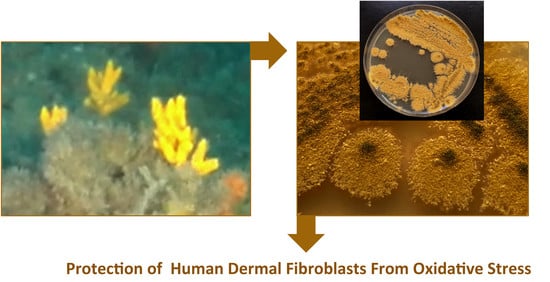
Marine Fungus Aspergillus chevalieri TM2-S6 Extract Protects Skin Fibroblasts from Oxidative Stress
Abstract
1. Introduction
2. Results
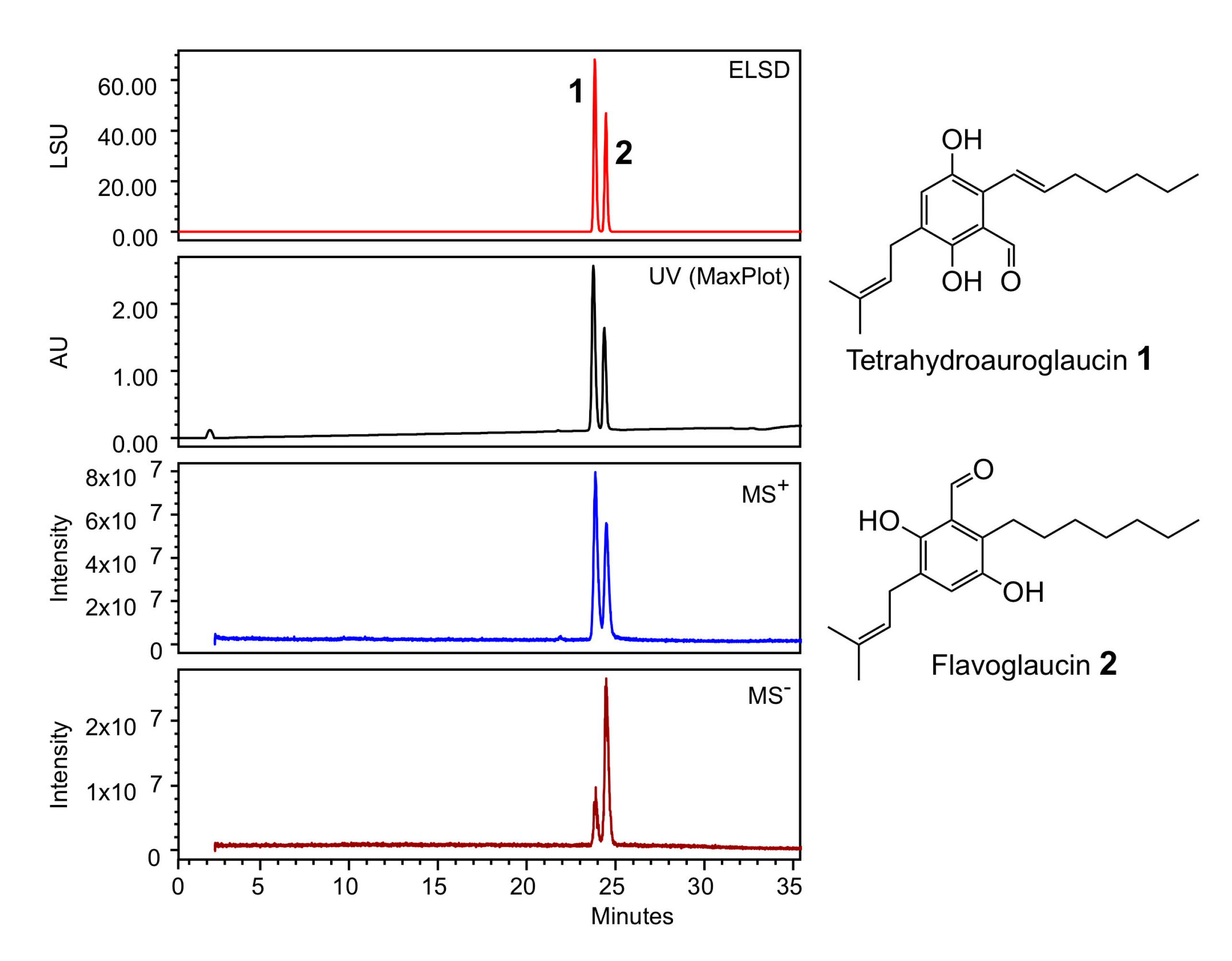
2.1. Phylogeny and Structural Investigation
2.2. Bioassays on Primary Human Fibroblasts
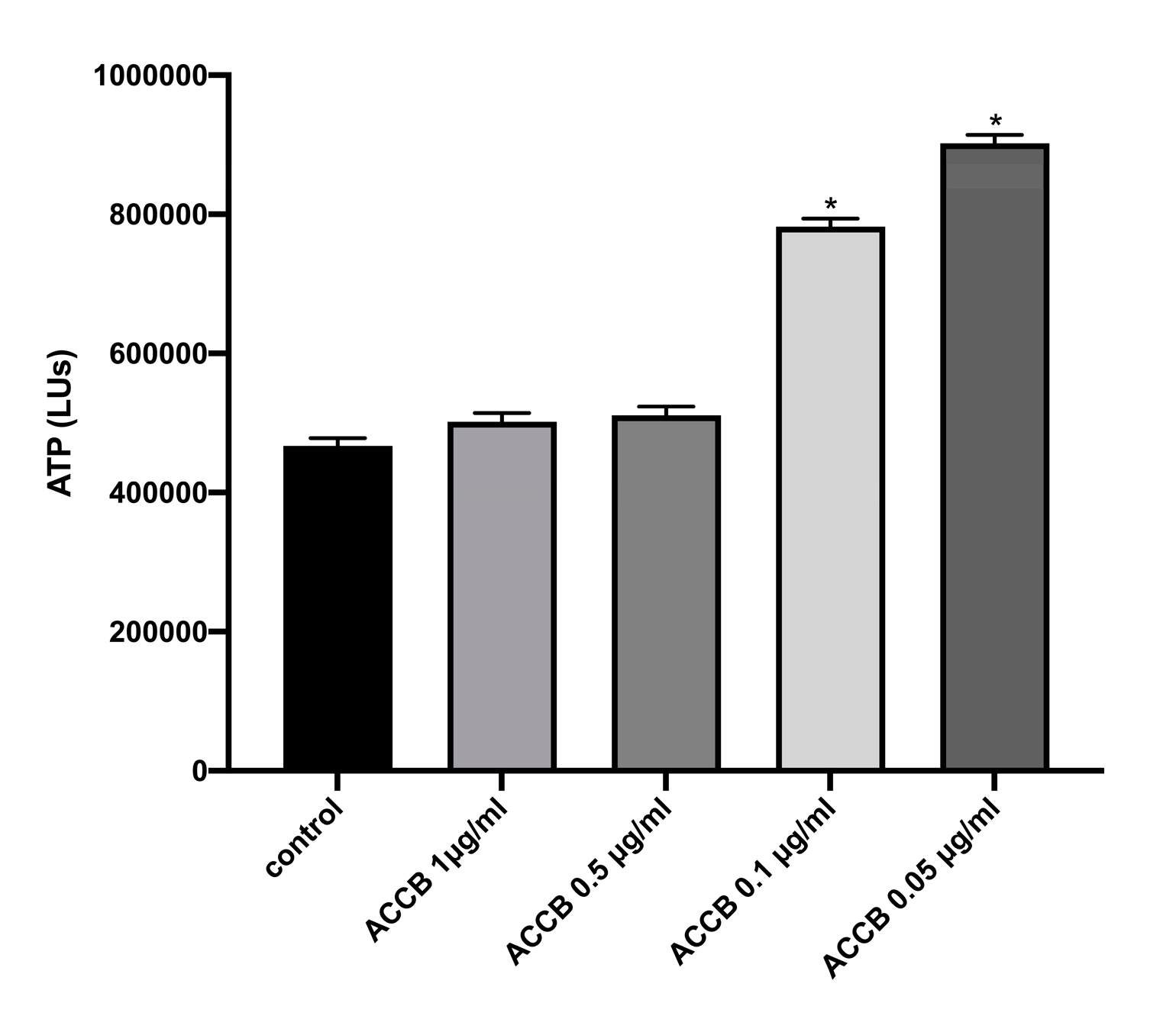
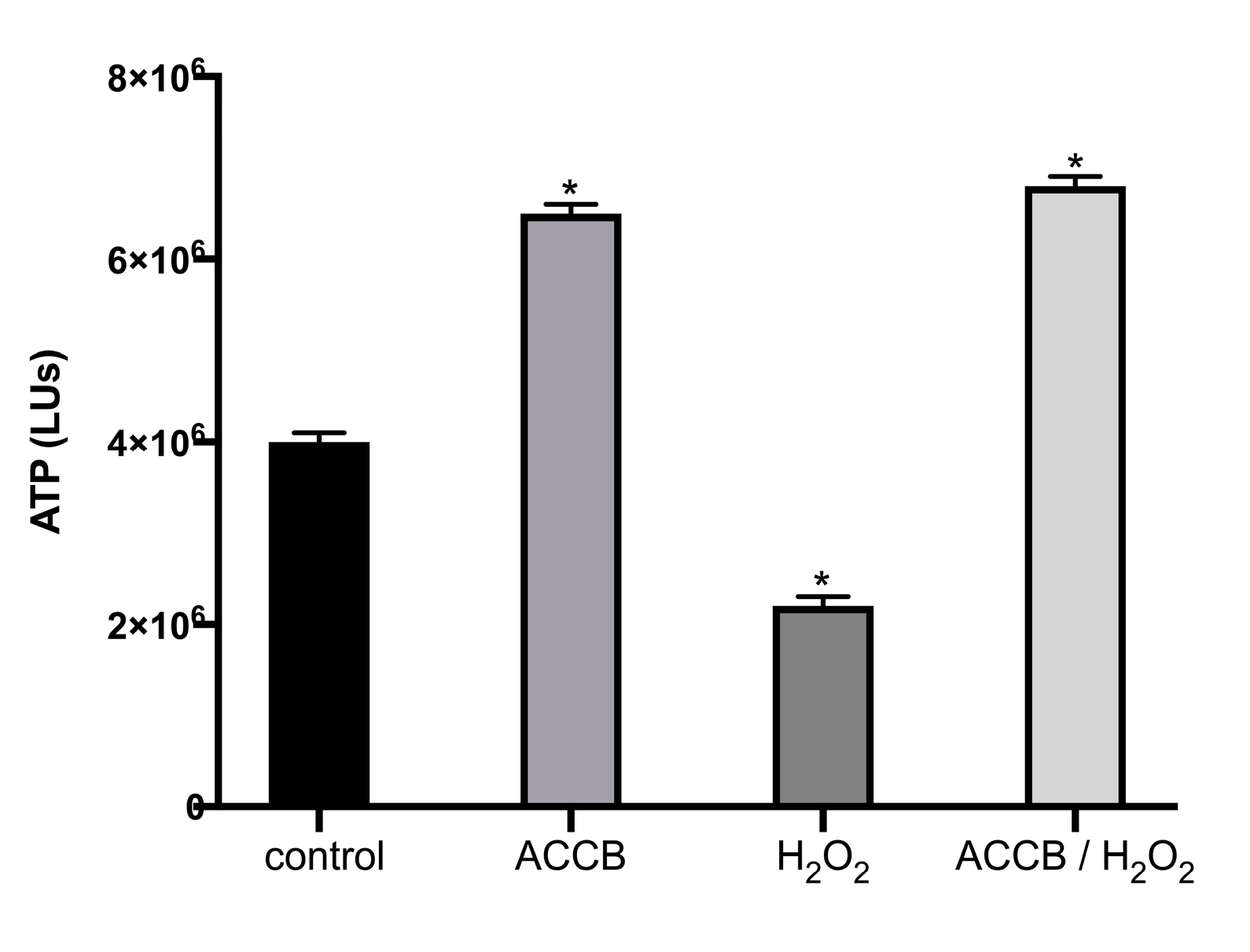
2.2.1. Cell Viability in Vitro
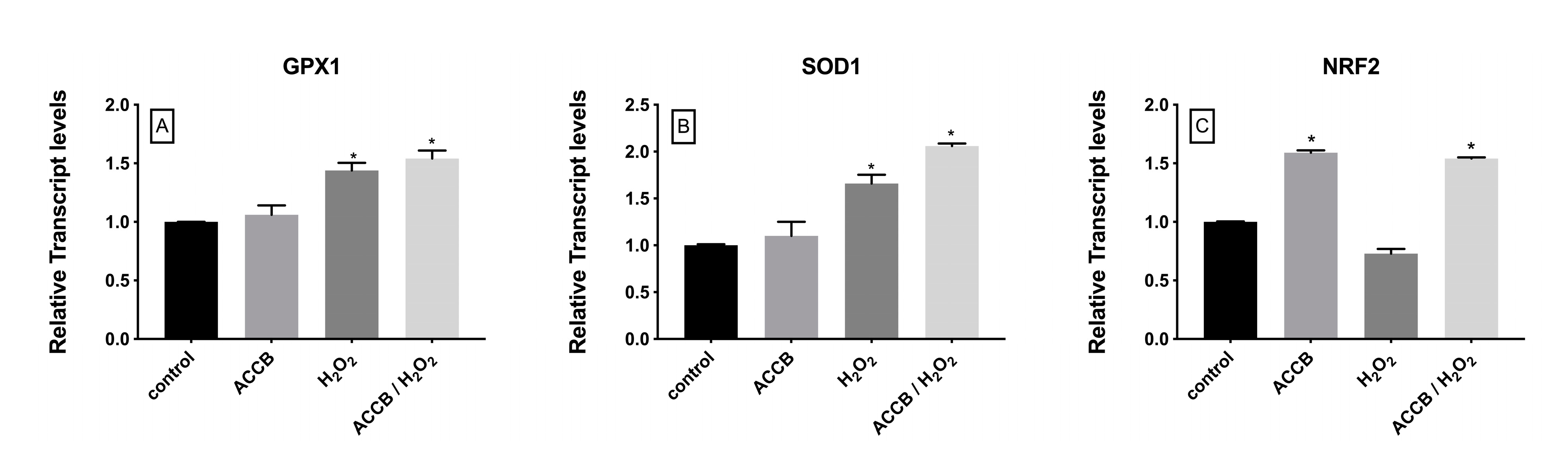
2.2.2. Genes Involved in Antioxidant Response Cell Pathway
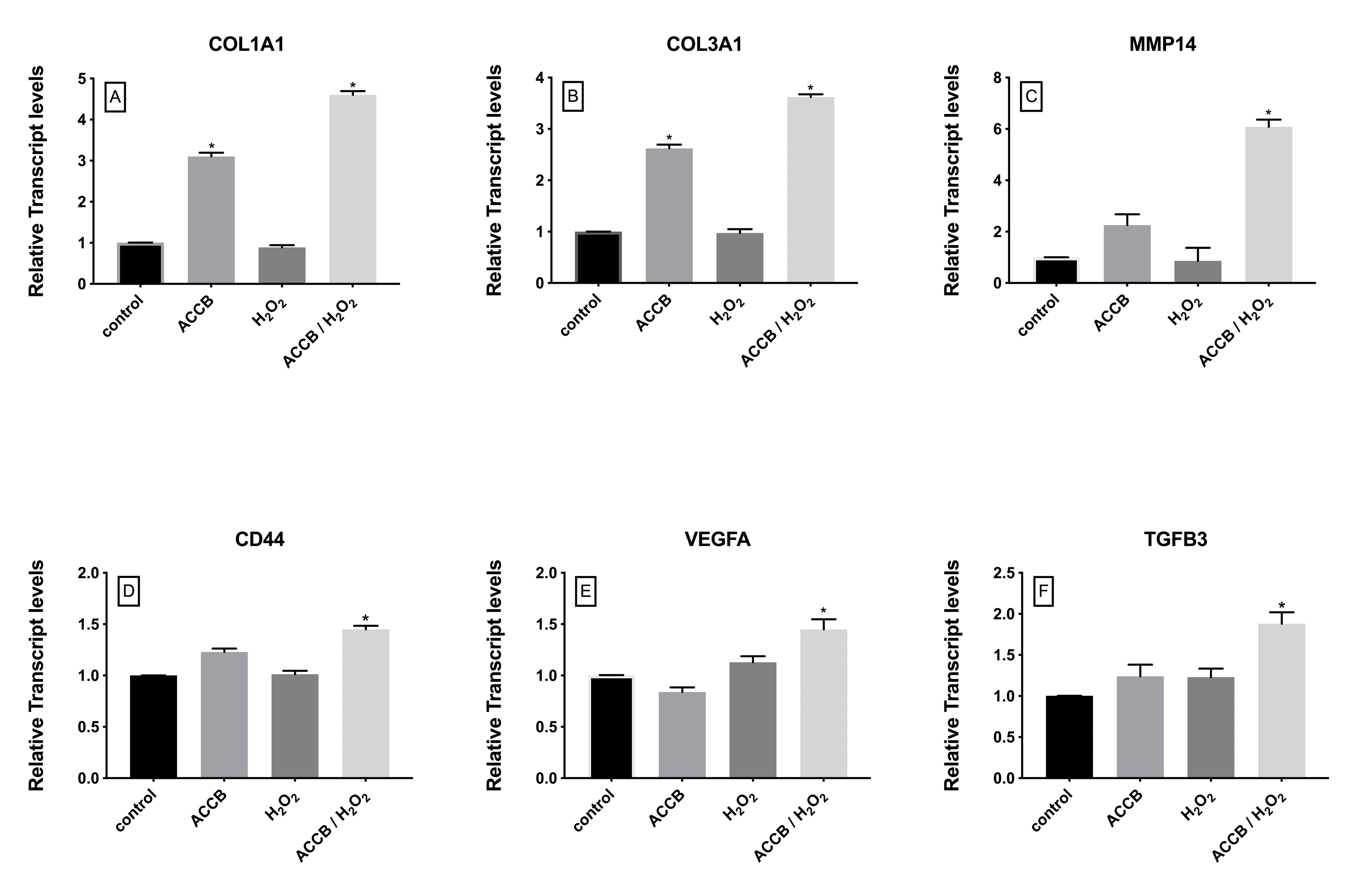
2.2.3. Genes Involved in Cell Proliferation and Extracellular Matrix Organization
2.2.4. Genes Involved in Cell Aging Pathway
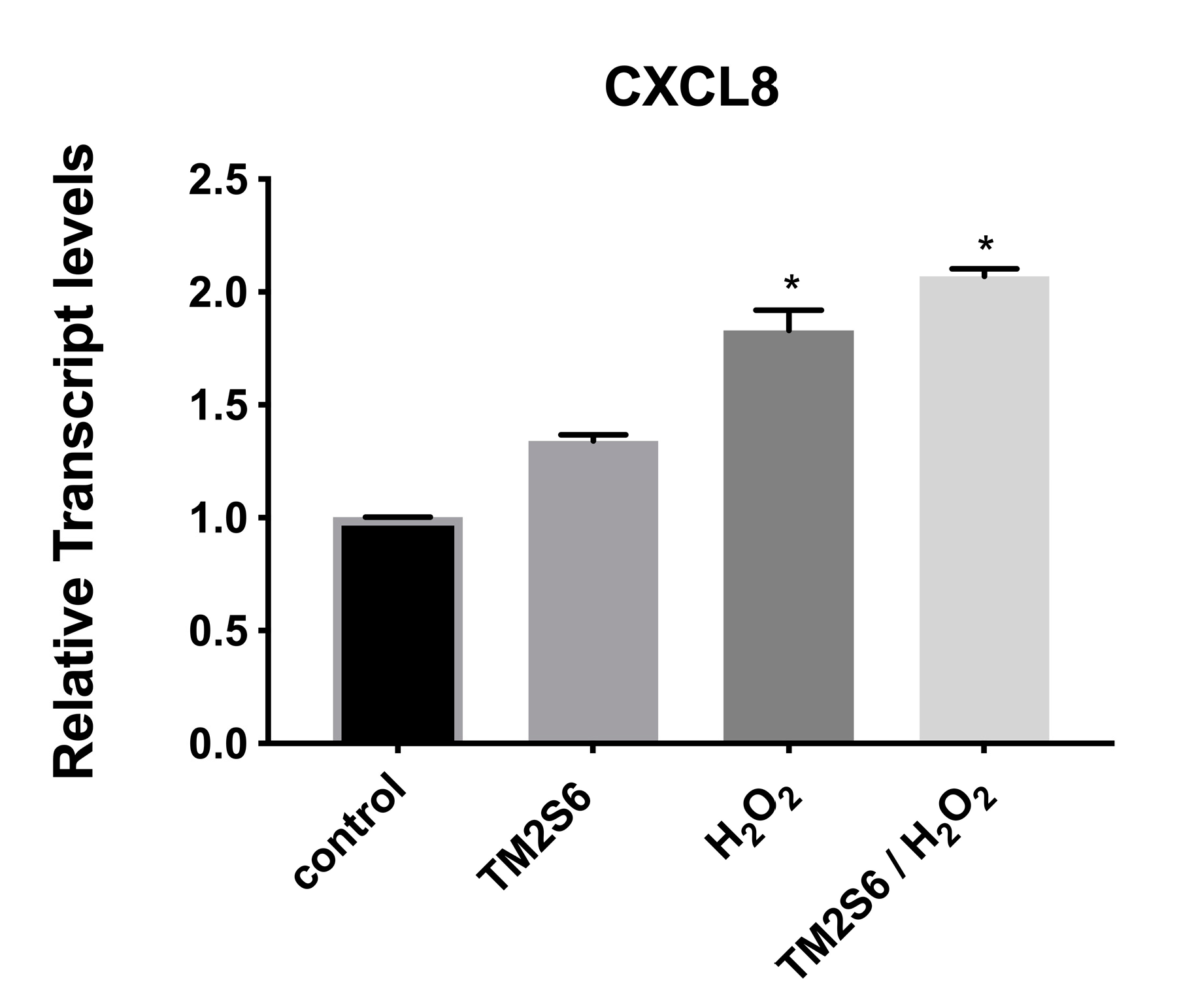
2.2.5. Genes Involved in Inflammation
3. Discussion
4. Materials and Methods
4.1. Strain Isolation and Identification
4.2. Phylogeny Investigation
4.3. Cultivation and Extract Preparation
4.4. Human Skin Cell Culture
4.5. Cell Viability Assay
4.6. H2O2 Treatment
4.7. Gene Expression Analysis by Real-Time RT-qPCR
4.7.1. RNA Extraction and cDNA Synthesis
4.7.2. RT-qPCR Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, T.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing. Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Beacham, D.A.; Amatangelo, M.D.; Cukierman, E. Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. Curr. Protoc. Cell. Biol. 2006, 33, 10.9.1–10.9.21. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. Natural Products from Marine Organisms and Their Associated Microbes. Chem. Biol. Chem. 2006, 7, 229–238. [Google Scholar] [CrossRef]
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine Drugs from Sponge-Microbe Association—A Review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine Microbial-Derived Molecules and Their Potential Use in Cosmeceutical and Cosmetic Products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Li, X.; Li, X.M.; Xu, G.M.; Li, C.S.; Wang, B.G. Antioxidant metabolites from marine alga-derived fungus Aspergillus wentii EN-48. Phytochem. Lett. 2014, 7, 120–123. [Google Scholar] [CrossRef]
- Song, F.; Ren, B.; Chen, C.; Yu, K.; Liu, X.; Zhang, Y.; Yang, N.; He, H.; Liu, X.; Dai, H.; et al. Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl. Microbiol. Biotechnol. 2014, 98, 3753–3758. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Kim, S.K.; Kang, J.S.; Choi, H.D.; Rho, J.R.; Son, B.W. Golmaenone, a New Diketopiperazine Alkaloid from the Marine-Derived Fungus Aspergillus sp. Chem. Pharm. Bull. (Tokyo) 2004, 52, 375–376. [Google Scholar] [CrossRef]
- Letsiou, S.; Bakea, A.; Le Goff, G.; Lopes, P.; Gardikis, Κ.; Alonso, C.; Alvarez, P.A.; Ouazzani, J. In vitro protective effects of marine-derived Aspergillus puulaauensis TM124-S4 extract on H2O2-stressed primary human fibroblasts. Toxicol. In Vitro 2020, 66, 104869. [Google Scholar] [CrossRef]
- Heydari, H.; Koc, A.; Simsek, D.; Gozcelioglu, B.; Altanlar, N.; Konuklugil, B. Isolation, identification and bioactivity screening of turkish marine-derived fungi. Farmacia 2019, 67, 780–788. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Morimoto, K.; Piamasaki, T. Flavoglaucin, a Metabolite of Eurotium cheva, lieri, its Antioxidation and Synergism with Tocopherol. JAOCS 1984, 61, 1864–1868. [Google Scholar] [CrossRef]
- Miyake, Y.; Ito, C.; Itoigawa, M.; Osawa, T. Antioxidants Produced by Eurotium herbariorum of Filamentous Fungi Used for the Manufacture of Karebushi, Dried Bonito (Katsuobushi). Biosci. Biotechnol. Biochem. 2009, 73, 1323–1327. [Google Scholar] [CrossRef]
- Nagarkatti, M.; Rieder, S.A.; Nagarkatti, P.S. Evaluation of Cell Proliferation and Apoptosis in Immunotoxicity Testing. Methods Mol. Biol. 2018, 1803, 209–230. [Google Scholar]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell. Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, K.; Liu, Q.; Quiles, J.L.; Filosa, R.; Kamal, M.A.; Wang, F.; Kay, G.; Zou, X.; Teng, H.; et al. Protective effects of raspberry on the oxidative damage in HepG2 cells through Keap1/Nrf2-dependent signaling pathway. Food Chem. Toxicol. 2019, 133, 110781. [Google Scholar] [CrossRef] [PubMed]
- Gurjala, A.N.; Liu, W.R.; Mogford, J.E.; Procaccini, P.S.A.; Mustoe, T.A. Age-dependent response of primary human dermal fibroblasts to oxidative stress: Cell survival, pro-survival kinases, and entrance into cellular senescence. Wound Repair Regen. 2005, 13, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Pohl, C.; Calles, C.; Marks, C.; Wild, S.; Krutmann, J. Cellular response to infrared radiation involves retrograde mitochondrial signaling. Free Radic. Biol. Med. 2007, 43, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.A.F.T.; Jubri, Z.; Rajab, N.; Rahim, K.A.; Yusof, Y.A.M.; Makpol, S. Gelam Honey Protects against Gamma-Irradiation Damage to Antioxidant Enzymes in Human Diploid Fibroblasts. Molecules 2013, 18, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, O.; Cousins, S.W.; Marin-Castaño, M.E. MMP-14 and TIMP-2 overexpression protects against hydroquinone-induced oxidant injury in RPE: Implications for extracellular matrix turnover. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5662–5670. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Korivi, M.; Lin, F.-Y.; Li, M.-L.; Lin, R.-W.; Wu, J.-J.; Yang, H.-L. Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblasts. J. Dermatol. Sci. 2018, 90, 123–134. [Google Scholar] [CrossRef]
- Qiang, M. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar]
- Lee, S.-H.; Lee, J.-H.; Lee, J.-H.; Min, K.-J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- Asparuhova, M.B.; Kiryak, D.; Eliezer, M.; Mihov, D.; Sculean, A. Activity of two hyaluronan preparations on primary human oral fibroblasts. J. Periodontal Res. 2019, 54, 33–45. [Google Scholar] [CrossRef]
- Stefani, M.; Markus, M.A.; Lin, R.C.Y.; Pinese, M.; Dawes, I.W.; Morris, B.J. The Effect of Resveratrol on a Cell Model of Human Aging. Ann. N. Y. Acad. Sci. 2007, 1114, 407–418. [Google Scholar] [CrossRef]
- Kyoung Kim, H.; Kyoung Kim, Y.; Song, I.-H.; Baek, S.-H.; Lee, S.-R.; Hye Kim, J.; Kim, J.-R. Down-Regulation of a Forkhead Transcription Factor, FOXO3a, Accelerates Cellular Senescence in Human Dermal Fibroblasts. J. Gerontol. Series A 2005, 60, 4–9. [Google Scholar] [CrossRef]
- Lu, Q.; Zhai, Y.; Cheng, Q.; Liu, Y.; Gao, X.; Zhang, T.; Wei, Y.; Zhang, F.; Yin, X. The Akt-FoxO3a-manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp. Physiol. 2013, 98, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J. A forkhead in the road to longevity: The molecular basis of lifespan becomes clearer. J. Hypertens. 2005, 23, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, R.J.; Voisin, S.; Russell, A.; Lamon, S. Recent advances in understanding the role of FOXO3. F1000Research. 2018, 31, 1372. [Google Scholar] [CrossRef]
- Fibbe, W.E.; Shi, Y. FOXO3, a Molecular Search for the Fountain of Youth. Cell Stem Cell 2019, 24, 351–352. [Google Scholar] [CrossRef]
- Watson, R.E.B.; Ogden, S.; Cotterell, L.F.; Bowden, J.J.; Bastrilles, J.Y.; Long, S.P.; Griffiths, C.E.M. A cosmetic ‘anti-ageing’ product improves photoaged skin: A double-blind, randomized controlled trial. Br. J. Dermatol. 2009, 161, 419–426. [Google Scholar] [CrossRef]
- Sharma, U.; Carrique, L.; Vadon-Le Goff, S.; Mariano, N.; Georges, R.-N.; Delolme, F.; Koivunen, P.; Myllyharju, J.; Moali, C.; Aghajari, N.; et al. Structural basis of homo- and heterotrimerization of collagen I. Nat. Commun. 2017, 8, 14671. [Google Scholar] [CrossRef]
- Remoué, N.; Molinari, J.; Andres, E.; Lago, J.C.; Barrichello, C.; Moreira, P.L. Development of an in vitro model of menopause using primary human dermal fibroblasts. Int. J. Cosmet. Sci. 2013, 35, 546–554. [Google Scholar] [CrossRef]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. Picardo, M, editor. PLoS ONE. 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.P.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Moens, S.; Goveia, J.; Stapor, P.C.; Cantelmo, A.R.; Carmeliet, P. The multifaceted activity of VEGF in angiogenesis—Implications for therapy responses. Cytokine Growth Factor Rev. 2014, 25, 473–482. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, E.; Alvarez, P.; Merino, R. TGFβ Superfamily Members as Regulators of B Cell Development and Function—Implications for Autoimmunity. Int. J. Mol. Sci. 2018, 19, 3928. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Morgan, C.; Nigam, Y. Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis 2013, 16, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Lee, U.; Kang, J.S.; Choi, H.C.; Son, B.W. A New Radical Scavenging Anthracene Glycoside, Asperflavin Ribofuranoside, and Polyketides from a Marine Isolate of the Fungus Microsporum. Chem. Pharm. Bull. 2006, 54, 882–883. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perronne, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-Rich Strawberry Extract Protects Human Dermal Fibroblasts against Hydrogen Peroxide Oxidative Damage and Improves Mitochondrial Functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Kalliampakou, K.; Gardikis, K.; Mantecon, L.; Infante, C.; Chatzikonstantinou, M.; Labrou, N.E.; Flemetakis, E. Skin Protective Effects of Nannochloropsis gaditana Extract on H2O2-Stressed Human Dermal Fibroblasts. Front. Mar. Sci. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Mahaseth, T.; Kuzminov, A. Potentiation of hydrogen peroxide toxicity: From catalase inhibition to stable DNA-iron complexes. Mutat. Res. 2017, 773, 274–281. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letsiou, S.; Bakea, A.; Goff, G.L.; Lopes, P.; Gardikis, K.; Weis, M.; Benayahu, Y.; Ouazzani, J. Marine Fungus Aspergillus chevalieri TM2-S6 Extract Protects Skin Fibroblasts from Oxidative Stress. Mar. Drugs 2020, 18, 460. https://doi.org/10.3390/md18090460
Letsiou S, Bakea A, Goff GL, Lopes P, Gardikis K, Weis M, Benayahu Y, Ouazzani J. Marine Fungus Aspergillus chevalieri TM2-S6 Extract Protects Skin Fibroblasts from Oxidative Stress. Marine Drugs. 2020; 18(9):460. https://doi.org/10.3390/md18090460
Chicago/Turabian StyleLetsiou, Sophia, Artemis Bakea, Géraldine Le Goff, Philippe Lopes, Konstantinos Gardikis, Michal Weis, Yehuda Benayahu, and Jamal Ouazzani. 2020. "Marine Fungus Aspergillus chevalieri TM2-S6 Extract Protects Skin Fibroblasts from Oxidative Stress" Marine Drugs 18, no. 9: 460. https://doi.org/10.3390/md18090460
APA StyleLetsiou, S., Bakea, A., Goff, G. L., Lopes, P., Gardikis, K., Weis, M., Benayahu, Y., & Ouazzani, J. (2020). Marine Fungus Aspergillus chevalieri TM2-S6 Extract Protects Skin Fibroblasts from Oxidative Stress. Marine Drugs, 18(9), 460. https://doi.org/10.3390/md18090460