Impact of Prevalence Ratios of Chondroitin Sulfate (CS)- 4 and -6 Isomers Derived from Marine Sources in Cell Proliferation and Chondrogenic Differentiation Processes
Abstract
1. Introduction
2. Results and Discussion
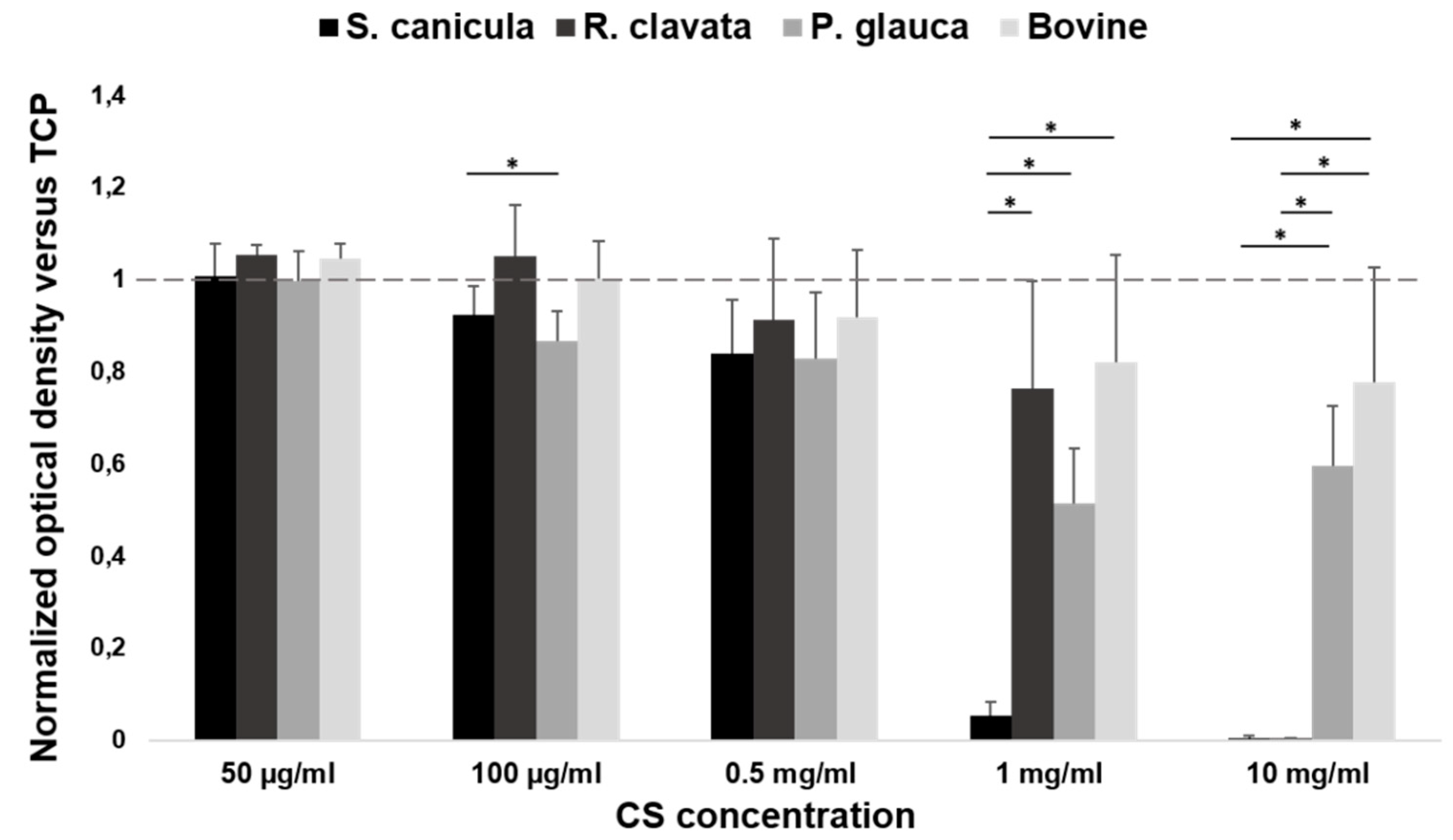
2.1. Viability in MG-63 Cell Line
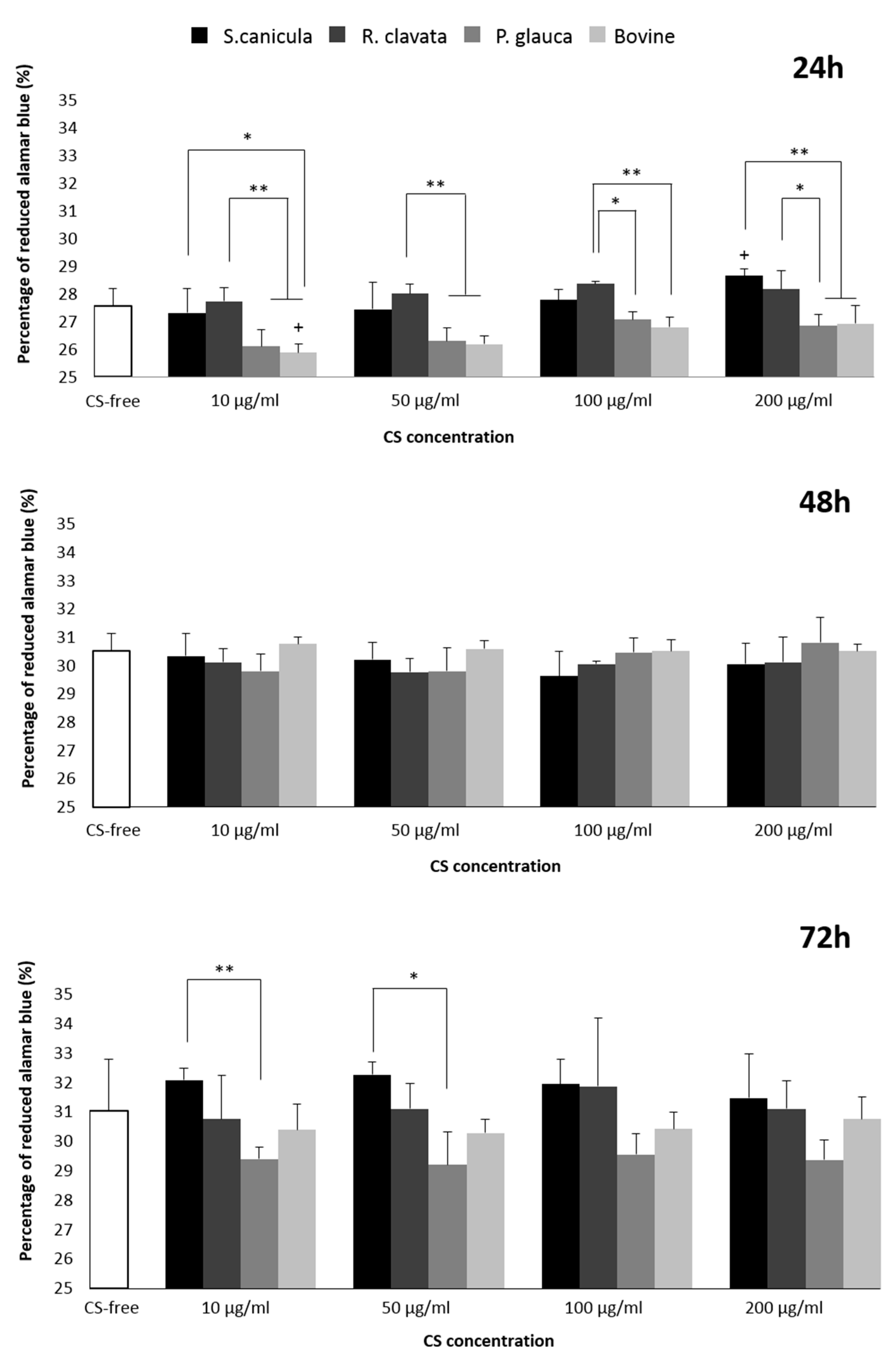
2.2. Viability and Proliferation in T/C-28a2 Cell Line
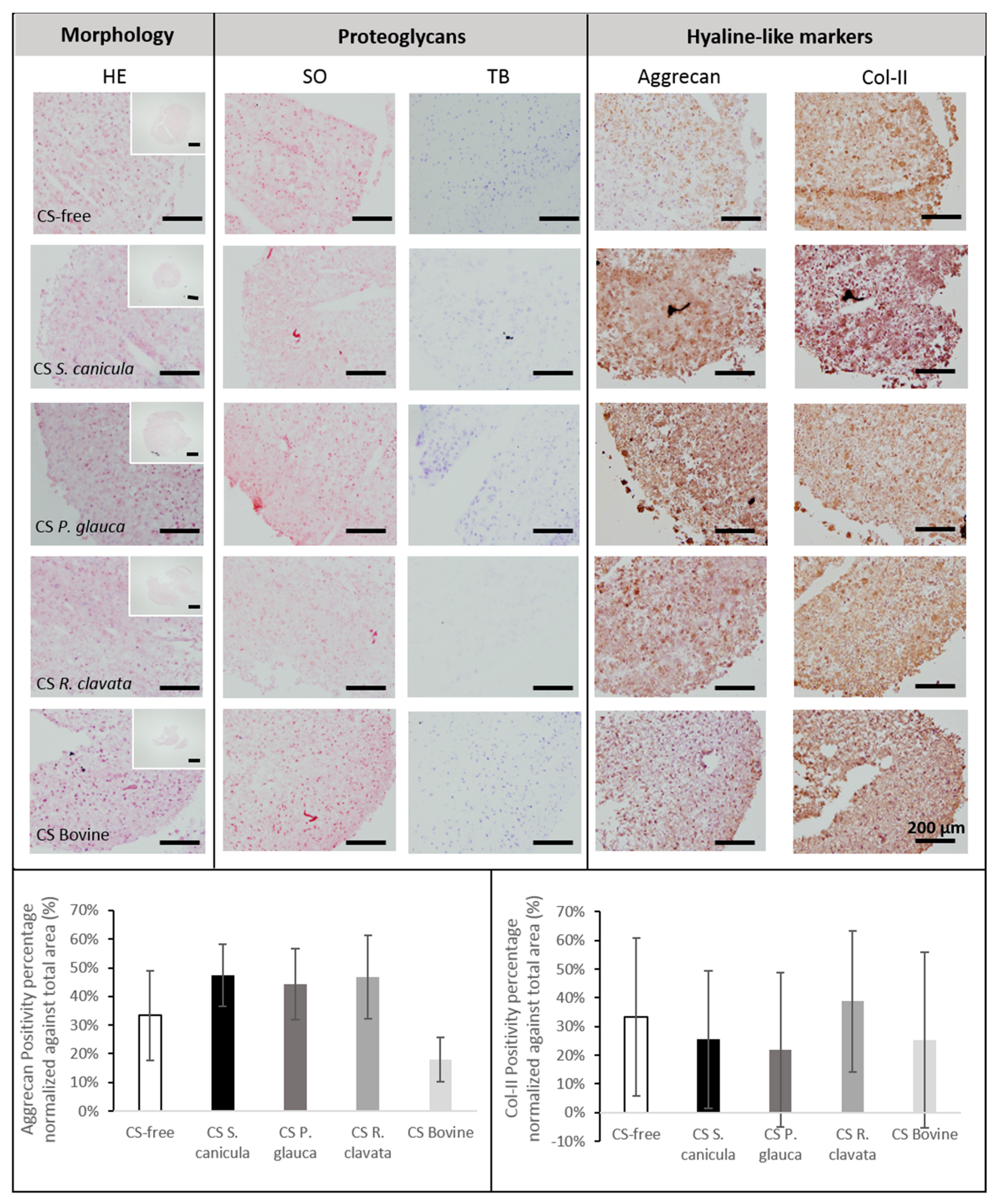
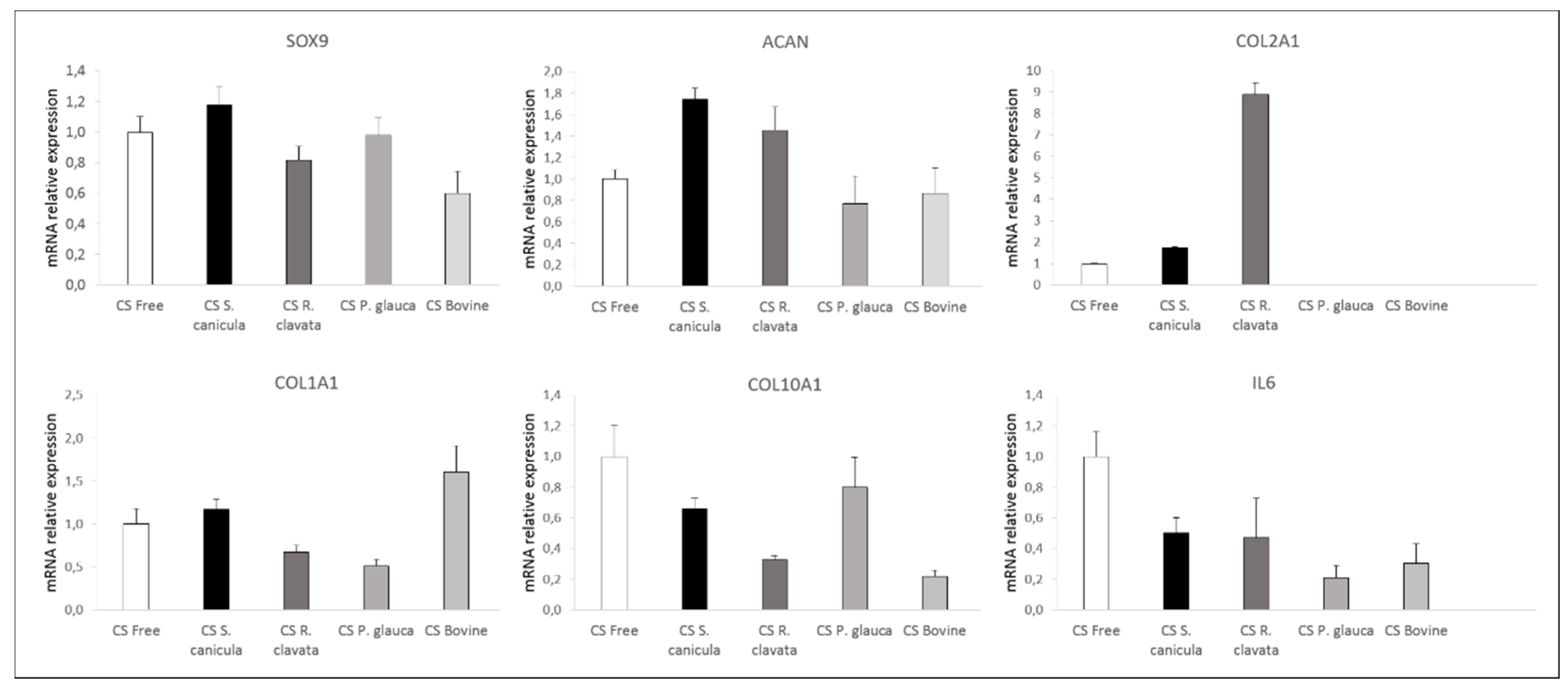
2.3. Chondrogenesis of Bone Marrow-Derived Human Mesenchymal Stem Cells (MSCs)
3. Materials and Methods
3.1. Chondroitin Sulfate
3.2. Cellular Studies
3.2.1. Viability in MG-63 Cell Line
3.2.2. Viability and Proliferation in T/C-28a2 Cell Line
3.2.3. Chondrogenesis of Bone Marrow-Derived Human MSCs
3.2.4. Molecular Expression
3.2.5. Histology and Immunohistochemistry
3.2.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suri, P.; Morgenroth, D.C.; Hunter, D.J. Epidemiology of osteoarthritis and associated comorbidities. PM R 2012, 4, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; Berenbaum, F.; Blanco, F.J.; Cosimo, d.B.; Lafeber, F.; Hauge, E.; Higginbottom, A.; Ioan-Facsinay, A.; Loughlin, J.; Meulenbelt, I.; et al. Translation of clinical problems in osteoarthritis into pathophysiological research goals. RMD Open 2016, 2, e000224. [Google Scholar] [CrossRef] [PubMed]
- Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin sulfate in the treatment of osteoarthritis: from in vitro studies to clinical recommendations. Ther. Adv. Musculoskelet. Dis. 2010, 2, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Jerosch, J. Effects of Glucosamine and Chondroitin Sulfate on Cartilage Metabolism in OA: Outlook on Other Nutrient Partners Especially Omega-3 Fatty Acids. Int. J. Rheumatol. 2011, 2011, 969012. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Martel-Pelletier, J.; Monfort, J.; Möller, I.; Castillo, J.R.; Arden, N.; Berenbaum, F.; Blanco, F.J.; Conaghan, P.G.; Doménech, G.; et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: A multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann. Rheum. Dis. 2016, 75, 37–44. [Google Scholar] [CrossRef]
- Calamia, V.; Mateos, J.; Fernández-Puente, P.; Lourido, L.; Rocha, B.; Fernández-Costa, C.; Montell, E.; Vergés, J.; Ruiz-Romero, C.; Blanco, F.J. A pharmacoproteomic study confirms the synergistic effect of chondroitin sulfate and glucosamine. Sci. Rep. 2014, 4, 5069. [Google Scholar] [CrossRef]
- Jordan, K.M.; Arden, N.K.; Doherty, M.; Bannwarth, B.; Bijlsma, J.W.; Dieppe, P.; Gunther, K.; Hauselmann, H.; Herrero-Beaumont, G.; Kaklamanis, P.; et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 2003, 62, 1145–1155. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Pomin, V.H. The Sea as a Rich Source of Structurally Unique Glycosaminoglycans and Mimetics. Microorganisms 2017, 5. [Google Scholar] [CrossRef]
- Thiele, H.; Sakano, M.; Kitagawa, H.; Sugahara, K.; Rajab, A.; Höhne, W.; Ritter, H.; Leschik, G.; Nürnberg, P.; Mundlos, S. Loss of chondroitin 6-O-sulfotransferase-1 function results in severe human chondrodysplasia with progressive spinal involvement. Proc. Natl. Acad. Sci. USA 2004, 101, 10155–10160. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, D.; Funakoshi, T.; Mizumoto, S.; Sugahara, K.; Iwasaki, N. Sulfation patterns of exogenous chondroitin sulfate affect chondrogenic differentiation of ATDC5 cells. J. Orthop. Sci. 2014, 19, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Goude, M.C.; McDevitt, T.C.; Temenoff, J.S. Chondroitin sulfate microparticles modulate transforming growth factor-β1-induced chondrogenesis of human mesenchymal stem cell spheroids. Cells Tissues. Organs. 2014, 199, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Stojsih, S.E.; Matthew, H.W. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 3499–3512. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Ochi, M.; Uchio, Y.; Adachi, N.; Matsusaki, M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J. Cell. Physiol. 1999, 179, 142–148. [Google Scholar] [CrossRef]
- Bayliss, M.T.; Osborne, D.; Woodhouse, S.; Davidson, C. Sulfation of chondroitin sulfate in human articular cartilage. The effect of age, topographical position, and zone of cartilage on tissue composition. J. Biol. Chem. 1999, 274, 15892–15900. [Google Scholar] [CrossRef]
- Orkoula, M.; Kontoyannis, C. Raman spectroscopy for the study of biological organisms (biogenic materials and biological tissues): A valuable analytical tool. Spectroscopy Europe 2014, 26, 18–21. [Google Scholar]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Pérez-Martín, R.; Blanco, M.; Sotelo, C.G.; Fassini, D.; Nunes, C.; Coimbra, M.A.; Silva, T.H.; Reis, R.L.; Vázquez, J.A. By-products of Scyliorhinus canicula, Prionace glauca and Raja clavata: A valuable source of predominantly 6S sulfated chondroitin sulfate. Carbohydr. Polym. 2017, 157, 31–37. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carvallal, R.; Reis, R.L.; Antelo, L.T.; Pérez-Martín, R.I.; Valcarcel, J. Isolation and Chemical Characterization of Chondroitin Sulfate from Cartilage By-Products of Blackmouth Catshark. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carballal, R.; Reis, R.L.; Pérez-Martín, R.I.; Valcarcel, J. Optimal isolation and characterisation of chondroitin sulfate from rabbit fish (Chimaera monstrosa). Carbohydr. Polym. 2019, 210, 302–313. [Google Scholar] [CrossRef] [PubMed]
- López-Álvarez, M.; López-Senra, E.; Valcárcel, J.; Vázquez, J.A.; Serra, J.; González, P. Quantitative evaluation of sulfation position prevalence in chondroitin sulphate by Raman spectroscopy. J. Raman Spectrosc. 2019, 50, 656–664. [Google Scholar] [CrossRef]
- Bansil, R.; Yannas, I.V.; Stanley, H.E. Raman Spectroscopy: A structural probe of glycosaminoglycans. BBA–Gen. Subj. 1978, 541, 535–542. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Silva, C.; Moller, S.; Schnabelrauch, M.; Reis, R.L.; Pashkuleva, I. Tunable nano-carriers from clicked glycosaminoglycan block copolymers. J. Mater. Chem. B 2014, 26, 4177–4184. [Google Scholar] [CrossRef]
- Vandrovcová, M.; Douglas, T.; Hauk, D.; Grössner-Schreiber, B.; Wiltfang, J.; Bačáková, L.; Warnke, P.H. Influence of collagen and chondroitin sulfate (CS) coatings on poly-(lactide-co-glycolide) (PLGA) on MG 63 osteoblast-like cells. Physiol. Res. 2011, 60, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, N.; Bianchi, D.; Trentin, A.; Volpi, N.; Soni, M. Safety assessment of non-animal chondroitin sulfate sodium: Subchronic study in rats, genotoxicity tests and human bioavailability. Food Chem. Toxicol. 2016, 93, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hineebusch, A.G. Regulation of translation initiation in Eukaryotes: mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Calamia, V.; Lourido, L.; Fernández-Puente, P.; Mateos, J.; Rocha, B.; Montell, E.; Vergés, J.; Ruiz-Romero, C.; Blanco, F.J. Secretome analysis of chondroitin sulfate-treated chondrocytes reveals anti-angiogenic, anti-inflammatory and anti-catabolic properties. Arthritis Res. Ther. 2012, 14, R202. [Google Scholar] [CrossRef]
- Magalhães, J.; Lebourg, M.; Deplaine, H.; Gómez Ribelles, J.L.; Blanco, F.J. Effect of the physicochemical properties of pure or chitosan-coated poly(L-lactic acid)scaffolds on the chondrogenic differentiation of mesenchymal stem cells from osteoarthritic patients. Tissue Eng. Part A 2015, 21, 716–728. [Google Scholar] [CrossRef]
- Diekman, B.O.; Guilak, F. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr. Opin. Rheumatol. 2013, 25, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.A.; Didiano, D.M.; Chu, C.R. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthr. Cartil. 2010, 18, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Scharstuhl, A.; Schewe, B.; Benz, K.; Gaissmaier, C.; Bühring, H.J.; Stoop, R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells 2007, 25, 3244–3251. [Google Scholar] [CrossRef]
- Varghese, S.; Hwang, N.S.; Canver, A.C.; Theprungsirikul, P.; Lin, D.W.; Elisseeff, J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008, 27, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Temenoff, J.S. The effect of desulfation of chondroitin sulfate on interactions with positively charged growth factors and upregulation of cartilaginous markers in encapsulated MSCs. Biomaterials 2013, 34, 5007–5018. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Takagi, M.; Wakitani, S.; Nihira, T.; Yoshida, T. Effect of chondroitin sulfate and hyaluronic acid on gene expression in a three-dimensional culture of chondrocytes. J. Biosci. Bioeng. 2005, 100, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Banu, N.; Tsuchiya, T. Markedly different effects of hyaluronic acid and chondroitin sulfate-A on the differentiation of human articular chondrocytes in micromass and 3-D honeycomb rotation cultures. J. Biomed. Mater. Res. A 2007, 80, 257–267. [Google Scholar] [CrossRef]
- Murado, M.A.; Fraguas, J.; Montemayor, M.I.; Vázquez, J.A.; González, P. Preparation of highly purified chondroitin sulphate from skate (Raja clavata) cartilage by-products. Process optimization including a new procedure of alkaline hydroalcoholic hydrolysis. Biochem. Eng. J. 2010, 49, 126–132. [Google Scholar] [CrossRef]
- Blanco, M.; Fraguas, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Vázquez, J.A. Production of Chondroitin Sulphate from Head, Skeleton and Fins of Scyliorhinus canicula By-Products by Combination of Enzymatic, Chemical Precipitation and Ultrafiltration Methodologies. Mar. Drugs 2015, 13, 3287–3308. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Blanco, M.; Fraguas, J.; Pastrana, L.; Pérez-Martín, R. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly processes. Food Chem. 2016, 198, 28–35. [Google Scholar] [CrossRef]
- Hermida-Gómez, T.; Fuentes-Boquete, I.; Gimeno-Longas, M.J.; Muiños-López, E.; Díaz-Prado, S.; de Toro, F.J.; Blanco, F.J. Bone marrow cells immunomagnetically selected for CD271+ antigen promote in vitro the repair of articular cartilage defects. Tissue Eng. Part A 2011, 17, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
| Bovine | P. glauca | R. clavata | S. canicula | |
|---|---|---|---|---|
| Ratio 1* (CS-4) | 0.8 ± 0.2 | 0.27 ± 0.07 | 0.46 ± 0.09 | 0.20 ± 0.07 |
| Ratio 2* (CS-6) | 0.22 ± 0.01 | 0.96 ± 0.09 | 0.67 ± 0.03 | 0.40 ± 0.03 |
| MW** | ≈ 20 kDa | 60 kDa | 44 kDa | 43–45 kDa |
| Gene | Forward | Reverse | Probes | Gene Bank A. Number |
|---|---|---|---|---|
| RPL13a | CAAGCGGATGAACACCAAC | TGTGGGGCAGCATACCTC | 28 | NM_012423.2 |
| SOX9 | GTACCCGCACTTGCACAAC | TCGCTCTCGTTCAGAAGTCTC | 61 | NM_000346 |
| ACAN | CGGTCTACCTCTACCCTAACCA | GAGAAGGAACCGCTGAAATG | 38 | NM_013227.3 |
| COL1A1 | CTGGCCCCATTGGTAATGT | ACCAGGGAAACCAGTAGCAC | 1 | NM_000088.3 |
| COL2A1 | TGGTGCTAATGGCGAGAAG | CCCAGTCTCTCCACGTTCAC | 4 | NM_001844.4 |
| COL10A1 | CACCTTCTGCACTGCTCATC | GGCAGCATATTCTCAGATGGA | 6 | NM_000493.3 |
| IL6 | GATGAGTACAAAAGTCCTGATCCA | CTGCAGCCACTGGTTCTGT | 40 | NM_000600.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Senra, E.; Casal-Beiroa, P.; López-Álvarez, M.; Serra, J.; González, P.; Valcarcel, J.; Vázquez, J.A.; Burguera, E.F.; Blanco, F.J.; Magalhães, J. Impact of Prevalence Ratios of Chondroitin Sulfate (CS)- 4 and -6 Isomers Derived from Marine Sources in Cell Proliferation and Chondrogenic Differentiation Processes. Mar. Drugs 2020, 18, 94. https://doi.org/10.3390/md18020094
López-Senra E, Casal-Beiroa P, López-Álvarez M, Serra J, González P, Valcarcel J, Vázquez JA, Burguera EF, Blanco FJ, Magalhães J. Impact of Prevalence Ratios of Chondroitin Sulfate (CS)- 4 and -6 Isomers Derived from Marine Sources in Cell Proliferation and Chondrogenic Differentiation Processes. Marine Drugs. 2020; 18(2):94. https://doi.org/10.3390/md18020094
Chicago/Turabian StyleLópez-Senra, Estefanía, Paula Casal-Beiroa, Miriam López-Álvarez, Julia Serra, Pío González, Jesus Valcarcel, José Antonio Vázquez, Elena F. Burguera, Francisco J. Blanco, and Joana Magalhães. 2020. "Impact of Prevalence Ratios of Chondroitin Sulfate (CS)- 4 and -6 Isomers Derived from Marine Sources in Cell Proliferation and Chondrogenic Differentiation Processes" Marine Drugs 18, no. 2: 94. https://doi.org/10.3390/md18020094
APA StyleLópez-Senra, E., Casal-Beiroa, P., López-Álvarez, M., Serra, J., González, P., Valcarcel, J., Vázquez, J. A., Burguera, E. F., Blanco, F. J., & Magalhães, J. (2020). Impact of Prevalence Ratios of Chondroitin Sulfate (CS)- 4 and -6 Isomers Derived from Marine Sources in Cell Proliferation and Chondrogenic Differentiation Processes. Marine Drugs, 18(2), 94. https://doi.org/10.3390/md18020094







