1. Introduction
The wide usage of antibiotics in the world has rapidly increased the risk of antibiotic-resistant pathogens. Many new and nonconventional anti-infective therapies have been developed and/or identified to solve this threat [
1]. Marine microorganisms are regarded as major sources of antimicrobial agents [
2].
Bacillus amyloliquefaciens, a widely distributed and environmentally friendly aerobic Gram-positive
Bacillus bacterium, is rich in metabolites and can secrete bacteriostatic substances, such as lipopeptides [
3,
4] and glycosidases [
5].
B. amyloliquefaciens has been used in animal feeding [
6] or the therapy of plant diseases due to its ability to produce broad-spectrum antibacterial activity [
7,
8]. The
B. amyloliquefaciens BTSS-3 isolated from a deep-sea shark (
Centroscyllium fabricii) has shown antimicrobial activity against pathogenic bacteria, including
Salmonella typhimurium,
Proteus vulgaris,
Clostridium perfringens, and
Staphylococcus aureus [
9]. Isolated from soil,
B. amyloliquefaciens MET0908 is used to cure cucumber anthracnose [
7].
B. amyloliquefaciens PPCB004 has inhibitory effects against the fungal pathogens of harvested citrus [
8].
B. amyloliquefaciens US 573 is reported as a feeding additive that promotes animal nutrient absorption and feed conversion ratio [
6].
The whitespotted bamboo shark (
Chiloscyllium plagiosum) is a demersal cartilage fish distributed from the Indian Ocean to the Western Pacific Ocean. The bacteria in the shark, particularly those in the digestive tract, produce inhibitory compounds responsible for controlling the colonization of potential pathogens in fish [
10,
11].
B. amyloliquefaciens-9 (GBacillus-9, also named
Bacillus sp. GFP-2), a strain of
B. amyloliquefaciens, was isolated from the intestinal tract of the whitespotted bamboo shark (
C. plagiosum) [
12]. The antimicrobial peptides (AMPs) and β-1,3-1,4-glucanase expressed in GBacillus-9 could partially contribute to inhibit Gram-negative/positive bacteria [
12]. In vivo, GBacillus-9 improves immunity by promoting the lysozyme content of the skin mucosa in hybrid sturgeon (
Acipenser sinensis), indicating GBacillus-9’s potential as a feed additive for aquaculture farmed fish [
13]. Furthermore, the data on Japanese eel support the idea of developing bacterial additives in fish raising by using GBacillus-9 [
14]. All these data suggest that the antibacterial material in GBacillus-9 can be used as a “drug” in the fish farm, which can help decrease the use of antibiotics in agriculture.
However, the idea of developing a “drug” by using GBacillus-9 is hindered by its low antibacterial material production. The optimization of fermentation conditions will benefit the use of GBacillus-9. The single-factor experiment, Plackett–Burman (PB), and central composite designs (CCDs) [
15,
16,
17,
18,
19] were used to optimize the fermentation conditions of GBacillus-9, achieve high antibacterial activity, and overcome the limited fermentation efficiency of GBacillus-9.
3. Discussion
The marine environment is a largely untapped source for the isolation of new microorganisms with the potential to produce bioactive compounds [
25]. The host organism synthesizes bioactive compounds as primary or secondary metabolites to protect or maintain homeostasis [
26]. GBacillus-9, which is isolated from the intestine of the whitespotted bamboo shark, has shown potential in the development of biological additives [
12,
13]. Thus, investigating the optimal fermentation conditions with high efficiency and low cost is an important strategy to develop biological additives. In the current study, PB design and CCD were applied to evaluate the fermentation efficiency of GBacillus-9 through various media, and the results showed efficient fermentation parameters with the improved yield of the antibacterial material in GBacillus-9.
The GBacillus-9 strain has shown a broad-spectrum antibacterial activity on Gram-negative and Gram-positive bacteria [
12]. In the current study, TG1 and BS168, which represented the Gram-negative and the Gram-positive bacteria, respectively, were used to detect antibacterial activity. The current data showed that the antibacterial material secreted by GBacillus-9 had a stronger activity against Gram-negative bacteria (TG1) than Gram-positive bacteria (BS168), which agreed with the fact that AMPs blocked the growth of Gram-negative bacteria by entering the membrane and disrupting the local electrostatic fields [
27]. The weak activity of GBacillus-9 against Gram-positive bacteria may be due to the inherent properties of Gram-positive bacteria, which contain more peptidoglycan than Gram-negative bacteria. The peptidoglycan hinders the entry of AMPs into cells, thereby reducing antibacterial activity [
28,
29].
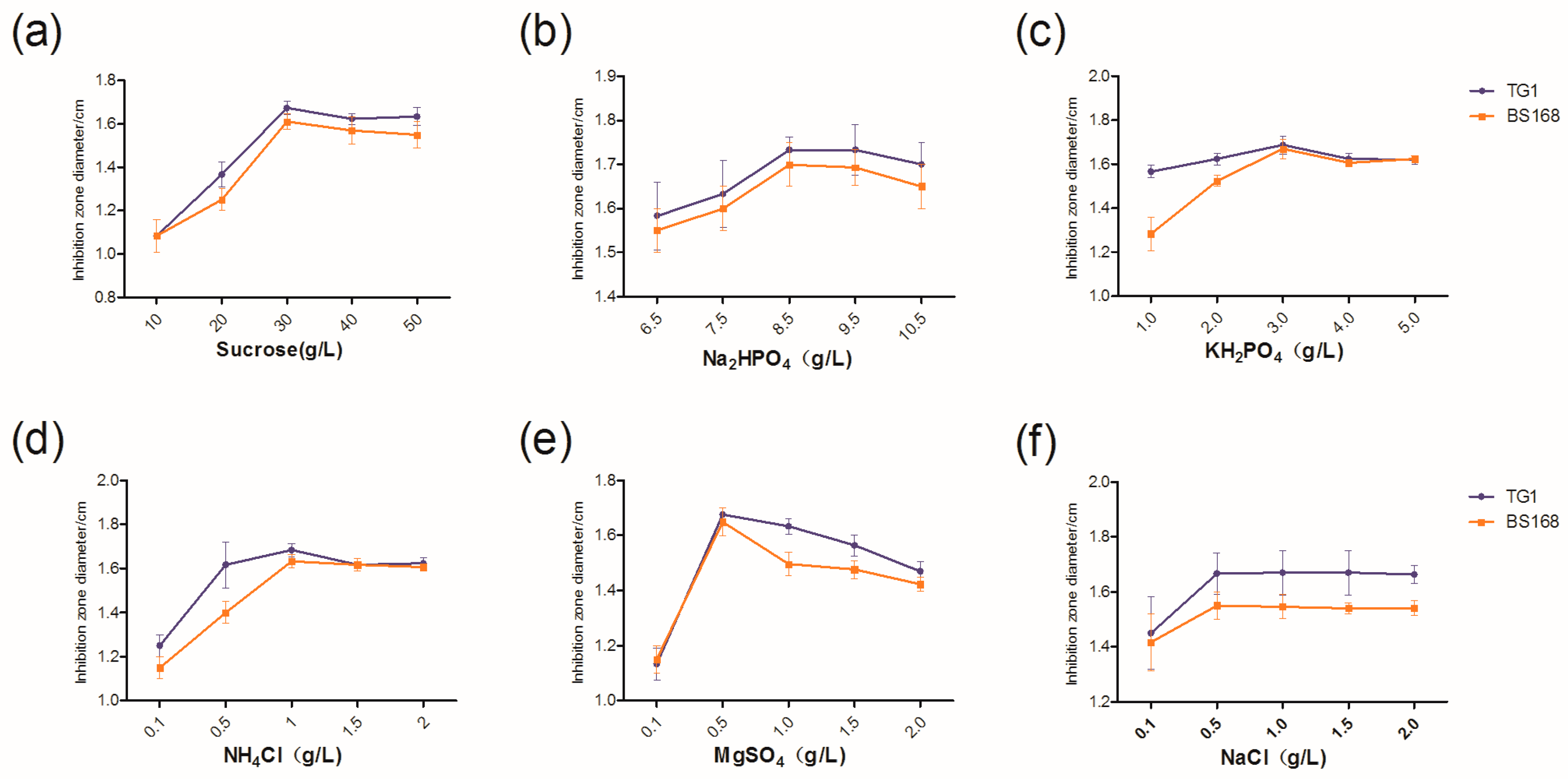
The medium most beneficial to the production of antibacterial substances in GBacillus-9 was obtained by screening different media. No evident relationship was found between the bacteriostatic activity and the number of bacteria. The increased antibacterial activity may be caused by a secondary metabolite secreted by GBacillus-9.
There have been many reports on the antibacterial substances of
B. subtilis, which mainly achieved the antibacterial effect by secreting peptides, lipids, and other substances [
30,
31]. Most of the
B. subtilis strains capable of secreting antibacterial substances are isolated from soil and plants [
6,
7,
8]. However, it is still relatively rare to isolate
Bacillus subtilis in the ocean, and the GBacillus-9 culture medium isolated in the present study has a strong antibacterial activity against Gram-positive and Gram-negative bacteria. Moreover, after optimizing the culture medium, the high activity remained even after spray drying, which was conducive to large-scale market applications.
Carbon and nitrogen sources play an important role in the growth and cellular metabolism of bacteria [
32,
33]. Although these carbon sources resulted in the similar OD value of GBacillus-9, sucrose, glucose, and mannitol had the highest antibacterial activity. The various antibacterial material products with different carbon sources agreed with the suggestion that high cell weight does not always lead to the increased production of antibacterial substances [
34,
35]. Sucrose was selected because it was cheaper than glucose and mannitol. In the present study, the effects of nine carbon sources and seven nitrogen sources on the secretion of GBacillus-9 antibacterial substances were screened. Unsurprisingly, sucrose and NH
4Cl were identified as the most important factors with respect to inhibition zone diameters of anti-TG1 and anti-BS168. Both sources met the bacterial mass and the production of antibacterial substances.
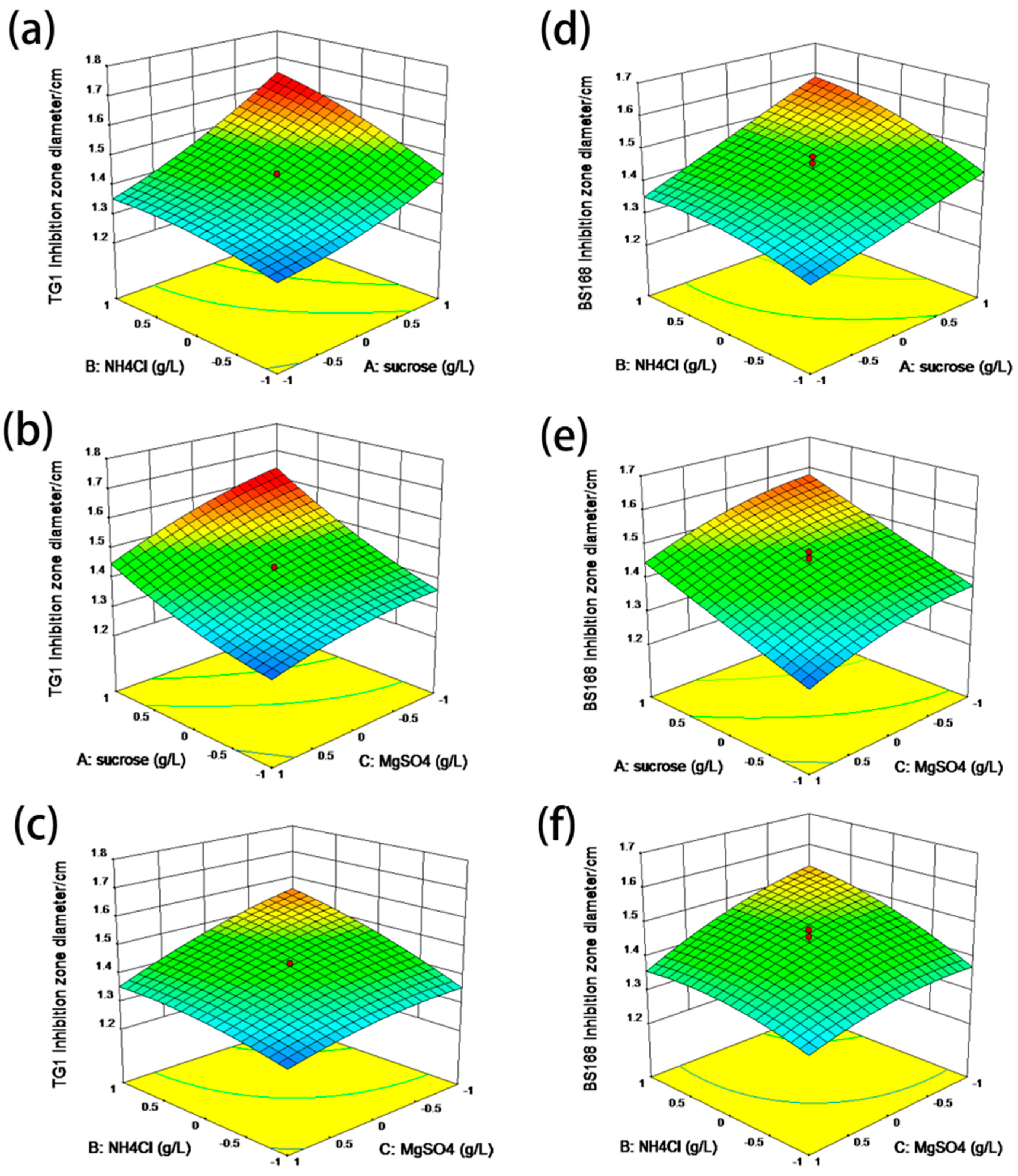
The PB design is a screening procedure that identifies essential variables in the production of a response parameter by analyzing the main effects, which allow multiple factors to be screened within one experiment. This design has been well used in assessing the nutritional requirements of bacteria to optimize enzyme production [
36,
37,
38]. In the present study, sucrose, NH
4Cl, and MgSO
4 were identified as three key factors affecting the yield of anti-TG1. However, only sucrose was identified as the key factor affecting the yield of anti-BS168. This difference was attributed to the intrinsic characteristics, e.g., the composition of cytoderm, and of Gram-positive and Gram-negative bacteria.
The increase in sucrose concentration increased the antibacterial activity of GBacillus-9, suggesting an increased production of bacteria when the concentration of sucrose was higher than 46.8 g L
−1. The reason for using sucrose at 46.8 g L
−1 in the culture medium was to avoid the viscosity caused by the higher concentration of sucrose in the subsequent spray drying for bacterial preparation [
39]. Furthermore, a high concentration of MgSO
4 inhibited the growth of GBacillus-9.
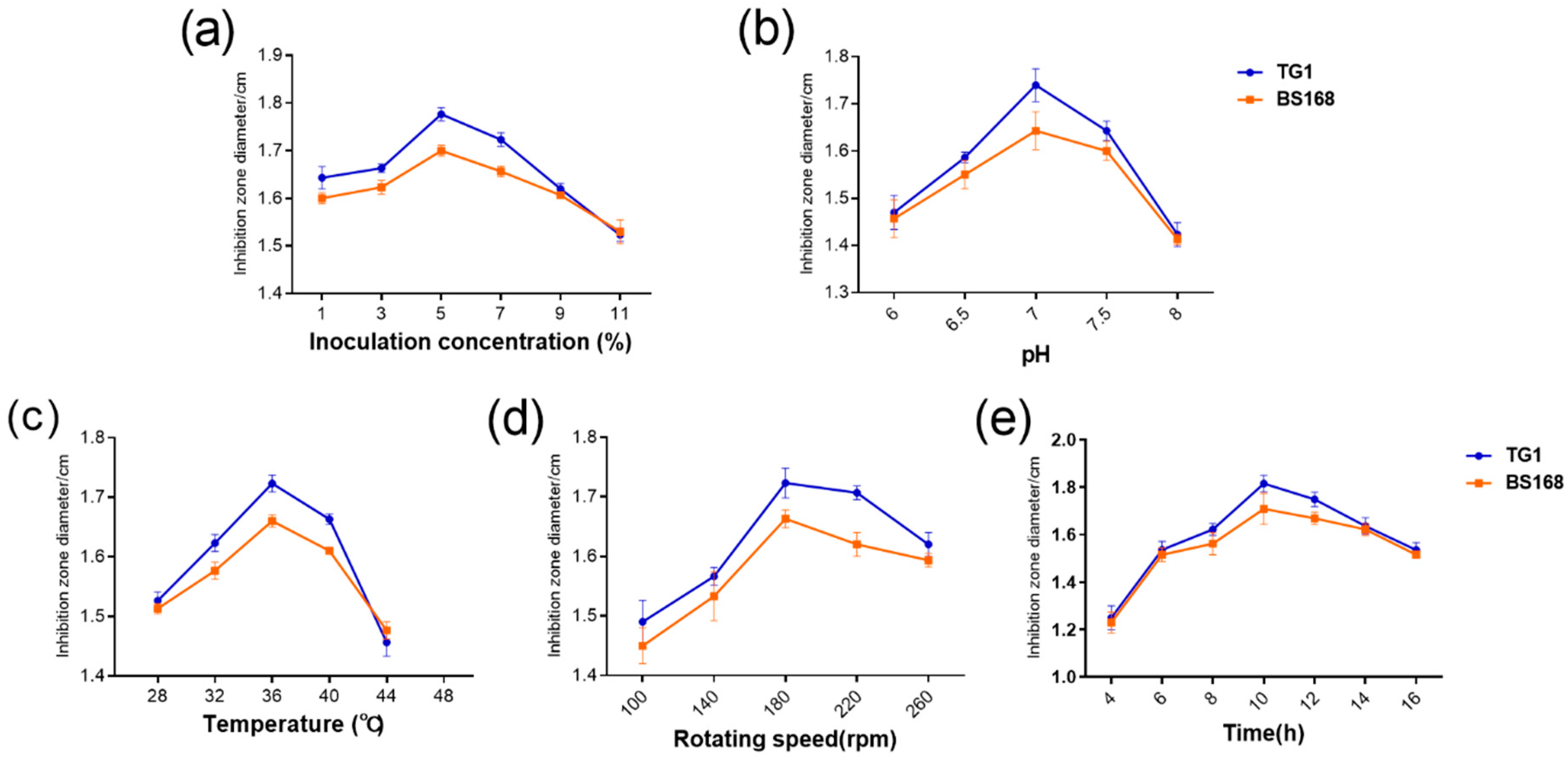
Aside from the cultured medium, the fermentation process also greatly affected the production. Moreover, pH can affect the cellular enzyme activity and the stability of microbial cell membranes, altering membrane permeability and nutrient absorption [
40,
41,
42]. Additionally, inoculation affects the growth of microorganisms and the accumulation of metabolites. Excessive inoculation accelerates the consumption of the medium, whereas low-inoculum cell concentrations prolong the log phase [
43,
44], both of which reduce the efficiency of fermentation. Besides, culture temperature, rotation speed, and culture time affect the secretion after fermentation in different degrees [
45]. In the present study, the optimal inoculum volume, temperature, rotation speed, and initial pH for GBacillus-9 were 5% (v/v), 36 °C, 180 rpm, and 7.0, respectively, which were consistent with those of other
B. amyloliquefaciens strains [
46,
47].
The limitation of the present study was that the antibacterial materials secreted by GBacillus-9 were not completely addressed. However, our previous data showed that β-1,3-1,4-glucanase and the antimicrobial peptides secreted by GBacillus-9 contributed to its antimicrobial activity [
12]. The detection of β-1,3-1,4-glucanase in the crude proteins from GBacillus-9 culture supernatant confirmed that GBacillus-9 could indeed express β-1,3-1,4-glucanase. Furthermore, the antimicrobial activity of purified recombinant β-1,3-1,4-glucanase cloned from the GBacillus-9 genome suggests that β-1,3-1,4-glucanase is a factor contributing to antimicrobial activity [
12]. The genome data indicated that GBacillus-9 contained genes encoding LCI (a 47 residue cationic antimicrobial peptide), yellowfin tuna GAPDH-related antimicrobial peptide, and human GAPDH-related AMPs [
12]. Beyond the potential AMPs, metabolites, such as difficidin, bacillibactin, bacilysin, surfactin, butirosin, macrolactin, bacillaene, and fengycin, and bacteriocins have been predicted in the genome of GBacillus-9 [
12], which may contribute to the antibacterial role. Collectively, these results suggest that the inhibitory effects of GBacillus-9 on Gram-negative/positive bacteria might be dependent on complex metabolites. More experiments are needed to address the antibacterial materials in GBacillus-9 in the future.
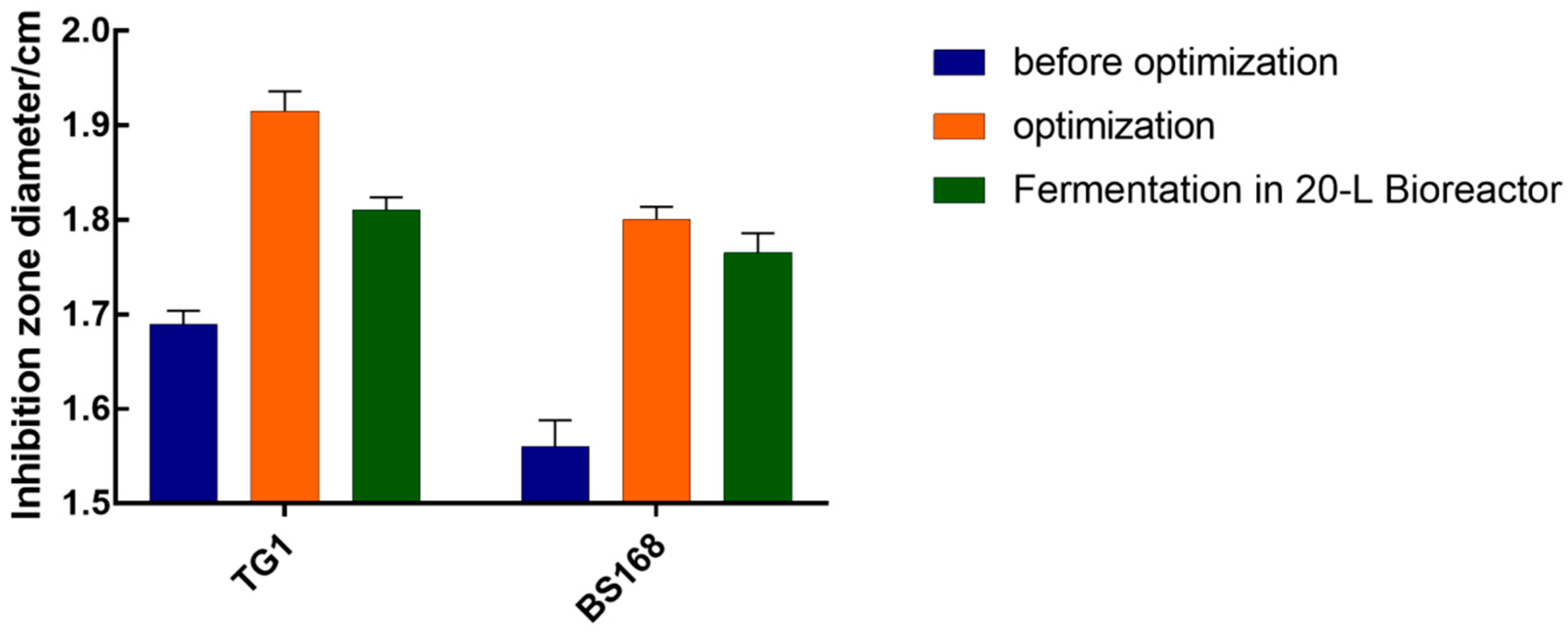
The inhibition zone diameter of the anti-TG1 and anti-BS168 in the optimal culture medium reached 1.82 and 1.78 cm, respectively, which were significantly improved compared to that in the LB medium. The finding that the bacteriostatic test of GBacillus-9 with 20 L was consistent with the predicted regression model suggested success in using the response surface method to improve the fermentation efficiency and that the factor effects were real.
In the present study, optimization studies using PB and response surface methodology identified the optimal nutritional factors involved in the maximal production of antibacterial compounds from GBacillus-9, supporting a fermenter to obtain a large number of GBacillus-9. Thus, two necessary market application requirements for the easily obtained and low-cost development of a “drug” were met. These data can enhance the process of developing biological additives. In addition, results showed the optimal fermentation time, temperature, inoculum volume, initial pH, and rotation speed for GBacillus-9. However, the antibacterial material of GBacillus-9 and specific mechanism behind its biological activity remain unknown. Hence, further research is required.