Antibacterial and Antiviral Activities of Local Thai Green Macroalgae Crude Extracts in Pacific White Shrimp (Litopenaeus vannamei)
Abstract
1. Introduction
2. Results
2.1. Chemical Content and Structural Analyses of Crude Polysaccharides from Three Local Thai Green Algae
2.2. Acute Toxicity of the HWCEs from Local Thai Green Macroalgae
2.3. Antibacterial Activity of the HWCEs from Local Thai Green Macroalgae
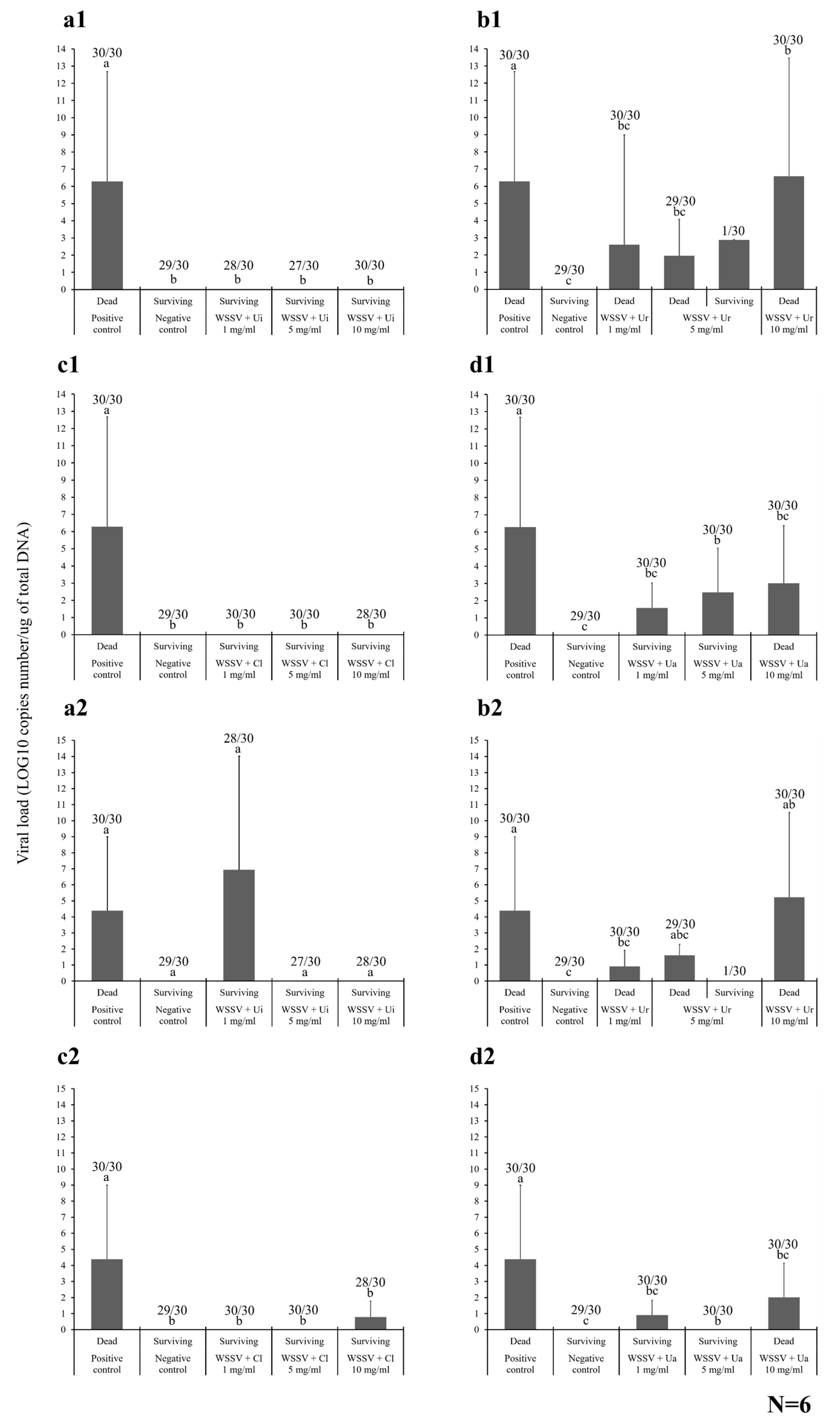
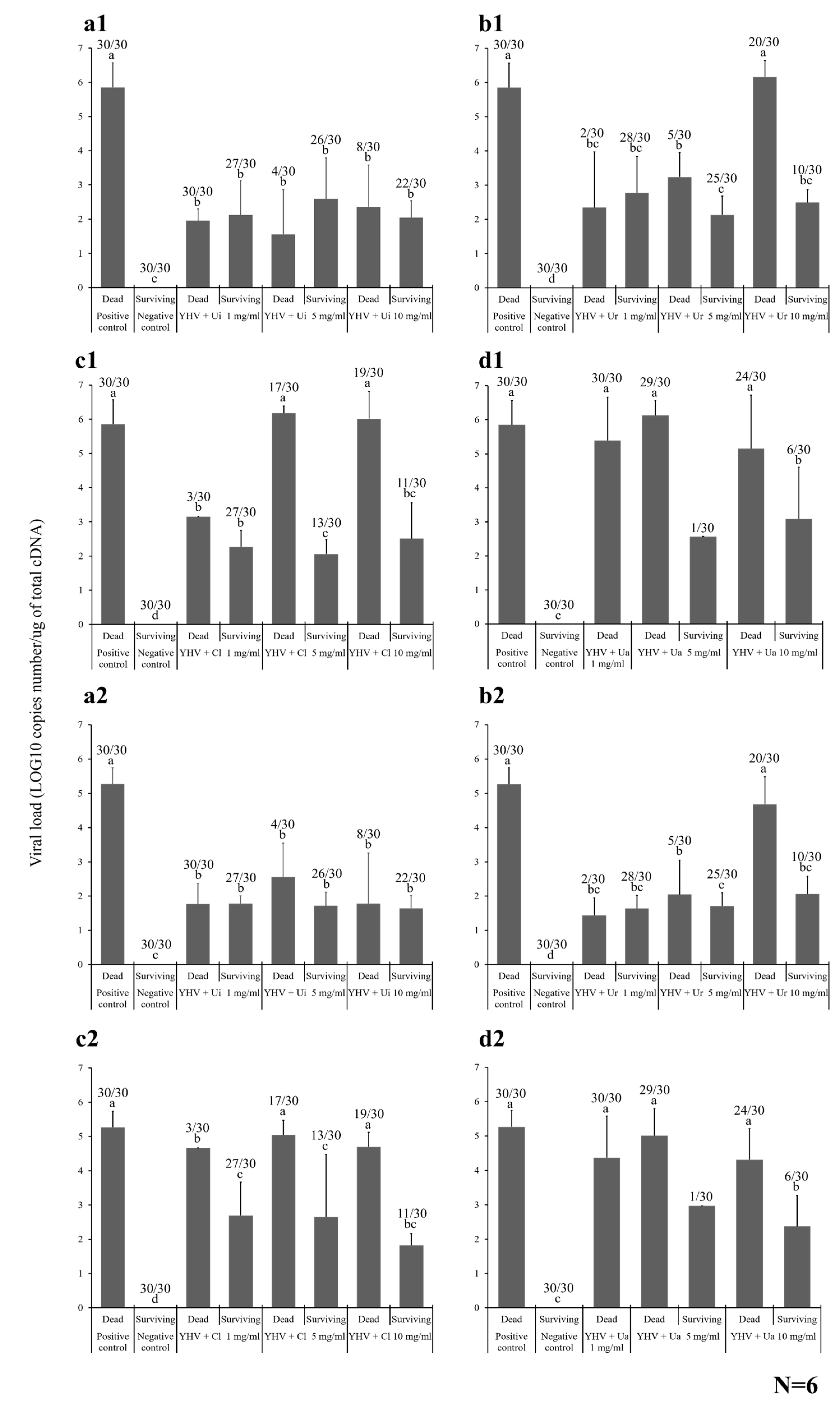
2.4. Antiviral Activity of the HWCEs from Local Thai Green Algae
2.4.1. Shrimp Mortality
2.4.2. Viral Loading Assay
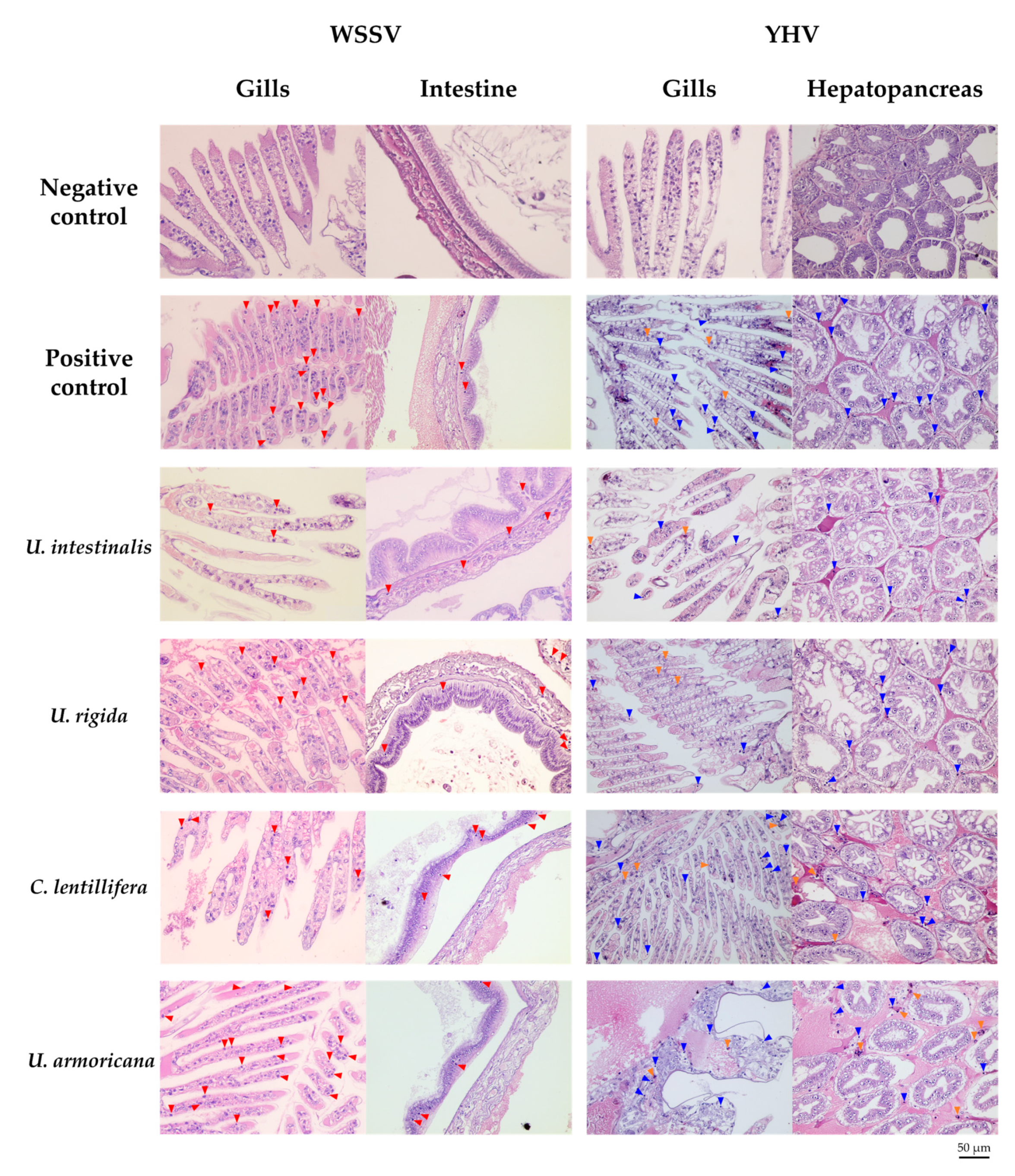
2.4.3. Histopathology
3. Discussion
4. Materials and Methods
4.1. Extraction of Hot Water Crude Extracts (HWCEs)
4.2. Chemical Analyses
4.3. Structure of HWCEs
4.4. Experimental Animals
4.5. Lethal Dose Analysis of HWCEs
4.6. Antibacterial Activity of HWCEs from Local Thai Green Algae
4.6.1. Preparation of Vibrio spp.
4.6.2. Minimal Inhibitory Concentrations (MICs) by Liquid Growth Inhibition Methods
4.7. Antiviral Activity of HWCEs from Local Thai Green Algae
4.7.1. Experimental Animals
4.7.2. Preparation of WSSV and YHV Inoculum
4.7.3. Median Lethal Dose Assay at 96 H (96-H LD50)
4.7.4. Antiviral Activity Analysis
4.7.5. Investigation of Viral Load in Target Tissues Using Quantitative Real-Time PCR for WSSV and Quantitative Real-Time RT-PCR for YHV
4.7.6. Histopathological Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The state of world fisheries and aquaculture. 2018. Available online: http://203.187.160.133:9011/www.fao.org/c3pr90ntc0td/3/i9540en/i9540en.pdf (accessed on 27 February 2020).
- Lightner, D.V. Virus diseases of farmed shrimp in the Western Hemisphere (the Americas): A review. J. Invert. Pathol. 2011, 106, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, J.G.; Aguirre-Guzmán, G.; Mejía-Ruíz, H. White Spot Syndrome Virus in cultured shrimp: A review. Aquac. Res. 2007, 38, 1339–1354. [Google Scholar] [CrossRef]
- Walker, P.J.; Mohan, C.V. Viral disease emergence in shrimp aquaculture: Origins, impact and the effectiveness of health management strategies. Rev. Aquac. 2009, 1, 125–154. [Google Scholar] [CrossRef]
- Flegel, T.W. A future vision for disease control in shrimp aquaculture. J. World Aquac. Soc. 2019, 50, 249–266. [Google Scholar] [CrossRef]
- Anantasomboon, G.; Poonkhum, R.; Sittidilokratna, N.; Flegel, T.W.; Withyachumnarnkul, B. Low viral loads and lymphoid organ spheroids are associated with yellow head virus (YHV) tolerance in whiteleg shrimp Penaeus vannamei. Dev. Comp. Immunol. 2008, 32, 613–626. [Google Scholar] [CrossRef]
- Selvin, J.; Manilal, A.; Sujith, S.; Kiran, G.S.; Lipton, A.P. Efficacy of marine green alga Ulva fasciata extract on the management of shrimp bacterial diseases. Lat. Am. J. Aquat. Res. 2011, 39, 197–204. [Google Scholar] [CrossRef]
- Dhar, A.K.; Piamsomboon, P.; Aranguren Caro, L.F.; Kanrar, S.; Adami, R.J.; Juan, Y.S. First report of acute hepatopancreatic necrosis disease (AHPND) occurring in the USA. Dis. Aquat. Org. 2019, 132, 241–247. [Google Scholar] [CrossRef]
- Farooqi, F.S.; Qureshi, W.U.H. Immunostimulants for aquaculture health management. J. Pharmacogn. Phytochem. 2018, 7, 1441–1447. [Google Scholar]
- Parmar, P.; Yusufzai, S.K. Therapeutic potentiality of florfenicol against vibriosis in Litopenaeus vannamei. J. Entomol. Zool. Stud. 2018, 6, 463–467. [Google Scholar]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, C.; Se-Kwon, K. Chapter 1: Introduction of marine algae extracts. In Marine algae extracts: Processes, product, and applications; Se-Kwon, K., Katarzyna, C., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 1, pp. 1–13. [Google Scholar]
- Praiboon, J.; Chirapart, A.; Soisarp, N. Principle and biological properties of sulfate polysaccharides from seaweed. In Marine Glycobiology Principles and Applications; Se-Kwon, K., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 85–120. [Google Scholar]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Seedevi, P.; Abirami, R.G.; Rajan, D.K. Application of marine-derived polysaccharides as immunostimulants in aquaculture: A review of current knowledge and further perspectives. Fish. Shellfish Immunol. 2019, 86, 1177–1193. [Google Scholar] [CrossRef]
- Aungtonya, C.M.; Liao, L. Marine flora (algae and seagrasses) in the reference collection of the phuket marine biological center, Thailand. Phuket Mar. Biol. Cent. Res. Bull. 2002, 64, 65–80. [Google Scholar]
- Ratana-arporn, P.; Chirapart, A. Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulata. Kasetsart J. Nat. Sci. 2006, 40, 75–83. [Google Scholar]
- Prathep, A.; Pongparadon, S.; Darakrai, A.; Wichachucherd, B.; Sinutok, S. Diversity and distribution of seaweed at Khanom-Mu Ko Thale Tai National Park, Nakhon Si Thammarat Province, Thailand. Songklanakarin J. Sci. Technol. 2011, 33, 633–640. [Google Scholar]
- Laungsuwon, R.; Chulalaksananukul, W. Antioxidant and anticancer activities of freshwater green algae, Cladophora glomerata and Microspora floccosa, from Nan River in northern Thailand. Maejo Int. J. Sci. Technol. 2013, 7, 181–188. [Google Scholar]
- Wongprasert, K.; Rudtanatip, T.; Praiboon, J. Immunostimulatory activity of sulfated galactans isolated from the red seaweed Gracilaria fisheri and development of resistance against white spot syndrome virus (WSSV) in shrimp. Fish. Shellfish Immunol. 2014, 36, 52–60. [Google Scholar] [CrossRef]
- Setthamongkol, P.; Tunkijjanukij, S.; Satapornvanit, K.; Salaenoi, J. Growth and nutrients analysis in marine macroalgae. Kasetsart J. Nat. Sci. 2015, 49, 211–218. [Google Scholar]
- Chen, X.; Sun, Y.; Liu, H.; Liu, S.; Qin, Y.; Li, P. Advances in cultivation, wastewater treatment application, bioactive components of Caulerpa lentillifera and their biotechnological applications. PeerJ 2019, 7, e6118. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Bertrand, D.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Determination of the chemical composition of ulvan, a cell wall polysaccharide from Ulva spp. (Ulvales, Chlorophyta) by FT-IR and chemometrics. J. Appl. Phycol. 2009, 21, 451–456. [Google Scholar] [CrossRef]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. J. Carbohyd. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.G.; Dabaghian, E.H.; Surayot, U. Water-soluble polysaccharides from Ulva intestinalis: Molecular properties, structural elucidation and immunomodulatory activities. J. Food Drug Anal. 2018, 26, 599–608. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T.V. Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef]
- Hernández-Garibay, E.; Zertuche-González, J.A.; Pacheco-Ruíz, I. Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J. Appl. Phycol. 2011, 23, 537–542. [Google Scholar] [CrossRef]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Algal polysaccharides, novel application, and outlook. In Algae based Polymers, Blends, and Composites Chemistry, Biotechnology and Material Sciences; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Cambridge, MA, USA, 2017; pp. 115–153. [Google Scholar]
- Paradossi, G.; Cavalieri, F.; Pizzoferrato, L.; Liquori, A.M. A physico-chemical study on the polysaccharide ulvan from hot water extraction of the macroalga Ulva. Int. J. Biol. Macromol. 1999, 25, 309–315. [Google Scholar] [CrossRef]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immuno. Pharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef]
- Robic, A.; Gaillard, C.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Ultrastructure of ulvan: A polysaccharide from green seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.F.; Dion, P.; Lerat, Y.; Lahaye, M. Seasonal variability of physicochemical and rheological properties of ulvan in two ulva species (chlorophyta) from the brittany coast. J. Phycol. 2009, 45, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Sulphated polysaccharides from Ulva clathrata and Cladosiphon okamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Tako, M.; Tamanaha, M.; Tamashiro, Y.; Uechi, S. Structure of ulvan isolated from the edible green seaweed, ulva pertusa. Adv. Biosci. Biotechnol. 2015, 6, 645–655. [Google Scholar] [CrossRef]
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J. Appl. Phycol. 2006, 18, 9–14. [Google Scholar] [CrossRef]
- Venugopal, V. Sulfated and non-sulfated polysaccharides from seaweeds and their uses: An overview. EC Nutrit. 2019, 14, 126–141. [Google Scholar]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Barbosa, J.D.S.; Costa, M.S.S.P.; Melo, L.F.M.D.; Medeiros, M.J.C.D.; Pontes, D.D.L.; Scortecci, K.C.; Rocha, H.A.O. In vitro immunostimulating activity of sulfated polysaccharides from Caulerpa cupressoides var. Flabellata. Mar. Drugs 2019, 17, 105. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. In vitro cytotoxicity assessment of ulvan, a polysaccharide extracted from green algae. Phytother. Res. 2012, 27, 1143–1148. [Google Scholar] [CrossRef]
- Baliano, A.P.; Pimentel, E.F.; Buzin, A.R.; Vieira, T.Z.; Romão, W.; Tose, L.V.; Lenz, D.; Andrade, T.U.; Fronza, M.; Kondratyuk, T.P.; et al. Brown seaweed Padina gymnospora is a prominent natural wound-care product. Rev. Bras. Farmacogn. 2016, 26, 714–719. [Google Scholar] [CrossRef]
- Silva, G.C.; Soares, R.C.; Carvalho, F.C.T.; Rocha, R.S.; Vieira, R.H.S.F.; Sousa, O.V. Antibacterial and cytotoxicity activity in macroalgae extracts: Perspectives for the use against pathogenic bacteria from shrimp farms (Litopenaeus vannamei). Acta Sci. Biol. Sci. 2018, 40, 1–9. [Google Scholar]
- De Lara-Isassi, G.; Álvarez-Hernández, S.; Collado-Vides, L. Ichtyotoxic activity of extracts from Mexican marine macroalgae. J. Appl. Phycol. 2000, 12, 45. [Google Scholar] [CrossRef]
- Targett, N.M.; Mitsui, A. Toxicity of subtropical marine algae using fish mortality and red blood cell hemolysis for bioassays. J. Phycol. 1979, 15, 181–185. [Google Scholar] [CrossRef]
- Lemée, R.; Pesando, D.; Durand-Clément, M.; Dubreuil, A.; Meinesz, A.; Guerriero, A.; Pietra, F. Preliminary survey of toxicity of the green alga Caulerpa taxifolia introduced into the Mediterranean. J. Appl. Phycol. 1993, 5, 485–493. [Google Scholar] [CrossRef]
- Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of some british green algae. Phytother. Res. 2010, 24, 1095–1098. [Google Scholar] [CrossRef]
- Ara, J.; Sultana, V.; Ehteshamul-Haque, S.; Qasim, R.; Ahmad, V.U. Cytotoxic activity of marine macro-algae on Artemia salina (brine shrimp). Phytother. Res. 1999, 13, 304–307. [Google Scholar] [CrossRef]
- Bansemir, A.; Blume, M.; Schröder, S.; Lindequist, U. Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture 2006, 252, 79–84. [Google Scholar] [CrossRef]
- Alghazeer, R.; Whida, F.; Abduelrhman, E.; Gammoudi, F.; Azwai, S. Screening of antibacterial activity in marine green, red and brown macroalgae from the western coast of Libya. Nat. Sci. 2013, 5, 7–14. [Google Scholar] [CrossRef]
- Mashjoor, S.; Yousefzadi, M.; Zolgharnain, H.; Kamrani, E.; Alishahi, M. Organic and inorganic nano-Fe3O4: Alga Ulva flexuosa-based synthesis, antimicrobial effects and acute toxicity to briny water rotifer Brachionus rotundiformis. Environ. Pollut. 2018, 237, 50–64. [Google Scholar] [CrossRef]
- Vidhya Hindu, S.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. A review on the impact of seaweed polysaccharide on the growth of probiotic bacteria and its application in aquaculture. Aquacult. Int. 2019, 27, 227–238. [Google Scholar] [CrossRef]
- Vatsos, I.N.; Rebours, C. Seaweed extracts as antimicrobial agents in aquaculture. J. Appl. Phycol. 2015, 27, 2017–2035. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Pushparaj, A.; Raubbin, R.S.; Balasankar, T. An antibacterial activity of the green seaweed Caulerpha sertularioides using five different solvents. Int. J. Pharm. Tech. Res. 2014, 6, 01–05. [Google Scholar]
- Li, Y.; Sun, S.; Pu, X.; Yang, Y.; Zhu, F.; Zhang, S.; Xu, N. Evaluation of Antimicrobial Activities of Seaweed Resources from Zhejiang Coast, China. Sustainability 2018, 10, 2158. [Google Scholar] [CrossRef]
- Esquer-Miranda, E.; Nieves-Soto, M.; Rivas-Vega, M.E.; Miranda-Baeza, A.; Piña-Valdez, P. Effects of methanolic macroalgae extracts from Caulerpa sertularioides and Ulva lactuca on Litopenaeus vannamei survival in the presence of Vibrio bacteria. Fish Shellfish Immunol. 2016, 51, 346–350. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Rajasekar, P.; Anjali, R.; Sathiyaraj, G.; Marudhupandi, T.; Selvam, S.; Prabhu, N.M.; You, S.G. Antibacterial efficacy of a fucoidan fraction (Fu-F2) extracted from Sargassum polycystum. Int. J. Biol. Macromol. 2019, 125, 485–495. [Google Scholar] [CrossRef]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Balasubramanian, V.; Palavesam, A. Effect of hot water extracts of brown seaweeds Sargassum spp. on growth and resistance to white spot syndrome virus in shrimp Penaeus monodon postlarvae. Aquac. Res. 2010, 41, e545–e553. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesama, A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef]
- Sivagnanavelmurugan, M.; Marudhupandi, T.; Palavesam, A.; Immanuel, G. Antiviral effect of fucoidan extracted from the brown seaweed, Sargassum wightii, on shrimp Penaeus monodon postlarvae against white spot syndrome virus. J. World Aquacult. Soc. 2012, 43, 697–706. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kitikiew, S.; Yeh, S.T.; Chen, J.C. White shrimp Litopenaeus vannamei that have received fucoidan exhibit a defense against Vibrio alginolyticus and WSSV despite their recovery of immune parameters to background levels. Fish Shellfish Immunol. 2016, 59, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Sinurat, E.; Saepudin, E.; Peranginangin, R.; Hudiyono, S. Immunostimulatory activity of brown seaweed-derived fucoidans at different molecular weights and purity levels towards white spot syndrome virus (WSSV) in shrimp Litopenaeus vannamei. J. Appl. Pharm. Sci. 2016, 6, 82–91. [Google Scholar] [CrossRef]
- Cantelli, L.; Goncalves, P.; Guertler, C.; Kayser, M.; Pilotto, M.R.; Barracco, M.A.; Perazzolo, L.M. Dietary supplementation with sulfated polysaccharides from Gracilaria birdiae promotes a delayed immunostimulation in marine shrimp challenged by the white spot syndrome virus. Aquac. Int. 2019, 27, 349–367. [Google Scholar] [CrossRef]
- Declarador, R.S.; Serrano, A.E., Jr.; Corre, V.L., Jr. Ulvan extract acts as immunostimulant against white spot syndrome virus (WSSV) in juvenile black tiger shrimp Penaeus monodon. Auqac. Aquar. Conserv. Legis. 2014, 7, 153–161. [Google Scholar]
- Lauzon, Q.D.; Serrano, A.E. Ulvan extract from Enteromorpha intestinalis enhances immune responses in Litopenaeus vannamei and Penaeus monodon juveniles. Anim. Biol. Anim. Husb. 2015, 7, 1–10. [Google Scholar]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Sotanon, N.; Saleeart, A.; Rattanarojpong, T.; Thanh Dong, H.; Senapin, S.; Wongprasert, K.; Sarikavanij, S.; Khunrae, P. C-terminal domain of WSSV VP37 is responsible for shrimp haemocytes binding which can be inhibited by sulfated galactan. Fish Shellfish Immunol. 2018, 77, 312–318. [Google Scholar] [CrossRef]
- Craigie, J.S.; Wen, Z.C.; van der Meer, J.P. Interspecific, intraspecific and nutritionally-determined variations in the composition of Agars from Gracilaria spp. Bot. Mar. 1984, 27, 55–61. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Cesaretti, M.; Luppi, E.; Maccari, F.; Volpi, N. A 96-well assay for uronic acid carbazole reaction. Carbohydr. Polym. 2003, 54, 59–61. [Google Scholar] [CrossRef]
- Srisapoome, P.; Klongklaew, N.; Areechon, N.; Wongpanya, R. Molecular and functional analyses of novel anti-lipopolysaccharide factors in giant river prawn (Macrobrachium rosenbergii, De Man) and their expression responses under pathogen and temperature exposure. Fish Shellfish Immunol. 2018, 80, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Molina-Aja, A.; Bolán-Mejía, C.; Gomez-Gil, B. In vitro susceptibility to 15 antibiotics of vibrios isolated from penaeid shrimps in Northwestern Mexico. Int. J. Antimicrob. Agents 2001, 17, 383–387. [Google Scholar] [CrossRef]
- Nunan, L.M.; Poulos, B.T.; Lightner, D.V. The detection of white spot syndrome virus (WSSV) and yellow head virus (YHV) in imported commodity shrimp. Aquaculture 1998, 160, 19–30. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests for Aquatic Animals 2017. Available online: https://www.oie.int/doc/ged/D6505.PDF (accessed on 27 February 2020).
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef]
- Balasubramanian, G.; Sarathi, M.; Kumar, S.R.; Hameed, A.S.S. Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture 2007, 263, 15–19. [Google Scholar] [CrossRef]
- Palanikumar, P.; Daffni Benitta, D.J.; Lelin, C.; Thirumalaikumar, E.; Michaelbabu, M.; Citarasu, T. Effect of Argemone mexicana active principles on inhibiting viral multiplication and stimulating immune system in Pacific white leg shrimp Litopenaeus vannamei against white spot syndrome virus. Fish Shellfish Immunol. 2018, 75, 243–252. [Google Scholar] [CrossRef]
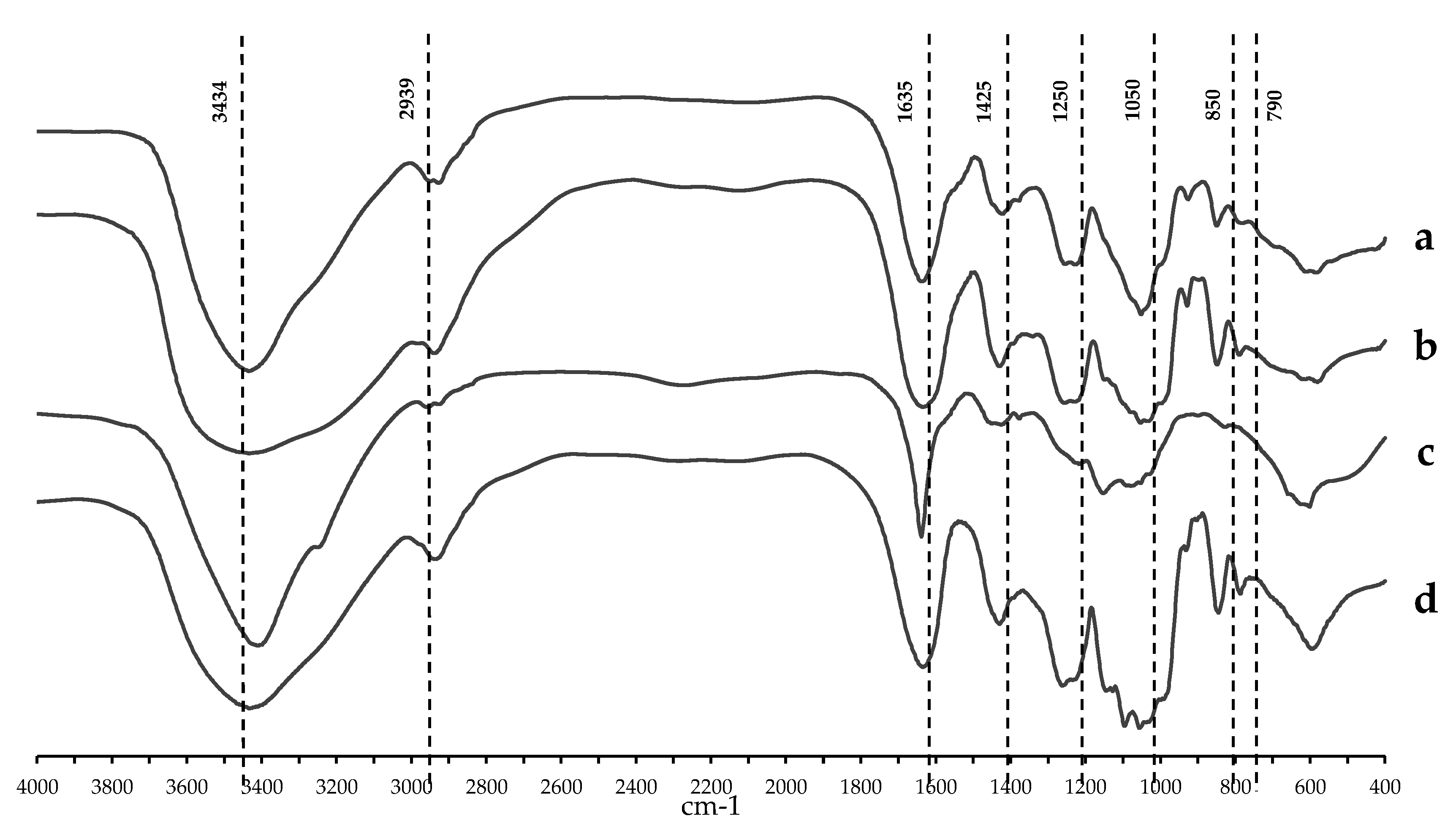
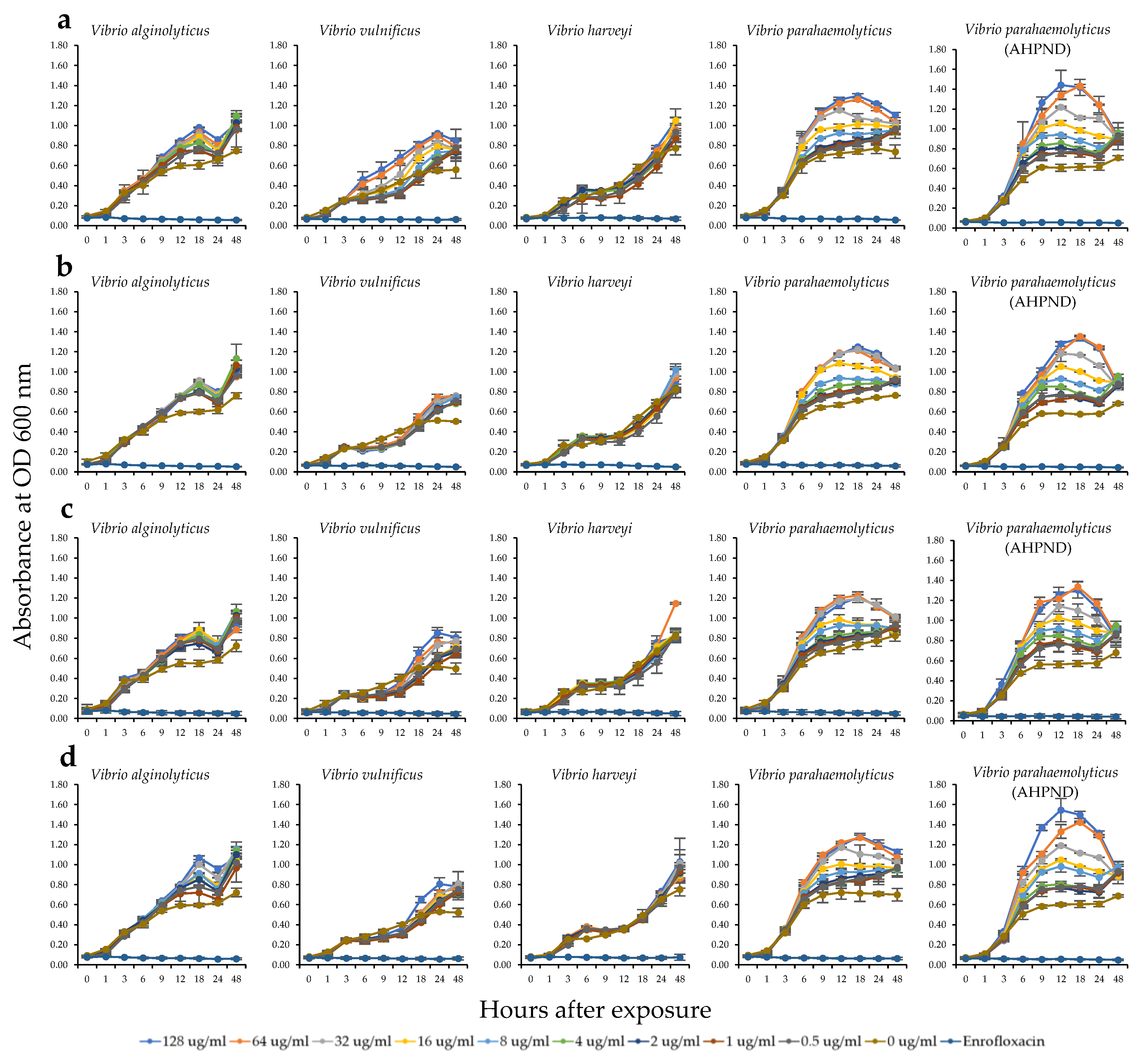
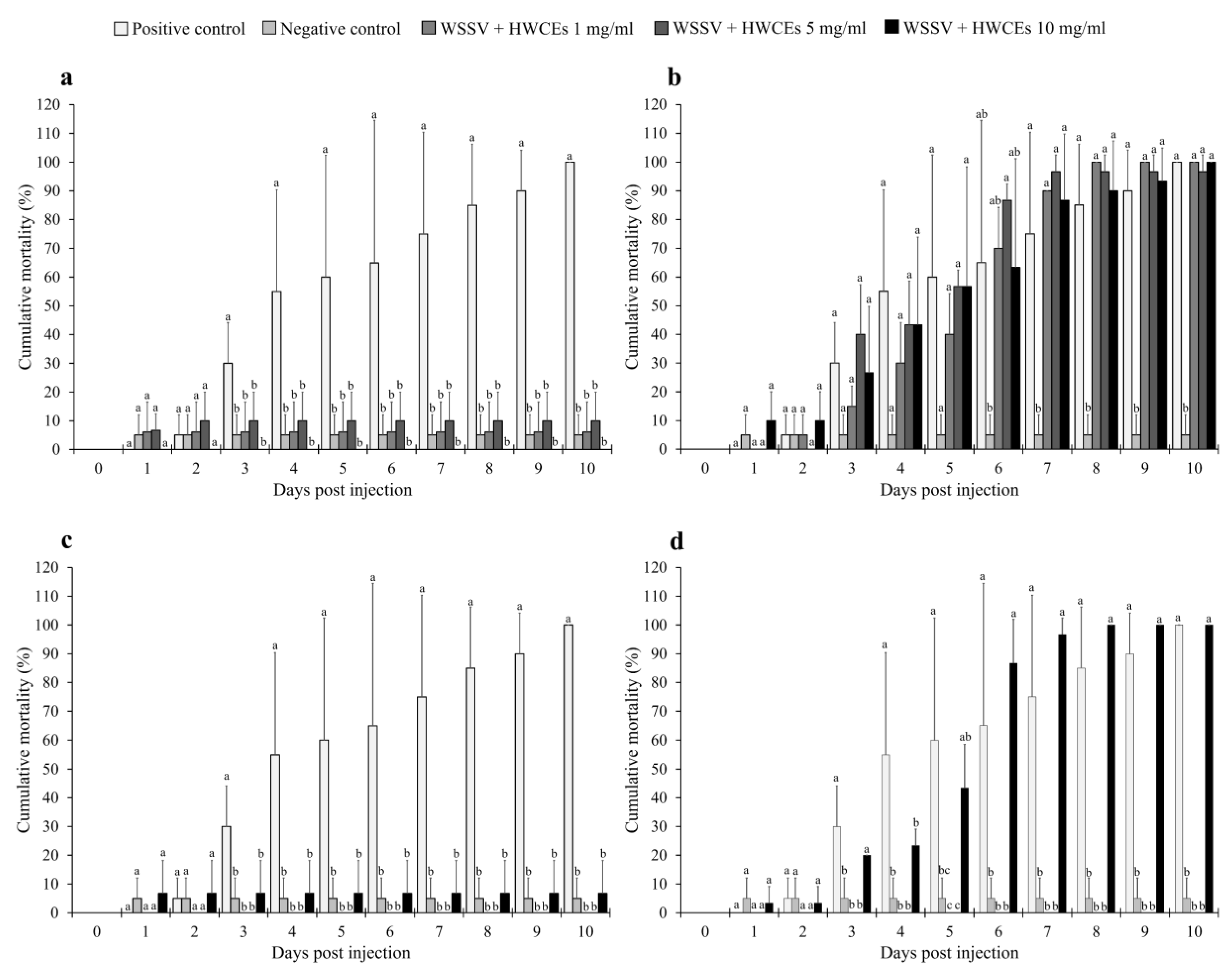
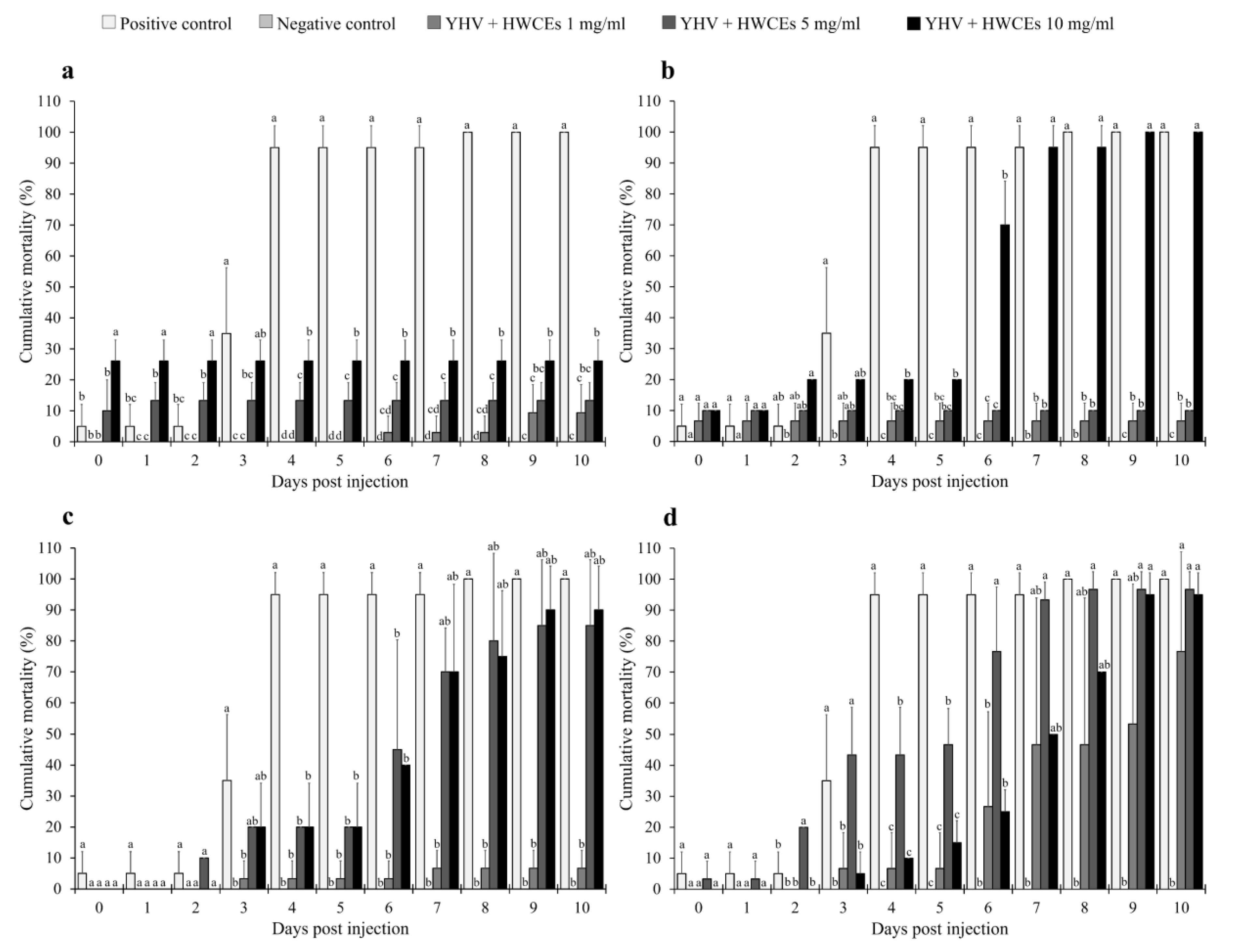
| Source of crude extract | Sulfate content (%) | Uronic acid content (%) | Carbohydrate content (%) |
|---|---|---|---|
| U. intestinalis (Ui) | 11.01 ± 0.29b | 29.58 ± 1.66b | 50.10 ± 9.18b |
| U. rigida (Ur) | 13.89 ± 0.38a | 32.76 ± 1.53b | 51.02 ± 3.72b |
| Caulopa lentillifera (Cl) | 7.29 ± 0.28c | 9.22 ± 0.56c | 6.85 ± 1.74c |
| U. armoricana (Ua) | 11.50 ± 0.44b | 41.47 ± 4.98a | 64.03 ± 2.75a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klongklaew, N.; Praiboon, J.; Tamtin, M.; Srisapoome, P. Antibacterial and Antiviral Activities of Local Thai Green Macroalgae Crude Extracts in Pacific White Shrimp (Litopenaeus vannamei). Mar. Drugs 2020, 18, 140. https://doi.org/10.3390/md18030140
Klongklaew N, Praiboon J, Tamtin M, Srisapoome P. Antibacterial and Antiviral Activities of Local Thai Green Macroalgae Crude Extracts in Pacific White Shrimp (Litopenaeus vannamei). Marine Drugs. 2020; 18(3):140. https://doi.org/10.3390/md18030140
Chicago/Turabian StyleKlongklaew, Nawanith, Jantana Praiboon, Montakarn Tamtin, and Prapansak Srisapoome. 2020. "Antibacterial and Antiviral Activities of Local Thai Green Macroalgae Crude Extracts in Pacific White Shrimp (Litopenaeus vannamei)" Marine Drugs 18, no. 3: 140. https://doi.org/10.3390/md18030140
APA StyleKlongklaew, N., Praiboon, J., Tamtin, M., & Srisapoome, P. (2020). Antibacterial and Antiviral Activities of Local Thai Green Macroalgae Crude Extracts in Pacific White Shrimp (Litopenaeus vannamei). Marine Drugs, 18(3), 140. https://doi.org/10.3390/md18030140






