LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Polyphenol Estimation (TPC, TFC and TTC)
2.2. Antioxidant Activities (ABTS, DPPH and FRAP)
2.3. LC-ESI-QTOF-MS/MS Characterization of The Phenolic Compounds
2.3.1. Phenolic Acids
Hydroxybenzoic Acids Derivatives
Hydroxycinnamic Acids and Other Phenolic Acid Derivatives
2.3.2. Flavonoids
Anthocyanins, Flavanols and Flavonols Derivatives
Isoflavonoids Derivatives
2.3.3. Other Polyphenols
Hydroxybenzaldehydes, hydroxycoumarins and hydroxyphenylpropenes Derivatives
Phenolic Terpenes Derivatives
Tyrosols and Other Polyphenols Derivatives
2.3.4. Lignans
Lignans Derivatives
2.4. HPLC Quantitative Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation and Extraction of Polyphenols
3.3. Estimation of Polyphenols and Antioxidant Assays
3.3.1. Total Phenolic Content (TPC)
3.3.2. Total Flavonoid Content (TFC)
3.3.3. Total Tannins Content (TTC)
3.3.4. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay
3.3.5. Ferric Reducing Antioxidant Power (FRAP) Assay
3.3.6. 2,2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) assay
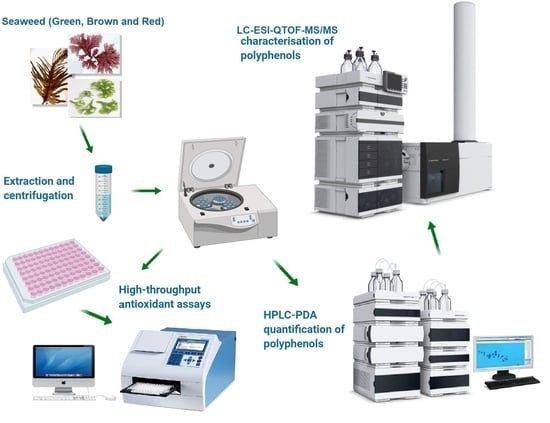
3.4. LC-ESI-QTOF-MS/MS Characterization of Phenolic Compounds
3.5. HPLC-PDA Quantitative Analysis of Individual Phenolic Compounds
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murua, P.; Yang, Z. The Global Status of Seaweed Production, Trade and Utilization; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Wyrepkowski, C.C.; Costa, D.L.; Sinhorin, A.P.; Vilegas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; dos Santos, L.C. Characterization and quantification of the compounds of the ethanolic extract from caesalpinia ferrea stem bark and evaluation of their mutagenic activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Mouritsen, J.D.; Johansen, M. Seaweeds: Edible, Available & Sustainable; The University of Chicago Press: Chicago, IL, USA; London, UK, 2013. [Google Scholar]
- Fleurence, J. Chapter 5–Seaweeds as food. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 149–167. [Google Scholar]
- Menon, V.V.; Lele, S.S. Nutraceuticals and bioactive compounds from seafood processing waste. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1405–1425. [Google Scholar]
- Suleria, H.A.; Masci, P.; Gobe, G.; Osborne, S. Current and potential uses of bioactive molecules from marine processing waste. J. Sci. Food Agric. 2016, 96, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown irish seaweed himanthalia elongata using lc-dad-esi-ms/ms. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Airanthi, M.K.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible japanese brown seaweeds. J. Food. Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (sargassum muticum). BioMed Res. Int. 2013, 2013, 604787. [Google Scholar] [CrossRef]
- Lopez, A.; Rico, M.; Rivero, A.; de Tangil, M.S. The effects of solvents on the phenolic contents and antioxidant activity of stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, P.; Ozogul, F.; Glew, R.; Ozogul, Y. Significance of antioxidants for seafood safety and human health. J. Agric. Food Chem. 2013, 61, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Kalita, P.; Tapan, B.K.; Pal, T.K.; Kalita, R. Estimation of total flavonoids content (tfc) and anti oxidant activities of methanolic whole plant extract of biophytum sensitivum linn. JDDT 2013, 3, 33–37. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, A.; Andrade, P.B.; Valentao, P.; Pereira, D.M.; Ferreres, F. Profiling phlorotannins from fucus spp. Of the northern portuguese coastline: Chemical approach by hplc-dad-esi/msn and uplc-esi-qtof/ms. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jäger, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of α-glucosidase inhibitors by combined use of high-resolution α-glucosidase inhibition profiling and hplc–hrms–spe–nmr. J. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef]
- García-Casal, M.N.; Ramirez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (ulva sp., sargassum sp. And porphyra sp.) in human subjects. J. Food Chem. 2008, 101, 79–85. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Mekinic, I.G.; Skroza, D.; Simat, V.; Hamed, I.; Cagalj, M.; Perkovic, Z.P. Phenolic content of brown algae (pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible irish seaweeds. J. Food Chem. 2010, 17, 205–220. [Google Scholar]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, gracilaria changii. J. Food Chem. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from icelandic seaweeds. J. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective effect of seaweeds with high antioxidant activity from the peniche coast (portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of abts, dpph, and orac assays. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Airanthi, M.K.; Hosokawa, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of extracts from indian seaweeds. J. Food Sci. Technol. 2010, 47, 94–99. [Google Scholar] [CrossRef]
- Ramon-Goncalves, M.; Gomez-Mejia, E.; Rosales-Conrado, N.; Leon-Gonzalez, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Lozano-Castellon, J.; Mardones, C.; Perez, A.J.; Saez, V.; Riquelme, S.; von Baer, D.; Vallverdu-Queralt, A. Phenolic profile of grape canes: Novel compounds identified by lc-esi-ltq-orbitrap-ms. Molecules 2019, 24, 3763. [Google Scholar]
- Rajauria, G. Optimization and validation of reverse phase hplc method for qualitative and quantitative assessment of polyphenols in seaweed. J. Pharm. Biomed. Anal. 2018, 148, 230–237. [Google Scholar] [CrossRef]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized lc-dad-esi/ms profiling method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-z.; Sun, G.; Zhang, A.; Fu, S.; Liu, J.-h. Screening and analyzing the potential bioactive components from rhubarb, using a multivariate data processing approach and ultra-high performance liquid chromatography coupled with time-of-flight mass spectrometry. Anal. Methods 2015, 7, 650–661. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, A.; Sun, H.; Zhang, Y. Chapter 23–Systematic characterization of the absorbed components of acanthopanax senticosus stem. In Serum Pharmacochemistry of Traditional Chinese Medicine; Wang, X., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 313–336. [Google Scholar]
- Al-Ayed, A.S. Integrated mass spectrometry approach to screening of phenolic molecules in hyphaene thebiaca fruits with their antiradical activity by thin-layer chromatography. Indian J. Chem. Technol. 2015, 22, 155–161. [Google Scholar]
- De Oliveira, D.N.; de Bona Sartor, S.; Damário, N.; Gollücke, A.P.; Catharino, R.R. Antioxidant activity of grape products and characterization of components by electrospray ionization mass spectrometry. J. Food Meas. Charact. 2014, 8, 9–14. [Google Scholar] [CrossRef]
- Lin, H.Q.; Zhu, H.L.; Tan, J.; Wang, H.; Wang, Z.Y.; Li, P.Y.; Zhao, C.F.; Liu, J.P. Comparative analysis of chemical constituents of moringa oleifera leaves from china and india by ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Molecules 2019, 24, 942. [Google Scholar] [CrossRef]
- Tong, T.; Li, J.; Ko, D.-O.; Kim, B.-S.; Zhang, C.; Ham, K.-S.; Kang, S.-G. In vitro antioxidant potential and inhibitory effect of seaweed on enzymes relevant for hyperglycemia. Food Sci. Biotechnol. 2014, 23, 2037–2044. [Google Scholar] [CrossRef]
- Geng, C.A.; Chen, H.; Chen, X.L.; Zhang, X.M.; Lei, L.G.; Chen, J.J. Rapid characterization of chemical constituents in saniculiphyllum guangxiense by ultra fast liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. Int. J. Mass Spectrom. 2014, 361, 9–22. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of chemical constituents of melastoma dodecandrum lour. By uplc-esi-q-exactive focus-ms/ms. Molecules 2017, 22, 476. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using lc-dad–esi-ms/ms. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; Garcia, P.; Gimenez, B.; Robert, P. Identification of polyphenols from chilean brown seaweeds extracts by lc-dad-esi-ms/ms. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Rajauria, G. In-vitro antioxidant properties of lipophilic antioxidant compounds from 3 brown seaweed. Antioxidants 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.A. Identification of Phenolic Compounds from Peanut Skin Using hplc-msn; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2009. [Google Scholar]
- Zhang, L.; Tu, Z.-C.; Wang, H.; Fu, Z.-F.; Wen, Q.-H.; Chang, H.-X.; Huang, X.-Q. Comparison of different methods for extracting polyphenols from ipomoea batatas leaves, and identification of antioxidant constituents by hplc-qtoe-ms2. Food Res. Int. 2015, 70, 101–109. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. Uflc-q-tof-ms/ms-based screening and identification of flavonoids and derived metabolites in human urine after oral administration of exocarpium citri grandis extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, S.; Liu, D.; Yang, M.; Wei, J. Analysis of flavonoids in dalbergia odorifera by ultra-performance liquid chromatography with tandem mass spectrometry. Molecules 2020, 25, 389. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Brahmbhatt, H.R.; Mishra, A. Phenolic, flavonoid, and amino acid compositions reveal that selected tropical seaweeds have the potential to be functional food ingredients. J. Food Process. Preserv. 2019, 43, e14266. [Google Scholar] [CrossRef]
- Wang, Y.; Vorsa, N.; Harrington, P.d.B.; Chen, P. Nontargeted metabolomic study on variation of phenolics in different cranberry cultivars using uplc-im-hrms. J. Agric. Food Chem. 2018, 66, 12206–12216. [Google Scholar] [CrossRef]
- Wu, S.-H.; Li, H.-B.; Li, G.-L.; Lv, N.; Qi, Y.-J. Metabolite identification of gut microflora-cassia seed interactions using uplc-qtof/ms. Exp. Ther. Med. 2020, 19, 3305–3315. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, Y.; Chen, Z.; Tan, J.; Bai, J.; Li, P.; Wang, Z.; Du, S. Rapid characterization of components in bolbostemma paniculatum by uplc/ltq-orbitrap msn analysis and multivariate statistical analysis for herb discrimination. Molecules 2018, 23, 1155. [Google Scholar] [CrossRef]
- Jesionek, W.; Majer-Dziedzic, B.; Horvath, G.; Moricz, A.M.; Choma, I.M. Screening of antibacterial compounds in salvia officinalis l. Tincture using thin-layer chromatography-direct bioautography and liquid chromatography-tandem mass spectrometry techniques. JPC J. Planar Chromatogr. Mod. TLC 2017, 30, 357–362. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Lettieri, A.; Nocera, P.; Bollino, F.; Catauro, M. A metabolic profiling approach to an italian-sage leaf extract (soa541) defines its antioxidant and anti-acetylcholinesterase properties. J. Funct. Foods 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Hermund, D.; Jacobsen, C.; Chronakis, I.S.; Pelayo, A.; Yu, S.; Busolo, M.; Lagaron, J.M.; Jónsdóttir, R.; Kristinsson, H.G.; Akoh, C.C. Stabilization of fish oil-loaded electrosprayed capsules with seaweed and commercial natural antioxidants: Effect on the oxidative stability of capsule-enriched mayonnaise. Eur. J. Lipd Sci. Technol. 2019, 121, 1800396. [Google Scholar] [CrossRef]
- Di Maio, I.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Veneziani, G.; Urbani, S.; Servili, M. Characterization of 3,4-dhpea-eda oxidation products in virgin olive oil by high performance liquid chromatography coupled with mass spectrometry. Food Chem. 2013, 138, 1381–1391. [Google Scholar] [CrossRef]
- Gomez-Alonso, S.; Salvador, M.D.; Fregapane, G. Phenolic compounds profile of cornicabra virgin olive oil. J. Agric. Food Chem. 2002, 50, 6812–6817. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.; El-Hawary, S.; Mousa, O.; El- Askary, N.; Esmat, A. In vivo tnf-α and il-1β inhibitory activity of phenolics isolated from trachelospermum jasminoides (lindl.) lem. Med. Plants Res. 2015, 9, 35–41. [Google Scholar]
- Yang, S.; Shan, L.; Luo, H.; Sheng, X.; Du, J.; Li, Y. Rapid classification and identification of chemical components of schisandra chinensis by uplc-q-tof/ms combined with data post-processing. Molecules 2017, 22, 1778. [Google Scholar] [CrossRef] [PubMed]
- Dhargalkar, V. Uses of seaweeds in the indian diet for sustenance and well-being. Sci. Cult. 2015, 80, 192–202. [Google Scholar]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Toledo, E.; Delgado-Rodriguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, J.; Kim, K.H. The therapeutic potential of piceatannol, a natural stilbene, in metabolic diseases: A review. J. Med. Food 2017, 20, 427–438. [Google Scholar] [CrossRef]
- Ma, C.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of phenolic compounds in palm fruits (jelly and fishtail palm) and their potential antioxidant activities. Antioxidants 2019, 8, 483. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms profiling of australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Yang, P.; Xu, F.; Li, H.F.; Wang, Y.; Li, F.C.; Shang, M.Y.; Liu, G.X.; Wang, X.; Cai, S.Q. Detection of 191 taxifolin metabolites and their distribution in rats using hplc-esi-it-tof-msn. Molecules 2016, 21, 764. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Application of ultra performance liquid chromatography-photodiode detector-quadrupole/time of flight-mass spectrometry (uplc-pda-q/tof-ms) method for the characterization of phenolic compounds of lepidium sativum l. Sprouts. Eur. Food Res. Technol. 2013, 236, 699–706. [Google Scholar] [CrossRef]
- Kim, I.; Lee, J. Variations in anthocyanin profiles and antioxidant activity of 12 genotypes of mulberry (morus spp.) fruits and their changes during processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Amoo, S.O.; Aremu, A.O.; Stirk, W.A.; Gruz, J.; Šubrtová, M.; Doležal, K.; Van Staden, J. Phenolic profiles, antioxidant capacity, and acetylcholinesterase inhibitory activity of eight south african seaweeds. J. Appl. Phycol. 2015, 27, 1599–1605. [Google Scholar] [CrossRef]
- Lever, J.; Brkljaca, R.; Kraft, G.; Urban, S. Natural products of marine macroalgae from south eastern australia, with emphasis on the port phillip bay and heads regions of victoria. Mar. Drugs. 2020, 18, 142. [Google Scholar] [CrossRef]
- Saunders, G.W.M.; Tanya, E. Refinements for the amplification and sequencing of red algal DNA barcode and redtol phylogenetic markers: A summary of current primers, profiles and strategies. Algae 2013, 28, 31–43. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; Soler-Villa, A.; Fitzgerald, R.J.; Brunton, N.P. Phenolic content and antioxidant activity of fractions obtained from selected irish macroalgae species (laminaria digitata, fucus serratus, gracilaria gracilis and codium fragile). J. Appl. Phycol. 2015, 27, 519–530. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.H.; Maki-Arvela, P.; Mikkola, J.P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Feng, Y.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Cherif, J.; Ayadi, M.T. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of halimium halimifolium (cistaceae). J. Appl. Pharm. Sci. 2014, 5, 52–57. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north borneo. J. Appl. Phycol. 2008, 20, 367. [Google Scholar] [CrossRef]
| Samples. | TPC (μg GAE/g) | TFC (μg QE/g) | TTC (μg CE/g) |
|---|---|---|---|
| Green seaweeds | |||
| Ulva sp. | 14.80 ± 0.54 d | 9.80 ± 1.96 de | - |
| Caulerpa sp. | 4.30 ± 0.45 d | 0.73 ± 0.08 f | 3.31 ± 7.02 b |
| Codium sp. | 2.29 ± 0.26 d | 1.11 ± 0.63 f | - |
| Red seaweeds * | |||
| Dasya sp. | 260.15 ± 2.25 c | 29.96 ± 0.48 c | 24.90 ± 3.46 b |
| Grateloupia sp. | 524.56 ± 0.46 b | 54.43 ± 0.74 a | - |
| Centroceras sp. | 49.31 ± 2.17 d | 42.55 ± 0.52 b | 4.45 ± 4.37 b |
| Brown seaweeds * | |||
| Ecklonia sp. | 1044.36 ± 2.54 a | 13.87 ± 1.18 d | 166.87 ± 23.24 a |
| Sargassum sp. | 22.27 ± 0.15 d | 3.88 ± 0.27 ef | 5.62 ± 0.01 b |
| Samples | ABTS (μg AAE/g) | DPPH (μg AAE/g) | FRAP (μg AAE/g) |
|---|---|---|---|
| Green seaweeds | |||
| Ulva sp. | 14.24 ± 0.93 d | - | 4.10 ± 1.45 bc |
| Caulerpa sp. | 20.93 ± 2.62 d | - | 0.53 ± 0.05 c |
| Codium sp. | 10.05 ± 6.65 d | - | 1.07 ± 0.62 c |
| Red seaweeds | |||
| Dasya sp. | 179.63 ± 9.3 c | 12.71 ± 0.83 b | 27.39 ± 1.47 bc |
| Grateloupia sp. | 243.06 ± 3.78 b | 19.12 ± 0.64 b | 35.05 ± 1.54 b |
| Centroceras sp. | 27.91 ± 3.79 d | 6.30 ± 0.73 b | 1.86 ± 1.15 c |
| Brown seaweeds * | |||
| Ecklonia sp. | 957.85 ± 0.36 a | 510.32 ± 3.38 a | 170.03 ± 2.04 a |
| Sargassum sp. | 42.62 ± 3.09 d | 13.71 ± 5.67 b | 4.76 ± 0.48 bc |
| No. | Proposed Compounds | Molecular Formula | RT (min) | Ionization (ESI+/ESI-) | Molecular Weight | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | MS/MS Product Ions | Seaweeds |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acid | ||||||||||
| Hydroxybenzoic acids | ||||||||||
| 1 | Vanillic acid 4-sulfate | C8H8O7S | 9.112 | [M − H]− | 247.9991 | 246.9918 | 246.9925 | 2.83 | 217, 203, 167 | * Sargassum sp., Centroceras sp., Ulva sp. |
| 2 | Gallic acid | C7H6O5 | 9.885 | ** [M − H]− | 170.0215 | 169.0142 | 169.0138 | −2.37 | 125 | Centroceras sp. |
| 3 | 4-Hydroxybenzoic acid 4-O-glucoside | C13H16O8 | 11.515 | [M − H]− | 300.0845 | 299.0772 | 299.0778 | 2.01 | 255, 137 | Sargassum sp. |
| 4 | Protocatechuic acid 4-O-glucoside | C13H16O9 | 13.546 | ** [M − H]− | 316.0794 | 315.0721 | 315.0719 | −0.63 | 153 | * Centroceras sp., Grateloupia sp. |
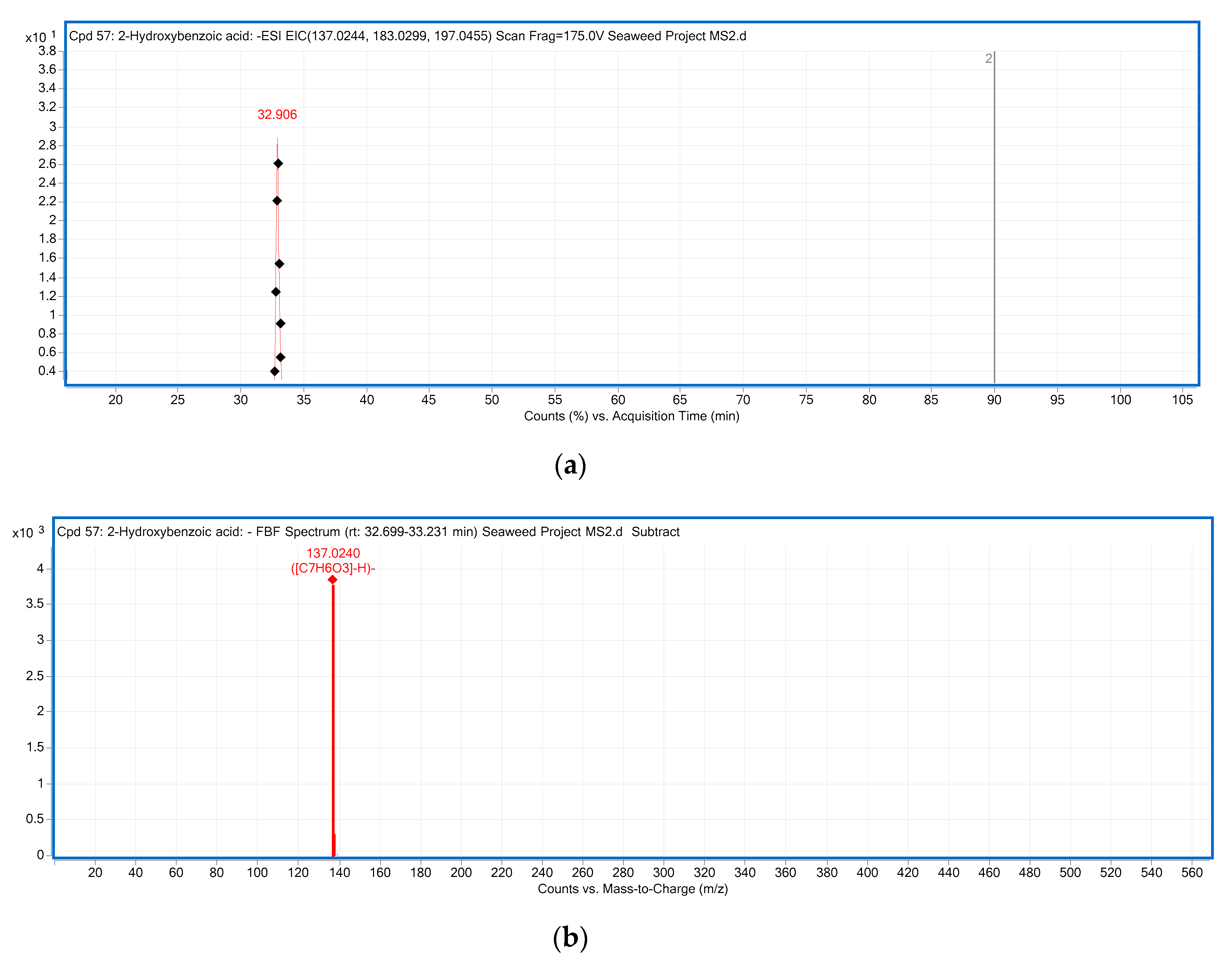
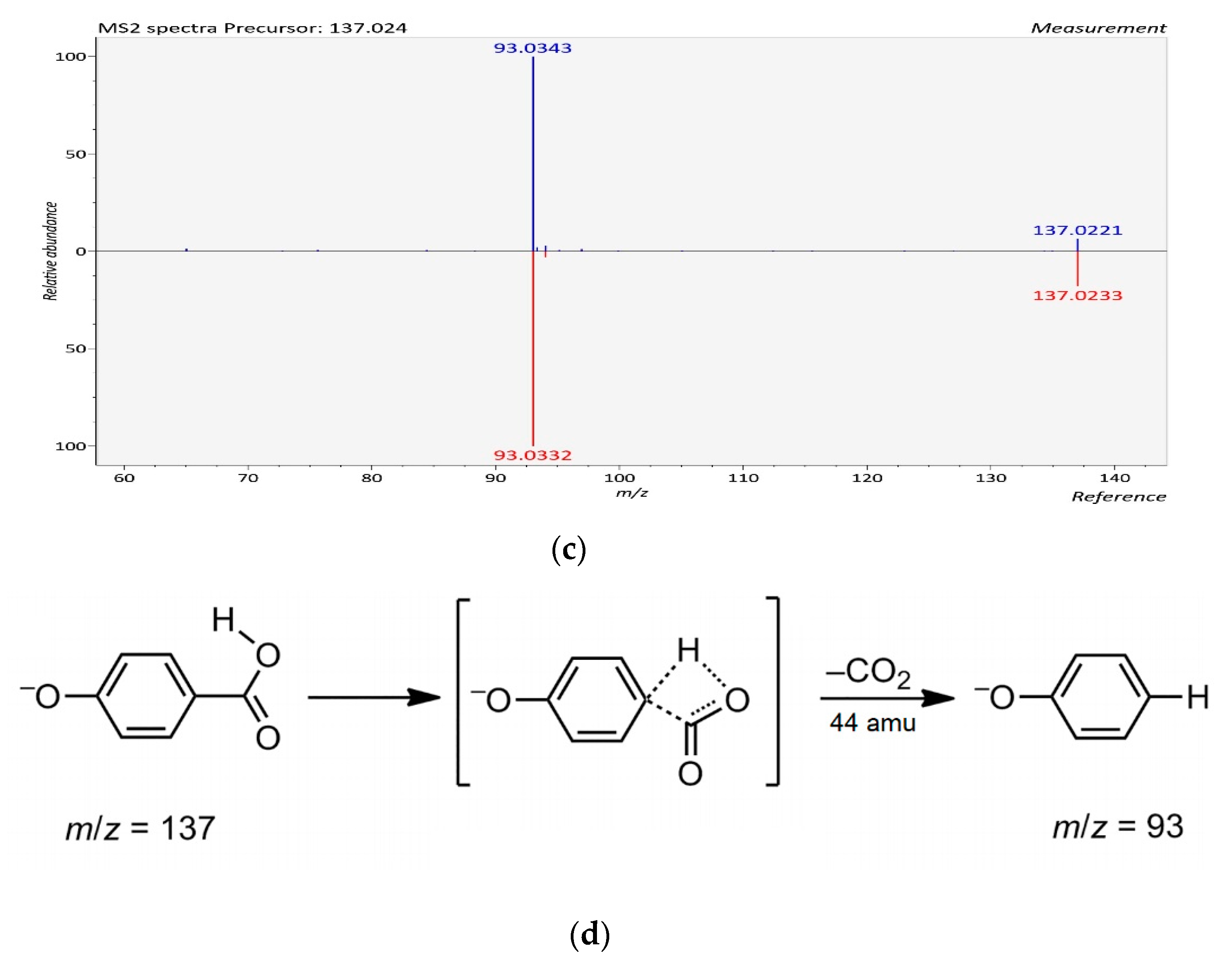
| 5 | p-Hydroxybenzoic acid | C7H6O3 | 32.906 | [M − H]− | 138.0317 | 137.0244 | 137.0240 | −2.91 | 93 | * Ulva sp., Caulerpa sp., Centroceras sp. |
| 6 | Ellagic acid glucoside | C20H16O13 | 38.451 | [M − H]− | 464.0591 | 463.0518 | 463.0518 | 0.01 | 301 | Ecklonia sp. |
| Hydroxycinnamic acids | ||||||||||
| 7 | 3-Sinapoylquinic acid | C18H22O10 | 7.005 | ** [M − H]− | 398.1213 | 397.1140 | 397.1144 | 1.01 | 223, 179 | * Centroceras sp., Ecklonia sp. |
| 8 | Cinnamoyl glucose | C15H18O7 | 8.861 | ** [M − H]− | 310.1053 | 309.098 | 309.0992 | 3.88 | 147, 131, 103 | * Codium sp., Ulva sp. |
| 9 | Caffeoyl glucose | C15H18O9 | 10.983 | ** [M − H]− | 342.0951 | 341.0878 | 341.0882 | 1.17 | 179, 161 | * Ecklonia sp., Centroceras sp. |
| 10 | Caffeic acid 3-O-glucuronide | C15H16O10 | 14.259 | ** [M − H]− | 356.0743 | 355.0670 | 355.0671 | 0.28 | 179 | Caulerpa sp. |
| 11 | Chlorogenic acid | C16H18O9 | 15.004 | ** [M − H]− | 354.0951 | 353.0878 | 353.0862 | −4.53 | 253, 190, 144 | * Centroceras sp., Caulerpa sp. |
| 12 | Caffeic acid | C9H8O4 | 18.274 | [M − H]− | 180.0423 | 179.0350 | 179.0350 | 0.01 | 151, 143, 133 | Caulerpa sp. |
| 13 | Caffeic acid 4-sulfate | C9H8O7S | 18.291 | [M − H]− | 259.9991 | 258.9918 | 258.9929 | 4.25 | 215, 179, 135 | Caulerpa sp. |
| 14 | Caffeoyl tartaric acid | C13H12O9 | 24.061 | ** [M − H]− | 312.0481 | 311.0408 | 311.0403 | −1.61 | 161 | * Grateloupia sp., Centroceras sp. |
| 15 | Isoferulic acid 3-sulfate | C10H10O7S | 24.520 | ** [M − H]− | 274.0147 | 273.0074 | 273.0086 | 4.4 | 193, 149 | Caulerpa sp. |
| 16 | Sinapic acid | C11H12O5 | 25.852 | ** [M − H]− | 224.0685 | 223.0612 | 223.0621 | 4.03 | 205, 179, 163 | * Ulva sp., Caulerpa sp., Grateloupia sp. |
| 17 | Ferulic acid | C10H10O4 | 32.604 | [M − H]− | 194.0579 | 193.0506 | 193.0513 | 3.63 | 178, 149, 134 | Caulerpa sp. |
| 18 | Coumaric acid | C9H8O3 | 33.797 | ** [M − H]− | 164.0473 | 163.0400 | 163.0406 | 3.68 | 119 | * Ulva sp., Ecklonia sp. |
| 19 | Sinapine | C16H24NO5 | 88.066 | [M + H]+ | 310.1652 | 310.1654 | 310.1646 | −2.58 | 251, 207, 175 | Codium sp. |
| Hydroxyphenylpentanoic acids | ||||||||||
| 20 | 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone 3-O-glucuronide | C17H20O10 | 14.855 | ** [M − H]− | 384.1056 | 383.0983 | 383.1001 | 4.70 | 221, 206, 191 | * Ecklonia sp., Codium sp. |
| 21 | 5-(3′,4′-dihydroxyphenyl)-valeric acid | C11H14O4 | 51.563 | ** [M − H]− | 210.0892 | 209.0819 | 209.0821 | 0.96 | 165, 150 | Caulerpa sp. |
| Hydroxyphenylacetic acids | ||||||||||
| 22 | 2-Hydroxy-2-phenylacetic acid | C8H8O3 | 6.18 | ** [M + H]+ | 152.0473 | 153.0546 | 153.055 | 2.61 | 125 | * Centroceras sp., Caulerpa sp., Sargassum sp. |
| Flavonoids | ||||||||||
| Anthocyanins | ||||||||||
| 23 | Delphinidin 3-O-sambubioside | C26H29O16 | 9.327 | [M + H]+ | 597.1464 | 597.1456 | 597.1473 | 2.85 | 303, 257, 229 | Grateloupia sp. |
| 24 | Isopeonidin 3-O-arabinoside | C21H21O10 | 41.658 | [M + H]+ | 433.1134 | 433.1135 | 433.1136 | 0.23 | 271, 253, 243 | Centroceras sp. |
| 25 | Malvidin 3-O-glucoside | C23H25O12 | 54.152 | [M + H]+ | 493.1343 | 493.1346 | 493.1343 | −0.61 | 331 | Centroceras sp. |
| Flavanols | ||||||||||
| 26 | Gallocatechin | C15H14O7 | 7.604 | ** [M − H]− | 306.0740 | 305.0667 | 305.0668 | 0.33 | 261, 219 | * Caulerpa sp., Ulva sp., Dasya sp., Ecklonia sp., Sargassum sp. |
| 27 | 3′-O-Methylcatechin | C16H16O6 | 17.857 | ** [M − H]− | 304.0947 | 303.0874 | 303.0886 | 3.96 | 271, 163 | Grateloupia sp. |
| 28 | Catechin (isomer) | C15H14O6 | 45.118 | [M − H]− | 290.0790 | 289.0717 | 289.0731 | 4.84 | 245, 205, 179 | Caulerpa sp. |
| Flavonols | ||||||||||
| 29 | Quercetin 3-O-(6”-malonyl-glucoside) | C24H22O15 | 9.902 | [M − H]− | 550.0959 | 549.0886 | 549.0887 | 0.18 | 463, 301, 161 | * Centroceras sp., Caulerpa sp. |
| 30 | 5,3′,4′-Trihydroxy-3-methoxy-6:7-methylenedioxyflavone 4’-O-glucuronide | C23H20O14 | 33.878 | [M − H]− | 520.0853 | 519.0780 | 519.0779 | −0.19 | 343 | Ecklonia sp. |
| 31 | 3,7-Dimethylquercetin | C17H14O7 | 80.642 | [M − H]− | 330.0740 | 329.0667 | 329.0674 | 2.13 | 314, 299, 271 | Centroceras sp. |
| Flavones | ||||||||||
| 32 | Rhoifolin | C27H30O14 | 44.036 | ** [M − H]− | 578.1636 | 577.1563 | 577.1588 | 4.33 | 413, 269 | Centroceras sp. |
| Isoflavonoids | ||||||||||
| 33 | Sativanone | C17H16O5 | 4.240 | [M − H]− | 300.0998 | 299.0925 | 299.0918 | −2.34 | 284, 269, 225 | Ecklonia sp. |
| 34 | Glycitein 7-O-glucuronide | C22H20O11 | 4.454 | ** [M − H]− | 460.1006 | 459.0933 | 459.0923 | −2.18 | 283, 268, 117 | Centroceras sp. |
| 35 | 3′,4′,5,7-Tetrahydroxyisoflavanone | C15H12O6 | 4.640 | ** [M − H]− | 288.0634 | 287.0561 | 287.0556 | −1.74 | 269, 259 | *Caulerpa sp., Grateloupia sp., Centroceras sp. |
| 36 | 3’-O-Methylequol | C16H16O4 | 4.803 | ** [M − H]− | 272.1049 | 271.0976 | 271.0972 | −1.48 | 147, 123, 121 | *Ecklonia sp., Grateloupia sp. |
| 37 | Dalbergin | C16H12O4 | 9.344 | ** [M − H]− | 268.0736 | 267.0663 | 267.0666 | 1.12 | 252, 224, 180 | * Grateloupia sp., Centroceras sp. |
| 38 | Dihydrobiochanin A | C16H14O5 | 80.715 | ** [M − H]− | 286.0841 | 285.0768 | 285.0771 | 1.05 | 270 | * Codium sp., Centroceras sp. |
| 39 | 3′-Hydroxydaidzein | C15H10O5 | 86.956 | [M − H]− | 270.0528 | 269.0455 | 269.0457 | 0.74 | 151, 117, 107 | * Grateloupia sp., Centroceras sp., Caulerpa sp., Ecklonia sp. |
| Other polyphenols | ||||||||||
| Hydroxybenzaldehydes | ||||||||||
| 40 | p-Hydroxybenzaldehyde | C7H6O2 | 15.921 | [M − H]− | 122.0368 | 121.0295 | 121.0295 | 0.01 | 92, 77 | * Dasya sp., Ecklonia sp., Codium sp. |
| Hydroxycoumarins | ||||||||||
| 41 | Urolithin A | C13H8O4 | 4.64 | [M − H]− | 228.0423 | 227.0350 | 227.0341 | −3.96 | 198, 182 | Grateloupia sp. |
| 42 | Scopoletin | C10H8O4 | 84.705 | ** [M − H]− | 192.0423 | 191.0350 | 191.0352 | 1.05 | 176, 147 | * Codium sp., Grateloupia sp., Sargassum sp. |
| Phenolic terpenes | ||||||||||
| 43 | Rosmanol | C20H26O5 | 24.965 | [M + H]+ | 346.1780 | 347.1853 | 347.1843 | −2.88 | 301, 231 | * Dasya sp., Ulva sp., Grateloupia sp., Ecklonia sp., Codium sp. |
| 44 | Carnosol | C20H26O4 | 85.931 | ** [M − H]− | 330.1831 | 329.1758 | 329.1747 | −3.34 | 287, 286, 285 | * Codium sp., Caulerpa sp. |
| 45 | Carnosic acid | C20H28O4 | 86.958 | ** [M − H]− | 332.1988 | 331.1915 | 331.1912 | −0.91 | 287, 269 | * Ecklonia sp., Dasya sp., Codium sp., Sargassum sp. |
| Tyrosols | ||||||||||
| 46 | Hydroxytyrosol 4-O-glucoside | C14H20O8 | 36.653 | ** [M − H]− | 316.1158 | 315.1085 | 315.1091 | 1.90 | 153, 123 | * Centroceras sp., Dasya sp., Grateloupia sp., Sargassum sp. |
| 47 | 3,4-DHPEA-EDA | C17H20O6 | 87.423 | [M − H]− | 320.1260 | 319.1187 | 319.1200 | 4.07 | 301, 275, 195 | Caulerpa sp. |
| Other polyphenols | ||||||||||
| 48 | 3,4-Dihydroxyphenylglycol | C8H10O4 | 7.005 | [M − H]− | 170.0579 | 169.0506 | 169.0503 | −1.77 | 141, 139, 123 | Centroceras sp. |
| 49 | Phloroglucinol | C6H6O3 | 14.793 | [M − H]− | 126.0317 | 125.0244 | 125.0242 | −1.59 | 97 | * Ecklonia sp., Sargassum sp. |
| 50 | Isopropyl 3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate | C12H16O5 | 24.882 | ** [M − H]− | 240.0998 | 239.0925 | 239.0919 | −2.51 | 195, 155, 99 | Dasya sp. |
| Lignans | ||||||||||
| Lignan derivatives | ||||||||||
| 51 | 2′-Hydroxyenterolactone | C18H18O5 | 7.781 | [M − H]− | 314.1154 | 313.1081 | 313.1082 | 0.32 | 295, 283 | Grateloupia sp. |
| 52 | Arctigenin | C21H24O6 | 8.131 | ** [M − H]− | 372.1573 | 371.1500 | 371.1509 | 2.42 | 356, 312, 295 | * Centroceras sp., Sargassum sp. |
| 53 | Dimethylmatairesinol | C22H26O6 | 83.663 | [M + H]+ | 386.1729 | 387.1802 | 387.1805 | 0.77 | 372, 369, 357, 329 | * Caulerpa sp., Dasya sp. |
| 54 | Deoxyschisandrin | C24H32O6 | 85.152 | ** [M + H]+ | 416.2199 | 417.2272 | 417.2286 | 3.36 | 402, 347, 316, 301 | * Ecklonia sp., Codium sp., Sargassum sp. |
| No. | Compound Name | Structure Formula | RT (min) | Concentration (μg/gf.w.) | Seaweed Samples |
|---|---|---|---|---|---|
| 1 | Gallic acid | C7H6O5 | 9.685 | 138.887 ± 0.02 | Centroceras sp. |
| 2 | Chlorogenic acid | C16H18O9 | 15.004 | 122.706 ± 0.01 | Centroceras sp. |
| 3 | Caffeic acid | C9H8O4 | 18.274 | 612.824 ± 0.02 | Caulerpa sp. |
| 4 | Caftaric acid | C13H12O9 | 24.532 | 19.667 ± 0.01 | Centroceras sp. |
| 5 | p-hydroxybenzoic acid | C7H6O3 | 32.906 | 846.083 ± 0.02 | Ulva sp. |
| 6 | Coumaric acid | C9H8O3 | 33.797 | 505.387 ± 0.03 | Ulva sp. |
| 7 | Catechin | C15H14O6 | 64.081 | 29.469 ± 0.03 | Caulerpa sp. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. https://doi.org/10.3390/md18060331
Zhong B, Robinson NA, Warner RD, Barrow CJ, Dunshea FR, Suleria HAR. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Marine Drugs. 2020; 18(6):331. https://doi.org/10.3390/md18060331
Chicago/Turabian StyleZhong, Biming, Nicholas A. Robinson, Robyn D. Warner, Colin J. Barrow, Frank R. Dunshea, and Hafiz A.R. Suleria. 2020. "LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential" Marine Drugs 18, no. 6: 331. https://doi.org/10.3390/md18060331
APA StyleZhong, B., Robinson, N. A., Warner, R. D., Barrow, C. J., Dunshea, F. R., & Suleria, H. A. R. (2020). LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Marine Drugs, 18(6), 331. https://doi.org/10.3390/md18060331