl. Sci. 2018, 8, 727–735. [Google Scholar]
- Hernández-Herrera, R.M.; Virgen-Calleros, G.; Ruiz-López, M.; Zañudo-Hernández, J.; Délano-Frier, J.P.; Sánchez-Hernández, C. Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus Alternaria solani. J. Appl. Phycol. 2014, 26, 1607–1614. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Ramsubhag, A.; Jayaraman, J. Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. J. Appl. Phycol. 2017, 29, 3235–3244. [Google Scholar] [CrossRef]
- Salim, D.; De Caro, P.; Merah, O.; Chbani, A. Control of Post-harvest Citrus Green Mold using Ulva lactuca Extracts as a Source of Active Substances. Int. J. Bio-Resour. Stress Manag. 2020, 11, 287–296. [Google Scholar] [CrossRef]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant. Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Esserti, S.; Smaili, A.; Makroum, K.; Belfaiza, M.; Rifai, L.A.; Koussa, T.; Kasmi, I.; Faize, M. Priming of Nicotiana benthamiana antioxidant defences using brown seaweed extracts. J. Phytopathol. 2018, 166, 86–94. [Google Scholar] [CrossRef]
- Ara, J.; Ehteshamul-Haque, S.; Sultana, V.; Qasim, R.; Ghaffar, A. Effect of Sargassum seaweed and microbial antagonists in the control of root rot disease of sunflower. Pakistan J. Bot. 1996, 28, 219–224. [Google Scholar]
- Khedia, J.; Dangariya, M.; Nakum, A.K.; Agarwal, P.; Panda, A.; Parida, A.K.; Gangapur, D.R.; Meena, R.; Agarwal, P.K. Sargassum seaweed extract enhances Macrophomina phaseolina resistance in tomato by regulating phytohormones and antioxidative activity. J. Appl. Phycol. 2020, 32, 4373–4384. [Google Scholar] [CrossRef]
- Ammar, N.; Jabnoun-Khiareddine, H.; Mejdoub-Trabelsi, B.; Nefzi, A.; Mahjoub, M.A.; Daami-Remadi, M. Pythium leak control in potato using aqueous and organic extracts from the brown alga Sargassum vulgare (C. Agardh, 1820). Postharvest Biol. Technol. 2017, 130, 81–93. [Google Scholar] [CrossRef]
- Ammar, N.; Nefzi, A.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Control of Fusarium Dry Rot Incited by Fusarium oxysporum f. sp. tuberosi Using Sargassum vulgare Aqueous and Organic Extracts. J. Microb. Biochem. Technol. 2017, 9, 200–208. [Google Scholar]
- Khompatara, K.; Pettongkhao, S.; Kuyyogsuy, A.; Deenamo, N.; Churngchow, N. Enhanced resistance to leaf fall disease caused by Phytophthora palmivora in rubber tree seedling by Sargassum polycystum extract. Plants 2019, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Sbaihat, L.; Takeyama, K.; Koga, T.; Takemoto, D.; Kawakita, K. Induced resistance in Solanum lycopersicum by algal elicitor extracted from Sargassum fusiforme. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Phytoelicitor activity of Sargassum vulgare and Acanthophora spicifera extracts and their prospects for use in vegetable crops for sustainable crop production. J. Appl. Phycol. 2020, 1–13. [Google Scholar] [CrossRef]
- Selvaraju, P.; Vijayakumar, A. Evaluation of antifungal activity of seaweed extract (Turbinaria conoides) against Fusarium oxysporum. J. Appl. Nat. Sci. 2016, 8, 60–62. [Google Scholar]
- Esserti, S.; Smaili, A.; Rifai, L.A.; Koussa, T.; Makroum, K.; Belfaiza, M.; Kabil, E.M.; Faize, L.; Burgos, L.; Alburquerque, N.; et al. Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. J. Appl. Phycol. 2017, 29, 1081–1093. [Google Scholar] [CrossRef]
- Boenisch, M.J.; Schäfer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant. Biol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Patel, J.S.; Selvaraj, V.; Gunupuru, L.R.; Rathor, P.K.; Prithiviraj, B. Combined application of Ascophyllum nodosum extract and chitosan synergistically activates host-defense of peas against powdery mildew. BMC Plant. Biol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Yunque, D.A.; Tibubos, K.; Critchley, A.T. Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J. Appl. Phycol. 2009, 21, 633–639. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Joe, M.; Sanares, R.C.; Fan, D.; Prithiviraj, B.; Critchley, A.T. Investigation of the application of Acadian Marine Plant Extract Powder (AMPEP) to enhance the growth, phenolic content, free radical scavenging, and iron chelating activities of Kappaphycus Doty (Solieriaceae, Gigartinales, Rhodophyta). J. Appl. Phycol. 2012, 24, 601–611. [Google Scholar] [CrossRef]
- Rothman, M.D.; Mattio, L.; Wernberg, T.; Anderson, R.J.; Uwai, S.; Mohring, M.B.; Bolton, J.J. A molecular investigation of the genus Ecklonia (Phaeophyceae, Laminariales) with special focus on the Southern Hemisphere. J. Phycol. 2015, 51, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.R.; Staden Van, J.V. Short Communication: 1-Aminocyc1opropane-1-carboxylic Acid in Seaweed Concentrate. Bot. Mar. 1985, 28, 415–417. [Google Scholar]
- Wang, J.; Zheng, J.; Huang, C.; Zhao, J.; Lin, J.; Zhou, X.; Naman, C.B.; Wang, N.; Gerwick, W.H.; Wang, Q.; et al. Eckmaxol, a Phlorotannin Extracted from Ecklonia maxima, Produces Anti-β-amyloid Oligomer Neuroprotective Effects Possibly via Directly Acting on Glycogen Synthase Kinase 3β. ACS Chem. Neurosci. 2018, 9, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Papenfus, H.B.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Seasonal variation in the polyamines of Ecklonia maxima. Bot. Mar. 2012, 55, 539–546. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Kulkarni, M.G.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Effect of a commercial seaweed extract (Kelpak®) and polyamines on nutrient-deprived (N, P and K) okra seedlings. Sci. Hortic. (Amsterdam) 2013, 151, 142–146. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Isolation and identification of cytokinins in a new commercial seaweed product made from Fucus serratus L. J. Appl. Phycol. 1997, 9, 327. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Comparison of cytokinin- and auxin-like activity in some commercially used seaweed extracts. J. Appl. Phycol. 1996, 8, 503–508. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Effect of seaweed concentrate on the establishment and yield of greenhouse tomato plants. J. Appl. Phycol. 1992, 4, 291–296. [Google Scholar] [CrossRef]
- Mattio, L.; Payri, C.E.; Verlaque, M. Taxonomic revision and geographic distribution of the subgenus Sargassum (Fucales, Phaeophyceae) in the western and central pacific islands based on morphological and molecular analyses. J. Phycol. 2009, 45, 1213–1227. [Google Scholar] [CrossRef]
- Tanniou, A.; Vandanjon, L.; Incera, M.; Serrano Leon, E.; Husa, V.; Le Grand, J.; Nicolas, J.L.; Poupart, N.; Kervarec, N.; Engelen, A.; et al. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Ammar, N.; Khiareddine, H.J.; Nefzia, A.; Mejdoub Trabelsi, B.; Daami Remadi, M. Extracts from the Brown Macroalga Sargassum vulgare for Postharvest Suppression of Potato Fusarium Dry Rot. Nat. Prod. Chem. Res. 2018, 9, 200–208. [Google Scholar] [CrossRef]
- Knight, H.; Knight, M.R. Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant. Sci. 2001, 6, 262–267. [Google Scholar] [CrossRef]
- Bürger, M.; Chory, J. Stressed Out About Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef]
- Bindu, M.S.; Levine, I.A. The commercial red seaweed Kappaphycus alvarezii-an overview on farming and environment. J. Appl. Phycol. 2011, 23, 789–796. [Google Scholar] [CrossRef]
- Mondal, D.; Ghosh, A.; Prasad, K.; Singh, S.; Bhatt, N.; Zodape, S.T.; Chaudhary, J.P.; Chaudhari, J.; Chatterjee, P.B.; Seth, A.; et al. Elimination of gibberellin from Kappaphycus alvarezii seaweed sap foliar spray enhances corn stover production without compromising the grain yield advantage. Plant. Growth Regul. 2015, 75, 657–666. [Google Scholar] [CrossRef]
- Patel, K.; Agarwal, P.; Agarwal, P.K. Kappaphycus alvarezii sap mitigates abiotic-induced stress in Triticum durum by modulating metabolic coordination and improves growth and yield. J. Appl. Phycol. 2018, 30, 2659–2673. [Google Scholar] [CrossRef]
- Prasad, K.; Das, A.K.; Oza, M.D.; Brahmbhatt, H.; Siddhanta, A.K.; Meena, R.; Eswaran, K.; Rajyaguru, M.R.; Ghosh, P.K. Detection and quantification of some plant growth regulators in a seaweed-based foliar spray employing a mass spectrometric technique sans chromatographic separation. J. Agric. Food Chem. 2010, 58, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.C.; Castro, J.Z.; Critchley, A.T.; Yokoya, N.S. Physiological responses of the red algae Gracilaria caudata (Gracilariales) and Laurencia catarinensis (Ceramiales) following treatment with a commercial extract of the brown alga Ascophyllum nodosum (AMPEP). J. Appl. Phycol. 2019, 31, 1883–1888. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y.A.C. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.K.; Pal, S.K.; Trivedi, K.; Yesuraj, D.; Singh, C.S.; Anand, K.G.V.; Chandramohan, M.; Patidar, R.; Kubavat, D.; et al. Sustainable enhancement in yield and quality of rain-fed maize through Gracilaria edulis and Kappaphycus alvarezii seaweed sap. J. Appl. Phycol. 2016, 28, 2099–2112. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef]
- Kuwada, K.; Wamocho, L.S.; Utamura, M.; Matsushita, I.; Ishii, T. Effect of red and green algal extracts on hyphal growth of arbuscular mycorrhizal fungi, and on mycorrhizal development and growth of papaya and passionfruit. Agron. J. 2006, 98, 1340–1344. [Google Scholar] [CrossRef]
- Satish, L.; Rameshkumar, R.; Rathinapriya, P.; Pandian, S.; Rency, A.S.; Sunitha, T.; Ramesh, M. Effect of seaweed liquid extracts and plant growth regulators on in vitro mass propagation of brinjal (Solanum melongena L.) through hypocotyl and leaf disc explants. J. Appl. Phycol. 2015, 27, 993–1002. [Google Scholar] [CrossRef]
- Torres, P.; Novaes, P.; Ferreira, L.G.; Santos, J.P.; Mazepa, E.; Duarte, M.E.R.; Noseda, M.D.; Chow, F.; dos Santos, D.Y.A.C. Effects of extracts and isolated molecules of two species of Gracilaria (Gracilariales, Rhodophyta) on early growth of lettuce. Algal Res. 2018, 32, 142–149. [Google Scholar] [CrossRef]
- Kumar, P.; Senthamil Selvi, S.; Govindaraju, M. Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against Candida spp. Appl. Nanosci. 2013, 3, 495–500. [Google Scholar] [CrossRef]
- Martínez-Aragón, J.F.; Hernández, I.; Pérez-Lloréns, J.L.; Vázquez, R.; Vergara, J.J. Biofiltering efficiency in removal of dissolved nutrients by three species of estuarine macroalgae cultivated with sea bass (Dicentrarchus labrax) waste waters 1. Phosphate. J. Appl. Phycol. 2002, 14, 365–374. [Google Scholar] [CrossRef]
- Copertino, M.D.S.; Tormena, T.; Seeliger, U. Biofiltering efficiency, uptake and assimilation rates of Ulva clathrata (Roth) J. Agardh (Clorophyceae) cultivated in shrimp aquaculture waste water. J. Appl. Phycol. 2009, 21, 31–45. [Google Scholar] [CrossRef]
- Robic, A.; Bertrand, D.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Determination of the chemical composition of ulvan, a cell wall polysaccharide from Ulva spp. (Ulvales, Chlorophyta) by FT-IR and chemometrics. J. Appl. Phycol. 2009, 21, 451–456. [Google Scholar] [CrossRef]
- Castelar, B.; Reis, R.P.; dos Santos Calheiros, A.C. Ulva lactuca and U. flexuosa (Chlorophyta, Ulvophyceae) cultivation in Brazilian tropical waters: Recruitment, growth, and ulvan yield. J. Appl. Phycol. 2014, 26, 1989–1999. [Google Scholar] [CrossRef]
- Rajesh, S.; Patric Raja, D.; Rathi, J.M.; Sahayaraj, K. Biosynthesis of silver nanoparticles using Ulva fasciata (Delile) ethyl acetate extract and its activity against Xanthomonas campestris pv. malvacearum. J. Biopestic. 2012, 5, 119–128. [Google Scholar]
- Saijo, Y.; Loo, E.P.I. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).