Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gelatin Characterization
2.1.1. Yield
2.1.2. Gel Strength
2.1.3. Amino Acid Composition
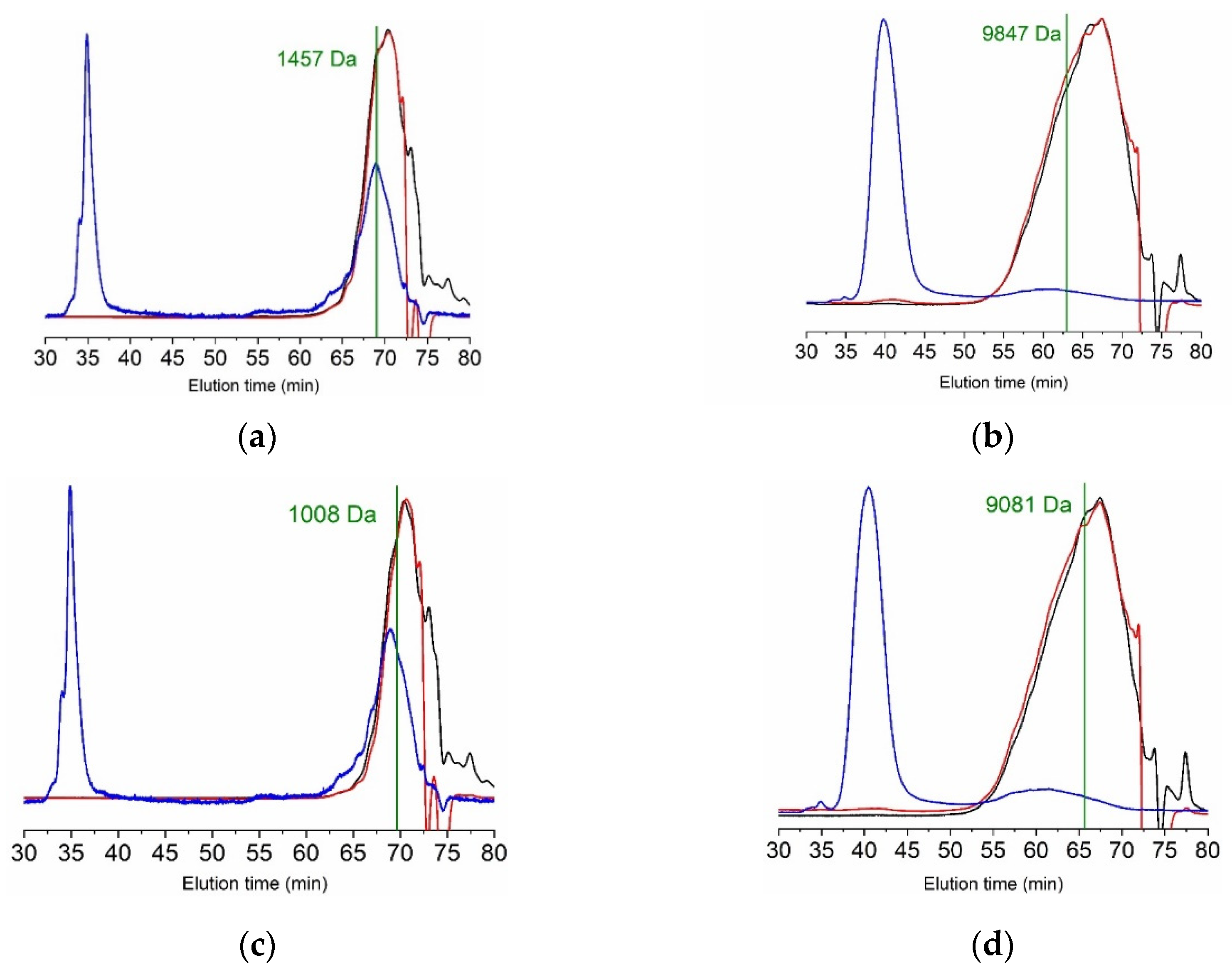
2.1.4. Molecular Weight Distribution Profiles
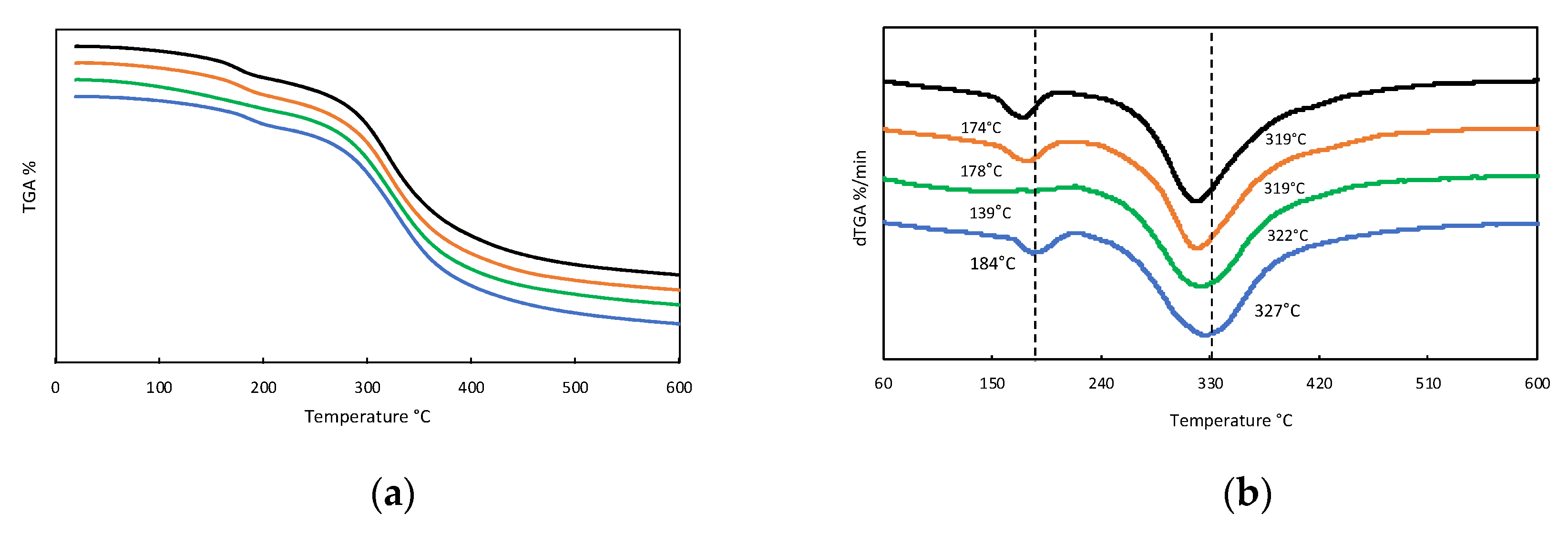
2.1.5. Thermal Stability
2.1.6. Infrared Spectroscopy
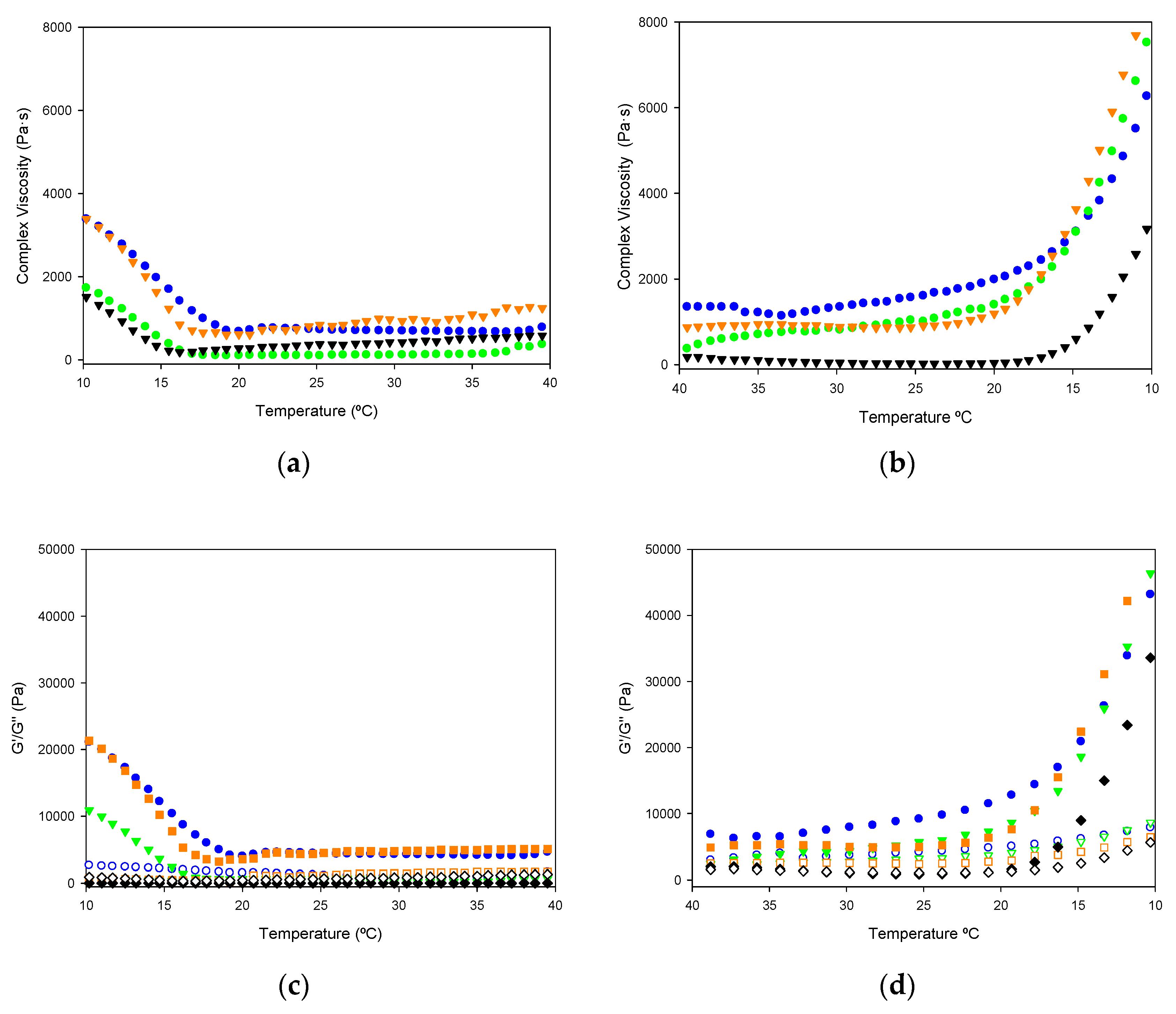
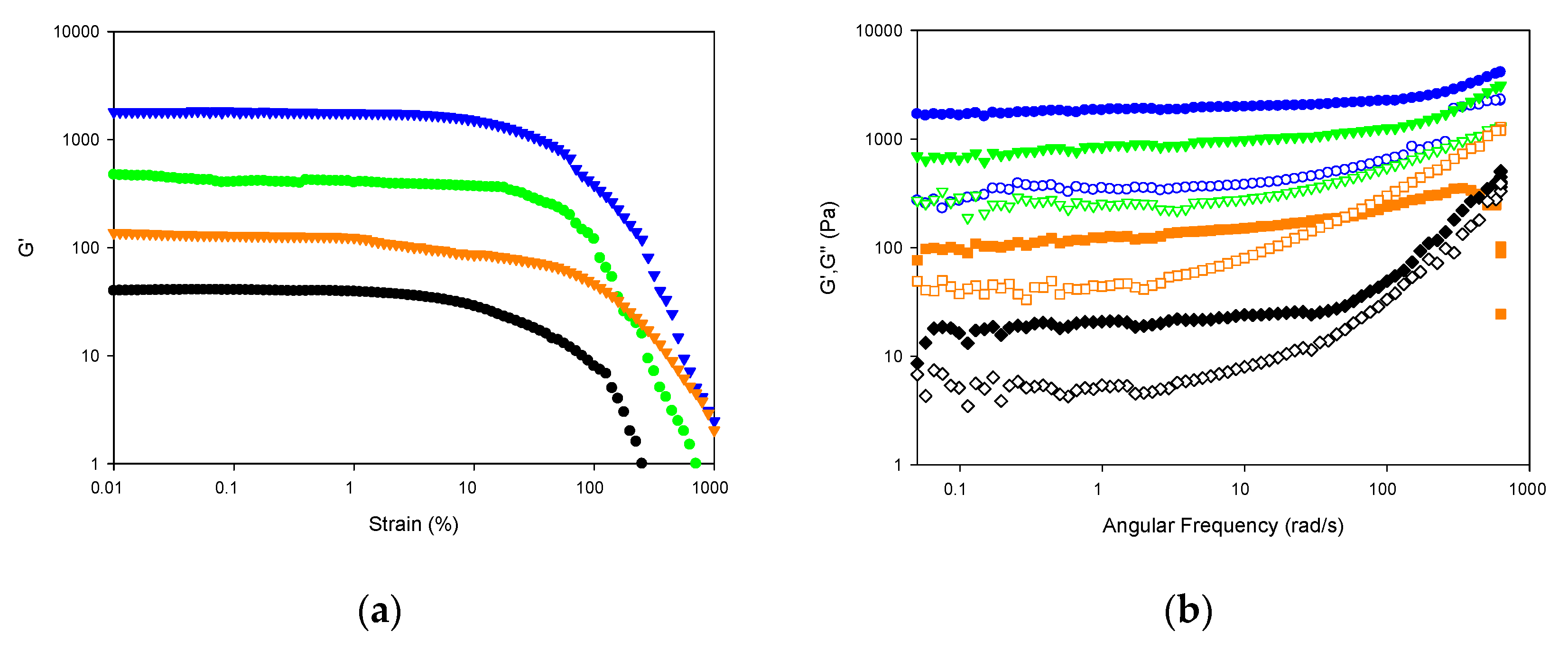
2.1.7. Viscoelastic Properties
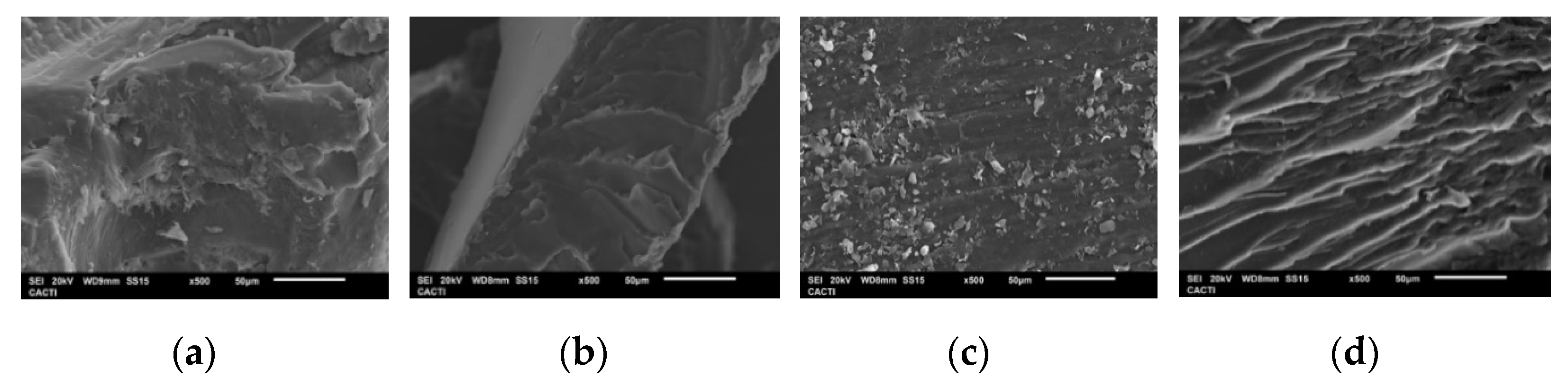
2.1.8. SEM
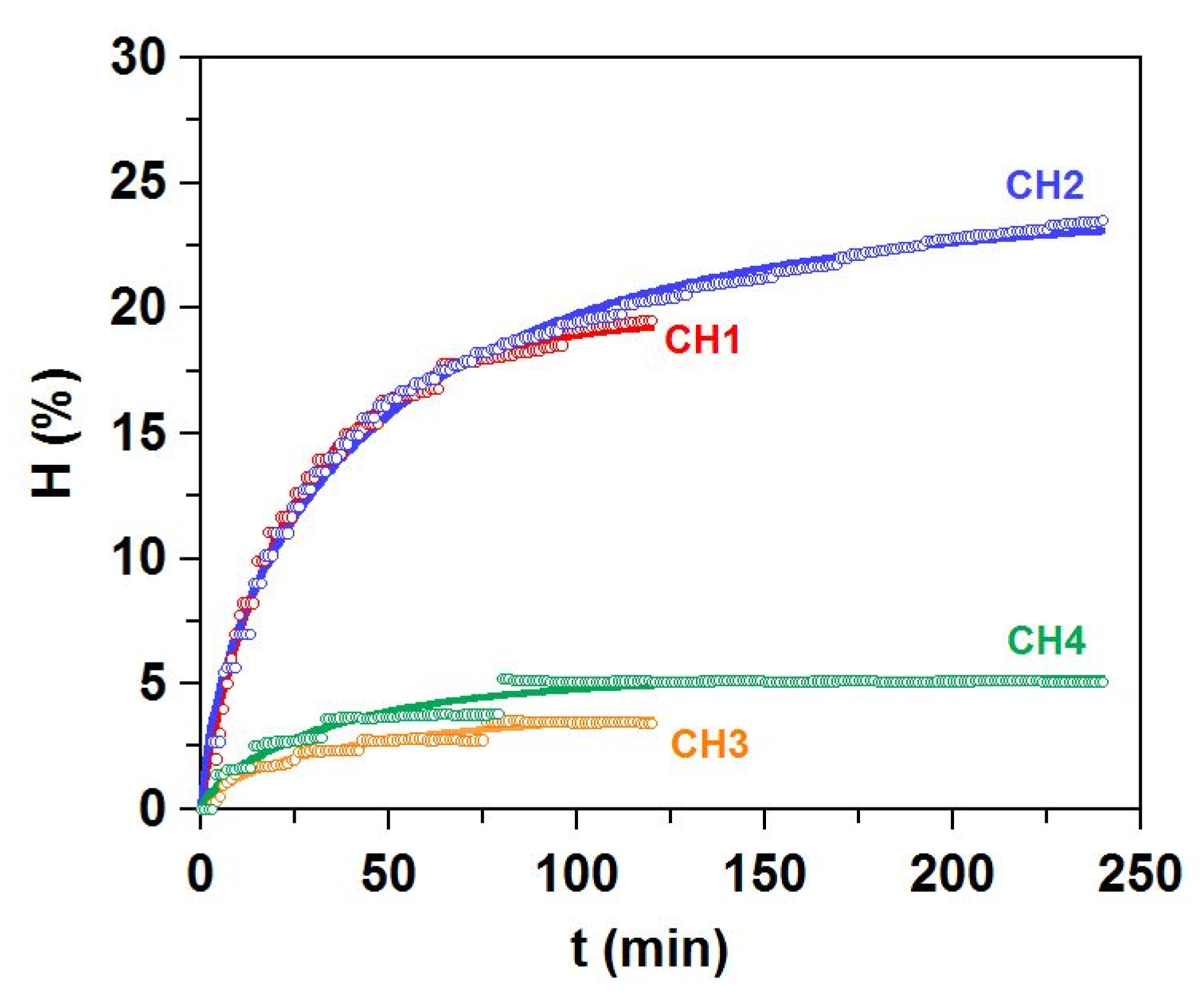
2.2. Production of Collagen Hydrolysates from Skin Waste after Gelatin Extraction
3. Materials and Methods
3.1. Skin By-Products from Aquaculture Fish
3.2. Production of Turbot Gelatin
3.3. Production of Collagen Hydrolysates (FPH)
3.4. Characterization
3.4.1. Chemical Composition and Bioactive Properties
3.4.2. Gel Strength
3.4.3. Molecular Weight
3.4.4. Infrared Spectroscopy
3.4.5. Thermogravimetric Analysis
3.4.6. Rheology
3.4.7. Scanning Electron Microscopy
3.5. Numerical Fitting and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel, J. Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules 2020, 10, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, B.; Sun, J.; Dong, P.; Xue, C.; Mao, X. Conversion of turbot skin wastes into valuable functional substances with an eco-friendly fermentation technology. J. Clean. Prod. 2017, 156, 367–377. [Google Scholar] [CrossRef]
- da Trindade Alfaro, A.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Brodsky, B.; Persikov, A.V. Molecular Structure of the Collagen Triple Helix. In Fibrous Proteins: Coiled-Coils, Collagen and Elastomers; Advances in Protein Chemistry; Academic Press: London, UK, 2005; Volume 70, pp. 301–339. [Google Scholar]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Sousa, S.C.; Vázquez, J.A.; Pérez-Martín, R.I.; Carvalho, A.P.; Gomes, A.M. Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins. Molecules 2017, 22, 1545. [Google Scholar] [CrossRef] [Green Version]
- Abdalbasit Adam Mariod, H.F. Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 134–147. [Google Scholar]
- Abedinia, A.; Ariffin, F.; Huda, N.; Nafchi, A.M. Extraction and characterization of gelatin from the feet of Pekin duck (Anas platyrhynchos domestica) as affected by acid, alkaline, and enzyme pretreatment. Int. J. Biol. Macromol. 2017, 98, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, Q.; Jia, H.; Xia, L.; Jin, W.; Shang, M.; Xu, C.; Dong, X. Physiochemical and functional properties of tiger puffer (Takifugu rubripes) skin gelatin as affected by extraction conditions. Int. J. Biol. Macromol. 2018, 109, 1045–1053. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Milovanovic, I.; Hayes, M. Marine Gelatine from rest raw materials. Appl. Sci. 2018, 8, 2407. [Google Scholar] [CrossRef] [Green Version]
- Hou, P.Z.; Regenstein, J.M. Optimization of Extraction Conditions for Pollock Skin Gelatin. J. Food Sci. 2004, 69, C393–C398. [Google Scholar] [CrossRef]
- Kuan, Y.-H.; Nafchi, A.M.; Huda, N.; Ariffin, F.; Karim, A.A. Effects of sugars on the gelation kinetics and texture of duck feet gelatin. Food Hydrocoll. 2016, 58, 267–275. [Google Scholar] [CrossRef]
- Jamilah, B.; Harvinder, K. Properties of gelatins from skins of fish_black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem. 2002, 77, 81–84. [Google Scholar] [CrossRef]
- Choi, S.-S.; Regenstein, J.M. Physicochemical and Sensory Characteristics of Fish Gelatin. J. Food Sci. 2000, 65, 194–199. [Google Scholar] [CrossRef]
- Kasankala, L.M.; Xue, Y.; Weilong, Y.; Hong, S.D.; He, Q. Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresour. Technol. 2007, 98, 3338–3343. [Google Scholar] [CrossRef] [PubMed]
- Songchotikunpan, P.; Tattiyakul, J.; Supaphol, P. Extraction and electrospinning of gelatin from fish skin. Int. J. Biol. Macromol. 2008, 42, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Dıaz, M.D.; Montero, P.; Gomez-Guillen, M.C. Gel properties of collagens from skins of cod (Gadus morhua) and hake (Merluccius merluccius) and their modification by the coenhancers magnesium sulphate, glycerol and transglutaminase. Food Chem. 2001, 74, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Arnesen, J.A.; Gildberg, A. Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Benjakul, S.; Ocen, D.; Yang, N.; Xu, B.; Zhang, L.; Xu, X. Physical and chemical properties of gelatin from the skin of cultured Amur sturgeon (Acipenser schrenckii). J. Appl. Ichthyol. 2013, 29, 943–950. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Herrick, D.Z.; Maziarz, E.P.; Liu, X.M. Analysis of gelatin using various separation and detection technologies. J. Anal. Pharm. Res. 2018, 7, 669–672. [Google Scholar]
- Zhou, P.; Mulvaney, S.J.; Regenstein, J.M. Properties of Alaska pollock skin gelatin: A comparison with tilapia and pork skin gelatins. J. Food Sci. 2006, 71, C313–C321. [Google Scholar] [CrossRef]
- Abd Elgadir, M.; Mirghani, M.E.S.; Adam, A. Fish gelatin and its applications in selected pharmaceutical aspects as alternative source to pork gelatin. J. Food Agric. Env. 2013, 11, 73–79. [Google Scholar]
- oint, F.A.O.; World Health Organization. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007; ISBN 9241209356. [Google Scholar]
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez-Amado, I.; Valcarcel, J. Valorization of Aquaculture By-Products of Salmonids to Produce Enzymatic Hydrolysates: Process Optimization, Chemical Characterization and Evaluation of Bioactives. Mar. Drugs 2019, 17, 676. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Pan, D.; Sun, Y.; Ou, C.; Wang, Y.; Cao, J. The modification of gelatin films: Based on various cross-linking mechanism of glutaraldehyde at acidic and alkaline conditions. Food Sci. Nutr. 2019, 7, 4140–4146. [Google Scholar] [CrossRef]
- Sazedul, H.M. Improvement of Properties of Edible Film Based on Gelatin from Cuttlefish (Sepia pharamois) Skin, Prince of Songkla University; Prince of Songkla University: Hat Yai, Songkhla, Thailand, 2011. [Google Scholar]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [Green Version]
- Casanova, F.; Mohammadifar, M.A.; Jahromi, M.; Petersen, H.O.; Sloth, J.J.; Eybye, K.L.; Kobbelgaard, S.; Jakobsen, G.; Jessen, F. Physico-chemical, structural and techno-functional properties of gelatin from saithe (Pollachius virens) skin. Int. J. Biol. Macromol. 2020, 156, 918–927. [Google Scholar] [CrossRef]
- Salem, A.; Fakhfakh, N.; Jridi, M.; Abdelhedi, O.; Nasri, M.; Debeaufort, F.; Zouari, N. Microstructure and characteristic properties of dogfish skin gelatin gels prepared by freeze/spray-drying methods. Int. J. Biol. Macromol. 2020, 162, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Friess, W.; Lee, G. Basic thermoanalytical studies of insoluble collagen matrices. Biomaterials 1996, 17, 2289–2294. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Taravel, M.N.; Domard, A. Collagen and its interaction with chitosan: II. Influence of the physicochemical characteristics of collagen. Biomaterials 1995, 16, 865–871. [Google Scholar] [CrossRef]
- Byler, D.; Brouillette, J.N.; Susi, H. Quantitative studies of protein structure by FTIR deconvolution and curve fitting. Spectroscopy 1986, 1, 29–332. [Google Scholar]
- Staroszczyk, H.; Pielichowska, J.; Sztuka, K.; Stangret, J.; Kołodziejska, I. Molecular and structural characteristics of cod gelatin films modified with EDC and TGase. Food Chem. 2012, 130, 335–343. [Google Scholar] [CrossRef]
- Holloway, P.W.; Mantsch, H.H. Structure of cytochrome b5 in solution by Fourier-transform infrared spectroscopy. Biochemistry 1989, 28, 931–935. [Google Scholar] [CrossRef]
- Yang, W.H.; Rao, M.A. Complex viscosity-temperature master curve of cornstarch dispersion during gelatinization. J. Food Process Eng. 1998, 21, 191–207. [Google Scholar] [CrossRef]
- Min Kim, J.; Eberle, A.P.R.; Kate Gurnon, A.; Porcar, L.; Wagner, N.J. The microstructure and rheology of a model, thixotropic nanoparticle gel under steady shear and large amplitude oscillatory shear (LAOS). J. Rheol. 2014, 58, 1301–1328. [Google Scholar] [CrossRef]
- Gordon, M.B.; Kloxin, C.J.; Wagner, N.J. The rheology and microstructure of an aging thermoreversible colloidal gel. J. Rheol. 2016, 61, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Murado, M.A.; Fraguas, J.; Montemayor, M.I.; Vázquez, J.A.; González, P. Preparation of highly purified chondroitin sulphate from skate (Raja clavata) cartilage by-products. Process optimization including a new procedure of alkaline hydroalcoholic hydrolysis. Biochem. Eng. J. 2010, 49, 126–132. [Google Scholar] [CrossRef]
- Valcarcel, J.; Sanz, N.; Vázquez, J.A. Optimization of the enzymatic protein hydrolysis of by-products from seabream (Sparus aurata) and seabass (Dicentrarchus labrax), chemical and functional characterization. Foods 2020, 9, 1503. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Menduíña, A.; Nogueira, M.; Durán, A.I.; Sanz, N.; Valcarcel, J. Optimal Production of Protein Hydrolysates from Monkfish By-Products: Chemical Features and Associated Biological Activities. Molecules 2020, 25, 4068. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Fraguas, J.; Mirón, J.; Valcárcel, J.; Pérez-Martín, R.I.; Antelo, L.T. Valorisation of fish discards assisted by enzymatic hydrolysis and microbial bioconversion: Lab and pilot plant studies and preliminary sustainability evaluation. J. Clean. Prod. 2020, 246, 119027. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Montero, P. Extraction of Gelatin from Megrim (Lepidorhombus boscii) Skins with Several Organic Acids. J. Food Sci. 2001, 66, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Benjakul, S.; Oungbho, K.; Visessanguan, W.; Thiansilakul, Y.; Roytrakul, S. Characteristics of gelatin from the skins of bigeye snapper, Priacanthus tayenus and Priacanthus macracanthus. Food Chem. 2009, 116, 445–451. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: Barking, UK, 1986; ISBN 0853343861. [Google Scholar]
- Vázquez, J.A.; Blanco, M.; Massa, A.E.; Amado, I.R.; Pérez-Martín, R.I. Production of fish protein hydrolysates from Scyliorhinus canicula discards with antihypertensive and antioxidant activities by enzymatic hydrolysis and mathematical optimization using response surface methodology. Mar. Drugs 2017, 15, 306. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fernández-Compás, A.; Blanco, M.; Rodríguez-Amado, I.; Moreno, H.; Borderías, J.; Pérez-Martín, R.I. Development of bioprocesses for the integral valorisation of fish discards. Biochem. Eng. J. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemistry. Methods of Analysis; AOAC: Rockville, MD, USA, 1997. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of amino acids on sulphonated polystyrene resins. An improved system. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Miller, E.; Bimbo, A.; Walters, D.E.; Barlow, S.; Sheridan, B. Determination of nitrogen solubility in dilute pepsin hydrochloric acid solution of fishmeal: Interlaboratory study. J. AOAC Int. 2002, 85, 1374–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estévez, N.; Fuciños, P.; Sobrosa, A.C.; Pastrana, L.; Pérez, N.; Luisa Rúa, M. Modeling the angiotensin-converting enzyme inhibitory activity of peptide mixtures obtained from cheese whey hydrolysates using concentration–response curves. Biotechnol. Prog. 2012, 28, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Amado, I.R.; Vázquez, J.A.; González, M.P.; Murado, M.A. Production of antihypertensive and antioxidant activities by enzymatic hydrolysis of protein concentrates recovered by ultrafiltration from cuttlefish processing wastewaters. Biochem. Eng. J. 2013, 76, 43–54. [Google Scholar] [CrossRef]
- Wainewright, F.W. Physical tests for gelatin and gelatin products. In The Science and Technology of Gelatin; Academic Press: New York, NY, USA, 1977; pp. 507–531. [Google Scholar]
- Blanco, M.; Sanz, N.; Valcarcel, J.; Pérez-Martín, R.I.; Sotelo, C.G. Does Subunit Composition Influence the Intermolecular Crosslinking of Fish Collagen? A Study with Hake and Blue Shark Skin Collagens. Polymers 2020, 12, 1734. [Google Scholar] [CrossRef]
- Hermida-Merino, C.; Pérez-Rodríguez, M.; Pereiro, A.B.; Piñeiro, M.M.; Pastoriza-Gallego, M.J. Tailoring Nanofluid Thermophysical Profile through Graphene Nanoplatelets Surface Functionalization. ACS Omega 2018, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.-C.; Huang, Q.-Y.; Ding, W.; Xiao, X.-H.; Zhang, H.-Y.; Xiong, L.-X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]


| Method | Yield
(%, w of Gelatin/w of Skin) | Gel Strength
(g) |
|---|---|---|
| M1 | 5.22 ± 0.08 c | 177.0 ± 5.9 a |
| M2 | 8.18 ± 0.09 a | 91.0 ± 21.6 c |
| M3 | 4.82 ± 0.98 c | 132.0 ± 2.0 b |
| M4 | 9.07 ± 1.26 a | 60.5 ± 16.7 c |
| M5 | 5.20 ± 0.27 c | 81.5 ± 6.9 c |
| M6 | 4.51 ± 0.51 c | 0.0 ± 0.0 |
| M7 | 6.97 ± 0.61 b | 0.0 ± 0.0 |
| Method | Peak Number | Rt (min) | Mw (kDa) | PDI | Peak Area (%) |
|---|---|---|---|---|---|
| M1 | 1-high Mw | 37.6–44.3 | - | - | 7.9 ± 0.3 |
| 2 | 45.3 ± 0.2 | 202.7 ± 0.5 | 1.044 | 31.5 ± 0.6 | |
| 3 | 48.4 ± 0.0 | 107.1 ± 2.6 | 1.023 | 32.0 ± 1,3 | |
| 4-low Mw | 50.3–65.2 | <100 | - | 28.6 ± 0.4 | |
| M2 | 1-high Mw | 37.7–44.5 | - | - | 4.7 ± 0.2 |
| 2 | 45.6 ± 0.0 | 208.1 ± 2.9 | 1.021 | 15.3 ± 0.9 | |
| 3 | 47.6 ± 0.0 | 150.3 ± 1.3 | 1.004 | 8.4 ± 018 | |
| 4 | 48.4 ± 0.0 | 111.6 ± 0.7 | 1.012 | 20.7 ± 0.9 | |
| 5-low Mw | 48.4–67.4 | <100 | - | 50.9 ± 3.7 | |
| M3 | 1-high Mw | 36.9–44.7 | - | - | 3.8 ± 0.3 |
| 2 | 45.6 ± 0.0 | 214.6 ± 4.3 | 1.024 | 11.5 ± 2.7 | |
| 3 | 47.5 ± 0.0 | 154.1 ± 1.8 | 1.004 | 6.1 ± 0.7 | |
| 4 | 48.4 ± 0.0 | 113.5 ± 0.7 | 1.011 | 21.7 ± 1.3 | |
| 5-low Mw | 49.3–6.6 | <100 | - | 57.0 ± 5.0 | |
| M4 | 1-high Mw | 47.5 | 200.8 | 1.065 | 7.6 |
| 2 | 48.9 | 104.5 | 1.021 | 20.7 | |
| 3-low Mw | 49.9–70.0 | <100 | - | 71.72 | |
| M5 | 1-high Mw | 36.0–44.9 | - | - | 0.6 ± 0.0 |
| 2 | 47.6 ± 0.1 | 200.4 ± 14.6 | 1.085 | 5.9 ± 4.2 | |
| 3 | 48.9 ± 0.4 | 113.1 ± 1.2 | 1.007 | 10.5 ± 3.1 | |
| 4-low Mw | 49.1–69.8 | <100 | - | 83.0 ± 7.4 | |
| M6 | 1-high Mw | 37.0–44.6 | - | - | 1.1 ± 1.0 |
| 2-low Mw | 45.3–70.4 | <100 | - | 98.9 ± 1.0 | |
| M7 | 1-high Mw | 34.5–46.4 | - | - | 0.6 ± 0.2 |
| 2-low Mw | 45.7–70.3 | <100 | - | 99.4 ± 0.2 |
| Secundary Structure Elements | WaveNumber (cm−1) |
|---|---|
| β-turn/ β-sheet | 1628–1632 |
| Random Coil | 1644–1650 |
| Triple α-Helix | 1660 |
| β-turn/ β-sheet | 1680–1690 |
| CH1 | CH2 | CH3 | CH4 | |
|---|---|---|---|---|
| Mass balance and hydrolysates characteristics | ||||
| Ydig (%) | 91.0 ± 0.2 a | 93.1 ± 0.2 b | 77.0 ± 0.03 c | 77.1 ± 0.5 c |
| Yoil (%) | 2.2 ± 0.1 a | 1.9 ± 0.2 a | 1.8 ± 0.2 a | 1.6 ± 0.6 a |
| Yskin (%) | 1.9 ± 0.3 a | 1.8 ± 0.9 a | 9.0 ± 0.6 b | 7.5 ± 0.1 c |
| Pr (g/L) | 45.5 ± 0.6 a | 46.8 ± 0.4 b | 41.4 ± 0.3 c | 39.9 ± 0.4 d |
| Hm (%) | 19.7 ± 0.3 a | 24.1 ± 0.3 b | 4.2 ± 0.5 c | 5.3 ± 0.1 d |
| vm (% min −1) | 0.35 ± 0.02 a | 0.21 ± 0.01 b | 0.04 ± 0.01 c | 0.07 ± 0.00 d |
| Amino acid composition of hydrolysates | ||||
| Gly (%) | 11.9 ± 0.2 a | 11.1 ± 0.1 b | 13.8 ± 0.4 c | 12.5 ± 0.5 a |
| Glu (%) | 12.6 ± 0.4 a | 12.7 ± 0.7 a | 13.0 ± 0.5 a | 13.0 ± 0.4 a |
| Pro (%) | 7.5 ± 0.3 a | 7.1 ± 0.5 a | 8.3 ± 0.7 a | 7.6 ± 0.1 a |
| OHPro (%) | 4.0 ± 0.1 a | 4.7 ± 0.1 b | 4.8 ± 0.2 b | 4.6 ± 0.1 b |
| TEAA/TAA (%) | 38.3 ± 0.4 a | 39.2 ± 0.4 b | 35.1 ± 0.4 c | 37.4 ± 2.1 a |
| Antihypertensive and digestibility properties | ||||
| Dig (%) | 90.3 ± 0.6 a | 94.2 ± 0.7 b | 82.3 ± 1.0 c | 86.3 ± 0.8 d |
| IACE (%) | 69.5 ± 3.5 a | 88.1 ± 1.8 b | 52.1 ± 4.7 c | 65.0 ± 0.7 d |
| IC50 (µg Pr/mL) | 131.2 ± 8.5 a | 40.3 ± 3.3 b | 976.1 ± 24.2 c | 462.1 ± 12.5 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valcarcel, J.; Fraguas, J.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Vázquez, J.A. Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities. Mar. Drugs 2021, 19, 491. https://doi.org/10.3390/md19090491
Valcarcel J, Fraguas J, Hermida-Merino C, Hermida-Merino D, Piñeiro MM, Vázquez JA. Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities. Marine Drugs. 2021; 19(9):491. https://doi.org/10.3390/md19090491
Chicago/Turabian StyleValcarcel, Jesus, Javier Fraguas, Carolina Hermida-Merino, Daniel Hermida-Merino, Manuel M. Piñeiro, and José Antonio Vázquez. 2021. "Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities" Marine Drugs 19, no. 9: 491. https://doi.org/10.3390/md19090491
APA StyleValcarcel, J., Fraguas, J., Hermida-Merino, C., Hermida-Merino, D., Piñeiro, M. M., & Vázquez, J. A. (2021). Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities. Marine Drugs, 19(9), 491. https://doi.org/10.3390/md19090491











