The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia reniformis (Nardo, 1847)
Abstract
1. Introduction
2. Results
2.1. General Observations
2.2. Natural Sponge Morphology
2.3. Effect of Depth and Transplantation on Morphology
2.4. Clearance and Respiration Rates
2.5. Survival and Growth Rates
2.6. Collagen Quantification
3. Discussion
3.1. Morphology and Bacterial Clearance Rates in Relation to Depth
3.2. Survival, Growth, and Collagen Content
4. Materials and Methods
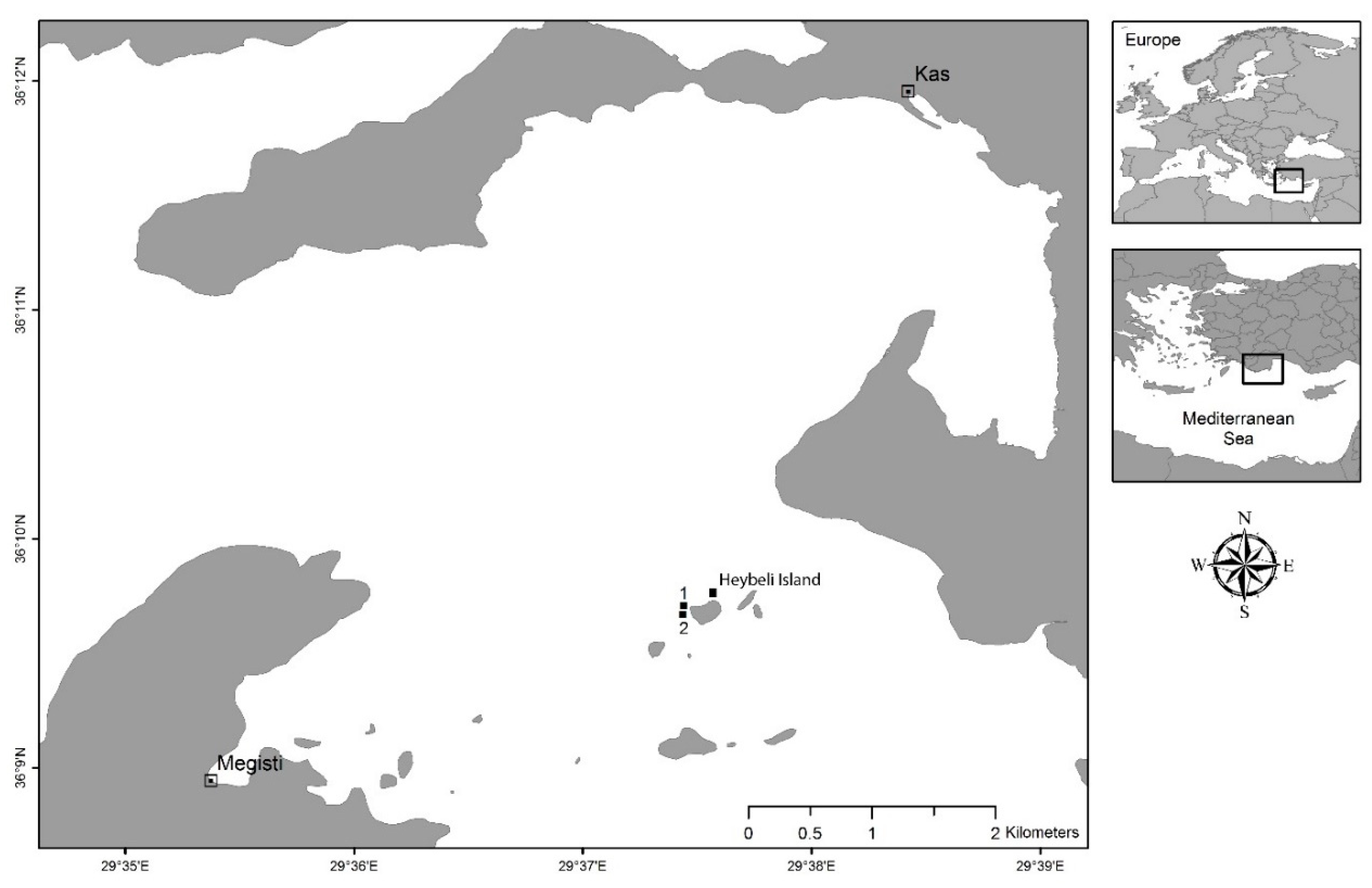
4.1. Study Location and Seawater Parameters
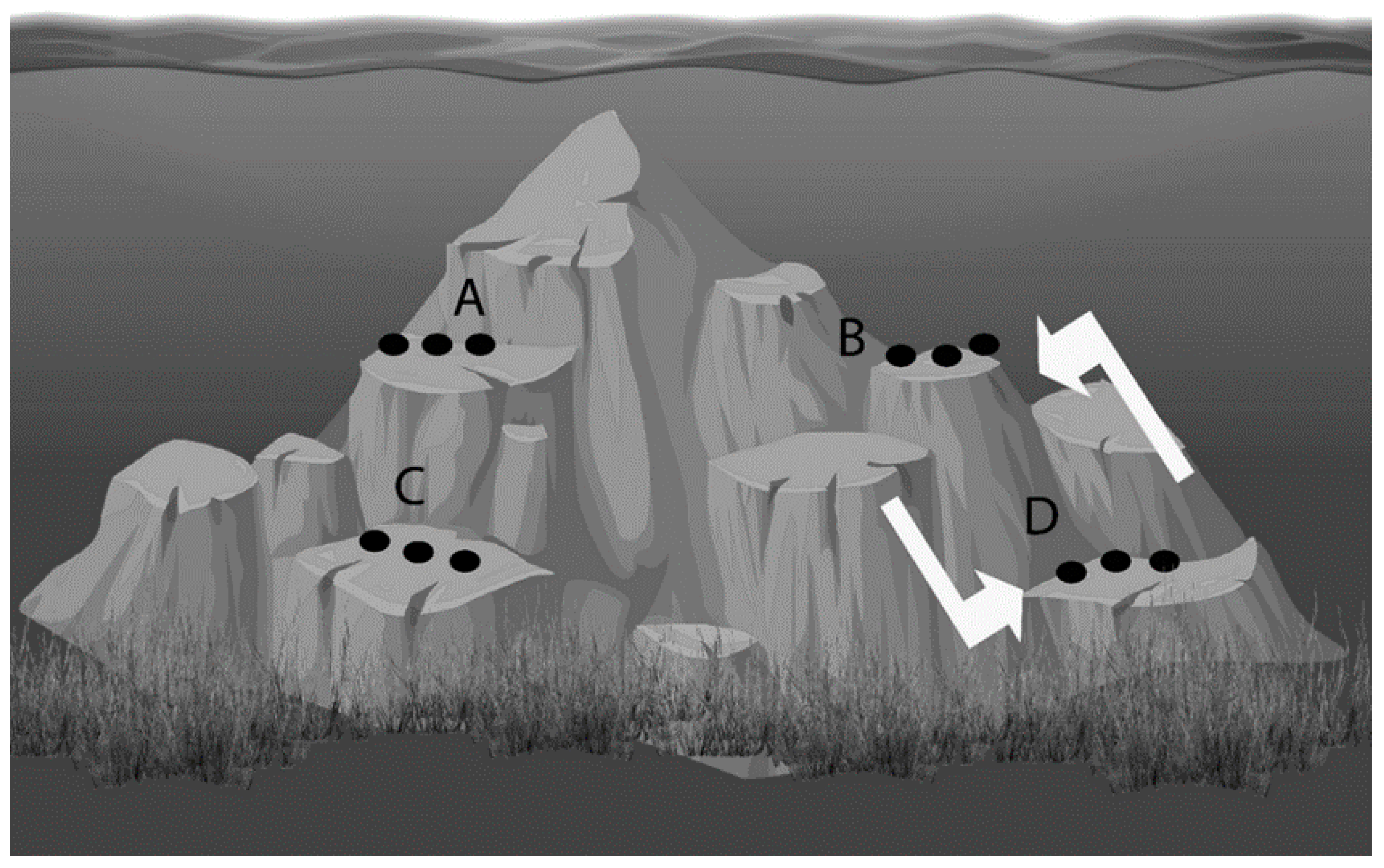
4.2. Sponge Collection, Seeding, and Transplantation
4.3. Determination of Sponge Size, Growth, and Morphological Characteristics
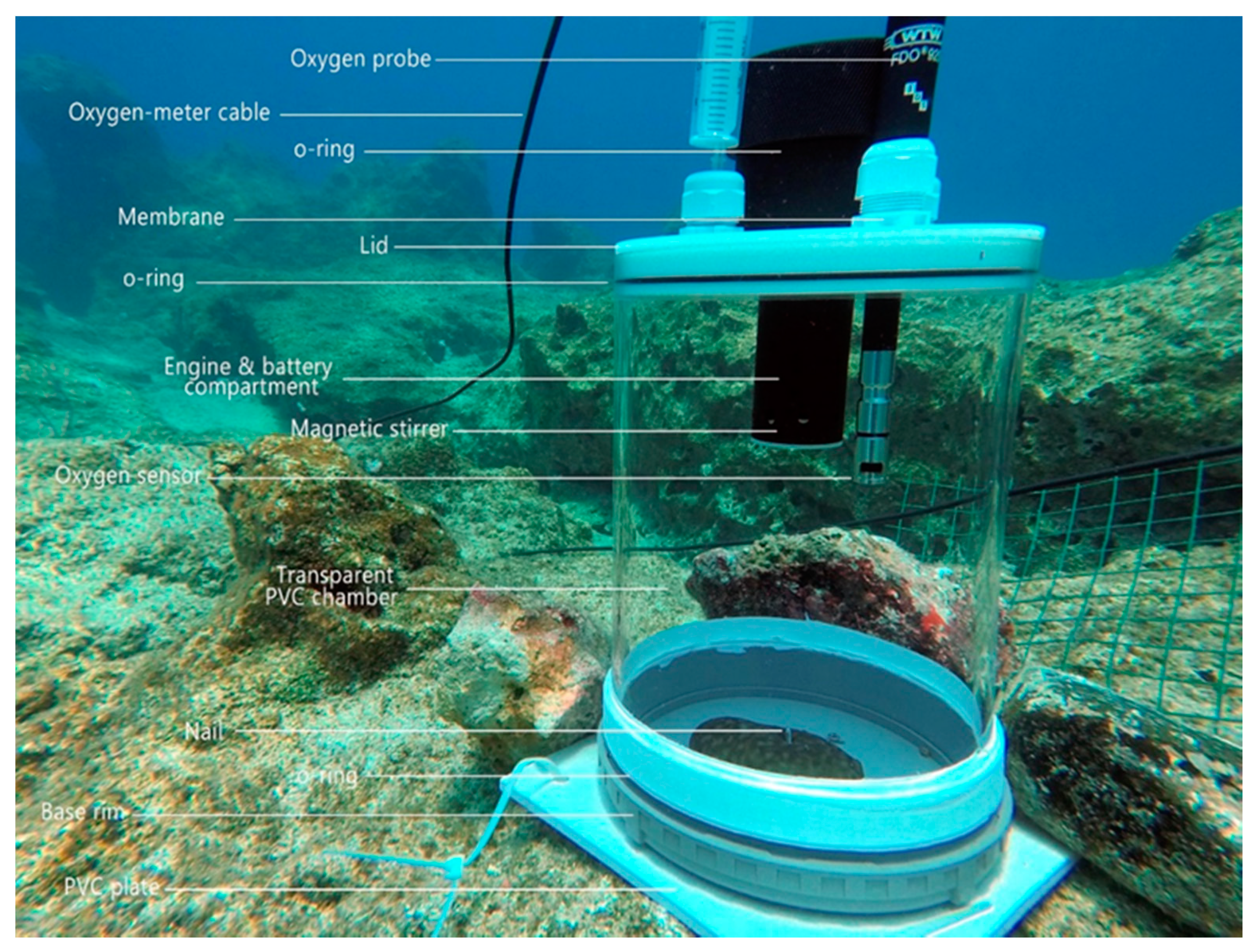
4.4. Clearance and Respiration Rates
4.5. Collagen Extraction
4.6. Collagen Quantification
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hooper, J.; van Soest, R. Systema Porifera: A Guide to the Classification of Sponges; Springer: Boston, MA, USA, 2002. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; De Voogd, N.J. Global diversity of sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed]
- Reiswig, H.M. In situ pumping activities of tropical Demospongiae. Mar. Biol. 1971, 9, 38–50. [Google Scholar] [CrossRef]
- Reiswig, H.M. The aquiferous systems of three marine Demospongiae. J. Morphol. 1975, 145, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Bell, J. Functional roles of sponges. Estuar. Estuar. Coast. Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Ribes, M.; Jiménez, E.; Yahel, G.; López-Sendino, P.; Diez, B.; Massana, R.; Sharp, J.H.; Coma, R. Functional convergence of microbes associated with temperate marine sponges. Environ. Microbiol. 2012, 14, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Tramper, J.; Wijffels, R. Cultivation of Marine Sponges. Mar. Biotechnol. 1999, 1, 509–532. [Google Scholar] [CrossRef]
- Weisz, J.B.; Lindquist, N.; Martens, C.S. Do associated microbial abundances impact marine demosponge pumping rates and tissue densities? Oecologia 2008, 155, 367–376. [Google Scholar] [CrossRef]
- Lavy, A.; Keren, R.; Yahel, G.; Ilan, M. Intermittent hypoxia and prolonged suboxia measured in situ in a marine sponge. Front. Mar. Sci. 2016, 3, 263. [Google Scholar] [CrossRef]
- Ludeman, D.A.; Reidenbach, M.A.; Leys, S.P. The energetic cost of filtration by demosponges and their behavioural response to ambient currents. J. Exp. Biol. 2017, 220, 4743–4744. [Google Scholar] [CrossRef]
- Jørgensen, C.B. Feeding-rates of sponges, lamellibranchs and ascidians. Nature 1949, 163, 912. [Google Scholar] [CrossRef]
- Jørgensen, C.B. Quantitative aspects of filter feeding in invertebrates. Biol. Rev. 1955, 30, 391–453. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Larsen, P. Comparative ecophysiology of active zoobenthic filter feeding, essence of current knowledge. J. Sea Res. 2000, 44, 169–193. [Google Scholar] [CrossRef]
- Ribes, M.; Coma, R.; Gili, J. Natural diet and grazing rate of the temperate sponge Dysidea avara (Demospongiae, Dendroceratida) throughout an annual cycle. Mar. Ecol. Prog. Ser. 1999, 176, 179–190. [Google Scholar] [CrossRef]
- Milanese, M.; Chelossi, E.; Manconi, R.; Sara, A.; Sidri, M.; Pronzato, R. The marine sponge Chondrilla nucula Schmidt, 1862 as an elective candidate for bioremediation in integrated aquaculture. Biomol. Eng. 2003, 20, 363–368. [Google Scholar] [CrossRef]
- Fu, W.T.; Sun, L.M.; Zhang, X.C.; Zhang, W. Potential of the marine sponge Hymeniacidon perlevis as a bioremediator of pathogenic bacteria in integrated aquaculture ecosystems. Biotechnol. Bioeng. 2006, 93, 1112–1122. [Google Scholar] [CrossRef]
- Stabili, L.; Mercurio, M.; Marzano, C.N.; Corriero, G. Filtering activity of Spongia officinalis var. adriatica (Schmidt) (Porifera, Demospongiae) on bacterioplankton: Implications for bioremediation of polluted seawater. Water Res. 2006, 40, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Xue, L.; Zhang, B.; Jin, M.; Fu, W. Bioremediation of bacteria pollution using the marine sponge Hymeniacidon perlevis in the intensive mariculture water system of turbot Scophthalmus maximus. Biotechnol. Bioeng. 2010, 105, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Pile, A.; Patterson, M.; Witman, J. In situ grazing on plankton 10 μm by the boreal sponge Mycale lingua. Mar. Ecol. Prog. Ser. 1996, 141, 95–102. [Google Scholar] [CrossRef]
- Hadas, E.; Marie, D.; Shpigel, M.; Ilan, M. Virus predation by sponges is a new nutrient-flow pathway in coral reef food webs. Limnol. Oceanogr. 2006, 51, 1548–1550. [Google Scholar] [CrossRef]
- Yahel, G.; Sharp, J.H.; Marie, D. In situ feeding and element removal in the symbiont–bearing sponge Theonella swinhoei: Bulk DOC is the major source for carbon. Limnol. Oceanogr. 2003, 48, 141–149. [Google Scholar] [CrossRef]
- De Goeij, J.M.; van Duyl, F.C. Coral cavities are sinks of dissolved organic carbon (DOC). Limnol. Oceanogr. 2007, 52, 2608–2617. [Google Scholar] [CrossRef]
- Alexander, B.E.; Liebrand, K.; Osinga, R.; van der Geest, H.G.; Admiraal, W.; Cleutjens, J.P.M. Cell turnover and detritus production in marine sponges from tropical and temperate benthic ecosystems. PLoS ONE 2014, 9, e109486. [Google Scholar] [CrossRef]
- Mueller, B.; de Goeij, J.M.; Vermeij, M.J.A.; Mulders, Y.; van der Ent, E.; Ribes, M. Natural diet of coral–excavating sponges consists mainly of dissolved organic carbon (DOC). PLoS ONE 2014, 9, e90152. [Google Scholar] [CrossRef] [PubMed]
- Gili, J.M.; Coma, R. Benthic suspension feeders: Their paramount role in littoral marine food webs. Trends Ecol. Evol. 1998, 13, 316–321. [Google Scholar] [CrossRef]
- Perez-Blázquez, A.; Davy, S.K.; Bell, J.J. Estimates of particulate organic carbon flowing from the pelagic environment to the benthos through sponge assemblages. PLoS ONE 2012, 7, e29569. [Google Scholar] [CrossRef]
- Maldonado, M.; Ribes, M.; van Duyl, F.C. Nutrient fluxes through sponges: Biology, budgets, and ecological implications. Adv. Mar. Biol. 2012, 62, 113–182. [Google Scholar] [CrossRef] [PubMed]
- De Goeij, J.M.; van Oevelen, D.; Vermeij, M.J.A.; Osinga, R.; Middelburg, J.J.; de Goeij, A.F.P.M.; Admiraal, W. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 2013, 342, 108–110. [Google Scholar] [CrossRef]
- Pronzato, R.; Bavestrello, G.; Cerrano, C.; Magnino, G.; Manconi, R.; Pantelis, J.; Sarà, A.; Sidri, M. Sponge farming in the Mediterranean Sea: New perspectives. Mem. Qld. Mus. 1999, 44, 485–491. [Google Scholar]
- Osinga, R.; Sidri, M.; Cerig, E.; Gokalp, S.Z.; Gokalp, M. Sponge Aquaculture Trials in the East–Mediterranean Sea: New Approaches to Earlier Ideas. Open Mar. Biol. J. 2010, 4, 74–81. [Google Scholar] [CrossRef]
- Gökalp, M.; Wijgerde, T.; Sarà, A.; de Goeij, J.M.; Osinga, R. Development of an Integrated Mariculture for the Collagen–Rich Sponge Chondrosia reniformis. Mar. Drugs 2019, 17, 29. [Google Scholar] [CrossRef]
- Naylor, R.; Goldburg, R.; Primavera, J. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef]
- Sarà, G.; Scilipoti, D.; Mazzola, A.; Modica, A. Effects of fish farming waste to sedimentary and particulate organic matter in a southern Mediterranean area (Gulf of Castellammare, Sicily): A multiple stable isotope study (??13C and ??15N). Aquaculture 2004, 234, 199–213. [Google Scholar] [CrossRef]
- Nimmo, F.; Cappell, R.; Huntington, T.; Grant, A. Does fish farming impact on tourism in Scotland? Aquac. Res. 2011, 42, 132–141. [Google Scholar] [CrossRef]
- Aguilar-Manjarrez, J.; Soto, D.; Brummett, R. Aquaculture Zoning, Site Selection and Area Management under the Ecosystem Approach to Aquaculture; A handbook; Report ACS18071; Rome, FAO, and World Bank Group: Washington, DC, USA, 2017. [Google Scholar]
- Silva, J.; Barros, A.; Aroso, I.; Fassini, D.; Silva, T.H.; Reis, R.L.; Duarte, A. Extraction of Collagen/Gelatin from the Marine Demosponge Chondrosia reniformis (Nardo, 1847) Using Water Acidified with Carbon Dioxide—Process Optimization. Ind. Eng. Chem. Res. 2016, 55, 6922–6930. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef]
- Fassini, D.; Duarte, A.R.C.; Reis, R.L.; Silva, T.H. Bioinspiring Chondrosia reniformis (Nardo, 1847) Collagen-Based Hydrogel: A New Extraction Method to Obtain a Sticky and Self-Healing Collagenous Material. Mar. Drugs. 2017, 15, 380. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs. 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Gerrodette, T.; Flechsig, A.O. Sediment–induced reduction in the pumping rate of the tropical sponge Verongia lacunosa. Mar. Biol. 1979, 55, 103–110. [Google Scholar] [CrossRef]
- Vogel, S. Current-Induced Flow through the Sponge, Halichondria. Biol. Bull. 1974, 147, 443–456. [Google Scholar] [CrossRef]
- Mendola, D.; De Caralt, S.; Uriz, M.; Van den End, F.; Van Leeuwen, J.L.; Wijffels, R.H. Environmental Flow Regimes for Dysidea avara Sponges. Mar. Biotechnol. 2008, 10, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Frost, T.M. Clearance rate determinations for the fresh–water sponge Spongilla–Lacustris effects of temperature, particle type and concentration and sponge size. Arch. Hydrobiol. 1978, 90, 330–356. [Google Scholar]
- Riisgård, H.U.; Thomassen, S.; Jakobsen, H.; Weeks, J.M.; Larsen, P.S. Suspension feeding in marine sponges Halichondria panicea and Haliclona urceolu: Effects of temperature on filtration rate and energy cost of pumping. Mar. Ecol. Prog. Ser. 1993, 96, 177–188. [Google Scholar] [CrossRef]
- Morganti, T.M.; Ribes, M.; Yahel, G.; Coma, R. Size Is the Major Determinant of Pumping Rates in Marine Sponges. Front. Physiol. 2019, 10, 1474. [Google Scholar] [CrossRef]
- Gökalp, M.; Kuehnhold, H.; De Goeij, J.M.; Osinga, R. Depth and turbidity affect in situ pumping activity of the Mediterranean sponge Chondrosia reniformis (Nardo, 1847). BioRxiv 2020, 2020, 009290. [Google Scholar] [CrossRef]
- Alexander, B.E.; Achlatis, M.; Osinga, R.; van der Geest, H.G.; Cleutjens, J.P.M.; Schutte, B.; de Goeij, J.M. Cell kinetics during regeneration in the sponge Halisarca caerulea: How local is the response to tissue damage? PeerJ 2015, 3, e820. [Google Scholar] [CrossRef]
- Nickel, M.; Brümmer, F. In vitro sponge fragment culture of Chondrosia reniformis (Nardo, 1847). J. Biotechnol. 2003, 100, 147–159. [Google Scholar] [CrossRef]
- Pozzolini, M.; Gallus, L.; Ghignone, S. Insights into the evolution of metazoan regenerative mechanisms: Roles of TGF superfamily members in tissue regeneration of the marine sponge Chondrosia reniformis. J. Exp. Biol. 2019, 222, jeb207894. [Google Scholar] [CrossRef]
- Morganti, T.M. In Situ Direct Study of filtration and Respiration Rate of Mediterranean Sponges. Ph.D. Thesis, Universidad Politécnica de Cataluña, Barcelona, Spain, 2015. Available online: https://digital.csic.es/bitstream/10261/141995/1/Morganti_Tesis_2016.pdf (accessed on 27 July 2016).
- Cebrian, E.; Agell, G.; Martí, R.; Uriz, M.J. Response of the Mediterranean sponge Chondrosia reniformis Nardo to copper pollution. Environ. Pollut. 2006, 141, 452–458. [Google Scholar] [CrossRef]
- Erwin, P.M.; Coma, R.; López-Sendino, P.; Serrano, E.; Ribes, M. Stable symbionts across the HMA-LMA dichotomy: Low seasonal and interannual variation in sponge-associated bacteria from taxonomically diverse hosts. FEMS Microbiol. Ecol. 2015, 91, 10. [Google Scholar] [CrossRef]
- Turon, X.; Galera, J.; Uriz, M.J. Clearance rates and aquiferous systems in two sponges with contrasting life-history strategies. J. Exp. Zool. 1997, 278, 22–36. [Google Scholar] [CrossRef]
- Caralt, S.D.; Uriz, M.J.; Wijffels, R.H. Grazing, differential size-class dynamics and survival of the Mediterranean sponge Corticium candelabrum. Mar. Ecol. Prog. Ser. 2008, 360, 97–106. [Google Scholar] [CrossRef]
- Ledda, F.D.; Pronzato, R.; Manconi, R. Mariculture for bacterial and organic waste removal: A field study of sponge filtering activity in experimental farming. Aquac. Res. 2014, 45, 1389–1401. [Google Scholar] [CrossRef]
- Wilkinson, C.; Vacelet, J. Transplantation of marine sponges to different conditions of light and current. J. Exp. Mar. Biol. Ecol. 1979, 37, 91–104. [Google Scholar] [CrossRef]
- Garrabou, J.; Zabala, M. Growth dynamics in four Mediterranean Demosponges. Estuar. Coast. Shelf Sci. 2001, 52, 293–303. [Google Scholar] [CrossRef]
- Van Treeck, P.; Eisinger, M.; Muller, J.; Paster, M.; Schuhmacher, H. Mariculture trials with Mediterranean sponge species—The exploitation of an old natural resource with sustainable and novel methods. Aquaculture 2003, 218, 439–455. [Google Scholar] [CrossRef]
- Page, M.J.; Handley, S.J.; Northcote, P.T.; Cairney, D.; Willan, R.C. Successes and pitfalls of the aquaculture of the sponge Mycale hentscheli. Aquaculture 2011, 312, 52–61. [Google Scholar] [CrossRef]
- Battershill, C.N.; Page, M.J. Sponge aquaculture for drug production. Aquac. Update. 1996, 5–6. [Google Scholar]
- Garrone, R.; Huc, A.; Junqua, S. Fine structure and physicochemical studies on the collagen of the marine sponge Chondrosia reniformis Nardo. J. Ultrastruct. Res. 1975, 52, 261–275. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Kellerman, J.; Muller, W.E.G.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface–pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Parma, L.; Bonasoro, F.; Bavestrello, G.; Cerrano, C.; Candia Carnevali, M.D. Mechanical adaptability of a sponge extracellular matrix: Evidence for cellular control of mesohyl stiffness in Chondrosia reniformis Nardo. J. Exp. Biol. 2006, 209, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Fassini, D.; Parma, L.; Wilkie, L.; Bavestrello, G.; Bonasoro, F.; Candia, D. Ecophysiology of mesohyl creep in the demosponge Chondrosia reniformis (Porifera: Chondrosida). J. Exp. Mar. Biol. Ecol. 2012, 428, 24–31. [Google Scholar] [CrossRef]
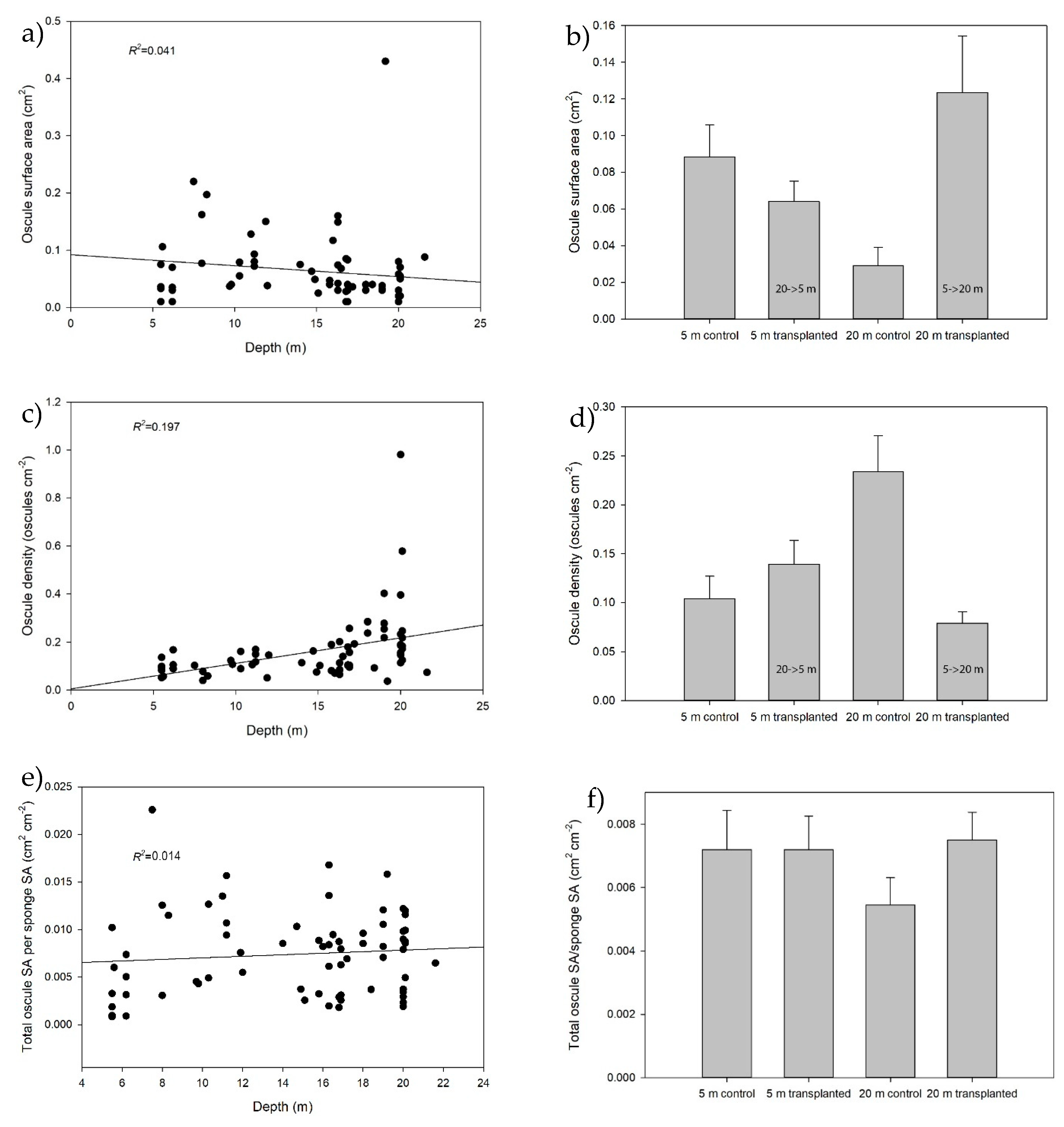
| Variable | F/t | df | Error | p |
|---|---|---|---|---|
| Oscule surface area (OSA) | ||||
| 3.391 | 3 | 30 | 0.031 * | |
| Planned contrasts with Bonferroni correction | ||||
| 5 m control versus 20 m control | 1.817 | 1 | 30 | 0.079 |
| 5 m control versus 5 m transplanted | 0.811 | 1 | 30 | 0.424 |
| 20 m control versus 20 m transplanted | 3.052 | 1 | 30 | 0.005 ** |
| Oscule density (OD) | ||||
| 7.322 | 3 | 32 | 0.001 ** | |
| Planned contrasts with Bonferroni correction | ||||
| 5 m control versus 20 m control | −3.557 | 1 | 32 | 0.001 ** |
| 5 m control versus 5 m transplanted | −1.012 | 1 | 32 | 0.319 |
| 20 m control versus 20 m transplanted | −4.464 | 1 | 32 | 0.000 *** |
| Total oscule surface area per unit of sponge surface area | ||||
| 0.821 | 3 | 31 | 0.492 | |
| Clearance rate | ||||
| 0.199 | 3 | 30 | 0.896 | |
| Respiration rate **** | ||||
| 0.143 | 1 | 15 | 0.711 | |
| % growth | ||||
| 1.447 | 3 | 32 | 0.248 | |
| Transplantation Experiment | Pooled | 5 m Control N = 8 | 5 m Transplant. N = 10 | 20 m Control N = 10 | 20 m Transplant. N = 10 |
|---|---|---|---|---|---|
| (8 weeks in culture) | |||||
| Survival Rates (%) | 95 | 80 | 100 | 100 | 100 |
| Growth Rates (%) | 63.6 ± 5.4 | 83.0 ± 13.2 | 52.5 ± 8.3 | 60.3 ± 11.1 | 70.2 ± 10.4 |
| Clearance Rate (mL cm−3 h−1) | 136.3 ± 21.1 | 164.8 ± 43.9 | 120.8 ± 53.3 | 131.4 ± 32.9 | 136.5 ± 43.9 |
| Respiration Rate (mg O2 cm−3 h−1) * | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.01 | ||
| Treatment | Collagen Yield (%) |
|---|---|
| 5 m control | 35.5 |
| 5 m transplanted | 14.5 |
| 20 m control | 18.4 |
| 20 m transplanted | 21.6 |
| Species | Method (Incubation) | Sponge Size # | Clearance Rate | Reference |
|---|---|---|---|---|
| Ex situ | ||||
| Crambe crambe | 0.25 h | 5 cm2 | 506–790 mL g−1 DM h−1 | Turon et al. 1997, [54] |
| Dysida avara | 0.25 h | 5 cm2 | 1380–3804 mL g−1 DM h−1 | Turon et al. 1997, [54] |
| Chondrilla nucula | 25 cm3 | 0.2 & 1.4 mL cm−3 h−1 | Milanese et al. 2003, [15] | |
| Spongia officinalis | 4 h, 1 L | 91.4 cm3 | 34–210 mL g−1 DM h−1 | Stabili et al. 2006, [17] |
| Corticium candelabrum | 1 h, 1 L, | 0.13–18.8 cm2 | 1000–10.000 mL g−1 DM h−1 | de Caralt et al. 2008, [55] |
| In situ | ||||
| Dysida avara | 1 or 3 L, 4 h | 25 cm3 | 104–2046 mL g−1 DM h−1 | Ribes et al. 1999a, [14] |
| Chondrosia reniformis | 4 L, 1 h | 50–340 | Cebrian et al. 2006, [49] * | |
| Ircinia variabilis | 1.5 h, 1 L | 50 cm3 | 15.96 mL cm−3 h−1 | Ledda et al. 2014, [56] |
| Agelas oroides | 1.5 h, 1 L | 35 cm3 | 20.0 mL cm−3 h−1 | Ledda et al. 2014, [56] |
| Chondrosia reniformis | 0.25 h, 6 L | 9–49 cm2 | 136.3 mL cm−3 h−1 639 mL g−1 DM h−1 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gökalp, M.; Kooistra, T.; Rocha, M.S.; Silva, T.H.; Osinga, R.; Murk, A.J.; Wijgerde, T. The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia reniformis (Nardo, 1847). Mar. Drugs 2020, 18, 358. https://doi.org/10.3390/md18070358
Gökalp M, Kooistra T, Rocha MS, Silva TH, Osinga R, Murk AJ, Wijgerde T. The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia reniformis (Nardo, 1847). Marine Drugs. 2020; 18(7):358. https://doi.org/10.3390/md18070358
Chicago/Turabian StyleGökalp, Mert, Tjitske Kooistra, Miguel Soares Rocha, Tiago H. Silva, Ronald Osinga, AlberTinka J. Murk, and Tim Wijgerde. 2020. "The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia reniformis (Nardo, 1847)" Marine Drugs 18, no. 7: 358. https://doi.org/10.3390/md18070358
APA StyleGökalp, M., Kooistra, T., Rocha, M. S., Silva, T. H., Osinga, R., Murk, A. J., & Wijgerde, T. (2020). The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia reniformis (Nardo, 1847). Marine Drugs, 18(7), 358. https://doi.org/10.3390/md18070358







