Advances in Research on the Bioactivity of Alginate Oligosaccharides
Abstract
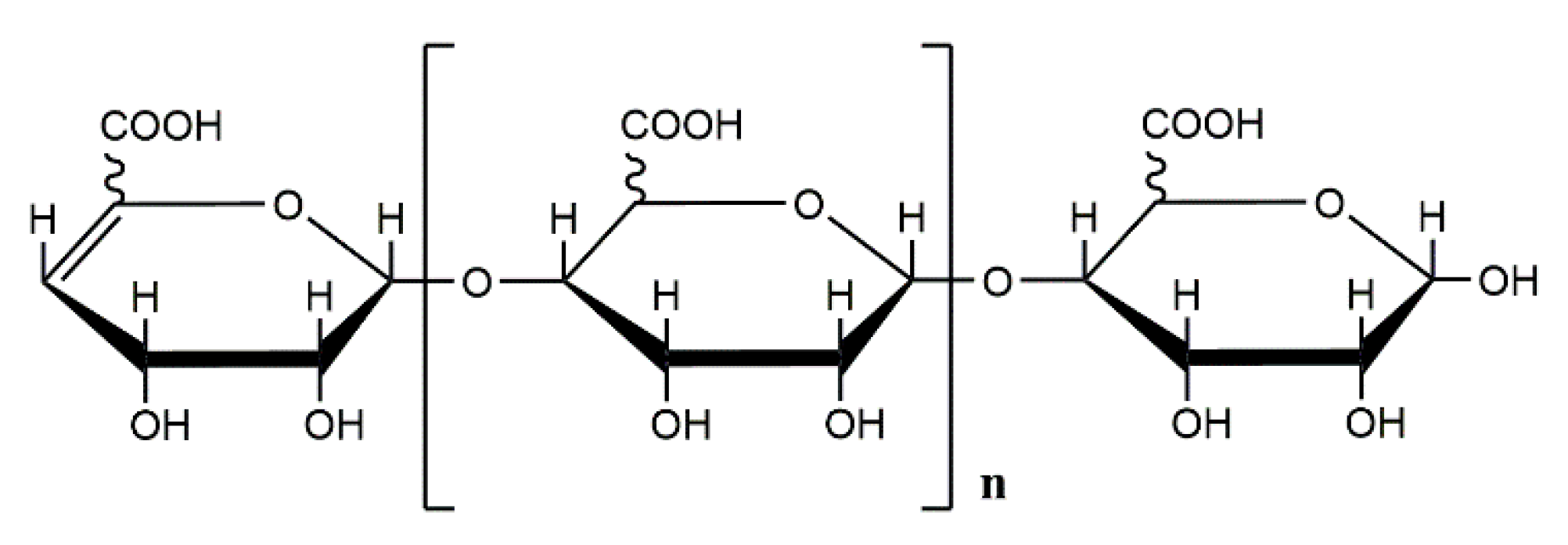
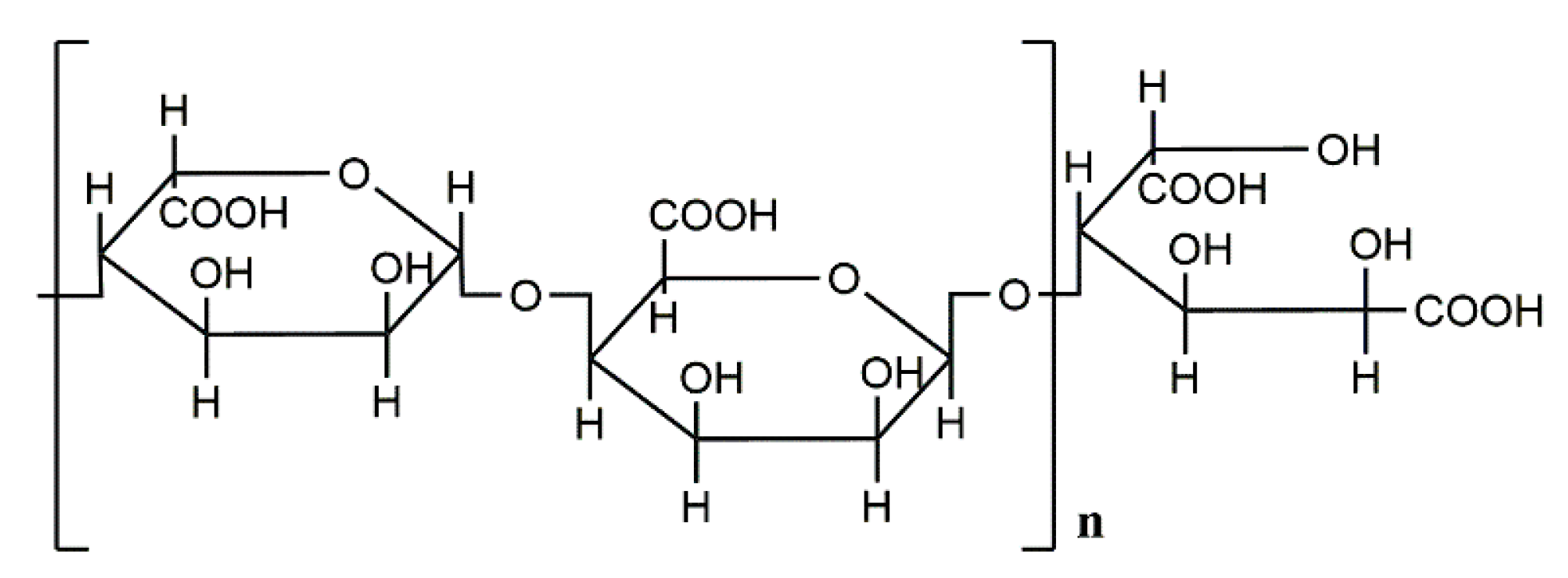
1. Introduction
2. Biological Activity of Alginate Oligosaccharides
2.1. Anti-Tumor Activity
2.2. Antioxidant Activity
2.3. Immunoregulatory Activity
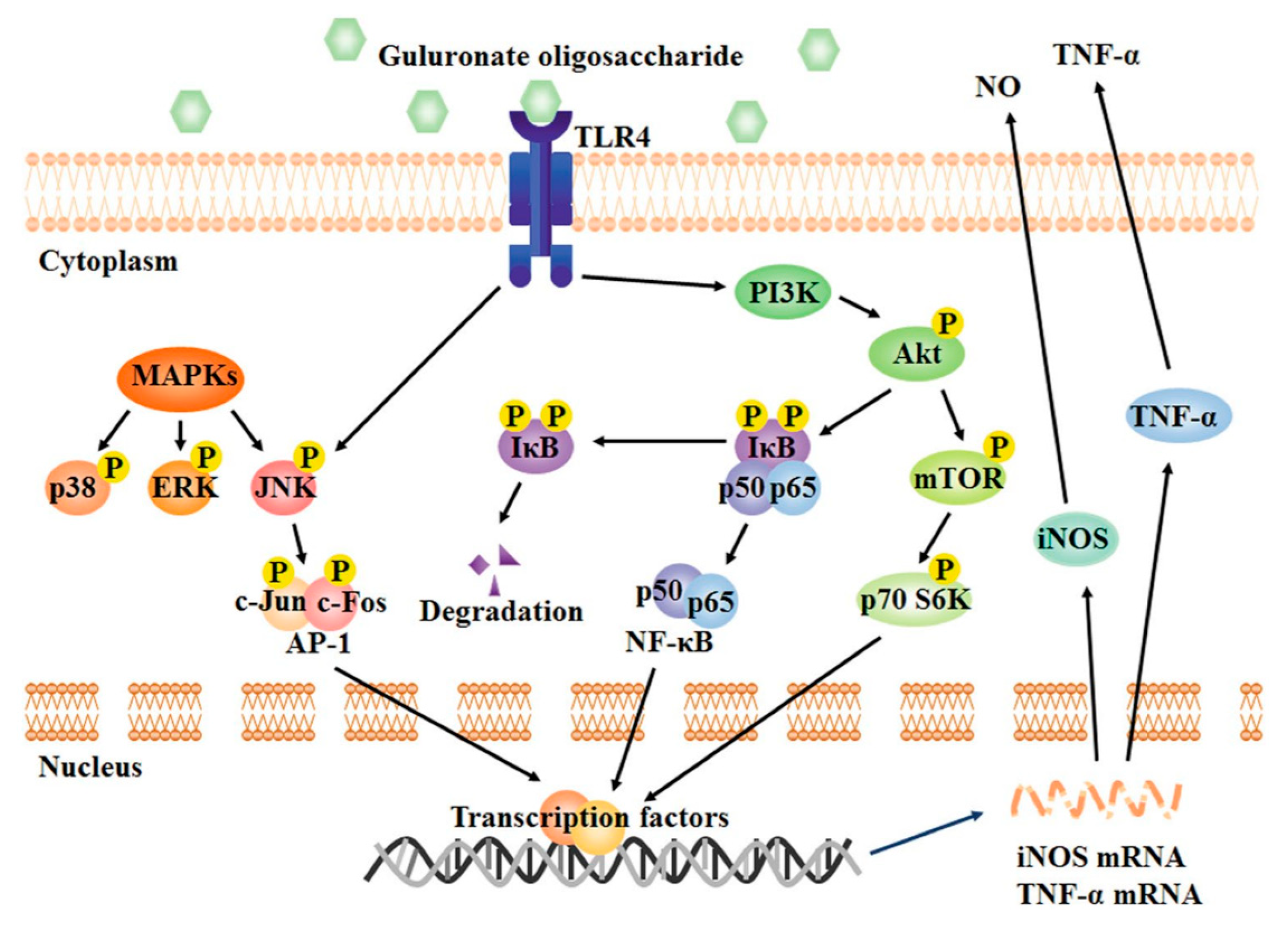
2.4. Anti-Inflammatory Activity
2.5. Neuroprotective Activity
2.6. Antibacterial Activity
2.7. Hypolipidemic Effect
2.8. Antihypertensive Activity
2.9. Suppression of Obesity
2.10. Hypoglycemia
2.11. Promotion of Cell Proliferation
2.12. Regulate Plant Growth
2.13. Other Activities
3. Concluding Remarks and Future Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 2012, 64, 194–205. [Google Scholar] [CrossRef]
- Remminghorst, U.; Rehm, B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Larsen, B.; Smidsrod, O. Uronic Acid Sequence in Alginate from Different Sources. Carbohydr. Res. 1974, 32, 217–225. [Google Scholar] [CrossRef]
- Schurks, N.; Wingender, J.; Flemming, H.C.; Mayer, C. Monomer composition and sequence of alginates from Pseudomonas aeruginosa. Int. J. Biol. Macromol. 2002, 30, 105–111. [Google Scholar] [CrossRef]
- Guo, W.B.; Song, C.J.; Kong, M.M.; Geng, W.T.; Wang, Y.Y.; Wang, S.F. Simultaneous production and characterization of medium-chain-length polyhydroxyalkanoates and alginate oligosaccharides by Pseudomonas mendocina NK-01. Appl. Microbiol. Biotechnol. 2011, 92, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Gorin, P.A.J.; Spencer, J.F.T. Exocellular Alginic Acid from Azotobacter Vinelandii. Can. J. Chem. 1966, 44, 993–998. [Google Scholar] [CrossRef]
- Pena, C.; Miranda, L.; Segura, D.; Nunez, C.; Espin, G.; Galindo, E. Alginate production by Azotobacter vinelandii mutants altered in poly-beta-hydroxybutyrate and alginate biosynthesis. J. Ind. Microbiol. Biotechnol. 2002, 29, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.C.; Kulkarni, S.S.; Zulueta, M.M.L.; Hung, S.C. Synthesis of Alginate Oligosaccharides Containing l-Guluronic Acids. Chem.-Asian J. 2009, 4, 386–390. [Google Scholar] [CrossRef]
- Skjakbraek, G.; Grasdalen, H.; Larsen, B. Monomer Sequence and Acetylation Pattern in Some Bacterial Alginates. Carbohydr. Res. 1986, 154, 239–250. [Google Scholar] [CrossRef]
- Cote, G.L.; Krull, L.H. Characterization of the Exocellular Polysaccharides from Azotobacter-Chroococcum. Carbohydr. Res. 1988, 181, 143–152. [Google Scholar] [CrossRef]
- Vreeland, V. Immunocytochemical localization of the extracellular polysaccharide alginic acid in the brown seaweed, Fucus distichus. J. Histochem. Cytochem. 1972, 20, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.D.T.; Nieduszynski, I.A.; Parker, K.D. Structural Components of Alginic Acid. 1. Crystalline-Structure of Poly-Beta-D-Mannuronic Acid—Results of X-Ray-Diffraction and Polarized Infrared Studies. Biopolymers 1973, 12, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Ni, F.; Sun, Y.; Ning, L.M.; Yao, Z. Elucidation of degrading pattern and substrate recognition of a novel bifunctional alginate lyase from Flammeovirga sp. NJ-04 and its use for preparation alginate oligosaccharides. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Ma, L.L.; Shi, H.T.; Zhu, J.B.; Wu, J.; Ding, Z.W.; An, Y.; Zou, Y.Z.; Ge, J.B. Alginate Oligosaccharide Prevents Acute Doxorubicin Cardiotoxicity by Suppressing Oxidative Stress and Endoplasmic Reticulum-Mediated Apoptosis. Mar. Drugs 2016, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Wang, S.L.; Zhang, Y.Y.; Chen, L.H. High-Level Expression of a Thermally Stable Alginate Lyase Using Pichia pastoris, Characterization and Application in Producing Brown Alginate Oligosaccharide. Mar. Drugs 2018, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Yu, X.; Wang, S.D.; Xu, C.Y.; Yin, H.; Wang, S.J. Alginate oligosaccharide attenuates alpha 2,6-sialylation modification to inhibit prostate cancer cell growth via the Hippo/YAP pathway. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Fang, W.S.; Bi, D.C.; Zheng, R.J.; Cai, N.; Xu, H.; Zhou, R.; Lu, J.; Wan, M.; Xu, X. Identification and activation of TLR4-mediated signalling pathways by alginate-derived guluronate oligosaccharide in RAW264.7 macrophages. Sci. Rep.-UK 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.Y.; Bi, D.C.; Fang, W.S.; Wei, G.B.; Xu, X. Alginate-Derived Oligosaccharide Inhibits Neuroinflammation and Promotes Microglial Phagocytosis of beta-Amyloid. Mar. Drugs 2015, 13, 5828–5846. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Khodagholi, F.; Abdi, A.; Maghsoudi, N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr. Polym. 2010, 79, 1063–1072. [Google Scholar] [CrossRef]
- Tondervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Khan, S.; Craine, K.M.; Onsoyen, E.; Rye, P.D.; et al. Alginate Oligosaccharides Inhibit Fungal Cell Growth and Potentiate the Activity of Antifungals against Candida and Aspergillus spp. PLoS ONE 2014, 9, e112518. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; Gao, Y.; Xue, C.H.; Li, R.W.; Tang, Q.J. Transcriptome analysis revealed anti-obesity effects of the Sodium Alginate in high-fat diet-induced obese mice. Int. J. Biol. Macromol. 2018, 115, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Terakado, S.; Ueno, M.; Tamura, Y.; Toda, N.; Yoshinaga, M.; Otsuka, K.; Numabe, A.; Kawabata, Y.; Murota, I.; Sato, N.; et al. Sodium Alginate Oligosaccharides Attenuate Hypertension and Associated Kidney Damage in Dahl Salt-Sensitive Rats Fed a High-Salt Diet. Clin. Exp. Hypertens. 2012, 34, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hiura, N.; Chaki, T.; Ogawa, H. Antihypertensive effects of sodium alginate oligosaccharides. Nippon Nogeik Kaishi 2001, 75, 783–785. [Google Scholar] [CrossRef]
- Zhang, D.D.; Fujii, I.; Lin, C.Z.; Ito, K.; Guan, H.S.; Zhao, J.E.; Shinohara, M.; Matsukura, M. The stimulatory activities of polysaccharide compounds derived from algae extracts on insulin secretion in vitro. Biol. Pharm. Bull. 2008, 31, 921–924. [Google Scholar] [CrossRef]
- Yokose, T.; Nishikawa, T.; Yamamoto, Y.; Yamasaki, Y.; Yamaguchi, K.; Oda, T. Growth-Promoting Effect of Alginate Oligosaccharides on a Unicellular Marine Microalga, Nannochloropsis oculata. Biosci. Biotechnol. Biochem. 2009, 73, 450–453. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Wu, L.Y.; Chen, Y.H.; Ni, H.; Xiao, A.F.; Cai, H.N. Characterization of an extracellular biofunctional alginate lyase from marine Microbulbifer sp ALW1 and antioxidant activity of enzymatic hydrolysates. Microbiol. Res. 2016, 182, 49–58. [Google Scholar] [CrossRef]
- Khotimchenko, Y.S. The antitumor properties of nonstarch polysaccharides: Carrageenans, alginates, and pectins. Russ. J. Mar. Biol. 2010, 36, 401–412. [Google Scholar] [CrossRef]
- Chen, J.Y.; Hu, Y.; Zhang, L.R.; Wang, Y.J.; Wang, S.C.; Zhang, Y.Z.; Guo, H.Y.; Ji, D.G.; Wang, Y.T. Alginate Oligosaccharide DP5 Exhibits Antitumor Effects in Osteosarcoma Patients following Surgery. Front. Pharmacol. 2017, 8, 623. [Google Scholar] [CrossRef]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. Bmc Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Jiang, X.L.; Guan, H.S.; Wang, P. Preparation, purification and characterization of alginate oligosaccharides degraded by alginate lyase from Pseudomonas sp. HZJ 216. Carbohydr. Res. 2011, 346, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Xu, X.; Tamura, T.; Oda, T.; Muramatsu, T. Enzymatically depolymerized alginate oligomers that cause cytotoxic cytokine production in human mononuclear cells. Biosci. Biotechnol. Biochem. 2003, 67, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.K.; Jiang, X.L.; Hwang, H.M.; Liu, S.L.; Guan, H.S. Antitumour activities of alginate-derived oligosaccharides and their sulphated substitution derivatives. Eur. J. Phycol. 2004, 39, 67–71. [Google Scholar] [CrossRef]
- Takahashi, K.; Watanuki, Y.; Yamazaki, M.; Abe, S. Local Induction of a Cyto-Toxic Factor in a Murine Tumor by Systemic Administration of an Antitumour Polysaccharide, Mga. Br. J. Cancer 1988, 57, 170–173. [Google Scholar] [CrossRef]
- Abe, S.; Takahashi, K.; Tsubouchi, J.; Aida, K.; Yamazaki, M.; Mizuno, D. Different Local Therapeutic Effects of Various Polysaccharides on Mh134 Hepatoma in Mice and Its Relation to Inflammation Induced by the Polysaccharides. Gann Jpn. J. Cancer Res. 1984, 75, 459–465. [Google Scholar]
- Nakajima, H.; Kita, Y.; Takashi, T.; Akasaki, M.; Yamaguchi, F.; Ozawa, S.; Tsukada, W.; Abe, S.; Mizuno, D. Immunopotentiation by a New Antitumor Polysaccharide, Dmg, a Degraded D-Manno-D-Glucan from Microellobosporia-Grisea Culture Fluid. Gann Jpn. J. Cancer Res. 1984, 75, 260–268. [Google Scholar]
- Fujihara, M.; Nagumo, T. The Effect of the Content of d-Mannuronic Acid and L-Guluronic Acid Blocks in Alginates on Antitumor-Activity. Carbohydr. Res. 1992, 224, 343–347. [Google Scholar] [CrossRef]
- Fujihara, M.; Nagumo, T. An Influence of the Structure of Alginate on the Chemotactic Activity of Macrophages and the Antitumor-Activity. Carbohydr. Res. 1993, 243, 211–216. [Google Scholar] [CrossRef]
- Hensel, A. gamma-Propoxy-sulfo-lichenin, an antitumor polysaccharide derived from lichenin. Pharm. Acta Helv. 1995, 70, 25–31. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.S.; Wertz, D.L.; Lyons, T.J.; Liou, L.L.; Goto, J.J.; Gralla, E.B. The dark side of dioxygen biochemistry. Curr. Opin. Chem. Biol. 1998, 2, 253–262. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S. Oxidized LDL and atherogenesis. Ann. N. Y. Acad. Sci. 1999, 874, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef]
- Nordberg, J.; Arner, E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Heilmann, J.; Merfort, I.; Weiss, M. Radical Scavenger Activity of Different 3’,4’-Dihydroxyflavonols and 1,5-Dicaffeoylquinic Acid Studied by Inhibition of Chemiluminescence. Planta Med. 1995, 61, 435–438. [Google Scholar] [CrossRef]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef]
- Guo, J.J.; Xu, F.Q.; Li, Y.H.; Li, J.; Liu, X.; Wang, X.F.; Hu, L.G.; An, Y. Alginate oligosaccharide alleviates myocardial reperfusion injury by inhibiting nitrative and oxidative stress and endoplasmic reticulum stress-mediated apoptosis. Drug Des. Dev. Ther. 2017, 11, 2387–2397. [Google Scholar] [CrossRef]
- Kelishomi, Z.H.; Goliaei, B.; Mandavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Mamashli, F.; Bigdeli, B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, B.F.; Xue, C.H.; Sun, L.P. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. J. Appl. Phycol. 2012, 24, 295–300. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Marin, E.; Martinez, A. Carbohydrates and Their Free Radical Scavenging Capability: A Theoretical Study. J. Phys. Chem. B 2012, 116, 9668–9675. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Induction of multiple cytokine secretion from RAW264.7 cells by alginate oligosaccharides. Biosci. Biotechnol. Biochem. 2007, 71, 238–241. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Stimulation of multiple cytokine production in mice by alginate oligosaccharides following intraperitoneal administration. Carbohydr. Res. 2007, 342, 1133–1137. [Google Scholar] [CrossRef]
- Xu, X.; Wu, X.T.; Wang, Q.Q.; Cai, N.; Zhang, H.X.; Jiang, Z.D.; Wan, M.; Oda, T. Immunomodulatory Effects of Alginate Oligosaccharides on Murine Macrophage RAW264.7 Cells and Their Structure-Activity Relationships. J. Agric. Food Chem. 2014, 62, 3168–3176. [Google Scholar] [CrossRef]
- Xu, X.; Bi, D.C.; Wu, X.T.; Wang, Q.Q.; Wei, G.B.; Chi, L.L.; Jiang, Z.D.; Oda, T.; Wan, M. Unsaturated guluronate oligosaccharide enhances the antibacterial activities of macrophages. Faseb J. 2014, 28, 2645–2654. [Google Scholar] [CrossRef]
- Yang, D.; Jones, K.S. Effect of alginate on innate immune activation of macrophages. J. Biomed. Mater. Res. A 2009, 90a, 411–418. [Google Scholar] [CrossRef]
- de Sousa, A.P.A.; Torres, M.R.; Pessoa, C.; de Moraes, M.O.; Rocha, F.D.; Alves, A.P.N.N.; Costa-Lotufo, L.V. In vivo growth-inhibition of Sarcoma 180 tumor by alginates from brown seaweed Sargassum vulgare. Carbohydr. Polym. 2007, 69, 7–13. [Google Scholar] [CrossRef]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. Febs Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef]
- Chen, J.Z.; Seviour, R. Medicinal importance of fungal beta-(1→3), (1→6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, W.Z.; Peng, Y.F.; Han, B.Q.; Yang, Y. Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. Int. Immunopharmacol. 2014, 23, 254–261. [Google Scholar] [CrossRef]
- Liu, Y.S.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases - regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef]
- Xu, X.; Bi, D.C.; Li, C.; Fang, W.S.; Zhou, R.; Li, S.M.; Chi, L.L.; Wan, M.; Shen, L.M. Morphological and Proteomic Analyses Reveal that Unsaturated Guluronate Oligosaccharide Modulates Multiple Functional Pathways in Murine Macrophage RAW264.7 Cells. Mar. Drugs 2015, 13, 1798–1818. [Google Scholar] [CrossRef]
- Sharifi, L.; Aghamohammadi, A.; Mohsenzadegan, M.; Rezaei, N.; Zavareh, F.T.; Moshiri, M.; Bokaie, S.; Barati, A.; Sayedi, S.J.; Azizi, G.; et al. Immunomodulation of TLR2 and TLR4 by G2013 (alpha-l-Guluronic acid) in CVID Patients. Int. J. Pediatr.-Massha 2017, 5, 5327–5337. [Google Scholar]
- Sharifi, L.; Mohsenzadegan, M.; Aghamohammadi, A.; Rezaei, N.; Zavareh, F.T.; Bokaie, S.; Moshiri, M.; Aghazadeh, Z.; Norouzbabaie, Z.; Azizi, G.; et al. Immunomodulatory Effect of G2013 (alpha-l-Guluronic Acid) on the TLR2 and TLR4 in Human Mononuclear Cells. Curr. Drug Discov. Technol. 2018, 15, 123–131. [Google Scholar] [CrossRef][Green Version]
- Calder, P.C.; Albers, R.; Antoine, J.M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, 1–45. [Google Scholar] [CrossRef]
- Pawelec, G.; Goldeck, D.; Derhovanessian, E. Inflammation, ageing and chronic disease. Curr. Opin. Immunol. 2014, 29, 23–28. [Google Scholar] [CrossRef]
- McInturff, J.E.; Modlin, R.L.; Kim, J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J. Investig. Dermatol. 2005, 125, 1–8. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Manfredi, A.A. How macrophages ring the inflammation alarm. Proc. Natl. Acad. Sci. USA 2014, 111, 2866–2867. [Google Scholar] [CrossRef]
- Levy, E.; Xanthou, G.; Petrakou, E.; Zacharioudaki, V.; Tsatsanis, C.; Fotopoulos, S.; Xanthou, M. Distinct Roles of TLR4 and CD14 in LPS-Induced Inflammatory Responses of Neonates. Pediatr. Res. 2009, 66, 179–184. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Biochem. 2012, 151, 65–74. [Google Scholar] [CrossRef]
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of Lipopolysaccharide (LPS)-Induced Inflammatory Responses by Sargassum hemiphyllum Sulfated Polysaccharide Extract in RAW 264.7 Macrophage Cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef]
- Niu, Y.G.; Shang, P.P.; Chen, L.; Zhang, H.; Gong, L.; Zhang, X.W.; Yu, W.J.; Xu, Y.H.; Wang, Q.; Yu, L.L. Characterization of a Novel Alkali-Soluble Heteropolysaccharide from Tetraploid Gynostemma pentaphyllum Makino and Its Potential Anti-inflammatory and Antioxidant Properties. J. Agric. Food Chem. 2014, 62, 3783–3790. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Shi, H.J.; Wang, Y.M.; Xue, C.H.; Tang, Q.J. Polymannuronic acid ameliorated obesity and inflammation associated with a high-fat and high-sucrose diet by modulating the gut microbiome in a murine model. Br. J. Nutr. 2017, 117, 1332–1342. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.Y.; Gao, Y.; Cai, N.; Jiang, Z.D.; Xu, X. Anti-inflammatory Activity of Guluronate Oligosaccharides Obtained by Oxidative Degradation from Alginate in Lipopolysaccharide-Activated Murine Macrophage RAW 264.7 Cells. J. Agric. Food Chem. 2015, 63, 160–168. [Google Scholar] [CrossRef]
- Nazeri, S.; Jamshidi, A.R.; Mahmoudi, M.; Vojdanian, M.; Azarian, S.K.; Afraei, S.; Mostafaei, S.; Hosseini, M.; Mirshafiey, A. The safety and efficacy of Guluronic acid (G2013) in ankylosing spondylitis: A randomized controlled parallel clinical trial. Pharmacol. Rep. 2019, 71, 393–398. [Google Scholar] [CrossRef]
- Mirshafiey, A.; Rehm, B.; Abhari, R.S.; Borzooy, Z.; Sotoude, M.; Razavi, A. Production of M2000(beta-d-mannuronic acid) and its therapeutic effect on experimental nephritis. Environ. Toxicol. Pharmacol. 2007, 24, 60–66. [Google Scholar] [CrossRef]
- Mirshafiey, A.; Cuzzocrea, S.; Rehm, B.; Mazzon, E.; Saadat, F.; Sotoude, M. Treatment of experimental arthritis with M2000, a novel designed non-steroidal anti-inflammatory drug. Scand. J. Immunol. 2005, 61, 435–441. [Google Scholar] [CrossRef]
- Mirshafiey, A.; Rehm, B.; Sotoude, M.; Razavi, A.; Abhari, R.S.; Borzooy, Z. Therapeutic approach by a novel designed anti-inflammatory drug, M2000, in experimental immune complex glomerulonephritis. Immunopharmacol. Immunotoxicol. 2007, 29, 49–61. [Google Scholar] [CrossRef]
- Aletaha, S.; Haddad, L.; Roozbehkia, M.; Bigdeli, R.; Asgary, V.; Mahmoudi, M.; Mirshafiey, A. M2000 (beta-d-Mannuronic Acid) as a Novel Antagonist for Blocking the TLR2 and TLR4 Downstream Signalling Pathway. Scand. J. Immunol. 2017, 85, 122–129. [Google Scholar] [CrossRef]
- Mortazavi-Jahromi, S.S.; Farazmand, A.; Motamed, N.; Navabi, S.S.; Mirshafiey, A. Effects of guluronic acid (G2013) on SHIP1, SOCS1 induction and related molecules in TLR4 signaling pathway. Int. Immunopharmacol. 2018, 55, 323–329. [Google Scholar] [CrossRef]
- Hajivalili, M.; Pourgholi, F.; Majidi, J.; Aghebati-Maleki, L.; Movassaghpour, A.A.; Kafil, H.S.; Mirshafiey, A.; Yousefi, M. G2013 modulates TLR4 signaling pathway in IRAK-1 and TARF-6 dependent and miR-146a independent manner. Cell. Mol. Biol. 2016, 62, 1–5. [Google Scholar]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Gotz, M.E.; Kunig, G.; Riederer, P.; Youdim, M.B.H. Oxidative Stress—Free-Radical Production in Neural Degeneration. Pharmacol. Ther. 1994, 63, 37–122. [Google Scholar] [CrossRef]
- Perskvist, N.; Long, M.; Stendahl, O.; Zheng, L.M. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-x(L) via an oxygen-dependent pathway. J. Immunol. 2002, 168, 6358–6365. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, J.F.; Li, J.; Yang, Z.; Xin, X.L.; Wang, J.; Ding, J.; Geng, M.Y. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci. Lett. 2005, 374, 222–226. [Google Scholar] [CrossRef]
- Hu, J.F.; Geng, M.Y.; Li, J.; Xin, X.L.; Wang, J.; Tang, M.K.; Zhang, J.T.; Zhang, X.; Ding, J. Acidic oligosaccharide sugar chain, a marine-derived acidic oligosaccharide, inhibits the cytotoxicity and aggregation of amyloid beta protein. J. Pharmacol. Sci. 2004, 95, 248–255. [Google Scholar] [CrossRef]
- Guo, X.L.; Xin, X.L.; Gan, L.; Nie, Q.; Geng, M.Y. Determination of the accessibility of acidic oligosaccharide sugar chain to blood-brain barrier using surface plasmon resonance. Biol. Pharm. Bull. 2006, 29, 60–63. [Google Scholar] [CrossRef]
- Morales, I.; Guzman-Martinez, L.; Cerda-Troncoso, C.; Farias, G.A.; Maccioni, R.B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Guan, H.S.; Tan, L. IL12/23 p40 Inhibition Ameliorates Alzheimer’s Disease-Associated Neuropathology and Spatial Memory in SAMP8 Mice. J. Alzheimers Dis. 2014, 38, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Frank-Cannon, T.C.; Mccoy, M.K.; Lee, J.K.; Martinez, T.N.; McAlpine, F.E.; Ruhn, K.A.; Tran, T.A. Neuroinflammation in Parkinson’s disease: Is there sufficient evidence for mechanism-based interventional therapy? Front. Biosci.-Landmrk 2008, 13, 709–717. [Google Scholar] [CrossRef]
- Moore, A.H.; O’Banion, M.K. Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2002, 54, 1627–1656. [Google Scholar] [CrossRef]
- Yao, L.L.; Kan, E.M.; Lu, J.; Hao, A.J.; Dheen, S.T.; Kaur, C.; Ling, E.A. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: Role of TLR4 in hypoxic microglia. J. Neuroinflamm. 2013, 10, 785. [Google Scholar] [CrossRef]
- Song, M.; Jin, J.; Lim, J.E.; Kou, J.; Pattanayak, A.; Rehman, J.A.; Kim, H.D.; Tahara, K.; Lalonde, R.; Fukuchi, K. TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2011, 8, 92. [Google Scholar] [CrossRef]
- Takeuchi, M.; Bucala, R.; Suzuki, T.; Ohkubo, T.; Yamazaki, M.; Koike, T.; Kameda, Y.; Makita, Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J. Neuropathol. Exp. Neurol. 2000, 59, 1094–1105. [Google Scholar] [CrossRef]
- Gasic-Milenkovic, J.; Loske, C.; Deuther-Conrad, W.; Munch, G. Protein “AGEing”—Cytotoxicity of a glycated protein increases with its degree of AGE-modification. Z. Gerontol. Geriatr. 2001, 34, 457–460. [Google Scholar] [CrossRef]
- Loske, C.; Neumann, A.; Cunningham, A.M.; Nichol, K.; Schinzel, R.; Riederer, P.; Munch, G. Cytotoxicity of advanced glycation endproducts is mediated by oxidative stress. J. Neural Transm. 1998, 105, 1005–1015. [Google Scholar] [CrossRef]
- Wong, A.; Luth, H.J.; Deuther-Conrad, W.; Dukic-Stefanovic, S.; Gasic-Milenkovic, J.; Arendt, T.; Munch, G. Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer’s disease. Brain Res. 2001, 920, 32–40. [Google Scholar] [CrossRef]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Khodagholi, F.; Moosavi-Movahedi, A.A.; Heli, H.; Hakimelahi, G.H. Alginate as an antiglycating agent for human serum albumin. Int. J. Biol. Macromol. 2007, 41, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Sun, G.Q.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.Q.; Chu, X.K.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.D.T.; Nieduszynski, I.A.; Parker, K.D.; Smolko, E.E. Structural Components of Alginic Acid. 2. Crystalline-Structure of Poly-Alpha-l-Guluronic Acid-Results of X-Ray-Diffraction and Polarized Infrared Studies. Biopolymers 1973, 12, 1879–1887. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Moskowitz, S.M.; Foster, J.M.; Emerson, J.; Burns, J.L. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 2004, 42, 1915–1922. [Google Scholar] [CrossRef]
- Powell, L.C.; Pritchard, M.F.; Ferguson, E.L.; Powell, K.A.; Patel, S.U.; Rye, P.D.; Sakellakou, S.M.; Buurma, N.J.; Brilliant, C.D.; Copping, J.M.; et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbiomes 2018, 4, 13. [Google Scholar] [CrossRef]
- Khan, S.; Tondervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Onsoyen, E.; Myrvold, R.; Howe, R.A.; Walsh, T.R.; Hill, K.E.; et al. Overcoming Drug Resistance with Alginate Oligosaccharides Able to Potentiate the Action of Selected Antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5134–5141. [Google Scholar] [CrossRef]
- Alkawash, M.A.; Soothill, J.S.; Schiller, N.L. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. Apmis 2006, 114, 131–138. [Google Scholar] [CrossRef]
- Wang, H.Z.; Song, Z.J.; Ciofu, O.; Onsoyen, E.; Rye, P.D.; Hoiby, N. OligoG CF-5/20 Disruption of Mucoid Pseudomonas aeruginosa Biofilm in a Murine Lung Infection Model. Antimicrob. Agents Chemother. 2016, 60, 2620–2626. [Google Scholar]
- Bales, P.M.; Renke, E.M.; May, S.L.; Shen, Y.; Nelson, D.C. Purification and Characterization of Biofilm-Associated EPS Exopolysaccharides from ESKAPE Organisms and Other Pathogens. PLoS ONE 2013, 8, e67950. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Govan, J.R.W.; Deretic, V. Microbial pathogenesis in cystic fibrosis: Mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996, 60, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Borgogna, M.; Skjak-Braek, G.; Paoletti, S.; Donati, I. On the Initial Binding of Alginate by Calcium Ions. The Tilted Egg-Box Hypothesis. J. Phys. Chem. B 2013, 117, 7277–7282. [Google Scholar] [CrossRef]
- Powell, L.C.; Sowedan, A.; Khan, S.; Wright, C.J.; Hawkins, K.; Onsoyen, E.; Myrvold, R.; Hill, K.E.; Thomas, D.W. The effect of alginate oligosaccharides on the mechanical properties of Gram-negative biofilms. Biofouling 2013, 29, 413–421. [Google Scholar] [CrossRef]
- Powell, L.C.; Pritchard, M.F.; Emanuel, C.; Onsoyen, E.; Rye, P.D.; Wright, C.J.; Hill, K.E.; Thomas, D.W. A Nanoscale Characterization of the Interaction of a Novel Alginate Oligomer with the Cell Surface and Motility of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Roberts, J.L.; Khan, S.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Onsoyen, E.; Myrvold, R.; Thomas, D.W.; Hill, K.E. An in vitro study of alginate oligomer therapies on oral biofilms. J. Dent. 2013, 41, 1307–1308. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Pritchard, M.F.; Jack, A.A.; Powell, L.C.; Sadh, H.; Rye, P.D.; Hill, K.E.; Thomas, D.W. Alginate oligosaccharides modify hyphal infiltration of Candida albicans in an invitro model of invasive human candidosis. J. Appl. Microbiol. 2017, 123, 625–636. [Google Scholar] [CrossRef]
- Yan, G.L.; Guo, Y.M.; Yuan, J.M.; Liu, D.; Zhang, B.K. Sodium alginate oligosaccharides from brown algae inhibit Salmonella Enteritidis colonization in broiler chickens. Poult. Sci 2011, 90, 1441–1448. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jiang, X.L.; Wang, P.; Hu, X.K. Effects of Alginate-Derived Oligosaccharides on Immune Ability of Farm-Cultured Shrimp Penaeus vannamei and Its Resistance to Vibrio harveyi. N. Am. J. Aquac. 2017, 79, 317–321. [Google Scholar] [CrossRef]
- An, Q.D.; Zhang, G.L.; Wu, H.T.; Zhang, Z.C.; Zheng, G.S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2009, 106, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Paxman, J.R.; Richardson, J.C.; Dettmar, P.W.; Corfe, B.M. Daily ingestion of alginate reduces energy intake in free-living subjects. Appetite 2008, 51, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.J.; Zhang, L.J.; Zhao, X.; He, X.X.; Li, M.M.; Zhao, X.L.; Wu, J.D.; Qiu, P.J.; Yu, G.L. Activated AMPK explains hypolipidemic effects of sulfated low molecular weight guluronate on HepG2 cells. Eur. J. Med. Chem. 2014, 85, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Mathers, J.C. Comparative gastrointestinal and plasma cholesterol responses of rats fed on cholesterol-free diets supplemented with guar gum and sodium alginate. Br. J. Nutr. 2001, 85, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Bang, M.A.; Jang, C.H.; Jo, G.H.; Jung, S.K.; Ki, S.H. Alginate oligosaccharide enhances LDL uptake via regulation of LDLR and PCSK9 expression. J. Nutr. Biochem. 2015, 26, 1393–1400. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, W.-g.; Han, F.; Lu, X.-z.; Gong, Q.-h.; Hu, X.-k.; Guan, H.-S. Effect of propylene glycol mannate sulfate on blood lipids and lipoprotein lipase in hyperlipidemic rat. Yaoxue Xuebao 2002, 37, 687–690. [Google Scholar]
- Gehrisch, S. Common mutations of the lipoprotein lipase gene and their clinical significance. Curr. Atheroscler. Rep. 1999, 1, 70–78. [Google Scholar] [CrossRef]
- de Man, F.H.A.F.; de Beer, F.; van der Laarse, A.; Jansen, H.; Leuven, J.A.G.; Souverijn, J.H.M.; Vroom, T.F.F.P.; Schoormans, S.C.M.; Fruchart, J.-C.; Havekes, L.M.; et al. The hypolipidemic action of bezafibrate therapy in hypertriglyceridemia is mediated by upregulation of lipoprotein lipase: No effects on VLDL substrate affinity to lipolysis or LDL receptor binding. Atherosclerosis 2000, 153, 363–371. [Google Scholar] [CrossRef]
- Wang, W.; Yoshie, Y.; Suzuki, T. Effect of alginate viscosity on digestibility and lipid metabolism in rats. Nippon Suisan Gakk 2003, 69, 72–79. [Google Scholar] [CrossRef]
- Lin, C.Z.; Guan, H.S.; Li, H.H.; Yu, G.L.; Gu, C.X.; Li, G.Q. The influence of molecular mass of sulfated propylene glycol ester of low-molecular-weight alginate on anticoagulant activities. Eur. Polym. J. 2007, 43, 3009–3015. [Google Scholar] [CrossRef]
- Xin, M.; Sun, Y.; Chen, H.J.; Li, Q.C.; Dun, Y.L.; Guan, H.S.; Hao, J.J.; Li, C.X. Propylene glycol guluronate sulfate (PGGS) reduces lipid accumulation via AMP-activated kinase activation in palmitate-induced HepG2 cells. Int. J. Biol. Macromol. 2018, 114, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Marounek, M.; Volek, Z.; Skrivanova, E.; Taubner, T.; Pebriansyah, A.; Duskova, D. Comparative study of the hypocholesterolemic and hypolipidemic activity of alginate and amidated alginate in rats. Int. J. Biol. Macromol. 2017, 105, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Moriya, C.; Shida, Y.; Yamane, Y.; Miyamoto, Y.; Kimura, M.; Huse, N.; Ebisawa, K.; Kameda, Y.; Nishi, A.; Du, D.D.; et al. Subcutaneous Administration of Sodium Alginate Oligosaccharides Prevents Salt-Induced Hypertension in Dahl Salt-Sensitive Rats. Clin. Exp. Hypertens. 2013, 35, 607–613. [Google Scholar] [CrossRef]
- Ji, W.; Chen, Y.Y.; Du, J.R.; Yu, D.K.; Zheng, X.Y.; Yang, F.; Yu, C.X.; Li, D.S.; Zhao, C.Y.; Qiao, K.Y. Antihypertensive effect and pharmacokinetics of low molecular mass potassium alginate. Sichuan Da Xue Xue Bao Yi Xue Ban J. Sichuan Univ. Med. Sci. Ed. 2009, 40, 694–696. [Google Scholar]
- Chen, Y.Y.; Ji, W.; Du, J.R.; Yu, D.K.; He, Y.; Yu, C.X.; Li, D.S.; Zhao, C.Y.; Qiao, K.Y. Preventive effects of low molecular mass potassium alginate extracted from brown algae on DOCA salt-induced hypertension in rats. Biomed. Pharmacother. 2010, 64, 291–295. [Google Scholar] [CrossRef]
- Uehara, Y.; Hirawa, N.; Takeda, T.; Numabe, A.; Kawabata, Y.; Nagoshi, H.; Gomi, T.; Ikegami, J.; Goto, A.; Omata, M. Possible linkage between renal injury and cardiac remodeling in Dahl salt-sensitive rats treated with the calcium channel antagonist benidipine. Hypertens. Res. 1995, 18, 245–253. [Google Scholar] [CrossRef]
- Ueno, M.; Tamura, Y.; Toda, N.; Yoshinaga, M.; Terakado, S.; Otsuka, K.; Numabe, A.; Kawabata, Y.; Murota, I.; Sato, N.; et al. Sodium Alginate Oligosaccharides Attenuate Hypertension in Spontaneously Hypertensive Rats Fed a Low-Salt Diet. Clin. Exp. Hypertens. 2012, 34, 305–310. [Google Scholar] [CrossRef]
- Haslam, D.; Sattar, N.; Lean, M. ABC of obesity—Obesity—Time to wake up. Br. Med. J. 2006, 333, 640–642. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Allen, A.; Pearson, J.P.; Dettmar, P.W.; Havler, M.E.; Atherton, M.R.; Onsoyen, E. Alginate as a source of dietary fiber. Crit. Rev. Food Sci. 2005, 45, 497–510. [Google Scholar] [CrossRef]
- Strugala, V.; Kennington, E.J.; Campbell, R.J.; Skjak-Braek, G.; Dettmar, P.W. Inhibition of pepsin activity by alginates in vitro and the effect of epimerization. Int. J. Pharm. 2005, 304, 40–50. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Hu, F.B.; Colditz, G.A.; Manson, J.E.; Willett, W.C.; Liu, S. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int. J. Obes. 2004, 28, 1569–1574. [Google Scholar] [CrossRef]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Hoad, C.L.; Rayment, P.; Spiller, R.C.; Marciani, L.; Alonso, B.D.; Traynor, C.; Mela, D.J.; Peters, H.P.F.; Gowland, P.A. In vivo imaging of intragastric gelation and its effect on satiety in humans. J. Nutr. 2004, 134, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.W.; Lai, C.S.; Kipnes, M.S.; Ataya, D.G.; Wheeler, K.B.; Zinker, B.A.; Garleb, K.A.; Firkins, J.L. Glycemic and insulinemic responses of nondiabetic healthy adult subjects to an experimental acid-induced viscosity complex incorporated into a glucose beverage. Nutrition 2002, 18, 621–626. [Google Scholar] [CrossRef]
- Williams, J.A.; Lai, C.S.; Corwin, H.; Ma, Y.Y.; Maki, K.C.; Garleb, K.A.; Wolf, B.W. Inclusion of guar gum and alginate into a crispy bar improves postprandial glycemia in humans. J. Nutr. 2004, 134, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Vaugelade, P.; Hoebler, C.; Bernard, F.; Guillon, F.; Lahaye, M.; Duee, P.H.; Darcy-Vrillon, B. Non-starch polysaccharides extracted from seaweed can modulate intestinal absorption of glucose and insulin response in the pig. Reprod. Nutr. Dev. 2000, 40, 33–47. [Google Scholar] [CrossRef]
- Kimura, Y.; Watanabe, K.; Okuda, H. Effects of soluble sodium alginate on cholesterol excretion and glucose tolerance in rats. J. Ethnopharmacol. 1996, 54, 47–54. [Google Scholar] [CrossRef]
- Anderson, J.W.; Allgood, L.D.; Turner, J.; Oeltgen, P.R.; Daggy, B.P. Effects of psyllium on glucose and serum lipid responses in men with type 2 diabetes and hypercholesterolemia. Am. J. Clin. Nutr. 1999, 70, 466–473. [Google Scholar] [CrossRef]
- Draget, K.I.; Braek, G.S.; Smidsrod, O. Alginic Acid Gels—The Effect of Alginate Chemical-Composition and Molecular-Weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Hao, C.; Hao, J.J.; Wang, W.; Han, Z.R.; Li, G.S.; Zhang, L.J.; Zhao, X.; Yu, G.L. Insulin Sensitizing Effects of Oligomannuronate-Chromium (III) Complexes in C2C12 Skeletal Muscle Cells. PLoS ONE 2011, 6, e24598. [Google Scholar] [CrossRef]
- Lan, Y.; Zeng, X.; Guo, Z.H.; Zeng, P.J.; Hao, C.; Zhao, X.; Yu, G.L.; Zhang, L.J. Polyguluronate sulfate and its oligosaccharides but not heparin promotes FGF19/FGFR1c signaling. J. Ocean Univ. China 2017, 16, 532–536. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hirshman, M.F.; Kurth, E.J.; Winder, W.W.; Goodyear, L.J. Evidence for 5′AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 1998, 47, 1369–1373. [Google Scholar] [PubMed]
- Kawada, A.; Hiura, N.; Tajima, S.; Takahara, H. Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch. Dermatol. Res. 1999, 291, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.W.; Zhu, R.; Ran, L.; Li, Y.Q.; Huang, K.; Peng, J.; He, W.; Zhou, C.L.; Wang, R.P. A novel non-contact communication between human keratinocytes and T cells: Exosomes derived from keratinocytes support superantigen-induced proliferation of resting T cells. Mol. Med. Rep. 2017, 16, 7032–7038. [Google Scholar] [CrossRef]
- Jung, S.; Lademann, J.; Darvin, M.E.; Richter, C.; Pedersen, C.B.; Richter, H.; Schanzer, S.; Kottner, J.; Blume-Peytavi, U.; Ropke, M.A. In vivo characterization of structural changes after topical application of glucocorticoids in healthy human skin. J. Biomed. Opt. 2017, 22, 76018. [Google Scholar] [CrossRef]
- Basler, K.; Galliano, M.F.; Bergmann, S.; Rohde, H.; Wladykowski, E.; Vidal, Y.S.S.; Guiraud, B.; Houdek, P.; Schuring, G.; Volksdorf, T.; et al. Biphasic influence of Staphylococcus aureus on human epidermal tight junctions. Ann. N. Y. Acad. Sci. 2017, 1405, 53–70. [Google Scholar] [CrossRef]
- Kawada, A.; Hiura, N.; Shiraiwa, M.; Tajima, S.; Hiruma, M.; Hara, K.; Ishibashi, A.; Takahara, H. Stimulation of human keratinocyte growth by alginate oligosaccharides, a possible co-factor for epidermal growth factor in cell culture. Febs Lett. 1997, 408, 43–46. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Yokose, T.; Nishikawa, T.; Kim, D.; Jiang, Z.D.; Yamaguchi, K.; Oda, T. Effects of alginate oligosaccharide mixtures on the growth and fatty acid composition of the green alga Chlamydomonas reinhardtii. J. Biosci. Bioeng. 2012, 113, 112–116. [Google Scholar] [CrossRef]
- Hu, X.K.; Jiang, X.L.; Hwang, H.M.; Liu, S.L.; Guan, H.S. Promotive effects of alginate-derived oligosaccharide on maize seed germination. J. Appl. Phycol. 2004, 16, 73–76. [Google Scholar] [CrossRef]
- Yun-hong, Z.; Li-shu, W.U.; Ming-jian, G.; Hong-qing, H.U.; Shan-xue, Z. Effects of Several Oligosaccharides on the Yield and Quality of Brassica chinensis. J. Huazhong Agric. Univ. 2009, 28, 164–168. [Google Scholar]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Yin, H.; Zhao, X.M.; Wang, W.X.; Du, Y.G.; He, A.L.; Sun, K.G. The promoting effects of alginate oligosaccharides on root development in Oryza sativa L. mediated by auxin signaling. Carbohydr. Polym. 2014, 113, 446–454. [Google Scholar] [CrossRef]
- Iwasaki, K.; Matsubara, Y. Purification of alginate oligosaccharides with root growth-promoting activity toward lettuce. Biosci. Biotechnol. Biochem. 2000, 64, 1067–1070. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, H.; Yin, H.; Wang, W.X.; Zhao, X.M.; Du, Y.G. Nitric oxide mediates alginate oligosaccharides-induced root development in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2013, 71, 49–56. [Google Scholar] [CrossRef]
- Terrile, M.C.; Paris, R.; Calderon-Villalobos, L.I.A.; Iglesias, M.J.; Lamattina, L.; Estelle, M.; Casalongue, C.A. Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J. 2012, 70, 492–500. [Google Scholar] [CrossRef]
- Bang, M.A.; Seo, J.H.; Seo, J.W.; Jo, G.H.; Jung, S.K.; Yu, R.; Park, D.H.; Park, S.J. Bacillus subtilis KCTC 11782BP-Produced Alginate Oligosaccharide Effectively Suppresses Asthma via T-Helper Cell Type 2-Related Cytokines. PLoS ONE 2015, 10, e0117524. [Google Scholar] [CrossRef] [PubMed]
- Taeb, M.; Mortazavi-Jahromi, S.; Jafarzadeh, A.; Mirzaei, M.R.; Mirshafiey, A. An in vitro evaluation of anti-aging effect of guluronic acid (G2013) based on enzymatic oxidative stress gene expression using healthy individuals PBMCs. Biomed. Pharmacother. 2017, 90, 262–267. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, H.N.; Yu, G.L.; Wang, J.X.; Li, X.J.; Guan, H.S. Preparation of oligoguluronate sulfates by solid phase acid degradation and analysis by negative-ion electrospray tandem mass spectrometry. Chem. J. Chin. Univ. 2008, 29, 1344–1348. [Google Scholar]
- Zhao, X.; Yu, G.L.; Guan, H.S.; Yue, N.; Zhang, Z.Q.; Li, H.H. Preparation of low-molecular-weight polyguluronate sulfate and its anticoagulant and anti-inflammatory activities. Carbohydr. Polym. 2007, 69, 272–279. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, G.; Yue, N.; Guan, H. Effects of low-molecular-weight polyguluronate sulfate on experimental urolithiasis in rats. Urol. Res. 2007, 35, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Liu, W.; Wang, W.; Zhao, X.; Wang, F.H. Polyguluronate sulfate (PGS) attenuates immunological liver injury in vitro and in vivo. Int. J. Biol. Macromol. 2018, 114, 592–598. [Google Scholar] [CrossRef]
- Wu, L.J.; Wang, W.; Zhang, X.S.; Zhao, X.; Yu, G.L. Anti-HBV activity and mechanism of marine-derived polyguluronate sulfate (PGS) in vitro. Carbohydr. Polym. 2016, 143, 139–148. [Google Scholar] [CrossRef]
- Zeng, X.; Lan, Y.; Zeng, P.; Guo, Z.; Hao, C.; Zhang, L. Polyguluronate sulfate, polymannuronate sulfate, and their oligosaccharides have antithrombin III- and heparin cofactor II-independent anticoagulant activity. J. Ocean Univ. China 2017, 16, 346–350. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. https://doi.org/10.3390/md18030144
Xing M, Cao Q, Wang Y, Xiao H, Zhao J, Zhang Q, Ji A, Song S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Marine Drugs. 2020; 18(3):144. https://doi.org/10.3390/md18030144
Chicago/Turabian StyleXing, Maochen, Qi Cao, Yu Wang, Han Xiao, Jiarui Zhao, Qing Zhang, Aiguo Ji, and Shuliang Song. 2020. "Advances in Research on the Bioactivity of Alginate Oligosaccharides" Marine Drugs 18, no. 3: 144. https://doi.org/10.3390/md18030144
APA StyleXing, M., Cao, Q., Wang, Y., Xiao, H., Zhao, J., Zhang, Q., Ji, A., & Song, S. (2020). Advances in Research on the Bioactivity of Alginate Oligosaccharides. Marine Drugs, 18(3), 144. https://doi.org/10.3390/md18030144




