Marine Fungal Cerebroside Flavuside B Protects HaCaT Keratinocytes against Staphylococcus aureus Induced Damage
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity of Flavuside B against SA
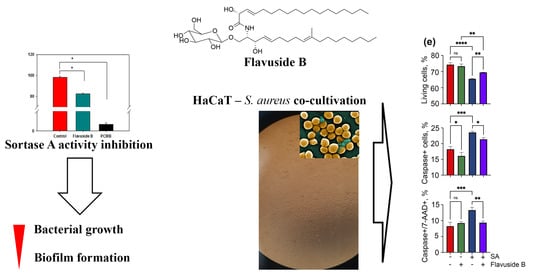
2.2. Sortase A Activity Inhibition
2.3. Influence of Flavuside B on HaCaT Cells Co-Cultivated with SA
2.3.1. The Viability of SA-Treated HaCaT Cells
2.3.2. Apoptotic Profile, Total Caspase Activity and Cell Cycle in SA-Treated HaCaT Cells
2.4. Influence on LPS-Induced HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Flavuside B
4.2. Antimicrobial Activity against Staphilococcus aureus
4.2.1. The Effect on Bacterial Growth
4.2.2. The Effect on Biofilm Formation
4.3. The Effect on Sortase A Enzymatic Activity
4.4. HaCaT Cell Culture
4.5. Co-Cultivation of HaCaT Cells with SA
4.6. The Treatment of HaCaT Cells with Lipopolysaccharide (LPS)
4.7. Lactate Dehydrogenase (LDH) Release Assay
4.8. Formazan Production (MTT) Assay
4.9. Flow Cytometry
4.9.1. Apoptosis
4.9.2. Total Caspase Activity
4.9.3. Cell Cycle
4.10. The NO Level Estimation
4.11. Statistical Data Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abril, A.G.; Villa, T.G.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Abbafati, C.; Machado, D.B.; Cislaghi, B.; Salman, O.M.; Karanikolos, M.; McKee, M.; Abbas, K.M.; Brady, O.J.; Larson, H.J.; Trias-Llimós, S.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Cai, S.; Gu, G.; Guo, Z.; Long, Z. Recent progress in the development of sortase A inhibitors as novel anti-bacterial virulence agents. RSC Adv. 2015, 5, 49880–49889. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, G.; Jensen, E.R.; Lenoy, E.; Schneewind, O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 2000, 97, 5510–5515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer Carol, L.; Drake David, R.; Dawson Deborah, V.; Blanchette Derek, R.; Brogden Kim, A.; Wertz Philip, W. Antibacterial Activity of Sphingoid Bases and Fatty Acids against Gram-Positive and Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2012, 56, 1157–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, H.; Mamadalieva, N.Z.; Ali, I.; Elizbit; Green, I.R.; Wang, D.; Zou, L.; Simal-Gandara, J.; Cao, H.; Xiao, J. Fungal glycosides: Structure and biological function. Trends Food Sci. Technol. 2021, 110, 611–651. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Sun, K.; Liu, P.; Yin, X.; Zhu, W. Cerebrosides and 2-pyridone alkaloids from the halotolerant fungus Penicillium chrysogenum grown in a hypersaline medium. J. Nat. Prod. 2011, 74, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Murshid, S.S.A.; Badr, J.M.; Youssef, D.T.A. Penicillosides A and B: New cerebrosides from the marine-derived fungus Penicillium species. Rev. Bras. Farmacogn. 2016, 26, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Sandjo, L.; Yun, K.; Leutou, A.S.; Kim, G.D.; Choi, H.D.; Kang, J.S.; Hong, J.; Son, B.W. Flavusides A and B, antibacterial cerebrosides from the marine-derived fungus Aspergillus Flavus. Chem. Pharm. Bull. 2011, 59, 1174–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurchenko, A.N.; Smetanina, O.F.; Ivanets, E.V.; Kirichuk, N.N.; Khudyakova, Y.V.; Yurchenko, E.A.; Afiyatullov, S.S. Metabolites from the Facultative Marine Fungus Penicillium Islandicum. Chem. Nat. Compd. 2016, 52, 365–367. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.C. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen. 2009, 17, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Celis, U.; López-Martínez, F.J.; Cervantes-Jiménez, R.; Ferríz-Martínez, R.A.; Blanco-Labra, A.; García-Gasca, T. Tepary Bean (Phaseolus acutifolius) Lectins Induce Apoptosis and Cell Arrest in G0/G1 by P53(Ser46) Phosphorylation in Colon Cancer Cells. Molecules 2020, 25, 1021. [Google Scholar] [CrossRef] [Green Version]
- Bedner, E.; Smolewski, P.; Amstad, P.; Darzynkiewicz, Z. Activation of Caspases Measured in Situ by Binding of Fluorochrome-Labeled Inhibitors of Caspases (FLICA): Correlation with DNA Fragmentation. Exp. Cell Res. 2000, 259, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.J.; Choi, H.G.; Chung, H.J.; Hong, C.K. Time course of expression of mRNA of inducible nitric oxide synthase and generation of nitric oxide by ultraviolet B in keratinocyte cell lines. Br. J. Dermatol. 2002, 147, 655–662. [Google Scholar] [CrossRef]
- De Kimpe, S.J.; Kengatharan, M.; Thiemermann, C.; Vane, J.R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 1995, 92, 10359–10363. [Google Scholar] [CrossRef] [Green Version]
- Kojima, H.; Nakatsubo, N.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Detection and Imaging of Nitric Oxide with Novel Fluorescent Indicators: Diaminofluoresceins. Anal. Chem. 1998, 70, 2446–2453. [Google Scholar] [CrossRef]
- Chen, J.H.; Cui, G.Y.; Liu, J.Y.; Tan, R.X. Pinelloside, an antimicrobial cerebroside from Pinellia ternata. Phytochemistry 2003, 64, 903–906. [Google Scholar] [CrossRef]
- Cateni, F.; Zilic, J.; Falsone, G.; Scialino, G.; Banfi, E. New cerebrosides from Euphorbia peplis L.: Antimicrobial activity evaluation. Bioorganic Med. Chem. Lett. 2003, 13, 4345–4350. [Google Scholar] [CrossRef]
- Shu, R.G.; Wang, F.W.; Yang, Y.M.; Liu, Y.X.; Tan, R.X. Antibacterial and xanthine oxidase inhibitory cerebrosides from Fusarium sp. IFB-121, an endophytic fungus in Quercus variabilis. Lipids 2004, 39, 667–673. [Google Scholar] [CrossRef]
- Cortés-Sánchez, A.d.J.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.R.; Xisto, M.I.; Pele, M.A.; Alviano, D.S.; Alviano, C.S.; Barreto-Bergter, E.; De Campos-Takaki, G.M. Monohexosylceramides from Rhizopus Species Isolated from Brazilian Caatinga: Chemical Characterization and Evaluation of Their Anti-Biofilm and Antibacterial Activities. Molecules 2018, 23, 1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, N.; Hu, C.; Zhang, Y.; Chen, S. Interaction of sortase A and lipase 2 in the inhibition of Staphylococcus aureus biofilm formation. Arch. Microbiol. 2009, 191, 879. [Google Scholar] [CrossRef] [PubMed]
- Thappeta, K.R.; Zhao, L.N.; Nge, C.E.; Crasta, S.; Leong, C.Y.; Ng, V.; Kanagasundaram, Y.; Fan, H.; Ng, S.B. In-Silico Identified New Natural Sortase A Inhibitors Disrupt S. aureus Biofilm Formation. Int. J. Mol. Sci. 2020, 21, 8601. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Shin, D.-S.; Oh, M.-N.; Chung, S.-C.; Lee, J.-S.; Chang, I.-M.; Oh, K.-B. Inhibition of Sortase, a Bacterial Surface Protein Anchoring Transpeptidase, by β-Sitosterol-3-O-glucopyranoside from Fritillaria verticillata. Biosci. Biotechnol. Biochem. 2003, 67, 2477–2479. [Google Scholar] [CrossRef]
- Nitulescu, G.; Margina, D.; Zanfirescu, A.; Olaru, O.T.; Nitulescu, G.M. Targeting bacterial sortases in search of anti-virulence therapies with low risk of resistance development. Pharmaceuticals 2021, 14, 415. [Google Scholar] [CrossRef]
- Reddersen, K.; Greber, K.E.; Korona-Glowniak, I.; Wiegand, C. The short lipopeptides (C10)2-kkkk-nh2 and (c12)2-kkkk-nh2 protect hacat keratinocytes from bacterial damage caused by staphylococcus aureus infection in a co-culture model. Antibiotics 2020, 9, 879. [Google Scholar] [CrossRef]
- de Carvalho Dias, K.; Barbugli, P.A.; de Patto, F.; Lordello, V.B.; de Aquino Penteado, L.; Medeiros, A.I.; Vergani, C.E. Soluble factors from biofilm of Candida albicans and Staphylococcus aureus promote cell death and inflammatory response. BMC Microbiol. 2017, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Juráňová, J.; Franková, J.; Ulrichová, J. The role of keratinocytes in inflammation. J. Appl. Biomed. 2017, 15, 169–179. [Google Scholar] [CrossRef]
- Haugwitz, U.; Bobkiewicz, W.; Han, S.-R.; Beckmann, E.; Veerachato, G.; Shaid, S.; Biehl, S.; Dersch, K.; Bhakdi, S.; Husmann, M. Pore-forming Staphylococcus aureus α-toxin triggers epidermal growth factor receptor-dependent proliferation. Cell. Microbiol. 2006, 8, 1591–1600. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Chingizova, E.A.; Girich, E.V.; Yurchenko, A.N.; Aminin, D.L.; Mikhailov, V.V. Cytoprotective Activity of p-Terphenyl Polyketides and Flavuside B from Marine-Derived Fungi against Oxidative Stress in Neuro-2a Cells. Molecules 2021, 26, 3618. [Google Scholar] [CrossRef]
- Pavicic, T.; Wollenweber, U.; Farwick, M.; Korting, H.C. Anti-microbial and -inflammatory activity and efficacy of phytosphingosine: An in vitro and in vivo study addressing acne vulgaris. Int. J. Cosmet. Sci. 2007, 29, 181–190. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Tao, H.; Peng, X.; Liu, P.; Zhu, W. Cerebrosides of the Halotolerant Fungus Alternaria raphani Isolated from a Sea Salt Field. J. Nat. Prod. 2009, 72, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-X.; Sun, Y.; Guo, W.-J.; Gu, Y.-H.; Wu, X.-F.; Tan, R.-X.; Xu, Q. Rebuilding the balance of STAT1 and STAT3 signalings by fusaruside, a cerebroside compound, for the treatment of T-cell-mediated fulminant hepatitis in mice. Biochem. Pharmacol. 2012, 84, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Sabutski, Y.E.; Menchinskaya, E.S.; Shevchenko, L.S.; Chingizova, E.A.; Chingizov, A.R.; Popov, R.S.; Denisenko, V.A.; Mikhailov, V.V.; Aminin, D.L.; Polonik, S.G. Synthesis and evaluation of antimicrobial and cytotoxic activity of oxathiine-fused quinone-thioglucoside conjugates of substituted 1,4-naphthoquinones. Molecules 2020, 25, 3577. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Luca, V.; Segev-Zarko Li-av, T.; Shai, Y.; Mangoni Maria, L. Temporins A and B Stimulate Migration of HaCaT Keratinocytes and Kill Intracellular Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 2520–2527. [Google Scholar] [CrossRef] [Green Version]
- Zhuravleva, O.I.; Antonov, A.S.; Oleinikova, G.K.; Khudyakova, Y.V.; Popov, R.S.; Denisenko, V.A.; Pislyagin, E.A.; Chingizova, E.A.; Afiyatullov, S.S. Virescenosides from the holothurian-associated fungus Acremonium striatisporum KMM 4401. Mar. Drugs 2019, 17, 616. [Google Scholar] [CrossRef] [Green Version]







| Concentration, µM | Inhibition of Bacterial Growth, % | Inhibition of Biofilm Formation, % |
|---|---|---|
| 10.0 | 27.05 ± 1.48 | 28.93 ± 2.17 |
| 25.0 | 28.44 ± 3.15 | 23.54 ± 1.47 |
| 50.0 | 38.02 ± 2.31 | 25.86 ± 1.09 |
| 100.0 | 49.13 ± 2.05 | 28.32 ± 3.05 |
| Time, min | Inhibition of SA Sortase A Activity, % | |
|---|---|---|
| Flavuside B Concentration, µM | ||
| 10.0 | 50.0 | |
| 5 | 17.15 ± 0.75 * | 15.43 ± 1.55 * |
| 10 | 17.35 ± 1.12 * | 12.29 ± 1.20 * |
| 15 | 15.84 ± 1.17 * | 9.75 ± 0.95 * |
| 20 | 13.82 ± 1.55 * | 8.12 ± 0.82 * |
| 25 | 12.78 ± 1.47 * | 8.63 ± 0.85 * |
| 30 | 11.80 ± 1.32 * | 9.81 ± 0.98 * |
| 35 | 11.15 ± 1.21 * | 10.13 ± 1.02 * |
| 40 | 11.30 ± 1.12 * | 8.87 ± 0.87 * |
| 45 | 10.73 ± 1.22 * | 7.23 ± 0.72 * |
| 50 | 10.34 ± 1.13 * | 6.10 ± 0.55 * |
| 55 | 9.77 ± 1.1 2* | 5.61 ± 0.52 * |
| 60 | 10.16 ± 1.06 * | 4.29 ± 0.39 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chingizova, E.A.; Menchinskaya, E.S.; Chingizov, A.R.; Pislyagin, E.A.; Girich, E.V.; Yurchenko, A.N.; Guzhova, I.V.; Mikhailov, V.V.; Aminin, D.L.; Yurchenko, E.A. Marine Fungal Cerebroside Flavuside B Protects HaCaT Keratinocytes against Staphylococcus aureus Induced Damage. Mar. Drugs 2021, 19, 553. https://doi.org/10.3390/md19100553
Chingizova EA, Menchinskaya ES, Chingizov AR, Pislyagin EA, Girich EV, Yurchenko AN, Guzhova IV, Mikhailov VV, Aminin DL, Yurchenko EA. Marine Fungal Cerebroside Flavuside B Protects HaCaT Keratinocytes against Staphylococcus aureus Induced Damage. Marine Drugs. 2021; 19(10):553. https://doi.org/10.3390/md19100553
Chicago/Turabian StyleChingizova, Ekaterina A., Ekaterina S. Menchinskaya, Artur R. Chingizov, Evgeny A. Pislyagin, Elena V. Girich, Anton N. Yurchenko, Irina V. Guzhova, Valery V. Mikhailov, Dmitry L. Aminin, and Ekaterina A. Yurchenko. 2021. "Marine Fungal Cerebroside Flavuside B Protects HaCaT Keratinocytes against Staphylococcus aureus Induced Damage" Marine Drugs 19, no. 10: 553. https://doi.org/10.3390/md19100553
APA StyleChingizova, E. A., Menchinskaya, E. S., Chingizov, A. R., Pislyagin, E. A., Girich, E. V., Yurchenko, A. N., Guzhova, I. V., Mikhailov, V. V., Aminin, D. L., & Yurchenko, E. A. (2021). Marine Fungal Cerebroside Flavuside B Protects HaCaT Keratinocytes against Staphylococcus aureus Induced Damage. Marine Drugs, 19(10), 553. https://doi.org/10.3390/md19100553