Anticancer Compounds Derived from Marine Diatoms
Abstract
1. Introduction
2. Characteristics
3. Anticancer Compounds from Diatoms
3.1. Monoacylglycerides (MAGs)
3.2. Oxylipins (OXLs)
3.3. Chrysolaminaran Polysaccharide
3.4. Fucoxanthin
3.5. Fatty Alcohol Ester (Nonyl 8-Acetoxy-6-Methyloctanoate, NAMO)
3.6. Adenosine and the Metabolites
3.7. Stigmasterol
3.8. Marennine
3.9. Haslene (Hasla-6(17),9,13,23-Tetraene) Lipid
3.10. Diatom Extracts
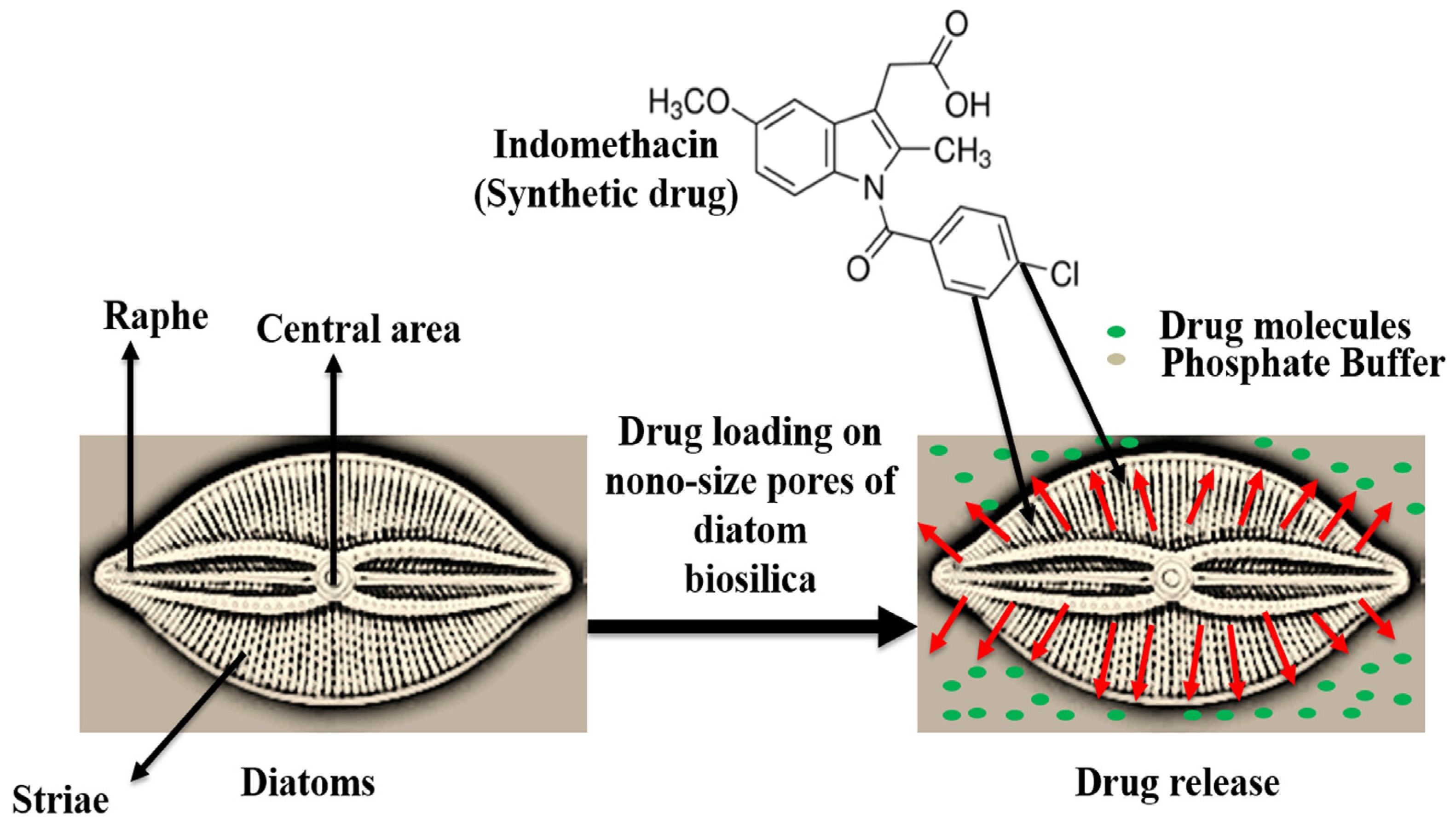
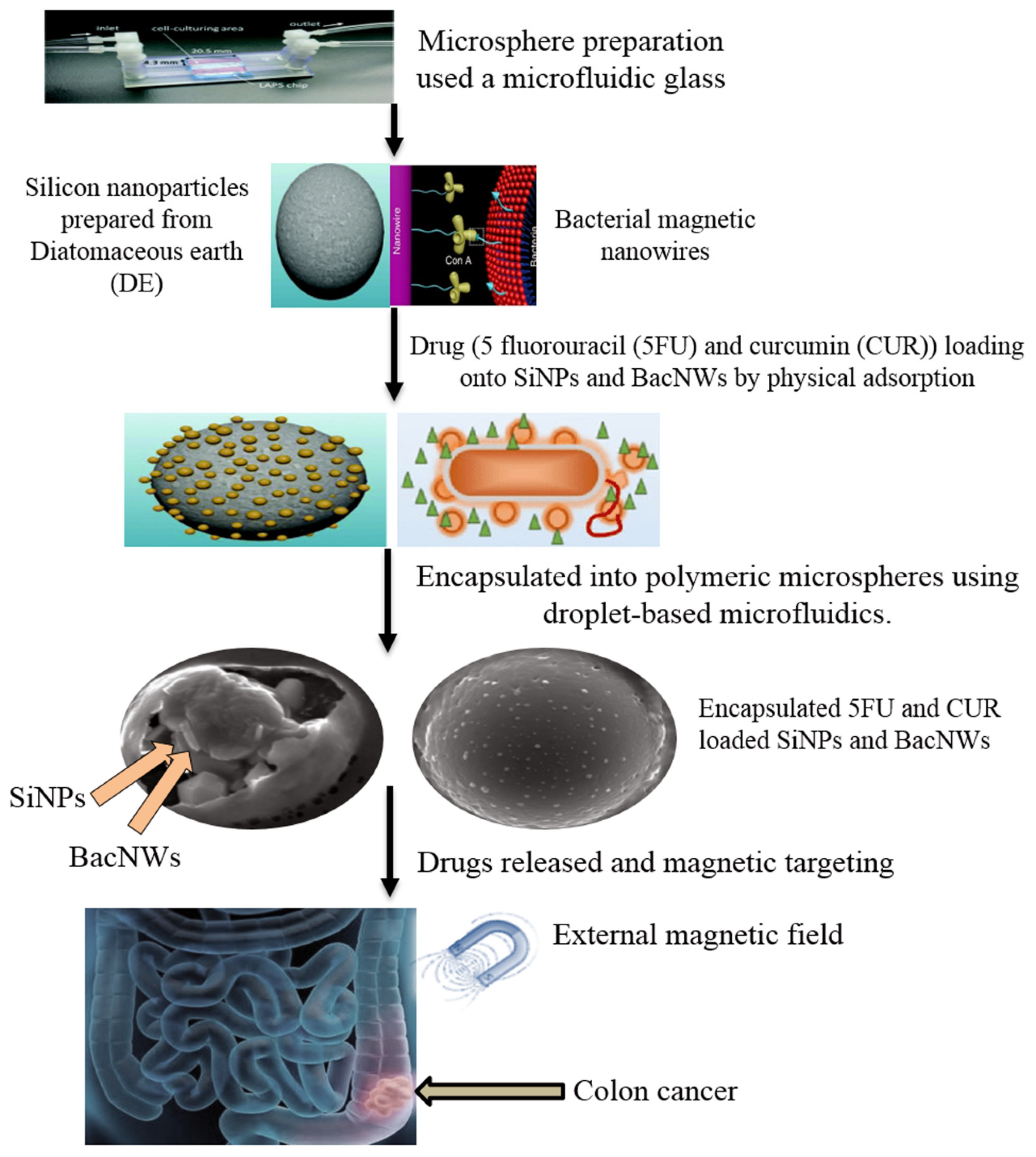
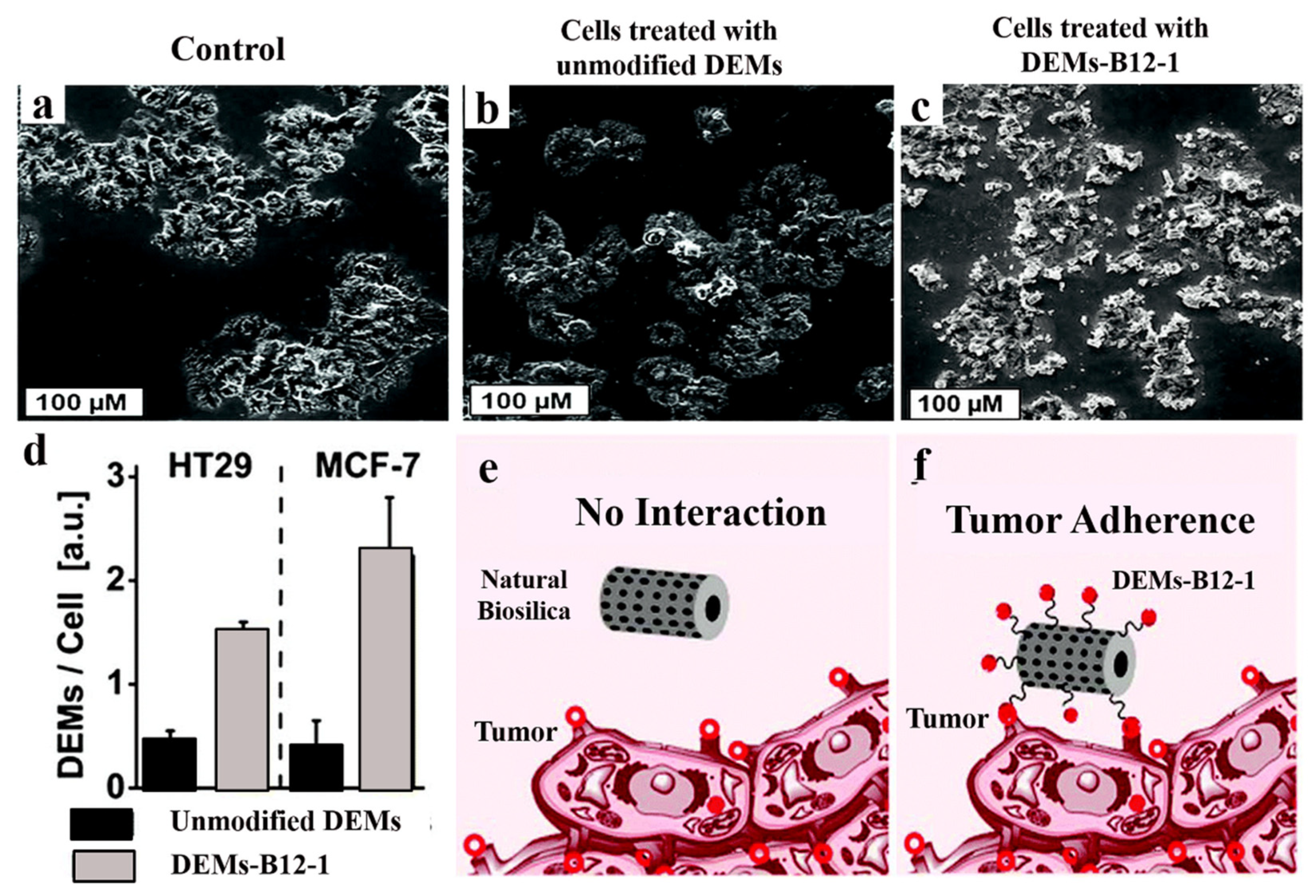
4. Diatoms for Drug Delivery Systems
4.1. Diatom-Based Nanoparticles (DNPs)
4.2. Diatoms-based Antibody
4.3. Diatoms-Based Vitamin B12
5. Conclusions and Future Work
Funding
Acknowledgments
Conflicts of Interest
References
- Cotas, J.; Marques, V.; Afonso, M.B.; Rodrigues, C.M.P.; Pereira, L. Antitumour potential of Gigartina pistillata Carrageenans against colorectal cancer stem cell-enriched tumourspheres. Mar. Drugs 2020, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Martínez Andrade, K.; Lauritano, C.; Romano, G.; Ianora, A. Marine microalgae with anti-cancer properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Ko, J.Y.; Lee, J.H.; Kwon, O.N.; Kim, S.W.; Jeon, Y.J. Apoptotic anticancer activity of a novel fatty alcohol ester isolated from cultured marine diatom, Phaeodactylum tricornutum. J. Funct. Foods 2014, 6, 231–240. [Google Scholar] [CrossRef]
- El-hack, M.E.A.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Mahgoub, A.; Elnesr, S.S.; Gebriel, M.G. Microalgae in modern cancer therapy: Current knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef]
- Folmer, F.; Jaspars, M.; Dicato, M.; Diederich, M. Photosynthetic marine organisms as a source of anticancer compounds. Phytochem. Rev. 2010, 9, 557–579. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Tomaz, A.C.D.A.; Souza, M.D.F.V.D.; Cunha, E.V.L.D.; Kiss, R.; Mathieu, V.; Ioannou, E.; Roussis, V. Halogenated diterpenes with In vitro antitumor activity from the red alga Sphaerococcus coronopifolius. Mar. Drugs 2020, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.Y.; Liao, L.; Park, S.H.; Kim, W.K.; Shin, J.; Lee, S.K. Antitumor activity of Asperphenin A, a lipopeptidyl benzophenone from marine-derived Aspergillus sp. fungus, by inhibiting tubulin polymerization in colon cancer cells. Mar. Drugs 2020, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Delasoie, J.; Rossier, J.; Haeni, L.; Rothen-Rutishauser, B.; Zobi, F. Slow-targeted release of a ruthenium anticancer agent from vitamin B12 functionalized marine diatom microalgae. Dalt. Trans. 2018, 47, 17221–17232. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Braca, A.; Ercolesi, E.; Romano, G.; Palumbo, A.; Casotti, R.; Francone, M.; Ianora, A. Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS ONE 2014, 9, e101220. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C. Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Mar. Drugs 2014, 12, 4883–4897. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, J.E.N.; de la Guardia, M. Applications of diatoms and silica nanotechnology in biosensing, drug and gene delivery, and formation of complex metal nanostructures. TrAC Trends Anal. Chem. 2011, 30, 1538–1548. [Google Scholar] [CrossRef]
- Bozarth, A.; Maier, U.G.; Zauner, S. Diatoms in biotechnology: Modern tools and applications. Appl. Microbiol. Biotechnol. 2009, 82, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.A.; Akbari, F.; Ghorakhlu, M.M.; De Guardia, M.; Khosroushahi, A.Y. Applications of diatoms as potential microalgae in nanobiotechnology. BioImpacts 2012, 2, 83–89. [Google Scholar] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Kusaikin, M.I.; Ermakova, S.P.; Shevchenko, N.M.; Isakov, V.V.; Gorshkov, A.G.; Vereshchagin, A.L.; Grachev, M.A.; Zvyagintseva, T.N. Structural characteristics and antitumor activity of a new chrysolaminaran from the diatom alga Synedra acus. Chem. Nat. Compd. 2010, 46, 1–4. [Google Scholar] [CrossRef]
- Lopez, P.J.; Descle’s, J.; Allen, A.E.; Bowler, C. Prospects in diatom research. Curr. Opin. Biotechnol. 2005, 16, 180–186. [Google Scholar] [CrossRef]
- Medarević, D.P.; Lošić, D.; Ibrić, S.R. Diatoms—Nature materials with great potential for bioapplications. Hem. Ind. 2015, 70, 613–627. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Soundharrajan, I.; Srigopalram, S.; Yusoff, M.M.; Maniam, G.P.; Govindan, N.; Choi, K.C. Potential pharmaceutical and biomedical applications of diatoms microalgae—An overview. Indian J. Geo. Mar. Sci. 2017, 46, 663–667. [Google Scholar]
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar]
- Cheng, J.; Li, Y.; Liang, J.; Gao, Y.; Wang, P. Morphological variability and genetic diversity in five species of Skeletonema (Bacillariophyta). Prog. Nat. Sci. 2008, 18, 1345–1355. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Biology of microalgae. In Microalgae in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2018; pp. 23–72. [Google Scholar]
- Palenzuela, J.M.T.; Vilas, L.G.; Bellas, F.M.; Garet, E.; González-Fernández, Á.; Spyrakos, E. Pseudo-nitzschia blooms in a coastal upwelling system: Remote sensing detection, toxicity and environmental variables. Water 2019, 11, 1954. [Google Scholar] [CrossRef]
- Narang, A.S.; Desai, D.S. Anticancer Drug Development Unique Aspects of Pharmaceutical Development; Springer: New York, NY, USA, 2009; pp. 49–92. [Google Scholar]
- Hussein, H.A.; Mohamad, H.; Ghazaly, M.M.; Laith, A.A.; Abdullah, M.A. Cytotoxic effects of Tetraselmis suecica chloroform extracts with silver nanoparticle co-application on MCF-7, 4 T1, and Vero cell lines. J. Appl. Phycol. 2020, 32, 127–143. [Google Scholar] [CrossRef]
- Miceli, M.; Cutignano, A.; Conte, M.; Ummarino, R.; Romanelli, A.; Ruvo, M.; Leone, M.; Mercurio, F.A.; Doti, N.; Manzo, E.; et al. Monoacylglycerides from the diatom Skeletonema marinoi induce selective cell death in cancer cells. Mar. Drugs 2019, 17, 625. [Google Scholar] [CrossRef] [PubMed]
- Khaddaj-mallat, R.; Morin, C.; Rousseau, E. Novel n-3 PUFA monoacylglycerides of pharmacological and medicinal interest: Anti-inflammatory and anti-proliferative effects. Eur. J. Pharmacol. 2016, 792, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Rodr, E.; Blier, P.U.; Fortin, S. Potential Application of Eicosapentaenoic acid monoacylglyceride in the management of colorectal cancer. Mar. Drugs 2017, 15, 283. [Google Scholar] [CrossRef] [PubMed]
- Alsufyani, T. Metabolite Profiling of the Chemosphere of the Macroalga Ulva (Ulvales, Chlorophyta) and its Associated Bacteria. Ph.D. Thesis, Thuringia University and State Library, Jena, Germany, June 2014. [Google Scholar]
- Yi, Z. Biotechnological Approaches to Enhance Fucoxanthin Production in a Model Diatom Phaeodactylum tricornutum. Ph.D. Thesis, Faculty of Life and Environmental Sciences, University of Iceland, Reykjavík, Iceland, November 2018. [Google Scholar]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Sarpal, S.; Sharma, K.; Scott, J.; Kumar, R.; Sugmaran, V.; Chopra, A.; Bansal, V.; Rajagopalan, N.K. Compositional analyses of oil extracts of microalgae biomasses by NMR and chromatographic techniques. J. Anal. Bioanal. Sep. Tech. 2016, 1, 17–41. [Google Scholar]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; Haramaty, L.; Vardi, A.; White, E.; Lutz, R.; Falkowski, P. Apoptosis—Inducing galactolipids from a cultured marine diatom Phaeodactylum tricornutum. J. Nat. Prod. 2008, 71, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Derwenskus, F.; Flister, V.F.; Schmid-staiger, U.; Hirth, T.; Bischo, S.C. Fucoxanthin, A carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Kim, Y.S.; Li, X.F.; Kang, K.H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef]
- Rowland, S.J.; Belt, S.T.; Wraige, E.J.; Masse, G.; Roussakis, C.; Robert, J. Effects of temperature on polyunsaturation in cytostatic lipids of Haslea ostrearia. Phytochemistry 2001, 56, 597–602. [Google Scholar] [CrossRef]
- Nappo, M.; Berkov, S.; Massucco, C.; Di Maria, V.; Bastida, J.; Codina, C.; Avila, C.; Messina, P.; Zupo, V.; Zupo, S. Apoptotic activity of the marine diatom Cocconeis scutellum and eicosapentaenoic acid in BT20 cells. Pharm. Biol. 2012, 50, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Nigjeh, S.E.; Yusoff, F.; Alitheen, N.B.M.; Rasoli, M.; Keong, Y.S.; Omar, A.R.B. Cytotoxic effect of ethanol extract of microalga, Chaetoceros calcitrans, and its mechanisms in inducing apoptosis in human breast cancer cell line. Biomed Res. Int. 2013, 2013, 8. [Google Scholar]
- Goh, S.H.; Alitheen, N.B.M.; Yusoff, F.M.; Yap, S.K.; Loh, S.P. Crude ethyl acetate extract of marine microalga, Chaetoceros calcitrans, induces apoptosis in MDA-MB-231 breast cancer cells. Pharmacogn. Mag. 2014, 10, 1–8. [Google Scholar]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.; Wu, C.; Wang, J. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Chan, K.W.; Khong, N.M.H.; Yau, S.K. Production of fucoxanthin-rich fraction (FxRF) from a diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Algal Res. 2015, 12, 26–32. [Google Scholar] [CrossRef]
- Moreau, D.; Tomasoni, C.; Jacquot, C.; Kaas, R.; Le, R.; Cadoret, J.; Muller-feuga, A.; Kontiza, I.; Vagias, C.; Roussis, V.; et al. Cultivated microalgae and the carotenoid fucoxanthin from Odontella aurita as potent anti-proliferative agents in bronchopulmonary and epithelial cell lines. Environ. Toxicol. Pharmacol. 2006, 22, 97–103. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Balasubramanian, M.P.; Nishigaki, I. Cancer preventive efficacy of marine carotenoid fucoxanthin: Cell cycle arrest and apoptosis. Nutrients 2013, 5, 4978–4989. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wang, W.; Zhang, H.; Zhang, X.; Han, C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evidence-based complement. Altern. Med. 2015, 2015, 1–15. [Google Scholar]
- Prestegard, S.K.; Oftedal, L.; Coyne, R.T.; Nygaard, G.; Skjærven, K.H.; Knutsen, G.; Døskeland, S.O.; Herfindal, L. Marine benthic diatoms contain compounds able to induce leukemia cell death and modulate blood platelet activity. Mar. Drugs 2009, 7, 605–623. [Google Scholar] [CrossRef]
- Bag, B.G.; Barai, A.C. Self-assembly of naturally occurring stigmasterol in liquids yielding a fi brillar network and gel. RSC Adv. 2020, 10, 4755–4762. [Google Scholar] [CrossRef]
- Gastineau, R.; Turcotte, F.; Pouvreau, J.B.; Morançais, M.; Fleurence, J.; Windarto, E.; Prasetiya, F.S.; Arsad, S.; Jaouen, P.; Babin, M.; et al. Marennine, promising blue pigments from a widespread Haslea diatom species complex. Mar. Drugs 2014, 12, 3161–3189. [Google Scholar] [CrossRef]
- Carbonnelle, D.; Pondaven, P.; Morançais, M.; Massé, G.; Bosch, S.; Jacquot, C.; Briand, G.; Robert, J.M.; Roussakis, C. Antitumor and antiproliferative effects of an aqueous extract from the marine diatom Haslea ostrearia (Simonsen) against solid tumors: Lung carcinoma (NSCLC-N6), kidney carcinoma (E39) and melanoma (M96) cell lines. Anticancer Res. 1999, 19, 621–624. [Google Scholar]
- Stonik, I.V.; Stonik, I. Low-molecular-weight metabolites from diatoms: Structures, biological roles and biosynthesis. Mar. Biotechnol. 2015, 13, 3672–3709. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1317. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-mensah, J.; Losic, D. Porous silica microshells from diatoms as biocarrier for drug delivery applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.A.; Mäkilä, E.M.; da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Biomaterials diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Taté, R.; Rendina, I.; et al. Diatomite biosilica nanocarriers for siRNA transport inside cancer cells. Biochim. Biophys. Acta 2014, 1840, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Zobi, F. Natural diatom biosilica as microshuttles in drug delivery systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef]
- Terracciano, M.; Shahbazi, M.A.; Correia, A.; Rea, I.; Lamberti, A.; Stefano, L.D.; Santos, H.A. Surface bioengineering of diatomite based nanovectors for efficient intracellular uptake and drug delivery. Nanoscale 2015, 7, 20063–20074. [Google Scholar] [CrossRef]
- He, Q.; Shi, J.; Chen, F.; Zhu, M.; Zhang, L. Biomaterials an anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials 2010, 31, 3335–3346. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, S.; Ma, Y.; Chen, X.; Zhang, A.; Zhang, X. Super-pH-sensitive mesoporous silica nanoparticle-based drug delivery system for effective combination cancer therapy. ACS Biomater. Sci. Eng. 2019, 5, 1878–1886. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Wang, Y.; Kaur, G.; Fathalla, D.; Fetih, G.; Santos, A.; Habib, F.; Evdokiou, A.; Losic, D. From the mine to cancer therapy: Natural and biodegradable theranostic silicon nanocarriers from diatoms for sustained delivery of chemotherapeutics. Adv. Healthc. Mater. 2016, 5, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Santos, A.; Kumeria, T.; Kaur, G.; Lambert, M.; Forward, P.; Evdokiou, A.; Losic, D. Multifunctional microspherical magnetic and pH responsive carriers for combination anticancer therapy engineered by droplet-based microfluidics. J. Mater. Chem. B 2017, 5, 4097–4109. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.G.; Losic, D.; Rosengarten, G.; Vinayak, V.; Rorrer, G.; De Stefano, L.; Kroger, N.; Voelcker, N.; Zhang, Y.X.; Santos, H.A. Diatom Nanotechnology Progress and Emerging Applications; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]









| Diatoms Species | Anticancer Compounds | Target Cells | IC50 | Time | References |
|---|---|---|---|---|---|
| Skeletonema marinoi | Monoacylglycerides (MAGs) | Haematological cancer cell line (U-937) | 5 µg/mL | 24 h | [28] |
| Colon cancer cell line (HCT-116) | 5 µg/mL | ||||
| MePR-2B normal cells | - | ||||
| Polyunsaturated aldehydes (PUAs 2-trans,4-trans-decadienal (DD)) | A549 | Not clarified | 48 h | [11] | |
| Colon adenocarcinoma metastatic ascites-deriving (COLO 205) | Not clarified | ||||
| Normal lung/brunch epithelial (BEAS-2B) | - | ||||
| Thalassiosira rotula Skeletonema costatum Pseudo-nitzschia delicatissima | Unsaturated aldehydes | Caco-2 cells | 11 ± 17 µg/mL | 48 h | [35] |
| Synedra acus | Chrysolaminaran | Human colon cancer cells (HT-29) | 54.5 µg/mL | 72 h | [18] |
| Colon cell line (DLD-1) | 47.7 µg/mL | ||||
| Phaeodactylum tricornutum | Nonyl 8-acetoxy-6-methyloctanoate (NAMO, fatty alcohol ester) | Human promyelocytic leukemia (HL-60) | 22.3 µg/mL | 48 h | [3] |
| Human lung carcinoma (A549) | 50 µg/mL | ||||
| Mouse melanoma (B16F10) | - | ||||
| Monogalactosyl diacylglycerols | Wild-type W2 Wild-type D3 | 52 µM 64 µM | 48 h | [36] | |
| Fucoxanthin | Caco-2 (derived from a human colon adenocarcinoma), Hep G2, and HeLa (derived from cervical cancer cells) | Not clarified | 48 h | [37] | |
| Navicula incerta | Stigmasterol (phytosterol) | Liver hepatocellular carcinoma (HepG2) | 8.25 µg/mL | 24 h | [38] |
| Haslea ostreria | hasla-6(17),9,13,23- tetraene | Human lung cancer (NSCLC-N6) | 3.8 µg/mL | 72 h | [39] |
| Marennine | Skin cancer (M96), lung cancer (NSCLC-N6), and kidney cancer (E39) | 30.2, 34.2, and 57.8 µg/mL | |||
| Cocconeis scutellum | Fraction 3 (eicosapentaenoic acid (EPA), diethyl ether extract) | Breast carcinoma (BT20) Human normal lymphocytes | Not clarified - | 24 h | [40] |
| Chaetoceros calcitrans | EtOH extract | MCF-7 | 3 µg/mL | 24 h | [41] |
| AcOEt extract | Breast adenocarcinoma (MDA-MB-231) | 60 µg/mL | 72 h | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, H.A.; Abdullah, M.A. Anticancer Compounds Derived from Marine Diatoms. Mar. Drugs 2020, 18, 356. https://doi.org/10.3390/md18070356
Hussein HA, Abdullah MA. Anticancer Compounds Derived from Marine Diatoms. Marine Drugs. 2020; 18(7):356. https://doi.org/10.3390/md18070356
Chicago/Turabian StyleHussein, Hanaa Ali, and Mohd Azmuddin Abdullah. 2020. "Anticancer Compounds Derived from Marine Diatoms" Marine Drugs 18, no. 7: 356. https://doi.org/10.3390/md18070356
APA StyleHussein, H. A., & Abdullah, M. A. (2020). Anticancer Compounds Derived from Marine Diatoms. Marine Drugs, 18(7), 356. https://doi.org/10.3390/md18070356






