Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Ultrasound on Extraction Yield, Total Polyphenols and Phlorotannin Content
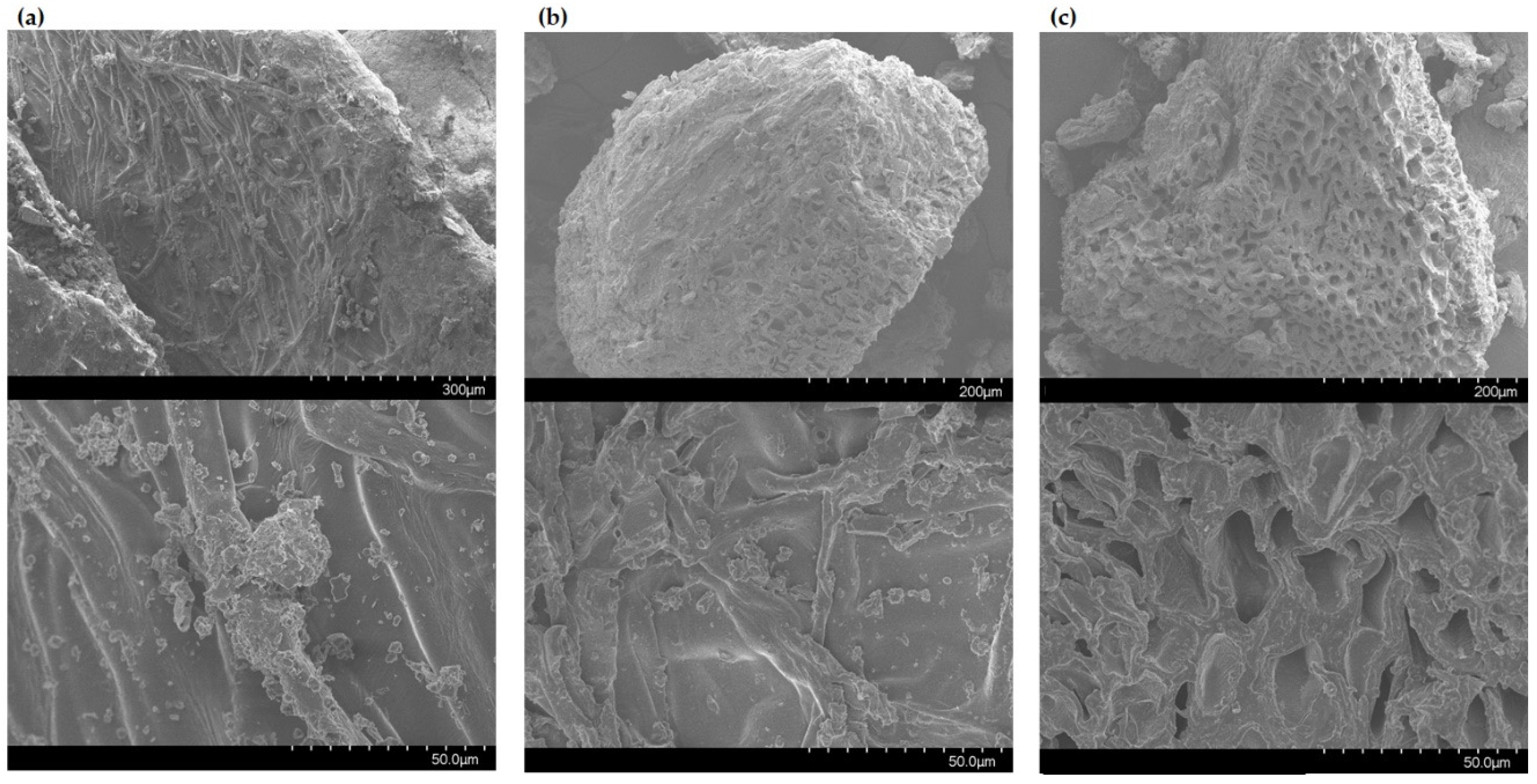
2.2. Scanning Electron Microscopic Analysis
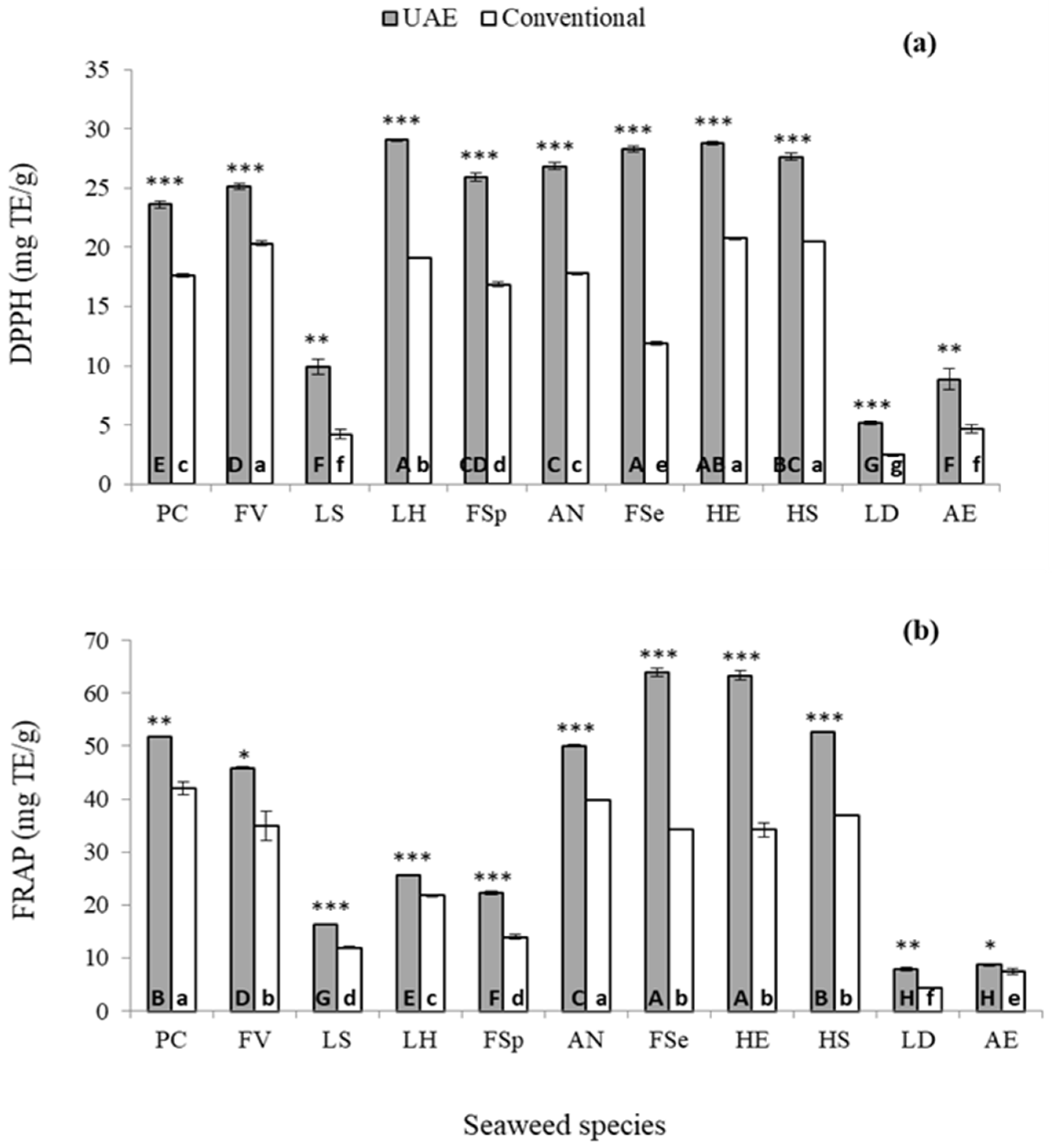
2.3. Comparison of Optimised UAE Conditions and Conventional Solvent Extraction
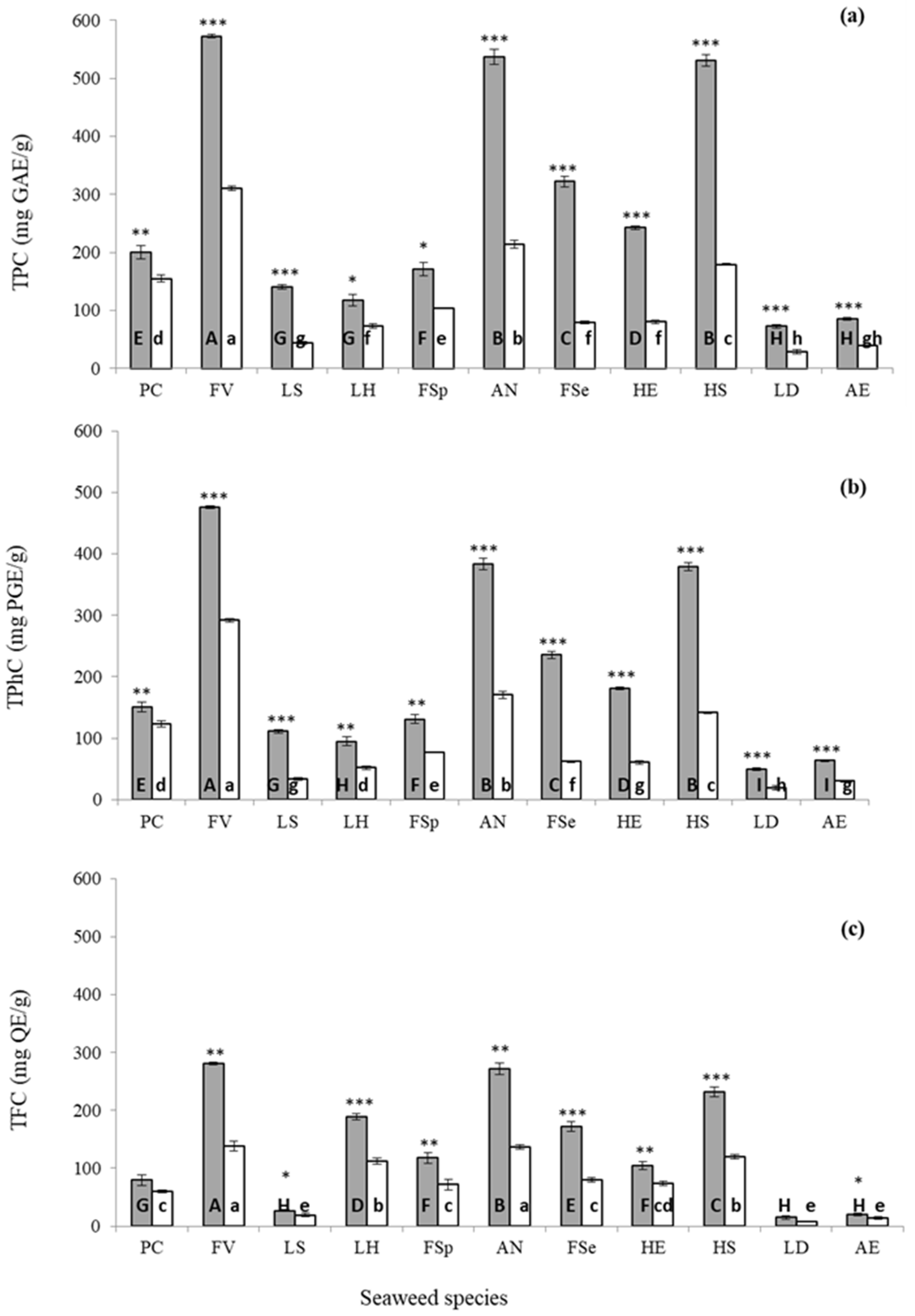
2.3.1. Extraction Yield and Phenolic Constituents
2.3.2. Antioxidant Capacity Determination
3. Materials and Methods
3.1. Chemicals
3.2. Seaweed Biomass
3.3. Ultrasound assisted Extraction (UAE) Procedures
3.4. Conventional Solvent Extraction
3.5. Scanning Electron Microscopy
3.6. Phenolic Composition and Antioxidant Capacity Analysis
3.6.1. Total Phenolic Content (TPC) and Total Phlorotannin Content (TPhC)
3.6.2. Total Flavonoid Content
3.6.3. DPPH Radical-scavenging Assay
3.6.4. Ferric Reducing Antioxidant Power (FRAP) Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & Mohr). J. Appl. Phycol. 2013, 25, 1777–1791. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Wang, L.; Park, Y.J.; Jeon, Y.J.; Ryu, B. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Bordoloi, A.; Goosen, N. Green and integrated processing approaches for the recovery of high-value compounds from brown seaweeds. Adv. Bot. Res. 2019. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum). BioMed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Déléris, P.; Nazih, H.; Bard, J.M. Seaweeds in human health. In Seaweed in Health and Disease Prevention, 1st ed.; Fleurence, J., Levine, I., Eds.; Elsevier: London, UK, 2016; Chapter 10; pp. 319–367. [Google Scholar]
- Fernando, I.S.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurised liquid extraction and solid–liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends. Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends. Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food. Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Chen, Y.; Liu, H.; Yuan, G.; Fan, Y.; Chen, K. Optimization of the microwave-assisted extraction of phlorotannins from Saccharina japonica Aresch and evaluation of the inhibitory effects of phlorotannin-containing extracts on HepG2 cancer cells. Chin. J. Oceanol. Limnol. 2013, 31, 1045–1054. [Google Scholar] [CrossRef]
- Liu, X.; Luo, G.; Wang, L.; Yuan, W. Optimization of antioxidant extraction from edible brown algae Ascophyllum nodosum using response surface methodology. Food Bioprod. Proc. 2019, 114, 205–215. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2015, 23, 308–316. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl.Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Kadam, S.; O’Donnell, C.; Rai, D.; Hossain, M.; Burgess, C.; Walsh, D.; Tiwari, B. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Benzie, I.F.; Szeto, Y. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Liu, S.C.; Lin, J.T.; Wang, C.K.; Chen, H.-Y.; Yang, D.J. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009, 114, 577–581. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
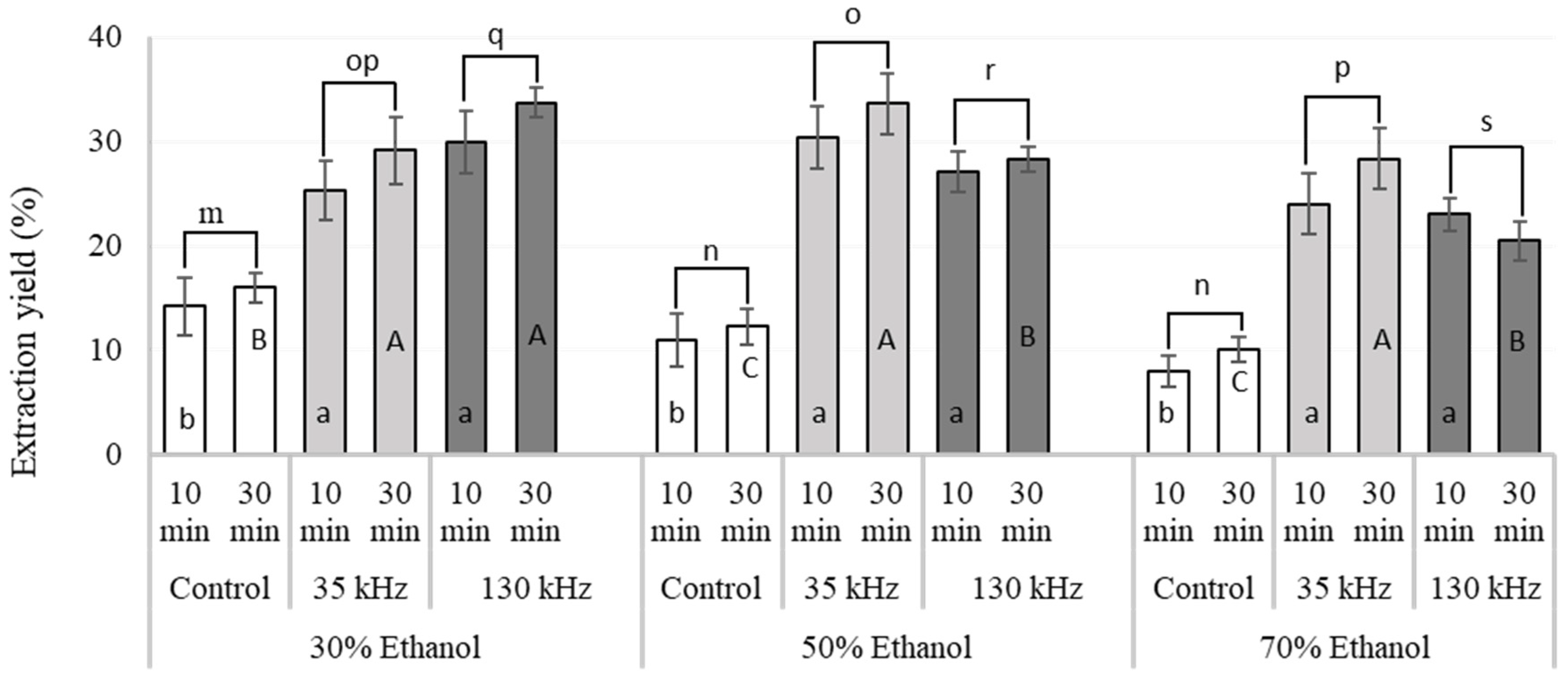
| Extraction Solvent | US Frequencies | Extraction Time (min) | TPC (Extract) (mg GAE/g) | TPC (Residue) (mg GAE/g) | TPC (Total) (mg GAE/g) | TPhC (Extract) (mg PGE/g) | TPhC (Residue) (mg PGE/g) | TPhC (Total) (mg PGE/g) |
|---|---|---|---|---|---|---|---|---|
| 30% ethanol | Control * | 10 | 314.5 ± 5.9 c | 262.9 ± 2.8 a | 577.5 ± 8.7 c | 222.0 ± 4.8 c | 179.9 ± 2.8 a | 401.9 ± 7.6 c |
| 30 | 340.3 ± 9.4 C | 247.8 ± 1.4 A | 588.1 ± 4.9 C | 248.7 ± 5.9 C | 178.9 ± 1.2 A | 427.7 ± 7.1 C | ||
| 35 kHz | 10 | 422.7 ± 4.4 b | 218.1 ± 2.8 b | 640.9 ± 7.2 b | 327.2 ± 7.2 b | 144.7 ± 2.3 b | 471.9 ± 9.5 a | |
| 30 | 463.7 ± 5.3 B | 201.2 ± 3.2 B | 664.9 ± 8.5 B | 392.3 ± 5.5 B | 122.1 ± 2.6 B | 514.4 ± 8.2 B | ||
| 130 kHz | 10 | 453.9 ± 6.7 a | 190.9 ± 2.9 c | 644.9 ± 9.7 a | 347.8 ± 4.4 a | 116.1 ± 2.4 c | 463.9 ± 6.8 b | |
| 30 | 579.7 ± 9.2 A | 125.2±0.4 C | 704.9 ± 9.6 A | 471.5 ± 7.5 A | 97.4 ± 2.9 C | 568.9 ± 9.9 A | ||
| 50% ethanol | Control * | 10 | 338.0 ± 4.9 b | 235.6 ± 1.8 a | 573.6 ± 6.8 b | 262.2 ± 4.0 c | 144.1 ± 1.5 a | 406.2 ± 5.6 c |
| 30 | 358.5 ± 5.3 C | 232.7 ± 3.9 A | 591.3 ± 9.2 C | 286.2 ± 4.4 C | 125.4 ± 3.2 A | 411.6 ± 7.6 C | ||
| 35 kHz | 10 | 464.7 ± 9.8 a | 179.6 ± 3.5 c | 644.3 ± 13.4 a | 408.7 ± 4.1 a | 121.7 ± 2.9 b | 530.4 ± 7.0 a | |
| 30 | 571.1 ± 10.0 A | 125.6 ± 1.1 C | 696.7 ± 11.7 A | 462.6 ± 2.1 A | 99.8 ± 2.9 B | 562.4 ± 5.1 A | ||
| 130 kHz | 10 | 458.6 ± 10.8 a | 190.0 ± 1.9 b | 648.7 ± 12.7 a | 350.1 ± 6.9 b | 144.6 ± 1.6 a | 494.8 ± 8.5 b | |
| 30 | 474.1 ± 12.7 B | 180.6 ± 1.1 B | 654.7 ± 13.9 B | 366.5 ± 4.0 B | 119.8 ± 1.9 A | 486.3 ± 6.0 B | ||
| 70% ethanol | Control * | 10 | 306.8 ± 7.3 c | 272.8 ± 3.2 a | 579.6 ± 10.5 c | 234.8 ± 5.0 c | 147.3 ± 2.5 a | 382.1 ± 7.5 b |
| 30 | 316.4 ± 6.1 C | 271.8 ± 0.9 A | 588.2 ± 6.9 C | 253.6 ± 5.0 C | 153.4 ± 1.7 A | 406.9 ± 6.7 C | ||
| 35 kHz | 10 | 436.9 ± 7.3 b | 195.6 ± 3.7 b | 632.5 ± 10.9 a | 362.4 ± 5.3 a | 119.5 ± 3.0 b | 481.9 ± 8.3 a | |
| 30 | 503.2 ± 9.2 A | 158.8 ± 3.3 B | 662.0 ± 12.5 A | 389.9 ± 3.5 A | 106.4 ± 2.9 C | 496.3 ± 6.4 A | ||
| 130 kHz | 10 | 453.2 ± 6.8 a | 154.2 ± 2.2 c | 607.4 ± 9.1 b | 347.2 ± 2.2 b | 122.7 ± 6.7 b | 469.9 ± 8.9 a | |
| 30 | 468.1 ± 10.4 B | 149.2 ± 2.6 B | 617.3 ± 13.0 B | 334.8 ± 2.2 B | 121.7 ± 2.1 B | 456.4 ± 4.4 B |
| Seaweed Species | Extraction Yield (%) | |
|---|---|---|
| UAE | Conventional | |
| Pelvetia caniculata | 20.5 ± 0.35 h | 14.0 ± 0.28 e |
| Fucus vesiculosus | 35.1 ± 0.33 b | 11.2 ± 0.29 g |
| Laminaria saccharina | 30.9 ± 0.41 d | 17.0 ± 0.47 c |
| Laminaria hyperborea | 36.9 ± 0.11 a | 19.3 ± 0.21 a |
| Fucus spiralis | 25.2 ± 0.50 f | 14.7 ± 0.45 de |
| Ascophyllum nodosum | 24.4 ± 0.31 f | 12.7 ± 0.15 f |
| Fucus serratus | 20.4 ± 0.19 h | 10.5 ± 0.12 g |
| Himanthalia elongata | 23.4 ± 0.30 g | 15.0 ± 0.26 d |
| Halidrys siliquosa | 29.0 ± 0.25 e | 14.5 ± 0.31 de |
| Laminaria digitata | 29.4 ± 0.16 e | 18.4 ± 0.21 b |
| Alaria esculenta | 33.0 ± 0.06 c | 17.2 ± 0.09 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. https://doi.org/10.3390/md18050250
Ummat V, Tiwari BK, Jaiswal AK, Condon K, Garcia-Vaquero M, O’Doherty J, O’Donnell C, Rajauria G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Marine Drugs. 2020; 18(5):250. https://doi.org/10.3390/md18050250
Chicago/Turabian StyleUmmat, Viruja, Brijesh K Tiwari, Amit K Jaiswal, Kevin Condon, Marco Garcia-Vaquero, John O’Doherty, Colm O’Donnell, and Gaurav Rajauria. 2020. "Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds" Marine Drugs 18, no. 5: 250. https://doi.org/10.3390/md18050250
APA StyleUmmat, V., Tiwari, B. K., Jaiswal, A. K., Condon, K., Garcia-Vaquero, M., O’Doherty, J., O’Donnell, C., & Rajauria, G. (2020). Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Marine Drugs, 18(5), 250. https://doi.org/10.3390/md18050250