Quorum Sensing Inhibitory and Antifouling Activities of New Bromotyrosine Metabolites from the Polynesian Sponge Pseudoceratina n. sp.
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Structure Elucidation
2.2. Biological Activities
2.2.1. QSi General Screening
2.2.2. Evaluation of the QS Inhibition of the Isolated Compounds
2.2.3. Antifouling Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Isolation of Bioactive Compounds
3.4. QSi Assay
3.5. Antifouling Assay
3.5.1. Assays Against Marine Bacteria
3.5.2. Assays Against Marine Microalgae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahboek, L.; Christophersen, C. The isoxazole alkaloids. In The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2001; Volume 57, pp. 185–233. [Google Scholar]
- Nunez, C.V.; de Almelda, E.V.R.; Granato, A.C.; Marques, S.O.; Santos, K.O.; Pereira, F.R.; Macedo, M.L.; Ferreira, A.G.; Hajdu, E.; Pinheiro, U.S.; et al. Chemical variability within the marine sponge Aplysina fulva. Biochem. Syst. Ecol. 2008, 36, 283–296. [Google Scholar] [CrossRef]
- Puyana, M.; Pawlik, J.; Blum, J.; Fehical, W. Metabolite variability Caribbean sponges of the genus Aplysina. Rev. Bras. Farmacogn. 2015, 25, 592–599. [Google Scholar] [CrossRef][Green Version]
- Konig, G.M.; Wright, A.D. Agelorin-A and Agelorin-B, And 11-Epi-Fistularin-3, 3 New Antibacterial Fistularin-3 Derivatives From The Tropical Marine Sponge Agelas oroides. Heterocycles 1993, 36, 1351–1358. [Google Scholar]
- Wang, Q.; Tang, X.L.; Luo, X.C.; de Voog, N.J.; Li, P.L.; Li, G.Q. Aplysinopsin-type and Bromotyrosine-derived Alkaloids from the South China Sea Sponge Fascaplysinopsis reticulata. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Hanssen, K.O.; Cervin, G.; Trepos, R.; Petitbois, J.; Haug, T.; Hansen, E.; Andersen, J.H.; Pavia, H.; Hellio, C.; Svenson, J. The Bromotyrosine Derivative Ianthelline Isolated from the Arctic Marine Sponge Stryphnus fortis Inhibits Marine Micro- and Macrobiofouling. Mar. Biotechnol. 2014, 16, 684–694. [Google Scholar] [CrossRef]
- Nicacio, K.J.; Ioca, L.P.; Froes, A.M.; Leomil, L.; Appolinario, L.R.; Thompson, C.C.; Thompson, F.L.; Ferreira, A.G.; Williams, D.E.; Andersen, R.J.; et al. Cultures of the Marine Bacterium Pseudovibrio denitrificans Ab134 Produce Bromotyrosine-Derived Alkaloids Previously Only Isolated from Marine Sponges. J. Nat. Prod. 2017, 80, 235–240. [Google Scholar] [CrossRef]
- Erpenbeck, D.; van Soest, R.W.M. Status and perspective of sponge chemosystematics. Mar. Biotechnol. 2007, 9, 2–19. [Google Scholar] [CrossRef]
- Shaker, K.H.; Zinecker, H.; Ghani, M.A.; Imhoff, J.F.; Schneider, B. Bioactive Metabolites from the Sponge Suberea sp. Chem. Biodivers. 2010, 7, 2880–2887. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Moriou, C.; Toullec, J.; Besson, M.; Soulet, S.; Schmitt, N.; Petek, S.; Lecchini, D.; Debitus, C.; Al-Mourabit, A. Bioactive Bromotyrosine-Derived Alkaloids from the Polynesian Sponge Suberea ianthelliformis. Mar. Drugs 2018, 16, 146. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.A.; Quinn, R.J. Psammaplysenes C and D, cytotoxic alkaloids from Psammoclemma sp. J. Nat. Prod. 2007, 70, 1827–1829. [Google Scholar] [CrossRef]
- Campos, P.-E.; Wolfender, J.-L.; Queiroz, E.F.; Marcourt, L.; Al-Mourabit, A.; De Voogd, N.; Illien, B.; Gauvin-Bialecki, A. Amphimedonoic acid and psammaplysene E, novel brominated alkaloids from Amphimedon sp. Tetrahedron Lett. 2017, 58, 3901–3904. [Google Scholar] [CrossRef]
- Kernan, M.R.; Cambie, R.C.; Bergquist, P.R. Chemistry of sponges 8. Anomoian-a, a bromotyrosine derivative from Anomoianthella popeae. J. Nat. Prod. 1990, 53, 720–723. [Google Scholar] [CrossRef]
- Tarazona, G.; Santamaria, G.; Cruz, P.G.; Fernandez, R.; Perez, M.; Martinez-Leal, J.F.; Rodriguez, J.; Jimenez, C.; Cuevas, C. Cytotoxic Anomoian B and Aplyzanzine B, New Bromotyrosine Alkaloids from Indonesian Sponges. ACS Omega 2017, 2, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Evan, T.; Rudi, A.; Ilan, M.; Kashman, Y. Aplyzanzine A, a new dibromotyrosine derivative from a Verongida sponge. J. Nat. Prod. 2001, 64, 226–227. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Magno, S. Archerine, a novel anti-histaminic bromotyrosine-derived compound from the Caribbean marine sponge Aplysina archeri. Eur. J. Org. Chem. 2001, 2001, 55–60. [Google Scholar] [CrossRef]
- Compagnone, R.S.; Avila, R.; Suarez, A.I.; Abrams, O.V.; Rangel, H.R.; Arvelo, F.; Pina, I.C.; Merentes, E. 11-deoxyfistularin-3, a new cytotoxic metabolite from the caribbean sponge Aplysina fistularis insularis. J. Nat. Prod. 1999, 62, 1443–1444. [Google Scholar] [CrossRef]
- Kurimoto, S.; Seino, S.; Fromont, J.; Kobayashi, J.; Kubota, T. Ma’edamines C and D, New Bromotyrosine Alkaloids Possessing a Unique Tetrasubstituted Pyridinium Moiety from an Okinawan Marine Sponge Suberea sp. Org. Lett. 2019, 21, 8824–8826. [Google Scholar] [CrossRef]
- Engel, S.; Jensen, P.R.; Fenical, W. Chemical ecology of marine microbial defense. J. Chem. Ecol. 2002, 28, 1971–1985. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R.; Sharp, K. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2011, 28, 345–387. [Google Scholar] [CrossRef]
- Puglisi, M.P.; Sneed, J.M.; Sharp, K.H.; Ritson-Williams, R.; Paul, V.J. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2019, 31, 1510–1553. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Saurav, K.; Costantino, V.; Venturi, V.; Steindler, L. Quorum Sensing Inhibitors from the Sea Discovered Using Bacterial N-acyl-homoserine Lactone-Based Biosensors. Mar. Drugs 2017, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Britstein, M.; Saurav, K.; Teta, R.; Della Sala, G.; Bar-Shalom, R.; Stoppelli, N.; Zoccarato, L.; Costantino, V.; Steindler, L. Identification and chemical characterization of N-acyl-homoserine lactone quorum sensing signals across sponge species and time. Fems Microbiology Ecology 2018, 94. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Simoes, M. Quorum Sensing Inhibition by Marine Bacteria. Mar. Drugs 2019, 17, 427. [Google Scholar] [CrossRef] [PubMed]
- Saurav, K.; Borbone, N.; Burgsdorf, I.; Teta, R.; Caso, A.; Bar-Shalom, R.; Esposito, G.; Britstein, M.; Steindler, L.; Costantino, V. Identification of Quorum Sensing Activators and Inhibitors in The Marine Sponge Sarcotragus spinosulus. Mar. Drugs 2020, 18, 127. [Google Scholar] [CrossRef]
- Aguila-Ramirez, R.N.; Hernandez-Guerrero, C.J.; Gonzalez-Acosta, B.; Id-Daoud, G.; Hewitt, S.; Pope, J.; Hellio, C. Antifouling activity of symbiotic bacteria from sponge Aplysina gerardogreeni. Int. Biodeterior. Biodegr. 2014, 90, 64–70. [Google Scholar] [CrossRef]
- Fang, S.T.; Yan, B.F.; Yang, C.Y.; Miao, F.P.; Ji, N.Y. Hymerhabdrin A, a novel diterpenoid with antifouling activity from the intertidal sponge Hymerhabdia sp. J. Antibiot. 2017, 70, 1043–1046. [Google Scholar] [CrossRef]
- Gribble, G.W. Biological Activity of Recently Discovered Halogenated Marine Natural Products. Mar. Drugs 2015, 13, 4044–4136. [Google Scholar] [CrossRef]
- Lira, N.S.; Montes, R.C.; Tavares, J.F.; da Silva, M.S.; da Cunha, E.V.L.; de Athayde, P.F.; Rodrigues, L.C.; Dias, C.D.S.; Barbosa, J.M. Brominated Compounds from Marine Sponges of the Genus Aplysina and a Compilation of Their C-13 NMR Spectral Data. Mar. Drugs 2011, 9, 2316–2368. [Google Scholar] [CrossRef]
- Shaala, L.A.; Bamane, F.H.; Badr, J.M.; Youssef, D.T.A. Brominated Arginine-Derived Alkaloids from the Red Sea Sponge Suberea mollis. J. Nat. Prod. 2011, 74, 1517–1520. [Google Scholar] [CrossRef]
- Xu, M.; Davis, R.A.; Feng, Y.J.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J. Ianthelliformisamines A-C, Antibacterial Bromotyrosine-Derived Metabolites from the Marine Sponge Suberea ianthelliformis. J. Nat. Prod. 2012, 75, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Thirionet, I.; Daloze, D.; Braekman, J.C. 5-Bromoverongamine, a novel antifouling tyrosine alkaloid from the sponge Pseudoceratina sp. Nat. Prod. Lett. 1998, 12, 209–214. [Google Scholar] [CrossRef]
- Trepos, R.; Cervin, G.; Hellio, C.; Pavia, H.; Stensen, W.; Stensvag, K.; Svendsen, J.S.; Haug, T.; Svenson, J. Antifouling Compounds from the Sub-Arctic Ascidian Synoicum pulmonaria: Synoxazolidinones A and C, Pulmonarins A and B, and Synthetic Analogues. J. Nat. Prod. 2014, 77, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Marmann, A.; Lin, W.H.; Proksch, P. Sponge Derived Bromotyrosines: Structural Diversity through Natural Combinatorial Chemistry. Nat. Prod. Commun. 2015, 10, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Putz, A.; Ortlepp, S.; Kjer, J.; Bayer, M. Bioactive natural products from marine sponges and fungal endophytes. Phytochem. Rev. 2010, 9, 475–489. [Google Scholar] [CrossRef]
- Andjouh, S.; Blache, Y. Screening of bromotyramine analogues as antifouling compounds against marine bacteria. Biofouling 2016, 32, 871–881. [Google Scholar] [CrossRef]
- Mani, L.; Jullian, V.; Mourkazel, B.; Valentin, A.; Dubois, J.; Cresteil, T.; Folcher, E.; Hooper, J.N.A.; Erpenbeck, D.; Aalbersberg, W.; et al. New Antiplasmodial Bromotyrosine Derivatives from Suberea ianthelliformis Lendenfeld, 1888. Chem. Biodivers. 2012, 9, 1436–1451. [Google Scholar] [CrossRef]
- Galeano, E.; Thomas, O.P.; Robledo, S.; Munoz, D.; Martinez, A. Antiparasitic Bromotyrosine Derivatives from the Marine Sponge Verongula rigida. Mar. Drugs 2011, 9, 1902–1913. [Google Scholar] [CrossRef]
- Lebouvier, N.; Jullian, V.; Desvignes, I.; Maurel, S.; Parenty, A.; Dorin-Semblat, D.; Doerig, C.; Sauvain, M.; Laurent, D. Antiplasmodial Activities of Homogentisic Acid Derivative Protein Kinase Inhibitors Isolated from a Vanuatu Marine Sponge Pseudoceratina sp. Mar. Drugs 2009, 7, 640–653. [Google Scholar] [CrossRef]
- Hirano, K.; Kubota, T.; Tsuda, M.; Watanabe, K.; Fromont, J.; Kobayashi, J. Ma’edamines A and B, cytotoxic bromotyrosine alkaloids with a unique 2(1H)pyrazinone ring from sponge Suberea sp. Tetrahedron 2000, 56, 8107–8110. [Google Scholar] [CrossRef]
- Tsuda, M.; Sakuma, Y.; Kobayashi, J. Suberedamines A and B, new bromotyrosine alkaloids from a sponge Suberea species. J. Nat. Prod. 2001, 64, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Purohit, H.J. Quenching the quorum sensing system: Potential antibacterial drug targets. Crit. Rev. Microbiol. 2011, 37, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.; Zhang, X.H. Quorum Quenching Agents: Resources for Antivirulence Therapy. Mar. Drugs 2014, 12, 3245–3282. [Google Scholar] [CrossRef] [PubMed]
- Toupoint, N.; Mohit, V.; Linossier, I.; Bourgougnon, N.; Myrand, B.; Olivier, F.; Lovejoy, C.; Tremblay, R. Effect of biofilm age on settlement of Mytilus edulis. Biofouling 2012, 28, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.A.; Sutcliffe, P.R.; Hooper, J.N.A.; Alencar, A.; Vacelet, J.; Pisera, A.; Petek, S.; Folcher, E.; Butscher, J.; Orempuller, J.; et al. Affinities of Sponges (Porifera) of the Marquesas and Society Islands, French Polynesia. Pac. Sci. 2013, 67, 493–511. [Google Scholar] [CrossRef]
- Petek, S.; Debitus, C.; Alencar, A.; Bourgeois, B.; Butscher, J.; Ekins, M.; Fleurisson, D.; Folcher, E.; Hall, K.A.; Hertrich, L.; et al. Sponges of Polynesia. Available online: https://sponges-polynesia.ird.fr (accessed on 21 April 2020).
- Boufridi, A.; Lachkar, D.; Erpenbeck, D.; Beniddir, M.A.; Evanno, L.; Petek, S.; Debitus, C.; Poupon, E. Ilimaquinone and 5-epi-Ilimaquinone: Beyond a Simple Diastereomeric Ratio, Biosynthetic Considerations from NMR-Based Analysis. Aust. J. Chem. 2017, 70, 743–750. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Rodrigues-Stien, A.D.; Petek, S.; Demoy-Schneider, M.; Hall, K.; Hooper, J.N.A.; Debitus, C.; Al-Mourabit, A. Cytotoxic Guanidine Alkaloids from a French Polynesian Monanchora n. sp Sponge. J. Nat. Prod. 2016, 79, 1929–1937. [Google Scholar] [CrossRef]
- Boufridi, A.; Petek, S.; Evanno, L.; Beniddir, M.A.; Debitus, C.; Buisson, D.; Poupon, E. Biotransformations versus chemical modifications: New cytotoxic analogs of marine sesquiterpene ilimaquinone. Tet. Lett. 2016, 57, 4922–4925. [Google Scholar] [CrossRef]
- Mai, T.; Tintillier, F.; Lucasson, A.; Moriou, C.; Bonno, E.; Petek, S.; Magre, K.; Al Mourabit, A.; Saulnier, D.; Debitus, C. Quorum sensing inhibitors from Leucetta chagosensis Dendy, 1863. Lett. Appl. Microbiol. 2015, 61, 311–317. [Google Scholar] [CrossRef]
- Kottakota, S.K.; Evangelopoulos, D.; Alnimr, A.; Bhakta, S.; McHugh, T.D.; Gray, M.; Groundwater, P.W.; Marrs, E.C.L.; Perry, J.D.; Spilling, C.D.; et al. Synthesis and Biological Evaluation of Purpurealidin E-Derived Marine Sponge Metabolites: Aplysamine-2, Aplyzanzine A, and Suberedamines A and B. J. Nat. Prod. 2012, 75, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Tsuda, M.; Ohizumi, Y.; Sasaki, T.; Kobayashi, J. Purealidin A, a New Cytotoxic Bromotyrosine-Derived Alkaloid from the Okinawan Marine Sponge Psammaplysilla purea. Experientia 1991, 47, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Pansini, M. Chemistry of Verongida Sponges.3. Constituents of a Caribbean Verongula sp. J. Nat. Prod. 1994, 57, 1564–1569. [Google Scholar] [CrossRef]
- Kobayashi, J.; Honma, K.; Tsuda, M.; Kosaka, T. Lipopurealin-D and Lipopurealin-E and Purealidin-H, New Bromotyrosine Alkaloids from the Okinawan Marine Sponge Psammaplysilla purea. J. Nat. Prod. 1995, 58, 467–470. [Google Scholar] [CrossRef]
- MarinLit Database: A Database of the Marine Natural Products Literature. Available online: http://pubs.rsc.org/marinlit (accessed on 21 April 2020).
- Jurek, J.; Yoshida, W.Y.; Scheuer, P.J.; Kelly-Borges, M. 3 New Bromotyrosine-Derived Metabolites of the Sponge Psammaplysilla purpurea. J. Nat. Prod. 1993, 56, 1609–1612. [Google Scholar] [CrossRef]
- Bassler, B.L.; Greenberg, E.P.; Stevens, A.M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 1997, 179, 4043–4045. [Google Scholar] [CrossRef]
- Henke, J.M.; Bassler, B.L. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 2004, 186, 6902–6914. [Google Scholar] [CrossRef]
- Henke, J.M.; Bassler, B.L. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 2004, 186, 3794–3805. [Google Scholar] [CrossRef]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Teasdale, M.E.; Liu, J.; Wallace, J.; Akhlaghi, F.; Rowley, D.C. Secondary Metabolites Produced by the Marine Bacterium Halobacillus salinus That Inhibit Quorum Sensing-Controlled Phenotypes in Gram-Negative Bacteria. Appl. Environ. Microbiol. 2009, 75, 567–572. [Google Scholar] [CrossRef]
- Long, R.A.; Qureshi, A.; Faulkner, D.J.; Azam, F. 2-n-pentyl-4-quinolinol produced by a marine Alteromonas sp and its potential ecological and biogeochemical roles. Appl. Environ. Microbiol. 2003, 69, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Manefield, M.; de Nys, R.; Kumar, N.; Read, R.; Givskov, M.; Steinberg, P.; Kjelleberg, S.A. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology-Sgm 1999, 145, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Skindersoe, M.E.; Ettinger-Epstein, P.; Rasmussen, T.B.; Bjarnsholt, T.; de Nys, R.; Givskov, M. Quorum sensing antagonism from marine organisms. Mar. Biotechnol. 2008, 10, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.; Brango-Vanegas, J.; Costa, G.M.; Castellanos, L.; Arevalo, C.; Duque, C. Marine organisms as source of extracts to disrupt bacterial communication: Bioguided isolation and identification of quorum sensing inhibitors from Ircinia felix. Rev. Bras. Farmacogn. 2015, 25, 199–207. [Google Scholar] [CrossRef]
- Kovalerchik, D.; Singh, R.P.; Schlesinger, P.; Mahajni, A.; Shefer, S.; Fridman, M.; Ilan, M.; Carmeli, S. Bromopyrrole Alkaloids of the Sponge Agelas oroides Collected Near the Israeli Mediterranean Coastline. J. Nat. Prod. 2020, 83, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Lianguo, C.; Lam, J.C.W. SeaNine 211 as antifouling biocide: A coastal pollutant of emerging concern. J. Environ. Sci. 2017, 61, 68–79. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Ceratinamides A and B: New antifouling dibromotyrosine derivatives from the marine sponge Pseudoceratina purpurea. Tetrahedron 1996, 52, 8181–8186. [Google Scholar] [CrossRef]
- Takada, N.; Watanabe, R.; Suenaga, K.; Yamada, K.; Ueda, K.; Kita, M.; Uemura, D. Zamamistatin, a significant antibacterial bromotyrosine derivative, from the Okinawan sponge Pseudoceratina purpurea. Tetrahedron. Lett. 2001, 42, 5265–5267. [Google Scholar] [CrossRef]
- Ortlepp, S.; Sjogren, M.; Dahlstrom, M.; Weber, H.; Ebel, R.; Edrada, R.; Thoms, C.; Schupp, P.; Bohlin, L.; Proksch, P. Antifouling activity of bromotyrosine-derived sponge metabolites and synthetic analogues. Mar. Biotechnol. 2007, 9, 776–785. [Google Scholar] [CrossRef]
- Debitus, C. TUAM 2011 Oceanographic Cruise Aboard RV Alis, 26 April–2 June 2011; Ifremer: Issy-les-Moulineaux, France, 2011. [Google Scholar] [CrossRef]
- Bovio, E.; Fauchon, M.; Toueix, Y.; Mehiri, M.; Varese, G.C.; Hellio, C. The Sponge-Associated Fungus Eurotium chevalieri MUT 2316 and its Bioactive Molecules: Potential Applications in the Field of Antifouling. Mar. Biotechnol. 2019, 21, 743–752. [Google Scholar] [CrossRef]
- Trepos, R.; Cervin, G.; Pile, C.; Pavia, H.; Hellio, C.; Svenson, J. Evaluation of cationic micropeptides derived from the innate immune system as inhibitors of marine biofouling. Biofouling 2015, 31, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.D.; Hellio, C.; Stokes, K.R.; Dennington, S.P.; Goodes, L.R.; Wood, R.J.K.; Walsh, F.C. Investigation of Chondrus crispus as a potential source of new antifouling agents. Int. Biodeterior. Biodegrad. 2011, 65, 939–946. [Google Scholar] [CrossRef]
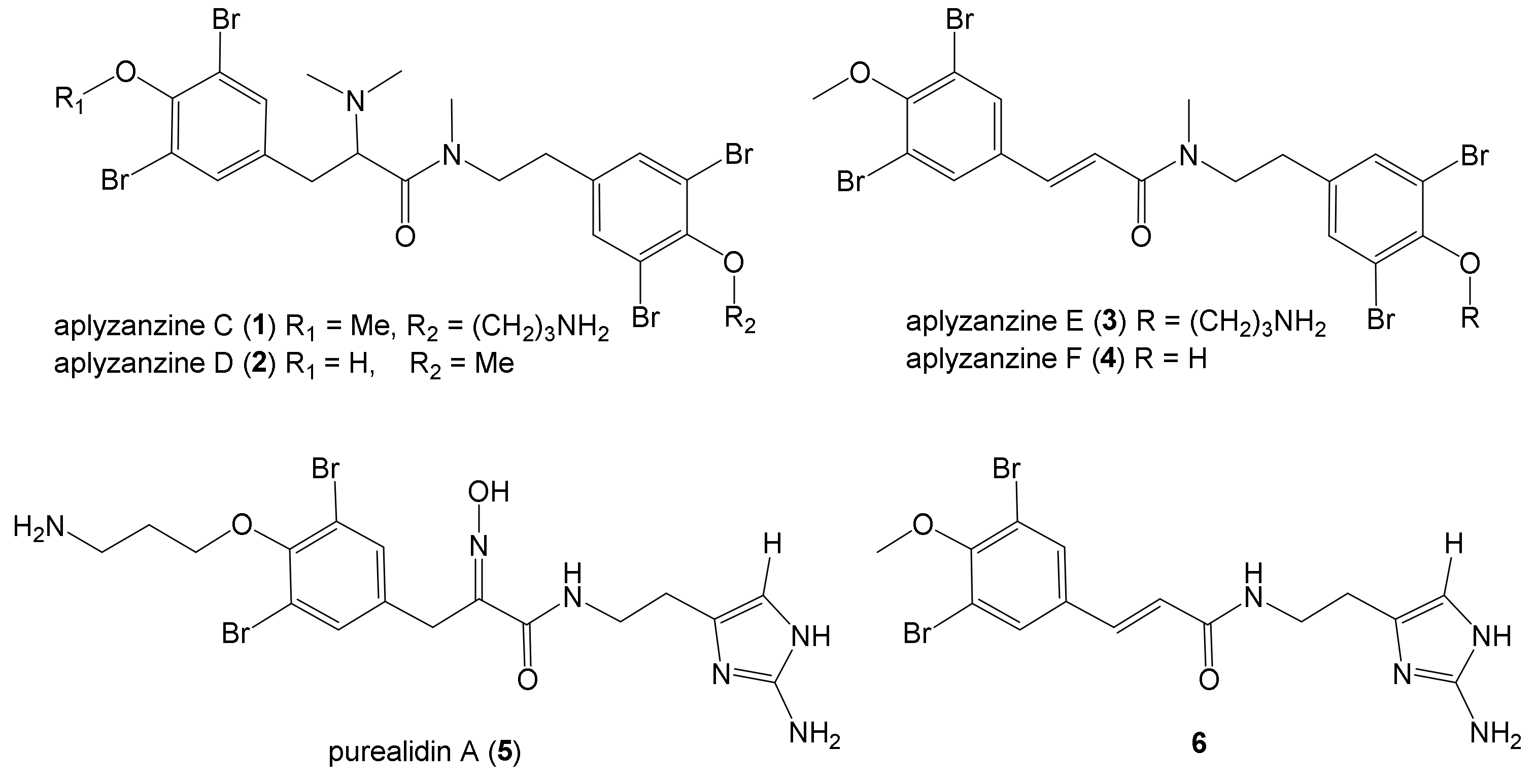
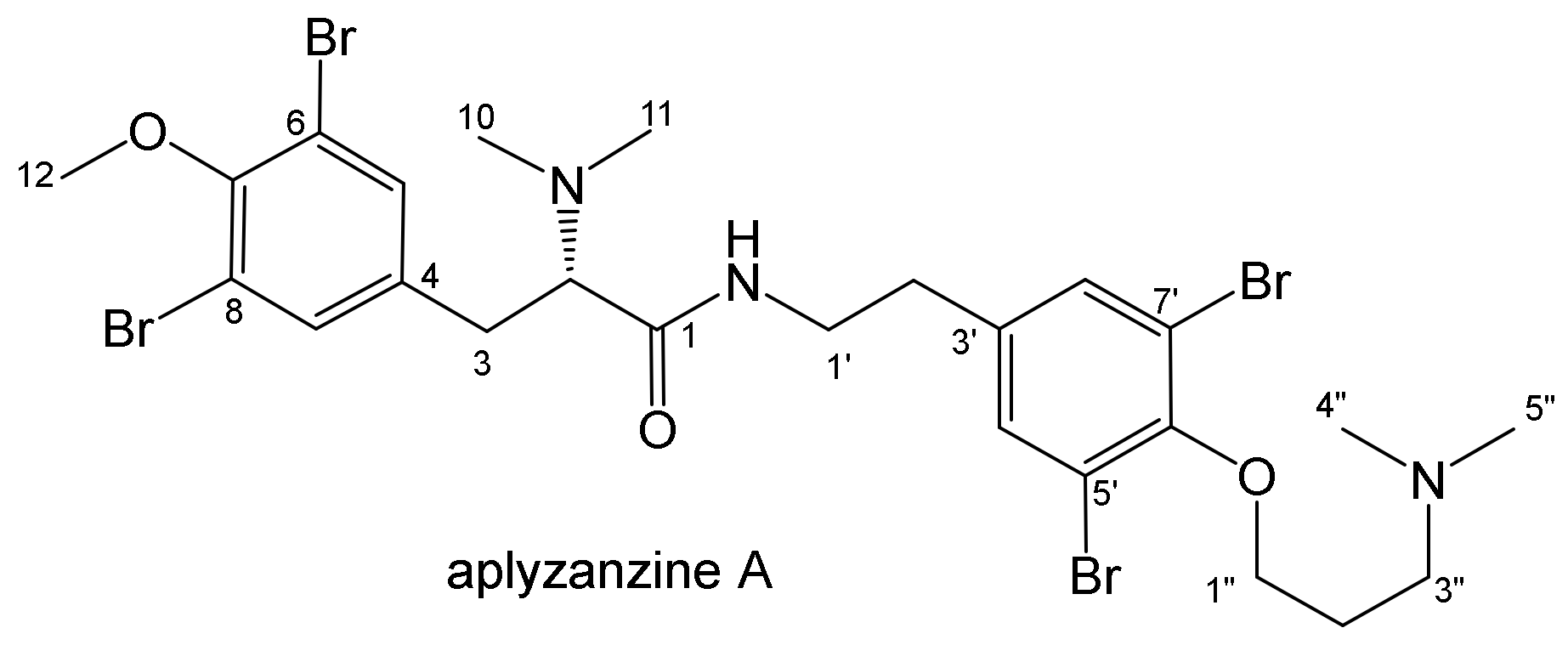

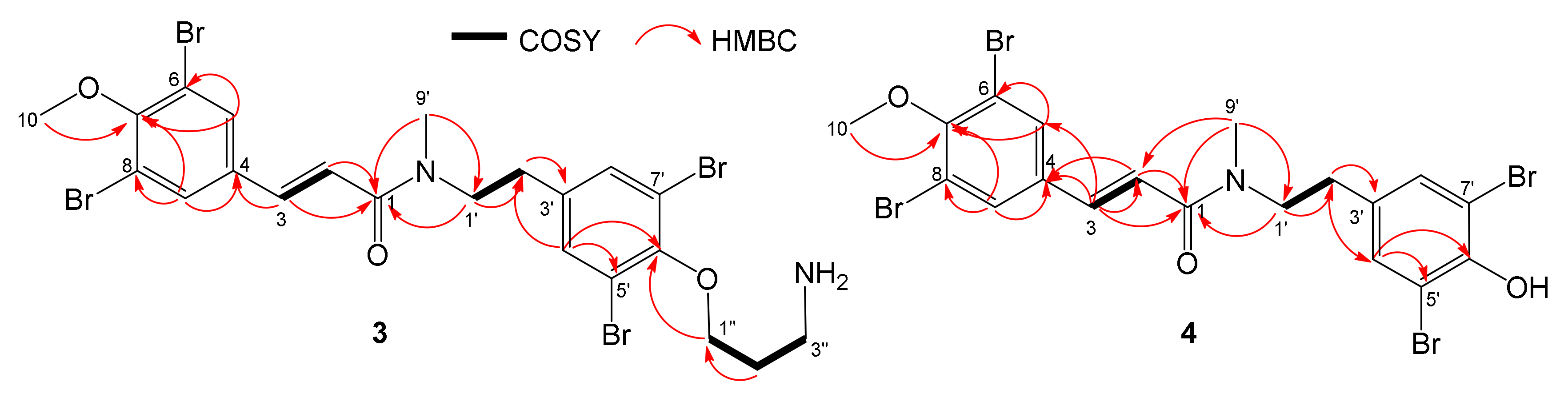
| Position | Aplyzanzine C (1) | Aplyzanzine D (2) | Aplyzanzine A | |||
|---|---|---|---|---|---|---|
| No. | δC, Type | δH, Mult | δC, Type | δH, Mult | δC, Type | δH, Mult (J in Hz) |
| 1 | 168.3, C | - | 167.2, C | - | 170.8, C | - |
| 2 | 66.3, CH | 4.65, m | 65.9, CH | 4.41, m | 69.8, CH | 3.14, dd (4.5, 8.8) |
| 3 | 35.1, CH2 | 2.97–3.47, m | 30.4, CH2 | 2.93–3.30, m | 31.6, CH2 | 2.71, dd (4.5, 13.8) 2.94, dd (8.8, 13.5) |
| 4 | 134.0, C | - | 132.0, C | - | 137.6, C | - |
| 5, 9 | 135.6, CH | 7.55, s | 133.6, CH | 7.36, s | 133.2, CH | 7.31, s |
| 6, 8 | 119.4, C | - | 110.5, C | - | 117.6, C | - |
| 7 | 155.7, C | - | 149.2, C | - | 152.3, C | - |
| 10, 11 | 42.7, CH3 | 2.91, s | 42.1, CH3 | 2.71, br s | 41.51, CH3 | 2.26, s |
| 12 | 61.4, CH3 | 3.84, s | 61.4, CH3 | 3.74, s | ||
| 1’ | 50.5, CH2 | 3.47, m | 50.4, CH2 | 3.54, m | 39.8, CH2 | 3.28, dt (2.8, 7.2) |
| 2’ | 32.8, CH2 | 2.57, m | 32.4, CH2 | 2.53, m | 34.2, CH2 | 2.54, m 2.27, m |
| 3’ | 139.5, C | - | 133.9, C | - | 137.9, C | - |
| 4’, 8’ | 134.5, CH | 7.51, s | 135.1, CH | 7.54, s | 132.8, CH | 7.23, s |
| 5’, 7’ | 119.1, C | - | 117.3, C | - | 117.7, C | - |
| 6’ | 152.7, C | - | 153.4, C | - | 150.9, C | - |
| 9’ | 35.8, CH3 | 2.65, s | 35.4, CH3 | 2.64, s | ||
| 10’ | 61.0, CH3 | 3.83, s | ||||
| 1” | 71.8, CH2 | 4.11, m | 69.7, CH2 | 3.96, t (5.5) | ||
| 2” | 29.2, CH2 | 2.21, m | 25.4, CH2 | 2.18, m | ||
| 3” | 39.0, CH2 | 3.27, m | 55.4, CH2 | 3.16, m | ||
| 4”, 5” | 42.92, CH3 | 2.67, s | ||||
| Position | Aplyzanzine E (3) | Aplyzanzine F (4) | ||
|---|---|---|---|---|
| No. | δC, Type | δH, Mult (J in Hz) | δC, Type 1 | δH, Mult (J in Hz) 1 |
| 1 | 168.9, C | - | 169.2/168.4, C | - |
| 2 | 120.7, CH | 6.60, d (15) | 120.7/121.2, CH | 6.64, d (16)/7.09, d (16) |
| 3 | 138.4, CH | 6.96, d (15) | 138.8/140.4, CH | 6.97, d (16)/7.38, d (16) |
| 4 | 133.3, C | - | 135.6, C | - |
| 5, 9 | 132.9, CH | 7.69, s | 133.2/133.4, CH | 7.68, s/7.90, s |
| 6, 8 | 119.9, C | - | 119.7, C | - |
| 7 | 156.5, C | - | 156.2, C | - |
| 10 | 61.1, CH3 | 3.88, s | 61.3, CH3 | 3.87, s |
| 1’ | 52.2, CH2 | 3.85, m | 52.6/51.5, CH2 | 3.78, t (6)/3.63, t (6) |
| 2’ | 34.2, CH2 | 2.86, m | 34.0/31.9, CH2 | 2.79, m/2.78, m |
| 3’ | 135.2, C | - | 133.1, C | - |
| 4’, 8’ | 134.9, CH | 7.40, s | 134.3/133.8, CH | 7.27, s/7.39, s |
| 5’, 7’ | 119.2, C | - | 112.5, C | - |
| 6’ | 152.7, C | - | 151.9, C | - |
| 9’ | 34.2, CH3 | 3.09, s | 34.7/36.9, CH3 | 3.05, s/3.16, s |
| 1” | 71.5, CH2 | 4.10, m | ||
| 2” | 29.1, CH2 | 2.19, m | ||
| 3” | 38.6, CH2 | 3.30, m | ||
| ____: Control without Product; - - -: 50 µg/mL; ……: 5 µg/mL | ||||
|---|---|---|---|---|
| No. | BB120 | JAF 375 (CAI-1 +) | JMH 597 (AI-2 +) | JMH 612 (HAI-1 +) |
| 1 |  | 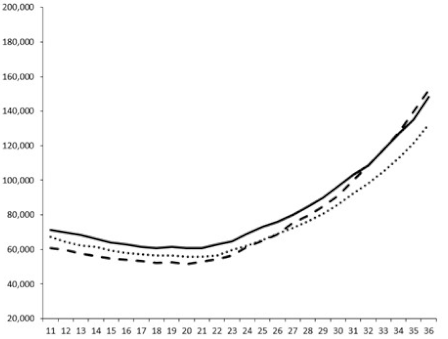 | 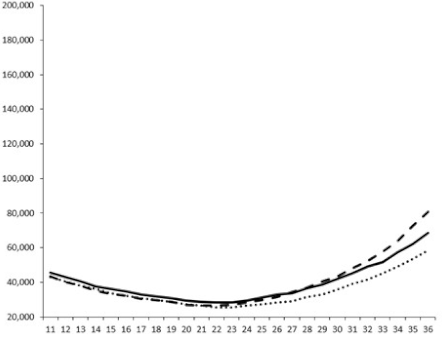 |  |
| 2 | 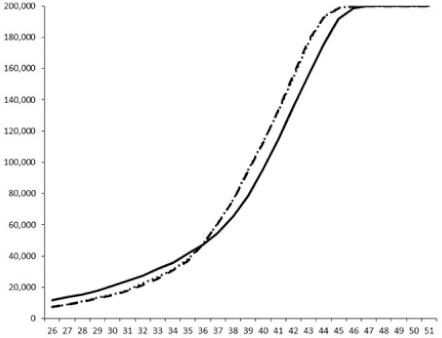 | 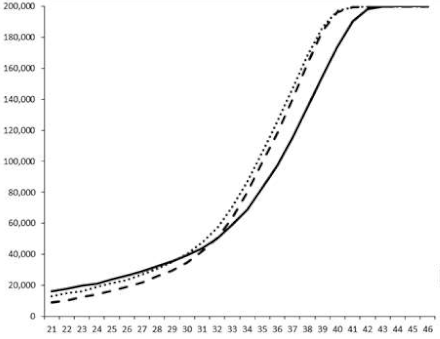 | 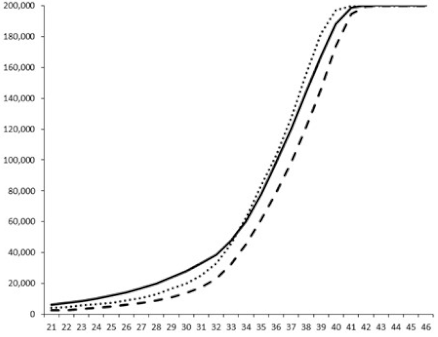 | 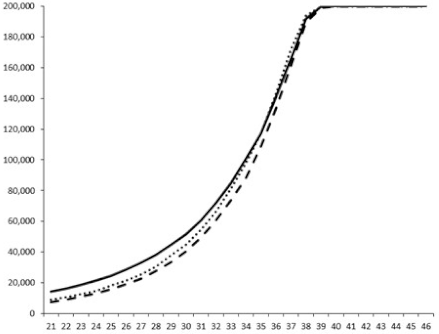 |
| 3 | 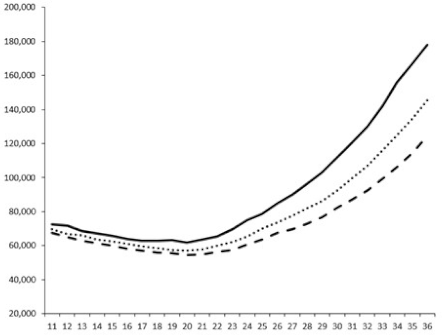 | 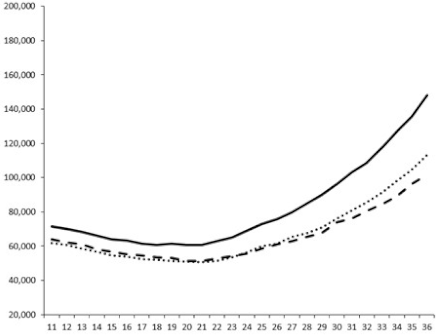 | 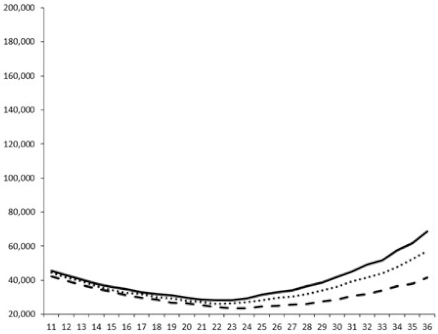 | 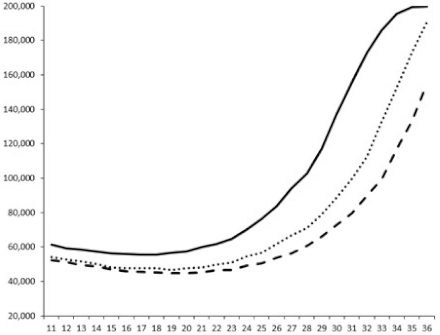 |
| 4 | 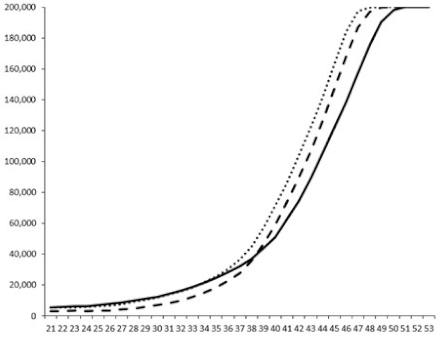 | 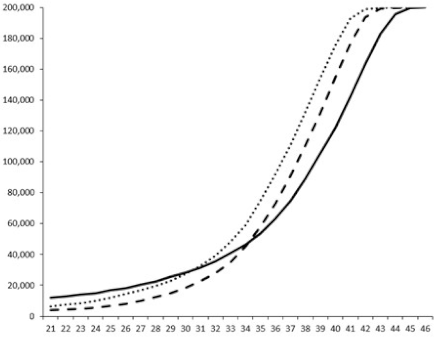 |  | 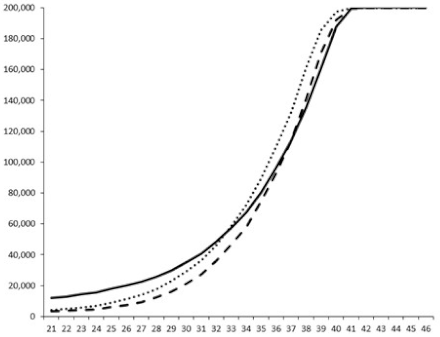 |
| 5 | 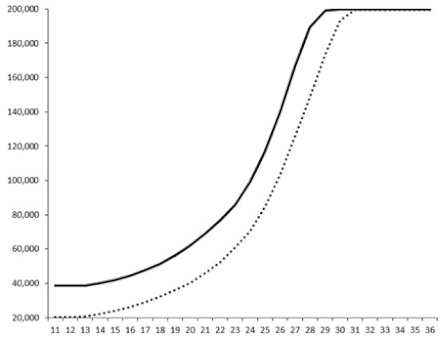 | nt 1 | nt | nt |
| 6 | 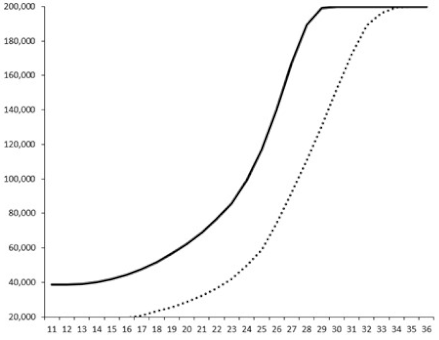 | nt | nt | nt |
| Microalgae | Marine Bacteria | |||||
|---|---|---|---|---|---|---|
| Mol. | P. purpureum | C. closterium | H. coffeaeformis | V. proteolyticus | V. aestuarianus | P. irgensii |
| Adhesion inhibition | ||||||
| 1 | 0.141 | 0.14 | 0.14 | - | - | - |
| 2 | - 2 | - | - | - | 1.48 | - |
| 3 | - | - | - | 0.15 | 0.15 | - |
| 4 | - | - | 0.02 | - | - | - |
| 5 | - | - | - | 0.02 | 0.19 | 0.19 |
| 6 | - | - | - | - | - | - |
| Seanine 3 | <0.03 | <0.03 | <0.03 | 0.03 | 3.5 | 3.5 |
| Syn. A 4 | - | 20 | 20 | - | - | 20 |
| Syn. C 5 | 0.2 | 2 | 2 | 2 | 2 | 20 |
| Pul. A 6 | - | - | 30 | - | 0.03 | - |
| Pul. B 7 | - | - | - | - | 20 | - |
| Ianthel. 8 | - | - | - | - | 0.2 | - |
| Growth inhibition | ||||||
| 1 | 0.14 | 0.14 | 0.14 | 1.37 | - | - |
| 2 | 1.48 | 1.48 | - | - | - | 1 |
| 3 | - | - | - | - | 0.01 | - |
| 4 | - | - | - | - | - | - |
| 5 | 19 | 1.9 | 0.02 | - | - | - |
| 6 | - | - | - | - | 0.22 | - |
| Seanine | <0.03 | <0.03 | <0.03 | 0.03 | <0.03 | 3.5 |
| Syn. A | 20 | 20 | 20 | 0.02 | 0.02 | - |
| Syn. C | 0.02 | 0.2 | 2 | 0.2 | 0.2 | 2 |
| Pul. A | 0.2 | - | - | - | - | 0.2 |
| Pul. B | - | - | - | - | - | - |
| Ianthel. | - | - | - | 21 | 0.2 | 2.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tintillier, F.; Moriou, C.; Petek, S.; Fauchon, M.; Hellio, C.; Saulnier, D.; Ekins, M.; Hooper, J.N.A.; Al-Mourabit, A.; Debitus, C. Quorum Sensing Inhibitory and Antifouling Activities of New Bromotyrosine Metabolites from the Polynesian Sponge Pseudoceratina n. sp. Mar. Drugs 2020, 18, 272. https://doi.org/10.3390/md18050272
Tintillier F, Moriou C, Petek S, Fauchon M, Hellio C, Saulnier D, Ekins M, Hooper JNA, Al-Mourabit A, Debitus C. Quorum Sensing Inhibitory and Antifouling Activities of New Bromotyrosine Metabolites from the Polynesian Sponge Pseudoceratina n. sp. Marine Drugs. 2020; 18(5):272. https://doi.org/10.3390/md18050272
Chicago/Turabian StyleTintillier, Florent, Céline Moriou, Sylvain Petek, Marilyne Fauchon, Claire Hellio, Denis Saulnier, Merrick Ekins, John N. A. Hooper, Ali Al-Mourabit, and Cécile Debitus. 2020. "Quorum Sensing Inhibitory and Antifouling Activities of New Bromotyrosine Metabolites from the Polynesian Sponge Pseudoceratina n. sp." Marine Drugs 18, no. 5: 272. https://doi.org/10.3390/md18050272
APA StyleTintillier, F., Moriou, C., Petek, S., Fauchon, M., Hellio, C., Saulnier, D., Ekins, M., Hooper, J. N. A., Al-Mourabit, A., & Debitus, C. (2020). Quorum Sensing Inhibitory and Antifouling Activities of New Bromotyrosine Metabolites from the Polynesian Sponge Pseudoceratina n. sp. Marine Drugs, 18(5), 272. https://doi.org/10.3390/md18050272





