Development of a Fast Liquid Chromatography Coupled to Mass Spectrometry Method (LC-MS/MS) to Determine Fourteen Lipophilic Shellfish Toxins Based on Fused–Core Technology: In-House Validation
Abstract
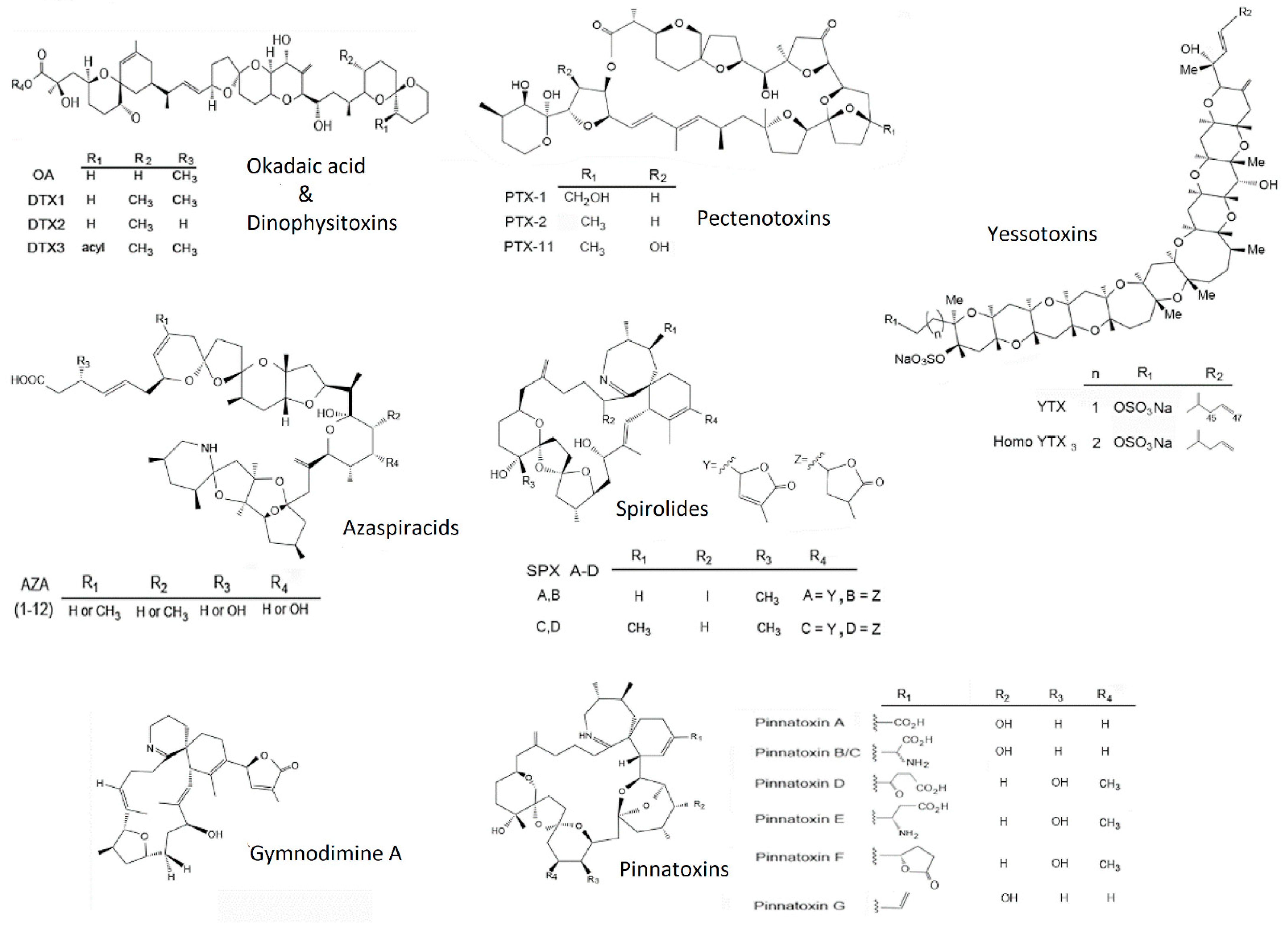
:1. Introduction
2. Results and Discussion
2.1. LOQ/LOD
2.2. Selectivity/Specificity
2.3. Linearity
2.4. Precision: Repeatability and Reproducibility
2.5. Accuracy
3. Materials and Methods
3.1. Chemicals
3.2. Reference Materials
3.3. Shellfish Samples
3.4. Sample Extraction and Hydrolysis
3.5. LC–MS/MS Procedure
3.6. Scope and Method Validation
3.6.1. LOQ/LOD
3.6.2. Selectivity/Specificity
3.6.3. Linearity
3.6.4. Precision: Repeatability and Reproducibility
3.6.5. Accuracy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Comission. Regulation (EU) No 786/2013 of 16 August 2013 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards the permitted limits of yessotoxins in live bivalve molluscs. Off. J. Eur. Union 2014, L 220, 14. [Google Scholar]
- European Comission. Regulation (EC) No 853/2004 of the European Parlamient and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L 139, 55. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on marine biotoxins in shellfish—Cyclic imines (spirolides, gymnodimines, pinnatoxins and pteriatoxins). EFSA J. 2010, 8, 1–39. [Google Scholar]
- Aasen, J.; Mackinnon, S.L.; Leblanc, P.; Walter, J.A.; Hovgaard, P.; Aune, T.; Quilliam, M.A. Detection and identification of spirolides in Norwegian shellfish and plankton. Chem. Res. Toxicol. 2005, 18, 509–515. [Google Scholar] [CrossRef]
- Rundberget, T.; Aasen, J.A.B.; Selwood, A.I.; Miles, C.O. Pinnatoxins and spirolides in Norwegian blue mussels and seawater. Toxicon 2011, 58, 700–711. [Google Scholar] [CrossRef]
- Hess, P.; Abadie, E.; Herve, F.; Berteaux, T.; Sechet, V.; Araoz, R.; Molgo, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambla-Alegre, M.; Miles, C.O.; de la Iglesia, P.; Fernández-Tejedor, M.; Jacobs, S.; Sioen, I.; Verbeke, W.; Samdal, I.A.; Sandvik, M.; Barbosa, V.; et al. Occurrence of cyclic imines in European commercial seafood and consumers risk assessment. Environ. Res. 2018, 161, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Ujevic, I.; Roje-Busatto, R.; Ezgeta-Balic, D. Comparison of amnesic, paralytic and lipophilic toxins profiles in cockle (Acanthocardia tuberculata) and smooth clam (Callista chione) from the central Adriatic Sea (Croatia). Toxicon 2019, 159, 32–37. [Google Scholar] [CrossRef]
- García-Altares, M.; Casanova, A.; Bane, V.; Diogene, J.; Furey, A.; de la Iglesia, P. Confirmation of pinnatoxins and spirolides in shellfish and passive samplers from Catalonia (Spain) by liquid chromatography coupled with triple quadrupole and high-resolution hybrid tandem mass spectrometry. Mar. Drugs 2014, 12, 3706–3732. [Google Scholar] [CrossRef]
- Villar González, A.; Rodríguez-Velasco, M.L.; Ben-Gigirey, B.; Botana, L.M. First evidence of spirolides in Spanish shellfish. Toxicon 2006, 48, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.P.; Arévalo, F.; Moroño, A.; Correa, J.; Muñíz, S.; Blanco, J. Detection and spatio-temporal distribution of pinnatoxins in shellfish from the Atlantic and Cantabrian coasts of Spain. Toxins 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero, P.; Miguens, N.; Rodríguez, I.; Botana, L.M. LC-MS/MS analysis of the emerging toxin pinnatoxin-G and high levels of esterified OA group toxins in Galician commercial mussels. Toxins 2019, 11, 394. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on marine biotoxins in shellfish—domoic acid. EFSA J. 2009, 1181, 1–61. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on marine biotoxins in shellfish—Emerging toxins: Brevetoxin group. EFSA J. 2010, 8, 1677. [Google Scholar] [CrossRef]
- Leyva-Valencia, I.; Hernández-Castro, J.E.; Band-Schmidt, C.J.; Turner, A.D.; O’Neill, A.; Núñez-Vázquez, E.J.; López-Cortés, D.J.; Bustillos-Guzmán, J.J.; Hernández-Sandoval, F.E. Lipophilic Toxins in Wild Bivalves from the Southern Gulf of California, Mexico. Mar. Drugs 2021, 19, 99. [Google Scholar] [CrossRef]
- Josić, D.; Rešetar, D.; Peršurić, Ž.; Martinović, T.; Kraljevic Pavelić, S. Detection of microbial toxins by -omics methods: A growing role of proteomics. In Proteomics for Microbial Toxin Identification; Elsevier: Amsterdam, The Netherlands, 2017; pp. 497–505. [Google Scholar]
- European Commission. Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls. Off. J. Eur. Union 2019, L 131, 51. [Google Scholar]
- EURLMB. EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS.Version 5. EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS.Version 5. Available online: https://www.aesan.gob.es/CRLMB/docs/docs/metodos_analiticos_de_desarrollo/EU-Harmonised-SOP-LIPO-LCMSMS_Version5.pdf (accessed on 30 June 2021).
- McNabb, P.; Selwood, A.I.; Holland, P.T. Multiresidue method for determination of algal toxins in shellfish: Single-laboratory validation and interlaboratory study. J. AOAC Int. 2005, 88, 761–772. [Google Scholar] [CrossRef] [Green Version]
- Gerssen, A.; Mulder, P.P.J.; McElhinney, M.A.; de Boer, J. Liquid chromatography–tandem mass spectrometry method for the detection of marine lipophilic toxins under alkaline conditions. J. Chromatogr. A 2009, 1216, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- McCarron, P.; Wright, E.; Quilliam, M.A. Liquid chromatography/mass spectrometry of domoic acid and lipophilic shellfish toxins with selected reaction monitoring and optional confirmation by library searching of product ion spectra. J. AOAC Int. 2014, 97, 316–324. [Google Scholar] [CrossRef]
- Brana-Magdalena, A.; Leao-Martins, J.M.; Glauner, T.; Gago-Martinez, A. Intralaboratory validation of a fast and sensitive UHPLC/MS/MS method with fast polarity switching for the analysis of lipophilic shellfish toxins. J. AOAC Int. 2014, 97, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Moreiras, G.; Leao, J.M.; Gago-Martinez, A. Analysis of Cyclic Imines in Mussels (Mytilus galloprovincialis) from Galicia (NW Spain) by LC-MS/MS. Int. J. Environ. Res. Public Health 2020, 17, 281. [Google Scholar] [CrossRef] [Green Version]
- Regueiro, J.; Rossignoli, A.E.; Álvarez, G.; Blanco, J. Automated on-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry for determination of lipophilic marine toxins in shellfish. Food Chem. 2011, 129, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Guiochon, G.; Gritti, F. Shell particles, trials, tribulations and triumphs. J. Chromatogr. A 2011, 1218, 1915–1938. [Google Scholar] [CrossRef]
- Rodríguez, I.; Alfonso, A.; González-Jartín, J.M.; Vieytes, M.R.; Botana, L.M. A single run UPLC-MS/MS method for detection of all EU-regulated marine toxins. Talanta 2018, 189, 622–628. [Google Scholar] [CrossRef]
- Yang, L.; Singh, A.; Lankford, S.K.; Stuart, J.; Rice, D.; Wu, W.H.; Hungerford, J.M. A rapid method for the detection of diarrhetic shellfish toxins and azaspiracid shellfish toxins in Washington State shellfish by liquid chromatography tandem mass spectrometry. J. AOAC Int. 2020, 103, 792–799. [Google Scholar] [CrossRef]
- O’Neill, A.; Morrell, N.; Turner, A.D.; Maskrey, B.H. Method performance verification for the combined detection and quantitation of the marine neurotoxins cyclic imines and brevetoxin shellfish metabolites in mussels (Mytilus edulis) and oysters (Crassostrea gigas) by UHPLC-MS/MS. J. Chromatogr. B 2021, 1179. [Google Scholar] [CrossRef]
- Fux, E.; Rode, D.; Bire, R.; Hess, P. Approaches to the evaluation of matrix effects in the liquid chromatography-mass spectrometry (LC-MS) analysis of three regulated lipophilic toxin groups in mussel matrix (Mytilus edulis). Food Addit. Contam. 2008, 25, 1024–1032. [Google Scholar] [CrossRef]
- Kilcoyne, J.; Fux, E. Strategies for the elimination of matrix effects in the liquid chromatography tandem mass spectrometry analysis of the lipophilic toxins okadaic acid and azaspiracid-1 in molluscan shellfish. J. Chromatogr. A 2010, 1217, 7123–7130. [Google Scholar] [CrossRef]
- Gerssen, A.; McElhinney, M.; Mulder, P.; Bire, R.; Hess, P.; de Boer, J. Solid phase extraction for removal of matrix effects in lipophilic marine toxin analysis by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 394, 1213–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar-González, A.; Rodríguez-Velasco, M.L.; Gago-Martínez, A. Determination of lipophilic toxins by LC/MS/MS: Single-laboratory validation. J. AOAC Int. 2011, 94, 909–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, J.; Arevalo, F.; Correa, J.; Morono, A. Lipophilic toxins in Galicia (NW Spain) between 2014 and 2017: Incidence on the main molluscan species and analysis of the monitoring efficiency. Toxins 2019, 11, 612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
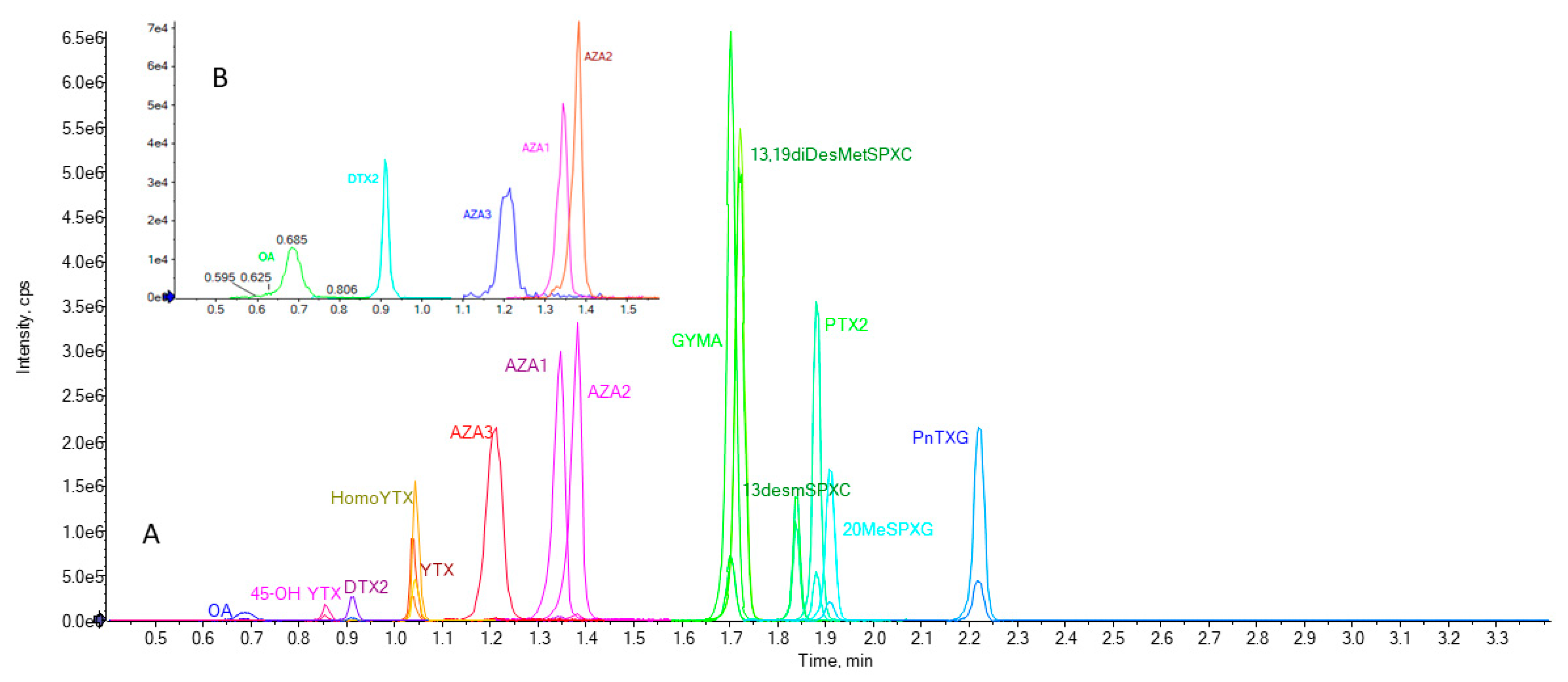
| Toxin | LOD (µg kg−1) | LOQ (µg kg−1) |
|---|---|---|
| OA | 3 | 10 |
| DTX2 | 9 | 29 |
| DTX1 | 9 | 29 |
| AZA1 | 9 | 29 |
| AZA2 | 9 | 29 |
| AZA3 | 9 | 29 |
| YTX | 22 | 72 |
| HomoYTX | 24 | 74 |
| PTX2 | 7.3 | 25 |
| 13desmSPXC | 0.2 | 0.67 |
| 13,19didesmSPXC | 0.035 | 0.12 |
| 20MethylSPXG | 1.1 | 3.8 |
| GYMA | 1.2 | 3.9 |
| PnTXG | 0.4 | 1.3 |
| OA | DTX2 | DTX1 | AZA1 | AZA2 | AZA3 | PTX2 | YTX | HomoYTX | 13desmSPXC | 13,19didesmSPXC | 20MethySPXG | GYMA | PnTXG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fortified levels (µg kg−1) | ||||||||||||||
| 30 * | 30 | 30 | 30 | 30 | 30 | 30 | 75 | 75 | 0.7 | 3 * | 3 ** | 3 ** | 3 * | |
| Matrix | Recovery (%) | |||||||||||||
| Mussel | 89.3 | 100.3 | 114.7 | 89.7 | 82.3 | 87.3 | 94.3 | 135.5 | 119.2 | 100 | 84.3 | 105.7 | 103.3 | 56.9 |
| Clam | 71 | 80.7 | 96.7 | 75.7 | 91.7 | 79.3 | 81.3 | 96.7 | 92 | 85.7 | 79.7 | 99.3 | 101.7 | 78.6 |
| Oyster | 94 | 105.7 | 122 | 103 | 99.7 | 95 | 100.3 | 137.2 | 111.1 | 142.9 a | 75.7 | 91.7 | 42.4 a | 57.7 |
| Mean (n = 3) | 84.8 | 95.6 | 111.1 | 89.5 | 91.2 | 87.2 | 92.0 | 123.1 | 107.4 | 92.9 (n = 2) | 79.9 | 98.9 | 102.5 (n = 2) | 64.4 |
| SD (n = 3) | 12.2 | 13.2 | 13.0 | 13.7 | 8.7 | 7.9 | 9.7 | 22.9 | 14.0 | 10.1 (n = 2) | 4.30 | 7.0 | 1.1 (n = 2) | 12.3 |
| RSDr, % | 14.3 | 13.8 | 11.7 | 15.3 | 9.6 | 9 | 10.6 | 18.6 | 13.0 | 10.9 | 5.4 | 7.1 | 1.1 | 19.1 |
| Toxin | r2 |
|---|---|
| OA | 0.9922 |
| DTX2 | 0.9917 |
| DTX1 | 0.9918 |
| AZA1 | 0.9972 |
| AZA2 | 0.9972 |
| AZA3 | 0.9966 |
| YTX | 0.9891 |
| HomoYTX | 0.9922 |
| PTX2 | 0.9969 |
| 13desmSPXC | 0.9969 |
| 13,19didesmSPXC | 0.9921 |
| 20MethylSPXG | 0.9965 |
| GYMA | 0.9970 |
| PnTXG | 0.9958 |
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RSDr, % (n = 9) | RSDR, % (n = 27) | RSDr, % (n = 9) | RSDR, % (n = 27) | RSDr, %(n = 9) | RSDR, % (n = 27) | RSDr, % (n = 9) | RSDR, % (n = 27) | RSDr, % (n = 9) | RSDR, % (n = 27) | |
| OA | 8.6 | 6.5 | 4.4 | 7.9 | 4.8 | 5.8 | 3.6 | 5.0 | ||
| DTX2 | 8.5 | 7.9 | 4.3 | 6.4 | 4.7 | 6.7 | 5.7 | 6.7 | ||
| DTX1 | 10.1 | 10.0 | 6.6 | 9.6 | 5.6 | 9.7 | 3.8 | 8.9 | ||
| AZA1 | 11.3 | 10.7 | 3.1 | 4.1 | 3.7 | 3.2 | 3.6 | 3.4 | ||
| AZA2 | 8.1 | 7.7 | 4.7 | 4.9 | 3.7 | 3.0 | 4.1 | 3.2 | ||
| AZA3 | 9.3 | 8.5 | 4.5 | 4.8 | 3.0 | 2.7 | 4.0 | 3.2 | ||
| YTX | 16.0 | 14.4 | 14.9 | 14.2 | 14.0 | 13.7 | 12.5 | 13.9 | ||
| HomoYTX | 13.0 | 11.1 | 14.0 | 13.6 | 12.8 | 12.7 | 12.9 | 13.9 | ||
| PTX2 | 8.1 | 8.5 | 9.3 | 7.2 | 7.5 | 5.1 | 5.8 | 5.0 | ||
| 13desmSPXC | 21.2 (n = 6) * | 30.6 (n = 18) * | 18.3 | 17.4 | 6.4 | 7.4 | 6.9 | 6.7 | 8.9 | 8.9 |
| 13,19didesmSPXC | 15.2 | 14.8 | 4.9 | 10.2 | 5.4 | 6.4 | 7.6 | 8.3 | ||
| 20MethylSPXG | 17.8 ** | 16.5 ** | 4.1 | 4.8 | 4.7 | 6.0 | 6.1 | 7.5 | ||
| GYMA | 36.0 ** | 30.9 ** | 7.4 | 7.2 | 5.9 | 5.4 | 7.0 | 7.3 | ||
| PnTXG | 21.9 | 14.8 | 4.9 | 5.1 | 6.3 | 7.0 | 7.5 | 8.7 | ||
| Sample | Matrix | Toxin | RSDr % (n = 5) | RSDR % (n = 10) |
|---|---|---|---|---|
| 508 | Mussel | Total OA | 10.1 | 11.7 |
| 509 | Mussel | Total OA | 8.6 | 14.5 |
| 510 | Mussel | Total OA | 7.9 | 13.0 |
| 512 | Mussel | Total OA | 9.7 | 10.3 |
| 513 | Mussel | Total OA | 8.9 | 12.5 |
| 1532 | Cockle | Total OA | 9.6 | 12.6 * |
| 1534 | Cockle | Total OA | 8.7 | 8.1 * |
| 1540 | Razor clam | Total OA | 11.2 | 10.0 * |
| 1541 | Cockle | Total OA | 11.3 | 8.7 * |
| 1547 | Clam | Total OA | 8.0 | 6.9 * |
| Type of Toxin | Mean (µg kg−1) | SD (µg kg−1) | RSDR, % (n = 8) | Certified Value | Recovery % |
|---|---|---|---|---|---|
| OA | 306.7 | 22.8 | 7.4 | 278.3 | 110.2 |
| DTX2 | 795.1 | 64.6 | 8.1 | 624.8 | 129.1 |
| DTX1 | 155.7 | 11.6 | 7.5 | 119.0 | 132.0 |
| AZA1 | 809.1 | 70.5 | 8.7 | 717.5 | 112.3 |
| AZA2 | 194.3 | 12.9 | 6.6 | 197.8 | 98.1 |
| AZA3 | 186.9 | 12.5 | 6.7 | 168 | 110.6 |
| YTX | 670.0 | 176.5 | 26.3 | 435.8 | 154.1 |
| PTX2 | 107.6 | 12.7 | 11.8 | 115.5 | 92.4 |
| 13desmSPXC | 509.0 | 35.4 | 6.9 | 472.5 | 108.0 |
| Recovery, % (Mean ± SD), n = 27 | |||||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | |
| OA | 84.8 ± 1.6 | 85.6 ± 5.4 | 91.7 ± 8.5 | 93.8 ± 14.9 | |
| DTX2 | 90.4 ± 2.1 | 92.5 ± 4.7 | 99.6 ± 10.6 | 101.0 ± 21.6 | |
| DTX1 | 100.7 ± 3.0 | 105.7 ± 8.2 | 110.7 ± 17.2 | 112.9 ± 32.3 | |
| AZA1 | 89.9 ± 2.9 | 91.2 ± 3.0 | 96.0 ± 4.9 | 98.7 ± 10.7 | |
| AZA2 | 90.8 ± 2.1 | 91.8 ± 3.4 | 97.0 ± 4.6 | 99.3 ± 10.0 | |
| AZA3 | 88.2 ± 2.3 | 91.0 ± 3.5 | 97.4 ± 4.20 | 99.8 ± 10.3 | |
| YTX | 107.9 ± 11.6 | 113.0 ± 32.2 | 121.1 ± 66.6 | 120.5 ± 134.2 | |
| HomoYTX | 96.2 ± 8.0 | 100.7 ± 27.3 | 107.8 ± 54.7 | 107.4 ± 119.0 | |
| PTX2 | 85.7 ± 2.1 | 84.9 ± 4.9 | 89.1 ± 7.2 | 91. 5 ± 14.6 | |
| 13desmSPXC | 100.8 ± 0.2 (n = 18) * | 71.1 ± 1.0 | 76.5 ± 1.3 | 83.4 ± 2.5 | 87.5 ± 6.9 |
| 13,19didesmSPXC | 72.8 ± 0.3 | 71.8 ± 2.2 | 67.1 ± 3.2 | 73.7 ± 9.2 | |
| 20MethylSPXG | 92.8 ** ± 0.5 | 98.9 ± 1.4 | 92.3 ± 4.1 | 101.7 ± 11.5 | |
| GYMA | 83.5 ** ± 0.8 | 97.2 ± 2.1 | 90.6 ± 3.7 | 99.3 ± 10.8 | |
| PnTXG | 62.0 ± 0.3 | 75.8 ± 1.2 | 70.3 ± 3.7 | 77.1 ± 10.0 | |
| Toxin | ESI | Q1 | Q3 | DEP (v) | EP (v) | CE (v) | CXP(v) |
|---|---|---|---|---|---|---|---|
| OA_DTX2 (qn) | NEG | 803.52 | 255.15 | −80 | −15 | −62 | −11 |
| OA_DTX2 (ql) | NEG | 803.52 | 563.40 | −80 | −15 | −60 | −11 |
| YTX (qn) | NEG | 570.43 | 467.40 | −80 | −15 | −42 | −11 |
| YTX (ql) | NEG | 570.43 | 396.40 | −80 | −15 | −42 | −11 |
| HomoYTX (qn) | NEG | 577.40 | 474.40 | −80 | −15 | −42 | −11 |
| HomoYTX (ql) | NEG | 577.40 | 403.40 | −80 | −15 | −42 | −11 |
| DTX1 (qn) | NEG | 817.50 | 255.15 | −80 | −15 | −60 | −11 |
| DTX1 (ql) | NEG | 817.50 | 563.45 | −80 | −15 | −52 | −11 |
| AZA3 (qn) | POS | 828.46 | 810.5 | 80 | 15 | 30 | 10 |
| AZA3 (ql) | POS | 828.46 | 658.4 | 80 | 15 | 43 | 10 |
| AZA1 (qn) | POS | 842.46 | 824.5 | 80 | 15 | 30 | 10 |
| AZA1 (ql) | POS | 842.46 | 672.4 | 80 | 15 | 43 | 10 |
| AZA2 (qn) | POS | 856.46 | 838.5 | 80 | 15 | 30 | 10 |
| AZA2 (ql) | POS | 856.46 | 672.4 | 80 | 15 | 43 | 10 |
| GYMA (qn) | POS | 508.33 | 490.2 | 80 | 15 | 50 | 10 |
| GYMA (ql) | POS | 508.33 | 136.00 | 80 | 15 | 50 | 10 |
| 13,19didesmSPXC (qn) | POS | 678.5 | 164.00 | 80 | 15 | 60 | 10 |
| 13,19didesmSPXC (ql) | POS | 678.50 | 430.3 | 80 | 15 | 50 | 10 |
| 13desmSPXC (qn) | POS | 692.5 | 164.3 | 80 | 15 | 60 | 10 |
| 13desmSPXC (ql) | POS | 692.50 | 444.3 | 80 | 15 | 55 | 10 |
| PTX2 (qn) | POS | 876.46 | 823.5 | 80 | 15 | 35 | 10 |
| PTX2 (ql) | POS | 876.46 | 213.1 | 80 | 15 | 50 | 10 |
| 20MethylSPXG (qn) | POS | 706.5 | 164.3 | 80 | 15 | 60 | 10 |
| 20MethylSPXG (ql) | POS | 706.50 | 348.3 | 80 | 15 | 55 | 10 |
| PnTXG (qn) | POS | 694.5 | 164.3 | 80 | 15 | 60 | 10 |
| PnTXG (ql) | POS | 694.5 | 440.3 | 80 | 15 | 50 | 10 |
| OA, DTXs, AZAs, PTX2 | YTX, HomoYTX | 13desmSPXC | 13,19didesmSPXC, 20MethylSPXG, GYMA, PnTXG |
|---|---|---|---|
| 2.1 | 5.2 | 0.19 | 0.47 |
| 6.3 | 15.7 | 0.6 | 0.9 |
| 10.6 | 26.5 | 1.8 | 1.9 |
| 16.9 | 42.1 | 3.0 | 3.75 |
| 21.6 | 53.9 | 4.7 | 7.5 |
| 31.7 | 79.2 | 6 | 15 |
| 45.7 | 114.29 | 8.8 | |
| 12.7 |
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | |
|---|---|---|---|---|---|
| OA | 30 | 80 | 160 | 320 | |
| DTXs | 30 | 80 | 160 | 320 | |
| AZAs | 30 | 80 | 160 | 320 | |
| PTX2 | 30 | 80 | 160 | 320 | |
| YTX | 75 | 200 | 400 | 800 | |
| HomoYTX | 75 | 200 | 400 | 800 | |
| 13desmSPXC | 0.7 | 8.3 | 22.2 | 44.4 | 89 |
| GYMA | 3 | 30 | 75 | 150 | |
| PnTXG | 3 | 30 | 75 | 150 | |
| 13,19didesmSPXC | 3 | 30 | 75 | 150 | |
| 20MethylSPXG | 3 | 30 | 75 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossignoli, A.E.; Mariño, C.; Martín, H.; Blanco, J. Development of a Fast Liquid Chromatography Coupled to Mass Spectrometry Method (LC-MS/MS) to Determine Fourteen Lipophilic Shellfish Toxins Based on Fused–Core Technology: In-House Validation. Mar. Drugs 2021, 19, 603. https://doi.org/10.3390/md19110603
Rossignoli AE, Mariño C, Martín H, Blanco J. Development of a Fast Liquid Chromatography Coupled to Mass Spectrometry Method (LC-MS/MS) to Determine Fourteen Lipophilic Shellfish Toxins Based on Fused–Core Technology: In-House Validation. Marine Drugs. 2021; 19(11):603. https://doi.org/10.3390/md19110603
Chicago/Turabian StyleRossignoli, Araceli E., Carmen Mariño, Helena Martín, and Juan Blanco. 2021. "Development of a Fast Liquid Chromatography Coupled to Mass Spectrometry Method (LC-MS/MS) to Determine Fourteen Lipophilic Shellfish Toxins Based on Fused–Core Technology: In-House Validation" Marine Drugs 19, no. 11: 603. https://doi.org/10.3390/md19110603
APA StyleRossignoli, A. E., Mariño, C., Martín, H., & Blanco, J. (2021). Development of a Fast Liquid Chromatography Coupled to Mass Spectrometry Method (LC-MS/MS) to Determine Fourteen Lipophilic Shellfish Toxins Based on Fused–Core Technology: In-House Validation. Marine Drugs, 19(11), 603. https://doi.org/10.3390/md19110603







