New Eunicellin-Type Diterpenes from the Panamanian Octocoral Briareum asbestinum
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of Diterpenes
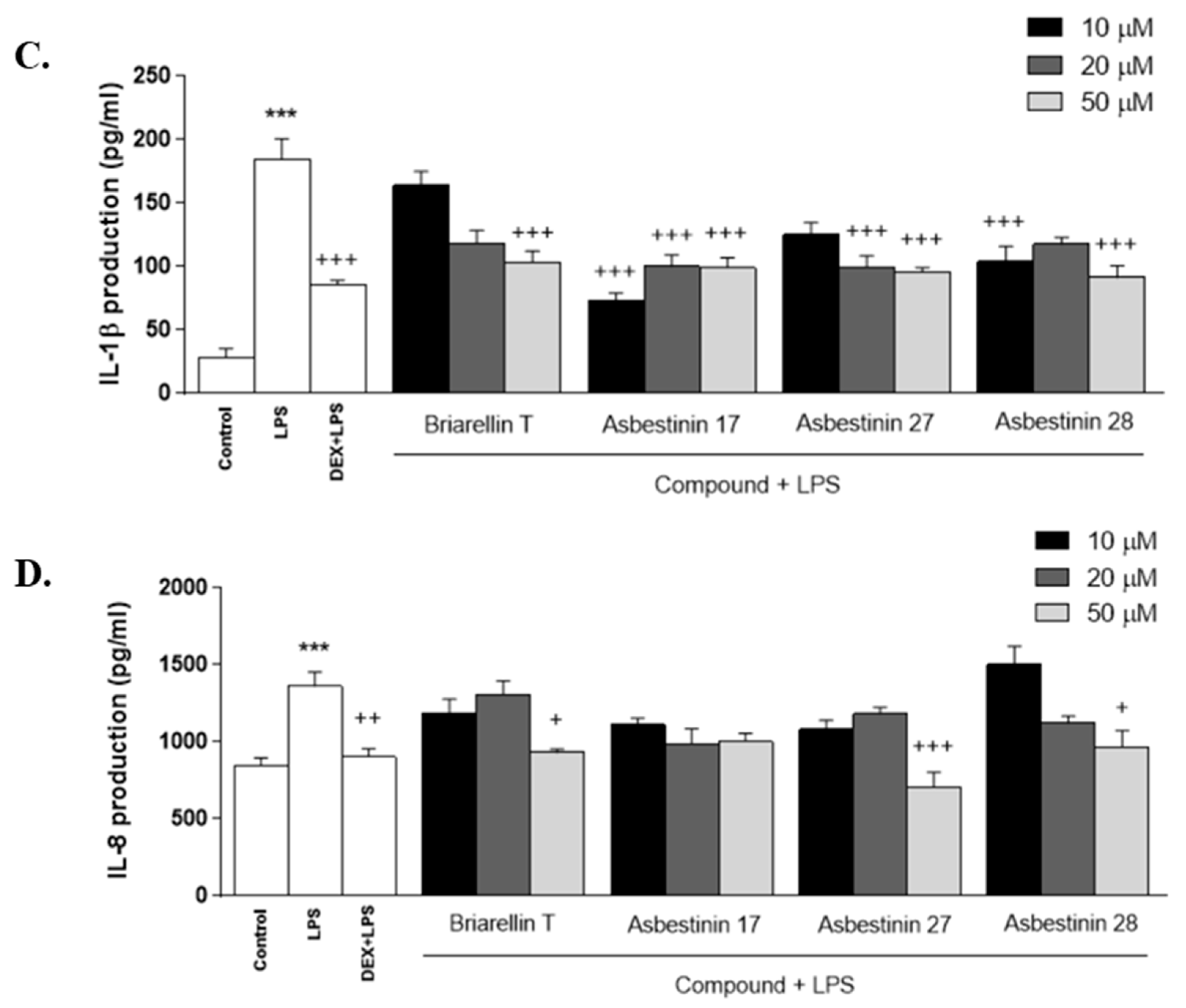
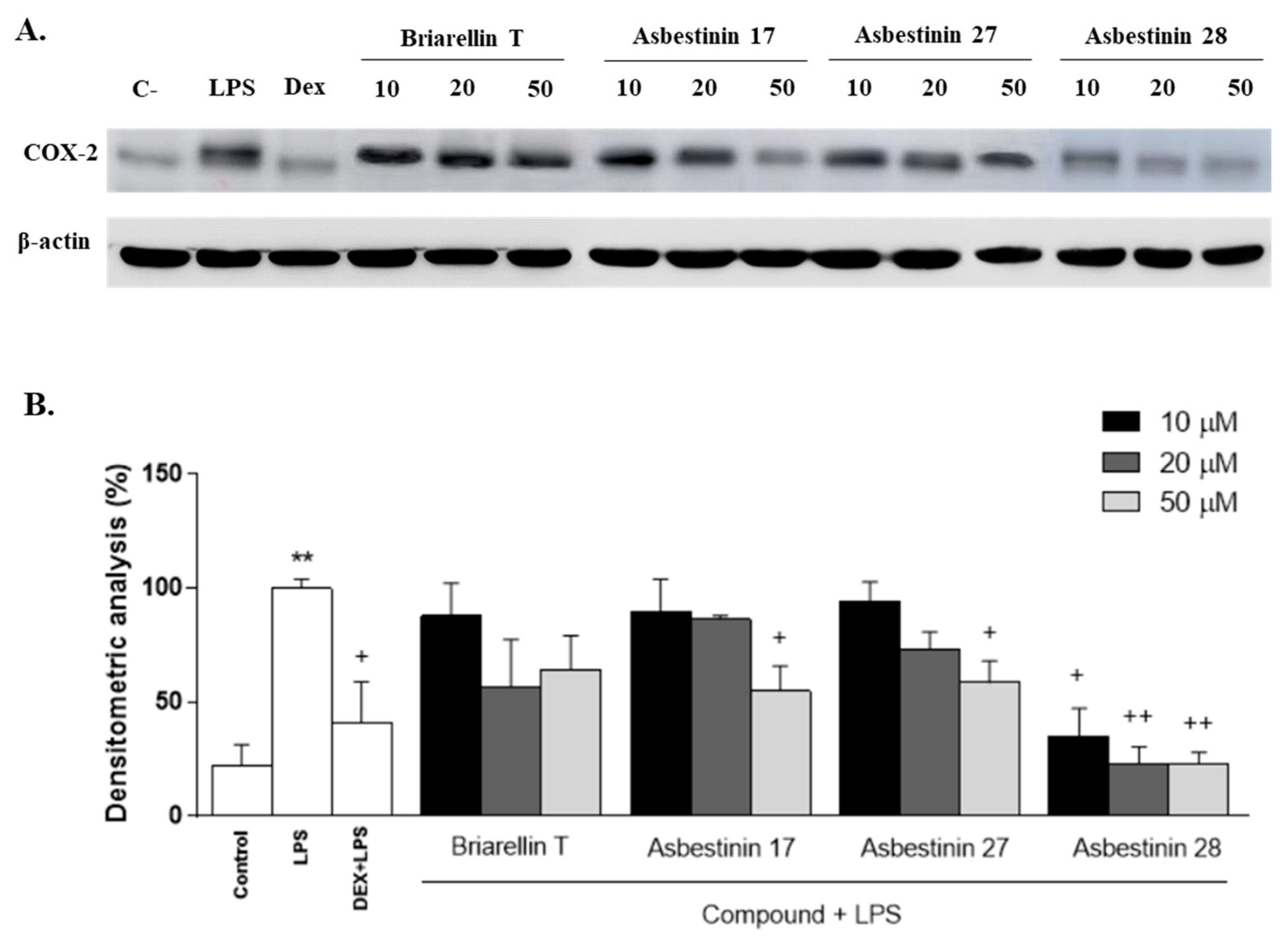
2.2. Anti-Inflammatory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. Cell Proliferation Assay
3.6. Determination of Cytokines Production
3.7. Isolation of Cytoplasmic Proteins and Analysis of Proteins Expression by Western Blot Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Terpenoids from Octocorals of the Genus Pachyclavularia. Mar. Drugs 2017, 15, 382. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Peng, B.-R.; Fang, L.-S.; Hwang, T.-L.; Su, J.-H.; Wu, Y.-C.; Sung, P.-J. Hydroperoxyditerpenoids from Octocorals. Isr. J. Chem. 2019, 59, 403–413. [Google Scholar] [CrossRef]
- Lei, H. Diterpenoids of Gorgonian Corals: Chemistry and Bioactivity. Chem. Biodivers. 2016, 13, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Berrue, F.; Kerr, R.G. Diterpenes from Gorgonian Corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D. The Natural Products Chemistry of West Indian Gorgonian Octocorals. Tetrahedron 1995, 51, 4571–4618. [Google Scholar]
- Sung, P.-J.; Chen, M.-C. The Heterocyclic Natural Products of Gorgonian Corals of Genus Briareum Exclusive of Briarane-Type Diterpenoids. Heterocycles 2002, 57, 1705–1715. [Google Scholar] [CrossRef]
- Putra, M.Y.; Wibowo, J.T.; Murniasih, T. A Review of Chemistry and Biological Activities of the Indonesian Octocorallia. J. Appl. Pharm. Sci. 2017, 7, 219–227. [Google Scholar]
- Gómez-Reyes, J.F.; Salazar, A.; Guzmán, H.M.; González, Y.; Fernández, P.L.; Ariza-Castolo, A.; Gutiérrez, M. Seco-briarellinone and Briarellin S, Two New Eunicellin-Based Diterpenoids from the Panamanian Octocoral Briareum asbestinum. Mar. Drugs 2012, 10, 2608–2617. [Google Scholar]
- Rodríguez, A.D.; Cóbar, O.M. Studies on the Minor Constituents of the Caribbean Gorgonian Octocoral Briareum asbestinum Pallas. Isolation and Structure Determination of the Eunicellin-Based Diterpenoids Briarellins E-I. Chem. Pharm. Bull. 1995, 43, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Ospina, C.A.; Rodríguez, A.D.; Ortega-Barria, E.; Capson, T.L. Briarellins J-P and Polyanthellin A: New Eunicellin-Based Diterpenes from the Gorgonian Coral Briareum Polyanthes and Their Antimalarial Activity. J. Nat. Prod. 2003, 66, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Stierle, D.B.; Carté, B.; Faulkner, D.J.; Tagle, B.; Clardy, J. The Asbestinins, a Novel Class of Diterpenes from the Gorgonian Briareum asbestinum. J. Am. Chem. Soc. 1980, 102, 5088–5092. [Google Scholar] [CrossRef]
- Welford, A.J.; Collins, I. The 2,11-Cyclized Cembranoids: Cladiellins, Asbestinins, and Briarellins (Period 1998-2010). J. Nat. Prod. 2011, 74, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Talero, E.; de Los Reyes, C.; García-Mauriño, S.; Motilva, V. Microalgae-Derived Oxylipins Decrease Inflammatory Mediators by Regulating the Subcellular Location of NFκB and PPAR-γ. Pharmacol. Res. 2018, 128, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Metryka, E.; Chibowska, K.; Gutowska, I.; Falkowska, A.; Kupnicka, P.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int. J. Mol. Sci. 2018, 19, 1813. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.F.; Shen, X.Y.; Lio, C.K.; Dai, Y.; Cheng, C.S.; Liu, J.X.; Yao, Y.D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A Rapid and Sensitive Method for The Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
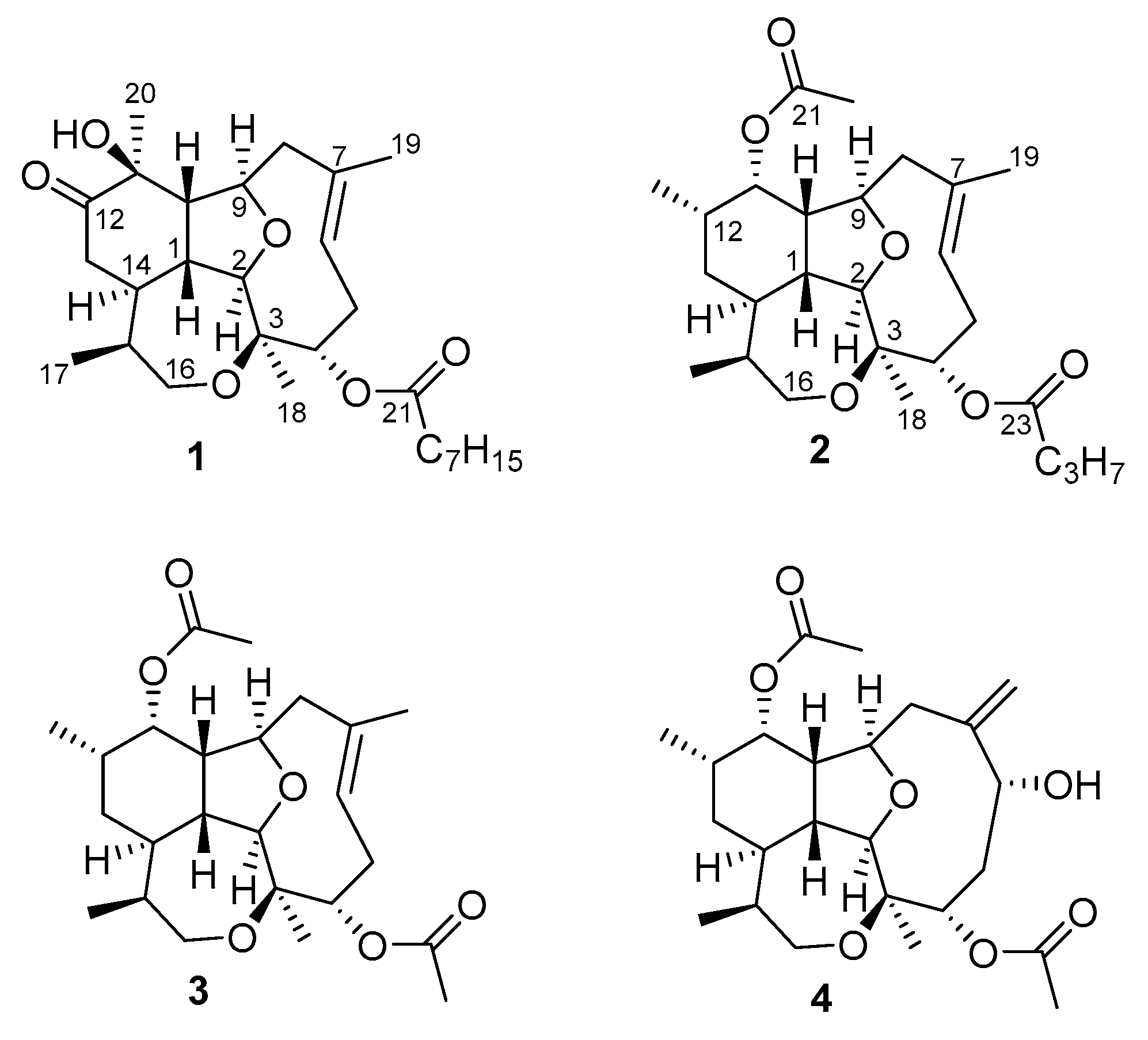
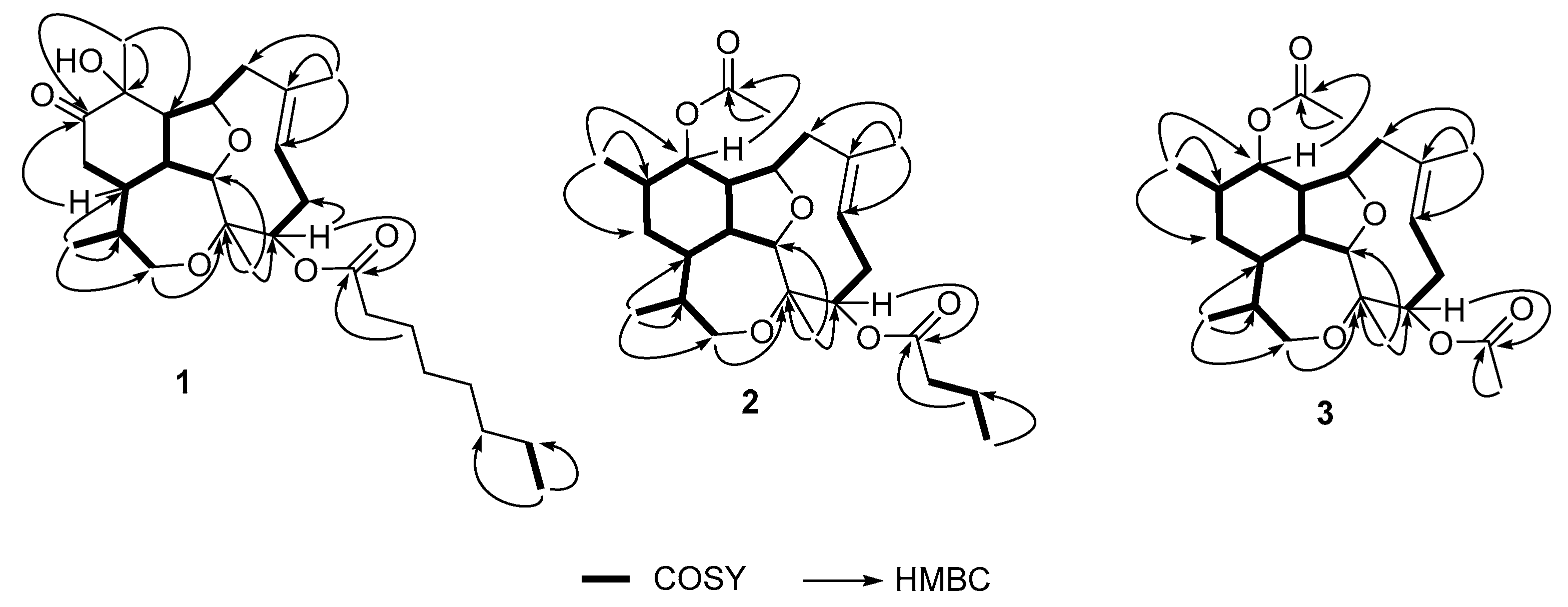
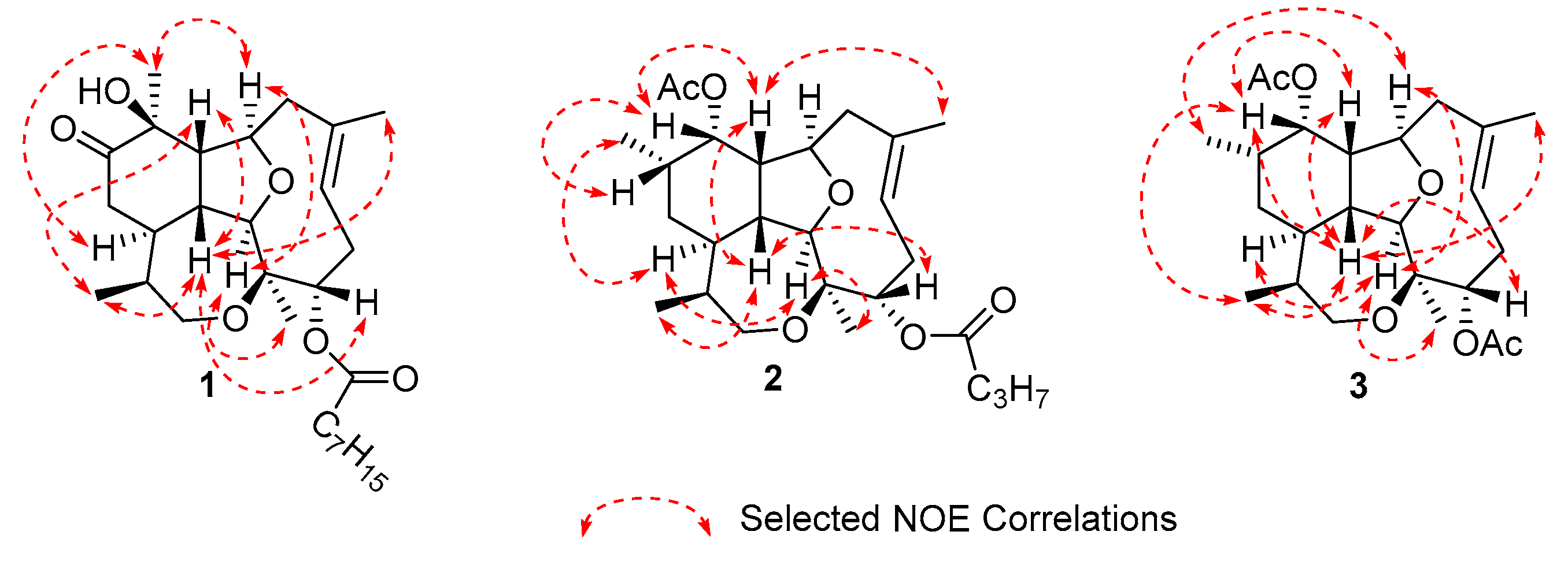

| Position | δC, mult. a | δH, mult. (J in Hz) b | HMBC | COSY |
|---|---|---|---|---|
| 1 | 37.8, CH | 2.53, dt (10.7, 8.5) | 11 | 2, 10, 14 |
| 2 | 95.3, CH | 4.09. d (8.5) | 4, 9, 14, 18 | 1 |
| 3 | 80.3, C a | |||
| 4 | 71.7, CH a,b | 5.33, m (overlap) | 5, 21 | 5 |
| 5a | 29.0, CH2 | 2.76, ddd (14.6, 9.2, 6.3) | 4, 6 | |
| 5b | 2.14, m (overlap) | |||
| 6 | 124.8, CH | 5.36, m (overlap) | 8, 19 | 5 |
| 7 | 129.9, C | |||
| 8a | 44.6, CH2 | 2.62, dd (13.5, 6.8) | 19 | 9 |
| 8b | 2.05, m (overlap) | |||
| 9 | 79.5, CH | 4.64, dd (6.6, 2.4) | 2, 7, 10 | 8, 10 |
| 10 | 51.9, CH | 2.32, dt (8.7, 8.0, 2.7) | 1, 9 | |
| 11 | 75.8, C | |||
| 12 | 214.3, C | |||
| 13a | 38.9, CH2 | 2.41, m | 12, 14 | 14 |
| 13b | 2.34, m | |||
| 14 | 38.0, CH | 2.32, m (overlap) | 1, 12, | 1, 13, 15 |
| 15 | 35.1, CH | 1.88, s | 14, 16, 17 | |
| 16a | 66.9, CH2 | 3.78, dd (13.7, 3.6) | 3, 17 | 15 |
| 16b | 3.30, dd, (13,7, 6.2) | |||
| 17 | 11.4, CH3 | 0.79, d (7.1) | 14, 15, 16 | 15 |
| 18 | 20.5, CH3 | 1.38, s | 2, 3, 4 | |
| 19 | 18.8, CH3 | 1.89, s | 6, 7, 8 | |
| 20 | 22.6, CH3 | 1.43, s | 10, 11, 12 | |
| 21 | 173.5, C | |||
| 22 | 34.6, CH2 | 2.34, m | ||
| 23 | 25.1, CH2 | 1.62, m | 21 | |
| 24 | 28.9 *, CH2 | 1.29, m | ||
| 25 | 28.9 *, CH2 | 1.29, m | ||
| 26 | 31.7, CH2 | 1.25, m | ||
| 27 | 22.6, CH2 | 1.27, m | 28 | |
| 28 | 14.0, CH3 | 0.88, t (7.0) | 26, 27 | 27 |
| Position | Asbestinin 27 (2) | Asbestinin 28 (3) | ||
|---|---|---|---|---|
| δC, mult. a | δH, mult. (J in Hz) b | δC, mult. | δH, mult. (J in Hz) | |
| 1 | 38.3, CH | 2.27, m | 38.3, CH | 2.27, dt (10.8, 8.3) |
| 2 | 94.6, CH | 4.04. d (8.5) | 94.6, CH | 4.01. m |
| 3 | 79.1, C | 79.1, C | ||
| 4 | 72.4, CH | 5.33, m | 72.7, CH | 5.31, m |
| 5a | 29.1, CH2 | 2.71, dt (14.9, 8.1) | 29.1, CH2 | 2.71, ddd (14.7, 9.3, 6.2) |
| 5b | 2.08, m | 2.08, m | ||
| 6 | 125.1, CH | 5.32, m | 124.9, CH | 5.31, m |
| 7 | 129.4, C | 129.4, C | ||
| 8a | 44.3, CH2 | 2.47, dd (13.4, 6.5) | 44.3, CH2 | 2.47, dd (13.3, 6.5) |
| 8b | 2.02, m | 2.02, m | ||
| 9 | 80.9, CH | 4.06, dd (8.9, 2.5) | 80.9, CH | 4.04, m |
| 10 | 48.4, CH | 2.09, m | 48.3, CH | 2.08, m |
| 11 | 73.8, CH | 5.30, m | 73.8, CH | 5.31, m |
| 12 | 31.0, CH | 1.97, m | 31.0, CH | 1.97, m |
| 13a | 31.3, CH2 | 1.47, dt (13.6, 9.8) | 31.3, CH2 | 1.47, dt (13.6, 9.8) |
| 13b | 0.98, m | 1.00, dt (13.6, 2.7) | ||
| 14 | 37.7, CH | 1.87, m | 37.7, CH | 1.90, m |
| 15 | 36.7, CH | 1.69, m | 36.7, CH | 1.73, m |
| 16a | 67.6, CH2 | 3.84, dd (13.3, 2.4) | 67.7, CH2 | 3.85, dd (13.5, 2.4) |
| 16b | 3.36, dd, (13.3, 5.1) | 3.37, dd, (13.5, 5.0) | ||
| 17 | 11.4, CH3 | 0.76, d (7.1) | 11.3, CH3 | 0.76, d (7.1) |
| 18 | 19.7, CH3 | 1.35, s | 19.7, CH3 | 1.34, s |
| 19 | 18.8, CH3 | 1.87, s | 18.8, CH3 | 1.86, s |
| 20 | 18.1, CH3 | 0.91, d (7.2) | 18.1, CH3 | 0.91, d (7.1) |
| 21 | 171.4, C | 171.4, C | ||
| 22 | 21.3, CH3 | 2.11, s | 21.4, CH3 | 2.11, s |
| 23 | 173.3, C | 170.7, C | ||
| 24 | 36.6, CH2 | 2.30, t (7.3) | 21.3, CH3 | 2.07, s |
| 25 | 18.6, CH2 | 1.66, m | ||
| 26 | 13.6 CH3 | 0.96, t (7.4) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, M.; Santamaría, R.; Gómez-Reyes, J.F.; Guzmán, H.M.; Ávila-Román, J.; Motilva, V.; Talero, E. New Eunicellin-Type Diterpenes from the Panamanian Octocoral Briareum asbestinum. Mar. Drugs 2020, 18, 84. https://doi.org/10.3390/md18020084
Gutiérrez M, Santamaría R, Gómez-Reyes JF, Guzmán HM, Ávila-Román J, Motilva V, Talero E. New Eunicellin-Type Diterpenes from the Panamanian Octocoral Briareum asbestinum. Marine Drugs. 2020; 18(2):84. https://doi.org/10.3390/md18020084
Chicago/Turabian StyleGutiérrez, Marcelino, Ricardo Santamaría, José Félix Gómez-Reyes, Héctor M. Guzmán, Javier Ávila-Román, Virginia Motilva, and Elena Talero. 2020. "New Eunicellin-Type Diterpenes from the Panamanian Octocoral Briareum asbestinum" Marine Drugs 18, no. 2: 84. https://doi.org/10.3390/md18020084
APA StyleGutiérrez, M., Santamaría, R., Gómez-Reyes, J. F., Guzmán, H. M., Ávila-Román, J., Motilva, V., & Talero, E. (2020). New Eunicellin-Type Diterpenes from the Panamanian Octocoral Briareum asbestinum. Marine Drugs, 18(2), 84. https://doi.org/10.3390/md18020084







