Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion
Abstract
1. Introduction
2. Results and Discussion
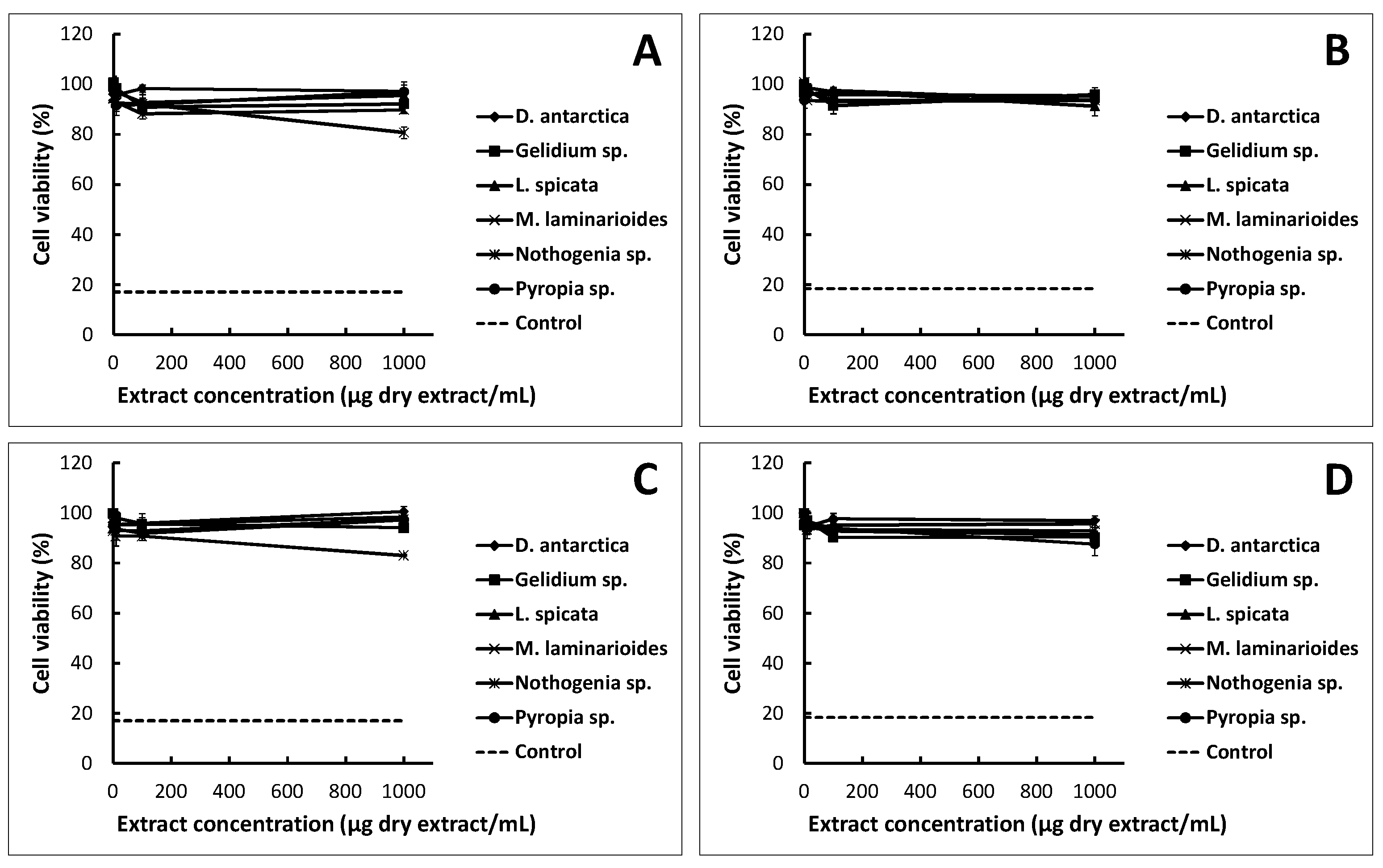
2.1. Cytotoxicity Assay
2.2. Total Polyphenol Content and Antioxidant Capacities
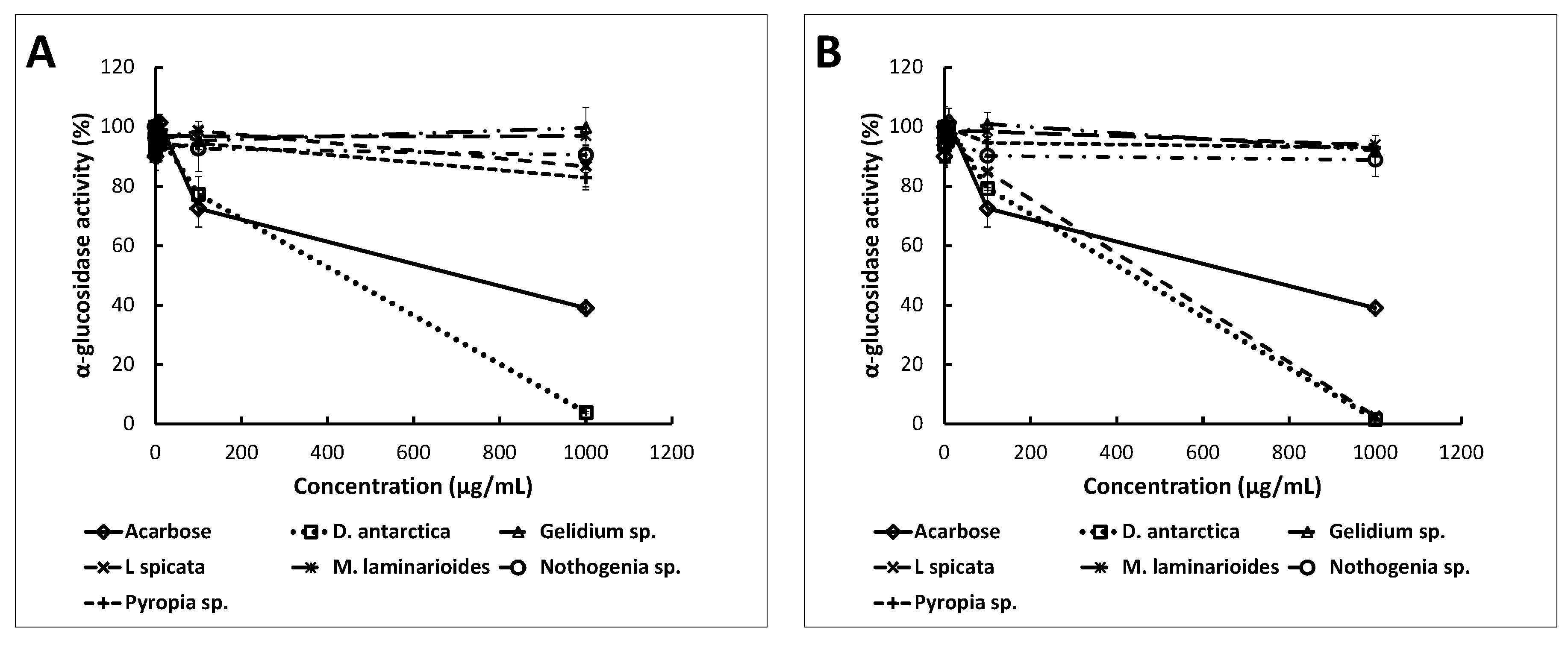
2.3. Anti-Enzymatic Activities
3. Materials and Methods
3.1. Chemicals and Cell Culture
3.2. Seaweed Collection and Identification
3.3. Cytotoxicity Assays
3.4. Polyphenol Extraction Methods
3.5. Total Polyphenol Content
3.6. Free Radical Scavenging Using DPPH Radical
3.7. Free Radical Scavenging by Oxygen Radical Absorbance Capacity (ORAC) Assay
3.8. Inhibition of α-Amylase Activity
3.9. Inhibitions of α-Glucosidase Activity
3.10. Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swamy, M.L.A. Marine algal sources for treating bacterial diseases. In Advances in Food and Nutrition Research, 1st ed.; Academic Press: Waltham, MA, USA, 2011; Volume 64, pp. 71–84. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2017. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, M.C.; Lopez, D.; Saiz, M.P.; Poquet, M.; Perez, J.; Puig-Parellada, P.; Màrmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wijesekara, I.; Li, Y.; Kim, S. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2013, 10, S2608–S2614. [Google Scholar] [CrossRef]
- Zhou, K.; Hogan, S.; Canning, C.; Sun, S. Inhibition of intestinal α-glucosidases and anti-postprandial hyperglycemic effect of grape seed extract. In Emerging Trends in Dietary Components for Preventing and Combating Disease; Patil, B.S., Jayaprakasha, G.K., Murthy, K.N., Seeram, N.P., Eds.; American Chemical Society: Washington, DC, USA, 2012; pp. 431–441. [Google Scholar] [CrossRef]
- Jayaraj, S.; Suresh, S.; Kadeppagari, R.; Kadeppagari, R. Amylase inhibitors and their biomedical applications. Starch 2013, 65, 535–542. [Google Scholar] [CrossRef]
- Kawamura-Konishi, Y.; Watanabe, N.; Saito, M.; Nakajima, N.; Sakaki, T.; Katayama, T.; Enomoto, T. Isolation of a new phlorotannin, a potent inhibitor of carbohydrate-hydrolyzing enzymes, from the brown alga sargassum patens. J. Agric. Food Chem. 2012, 60, 5565–5570. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.E. Algas Marinas Bentónicas. In Biodiversidad de Chile, Patrimonio y Desafíos, Ministerio del Medio Ambiente, 3rd ed.; Gobieron de Chile: Santiago, Chile, 2018; Volume I. [Google Scholar]
- Naquvi, K.J.; Ahamad, J.; Mir, S.R.; Ali, M.; Shuaib, M. Review on role of natural alpha-glucosidase inhibitors for management of diabetes mellitus. Int. J. Biomed. Res. 2011, 2. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Gulati, V.; Harding, I.H.; Palombo, E.A.; Palombo, E.A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement. Altern. Med. 2012, 12, 77. [Google Scholar] [CrossRef]
- Khalid, S.; Abbas, M.; Saeed, F.; Bader-Ul-Ain, H.; Rasul, H. Therapeutic Potential of Seaweed Bioactive Compounds. In Seaweed Biomaterials; IntechOpen: London, UK, 2018; pp. 7–25. [Google Scholar] [CrossRef]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit α-Amylase in Vitro: Identifying active components in rowanberry and raspberry. J. Agric. Food. Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Cytotoxicity of African medicinal plants against normal animal and human cells. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Academic Press: Waltham, MA, USA, 2014. [Google Scholar] [CrossRef]
- Martinez-Maqueda, D.; Millares, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Eds.; Springer: London, UK, 2015; pp. 113–130. [Google Scholar]
- Galindo, C.V.; Castell, E.C.; Montell, L.T.; Segura, E.S.; Baeza, M.M.R.; Guilen, G. Evaluación de la citotoxicidad y bioseguridad de un extracto de polifenoles de huesos de aceitunas. Nutr. Hosp. 2014, 29, 1388–1393. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Aladedunye, F.; Rodriguez-Juarez, R.; Li, D.; Thomas, J.; Kovalchuk, O.; Przybylski, R. Novel antioxidants are not toxic to normal tissues but effectively kill cancer cells. Cancer Biol. Ther. 2013, 14, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Screening of antioxidant activity in Icelandic seaweed. Available online: http://www.avs.is/media/avs/Screening_of_antioxidant_activity_final_II.pdf (accessed on 6 June 2020).
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar] [CrossRef]
- Miranda-Delgado, A.; Montoya, M.J.; Paz-Araos, M.; Mellado, M.; Villena, J.; Arancibia, P.; Madrid, A.; Jara-Gutiérrez, C. Antioxidant and anti-cancer activities of brown and red seaweed extracts from Chilean coasts. Lat. Am. J. Aquat. Res. 2018, 46, 301–313. [Google Scholar] [CrossRef]
- Sanz-Pintos, N.; Pérez-Jiménez, J.; Buschmann, A.H.; Vergara-Salinas, J.R.; Pérez-Correa, J.R.; Saura-Calixto, F. Macromolecular antioxidants and dietary fiber in edible seaweeds. J. Food. Sci. 2017, 82, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Brown and red seaweeds as potential sources of antioxidant nutraceuticals. J. Appl. Phycol. 2012, 24, 1123–1132. [Google Scholar] [CrossRef]
- Jacobsen, C.; Sørensen, A.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Manap, M.Y.A.; Khatib, A.; Razis, A.F.A. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. Biofactors 2005, 23, 189–195. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. Enzyme J. Enzyme Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit a -glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Food Sci. Nutr. 2014, 7486, 1–8. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, Y.; Wang, M.; Lee, W.; Rhee, H. Inhibitory effect of pine extract on a-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef]
- Bischoff, H. Pharmacology of α-glucosidase inhibition. Eur. J. Clin. Invest. 1994, 24, 3–10. [Google Scholar] [CrossRef]
- Oboh, G.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Inhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitro. Food Nutr. Res. 2010, 49, 14–20. [Google Scholar]
- Kajaria, D.; Ranjana, J.T.; Tripathi, Y.B.; Tiwari, S. In-vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug—Shirishadi. J. Adv. Pharm. Technol. Res. 2013, 4, 2–5. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Diferential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurised liquid extraction and solid–liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R. Measurement of Oxygen Radical Absorbance in Biological Samples. In Methods in Enzymology; Academic Press: Waltham, MA, USA, 1999; Volume 299, pp. 50–62. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef] [PubMed]


| Species | Type | Total Polyphenolsmg GAE/g Dry Seaweed | DPPH μmol ET/g Dry Seaweed | ORAC μmol ET/g Dry Seaweed | |||
|---|---|---|---|---|---|---|---|
| Ethanol | Acetone | Ethanol | Acetone | Ethanol | Acetone | ||
| Durvillaea antarctica | Brown | 7.4 ± 0.2 b | 6.7 ± 0.7 a | 48.5 ± 4.2 a | 27.8 ± 2.2a | 680.1 ± 11.6 a | 64.7 ± 0.0 a |
| Gelidium sp. | Red | 3.2 ± 0.3 a | 3.4 ± 0.2 a | 4.8 ± 0.4 b | 4.7 ± 0.2 c | 277.8 ± 15.5 bc | 6.9 ± 0.5 c |
| Lessonia spicata | Brown | 3.3 ± 0.2 b | 3.8 ± 0.1 a | 6.6 ± 0.7 a | 10.7 ± 0.6 a | 448.3 ± 33.4 a | 21.3 ± 1.3 b |
| Nothogenia sp. | Red | 4.8 ± 0.3 b | 6.0 ± 0.3 a | 5.4 ± 0.3 ab | 6.9 ± 0.1 bc | 371.6 ± 12.3 ab | 18.1 ± 0.9 b |
| Mazzaella laminarioides | Red | 1.9 ± 0.1 a | 3.1 ± 0.1 a | 2.2 ± 0.1 c | 7.05 ± 0.8 b | 208.1 ± 10.4 c | 8.7 ± 0.6 c |
| Pyropia sp. | Red | 2.2 ± 0.0 b | 3.1 ± 0.1 a | 6.5 ± 0.4 ab | 10.6 ± 0.9 a | 455.3 ± 3.4 a | 30.7 ± 2.9 a |
| Species | Extract Type | |
|---|---|---|
| Ethanol | Acetone | |
| D. antarctica | 473.4 ± 0.9 b | 466 ± 1.3 a |
| L. spicate | 5317.6 ± 0.75 e | 479.2 ± 1.7 c |
| Acarbose | 797.85 ± 1.1 d | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. https://doi.org/10.3390/md18070353
Pacheco LV, Parada J, Pérez-Correa JR, Mariotti-Celis MS, Erpel F, Zambrano A, Palacios M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Marine Drugs. 2020; 18(7):353. https://doi.org/10.3390/md18070353
Chicago/Turabian StylePacheco, Luz Verónica, Javier Parada, José Ricardo Pérez-Correa, María Salomé Mariotti-Celis, Fernanda Erpel, Angara Zambrano, and Mauricio Palacios. 2020. "Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion" Marine Drugs 18, no. 7: 353. https://doi.org/10.3390/md18070353
APA StylePacheco, L. V., Parada, J., Pérez-Correa, J. R., Mariotti-Celis, M. S., Erpel, F., Zambrano, A., & Palacios, M. (2020). Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Marine Drugs, 18(7), 353. https://doi.org/10.3390/md18070353








