Mycosporine-Like Amino Acids (MAAs) in Zooplankton
Abstract
1. Introduction
2. Results and Discussion
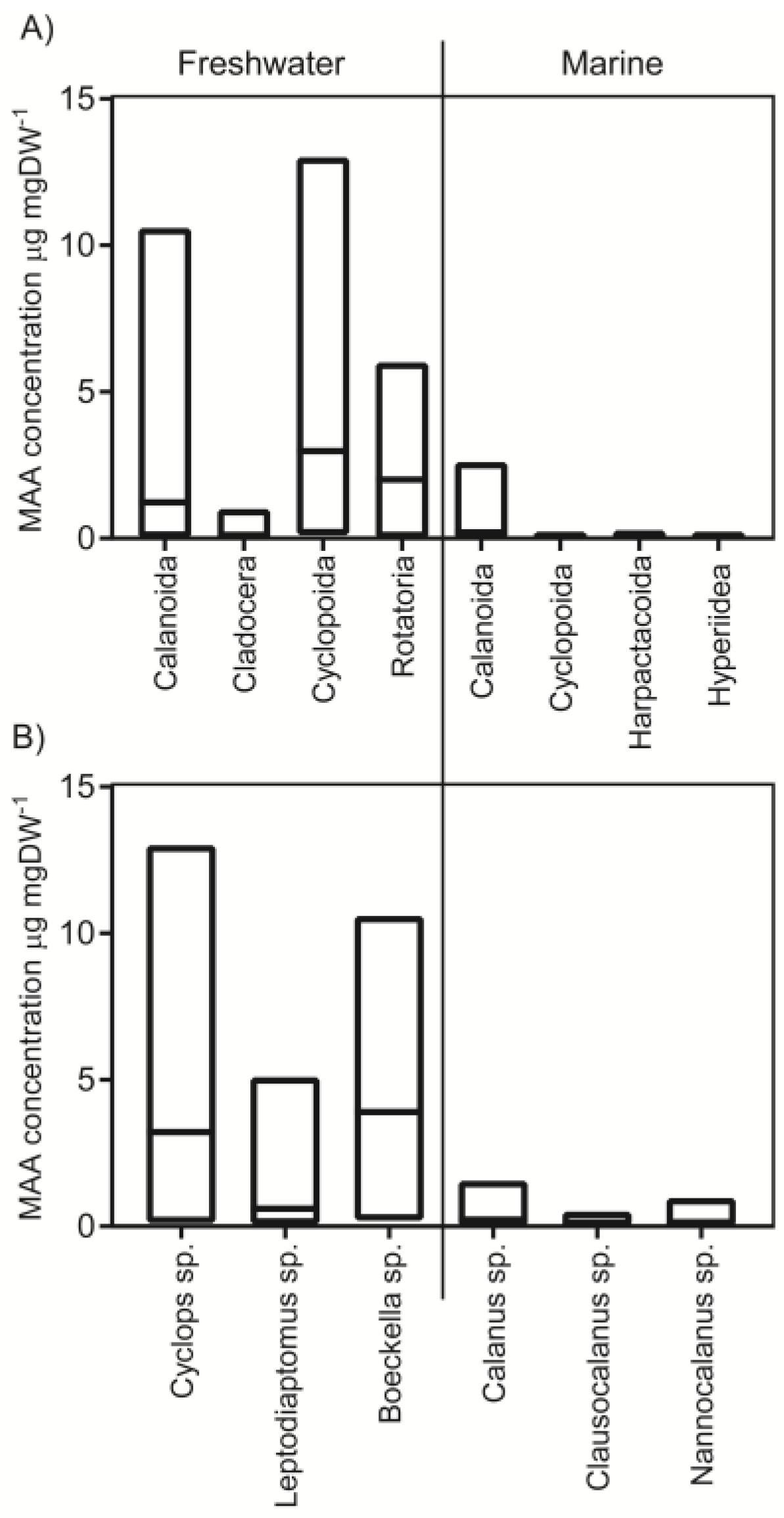
2.1. Concentrations in Different Taxa
2.2. Mechanism for Gaining the MAAs (Feeding, Symbiosis, Production)
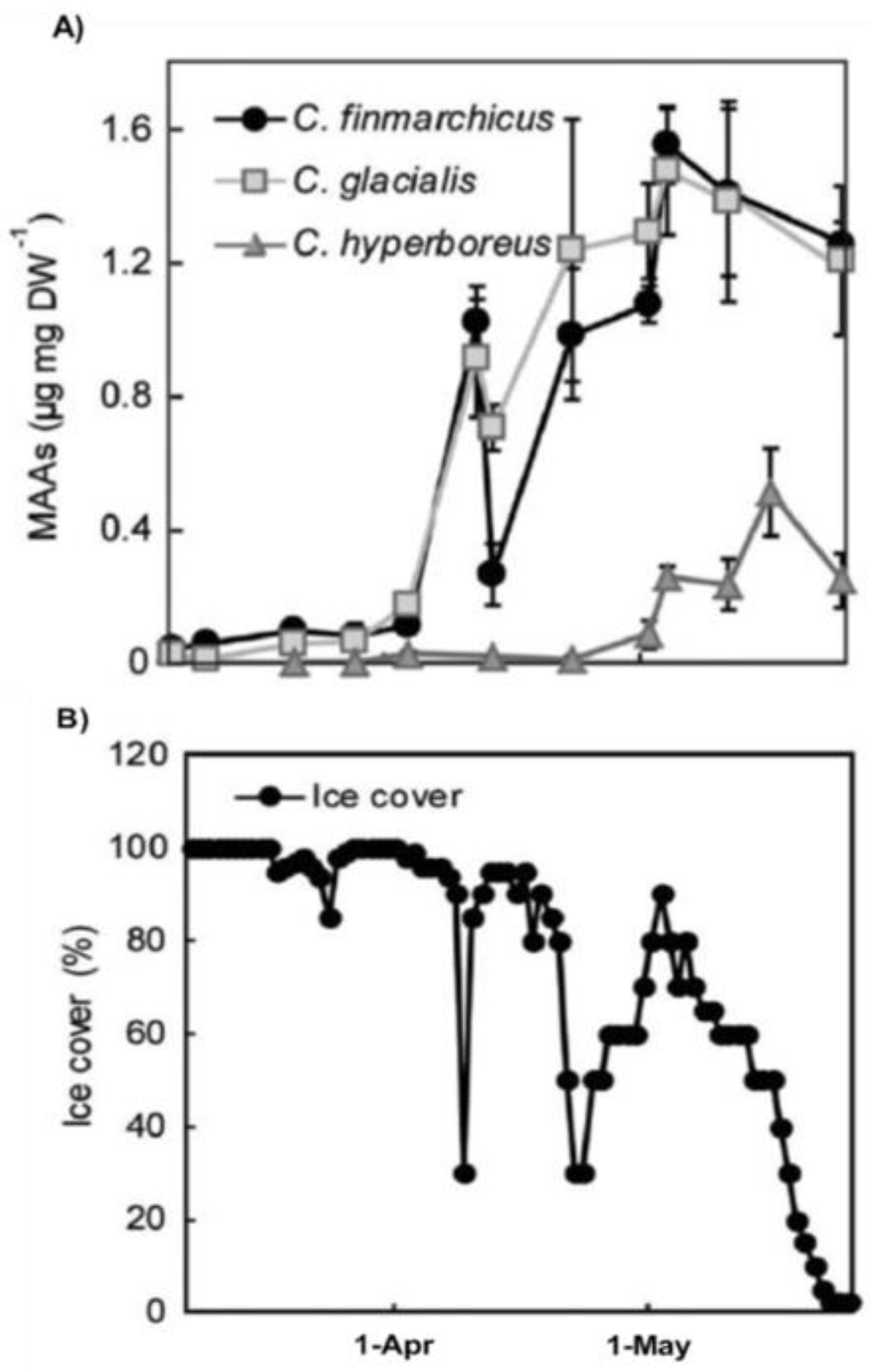
2.3. Seasonal Variation
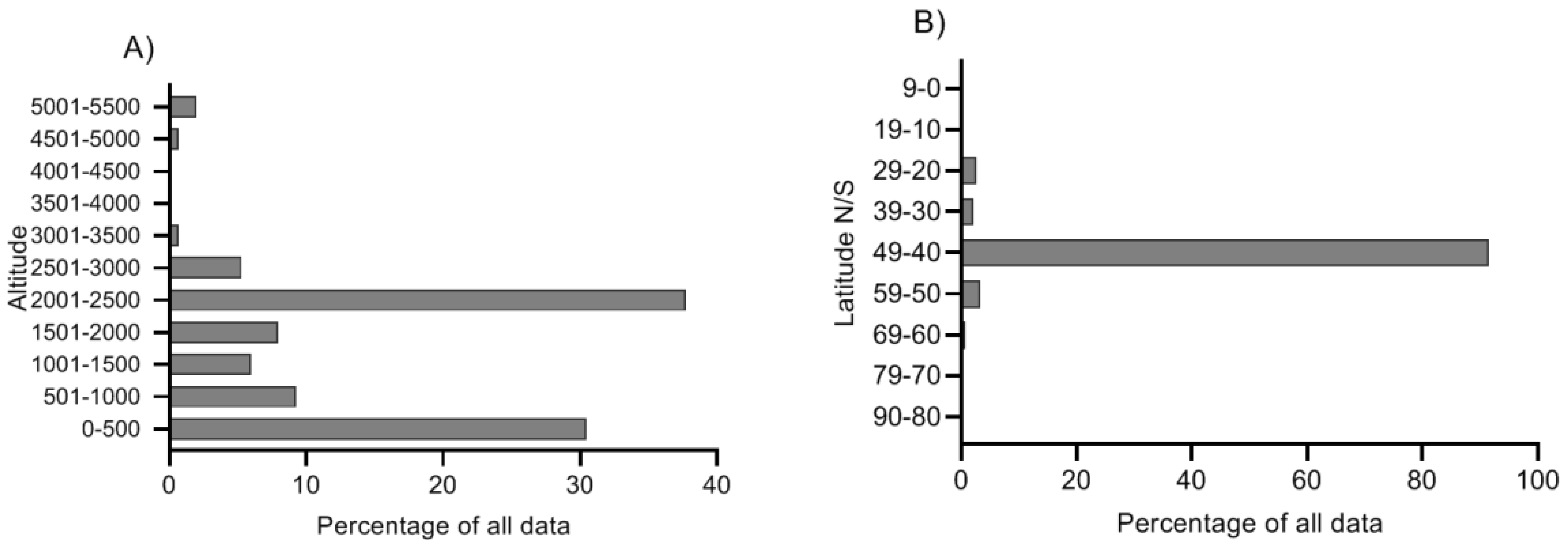
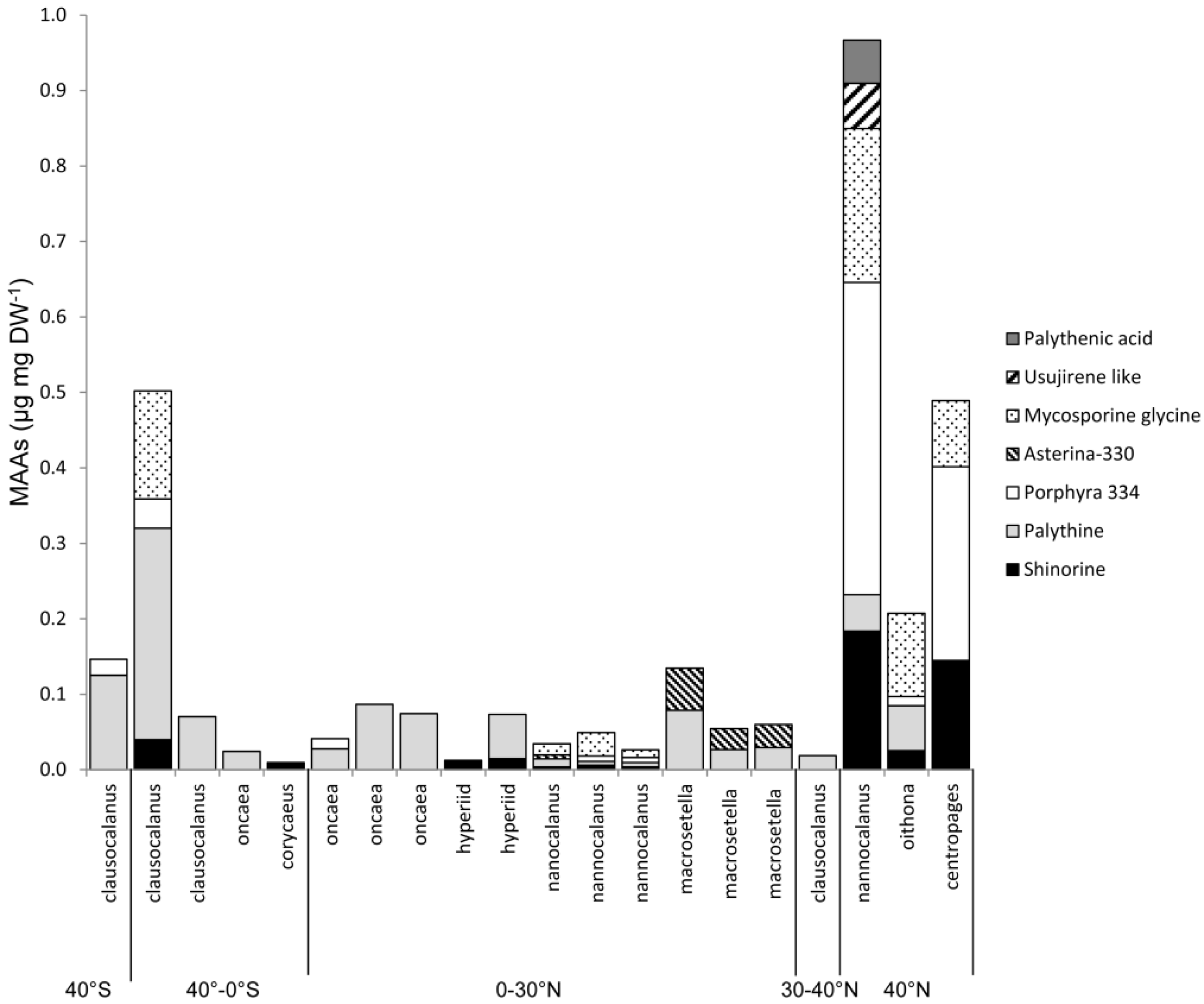
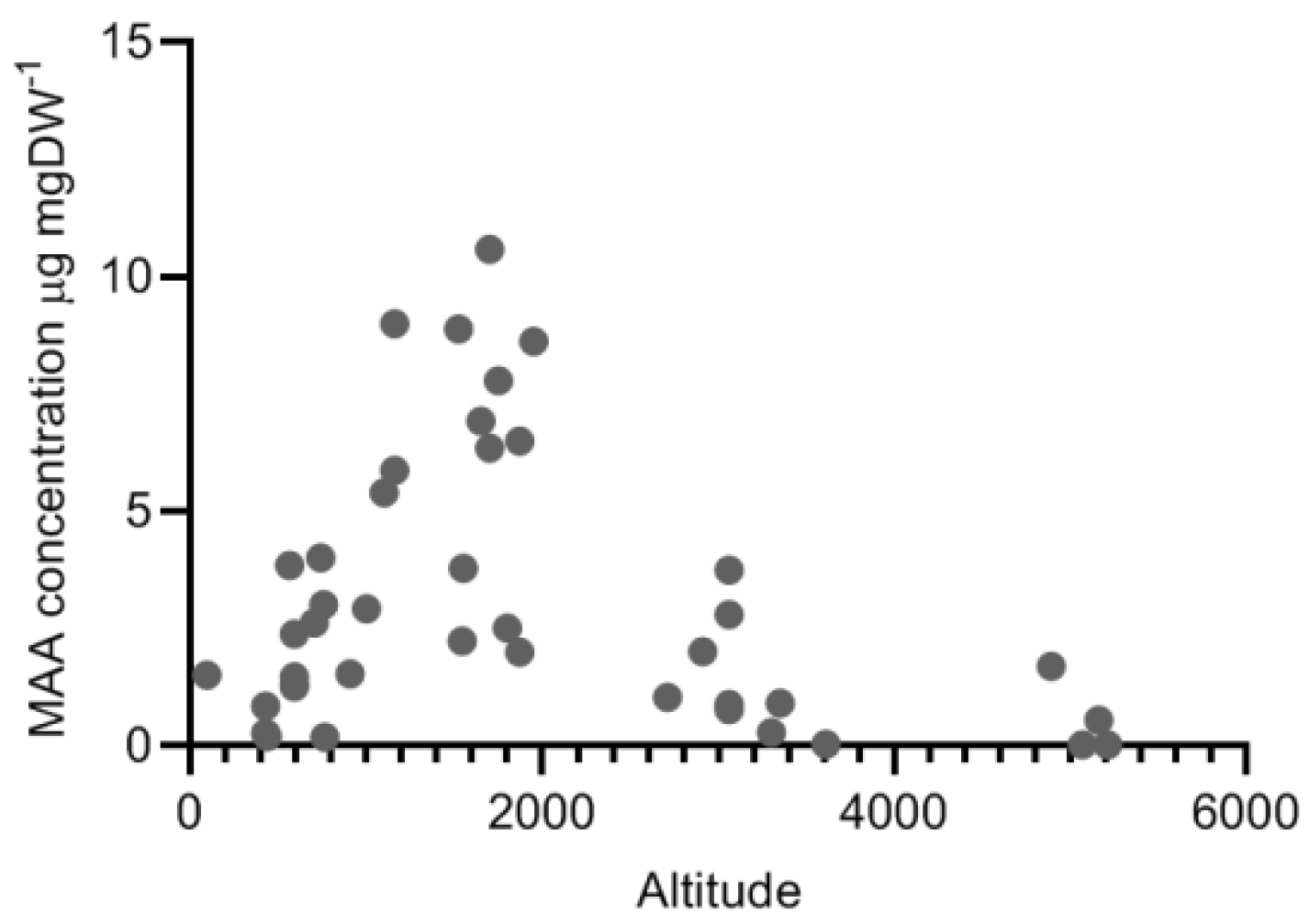
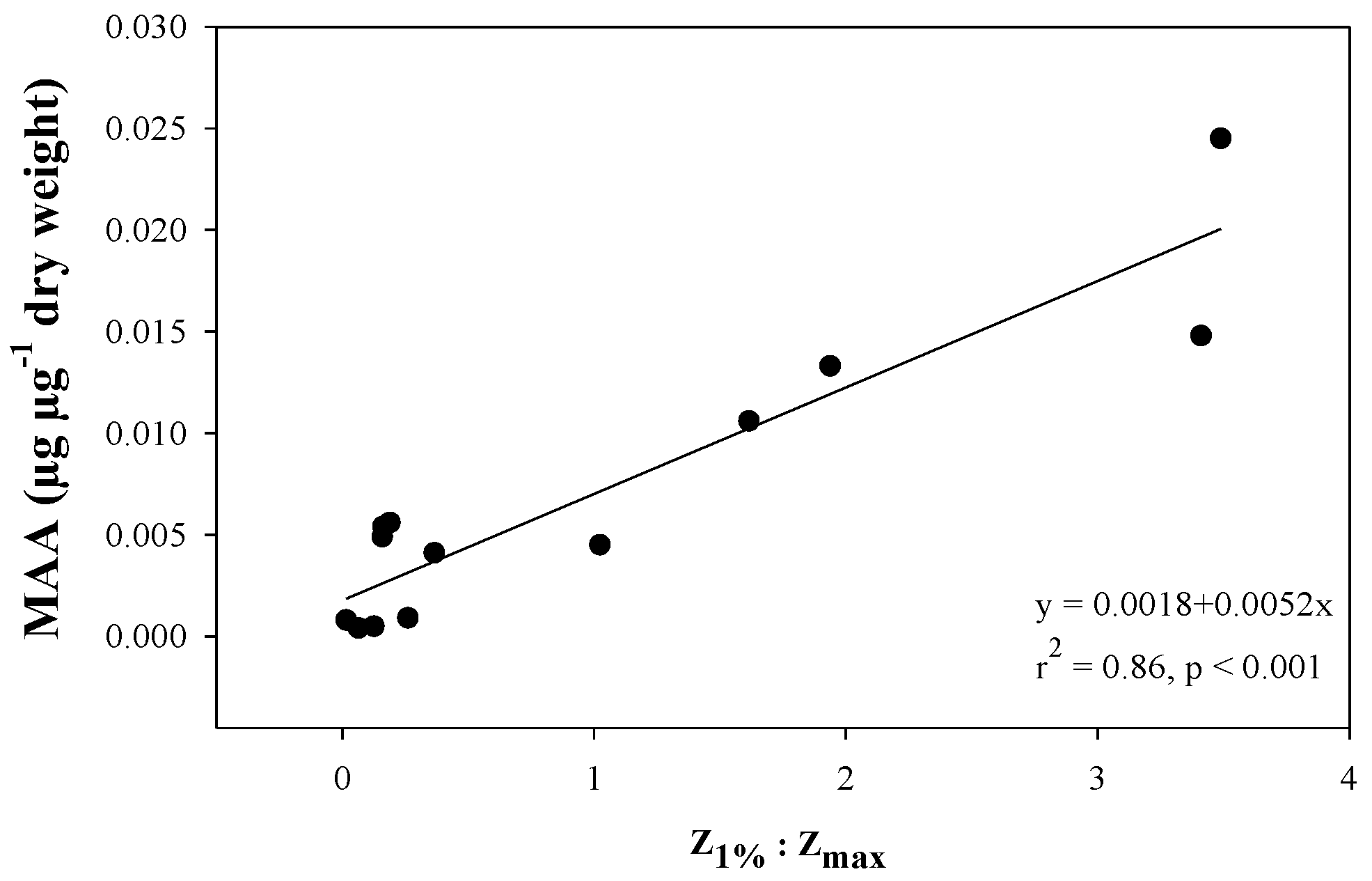
2.4. Variation along Different Environmental Gradients
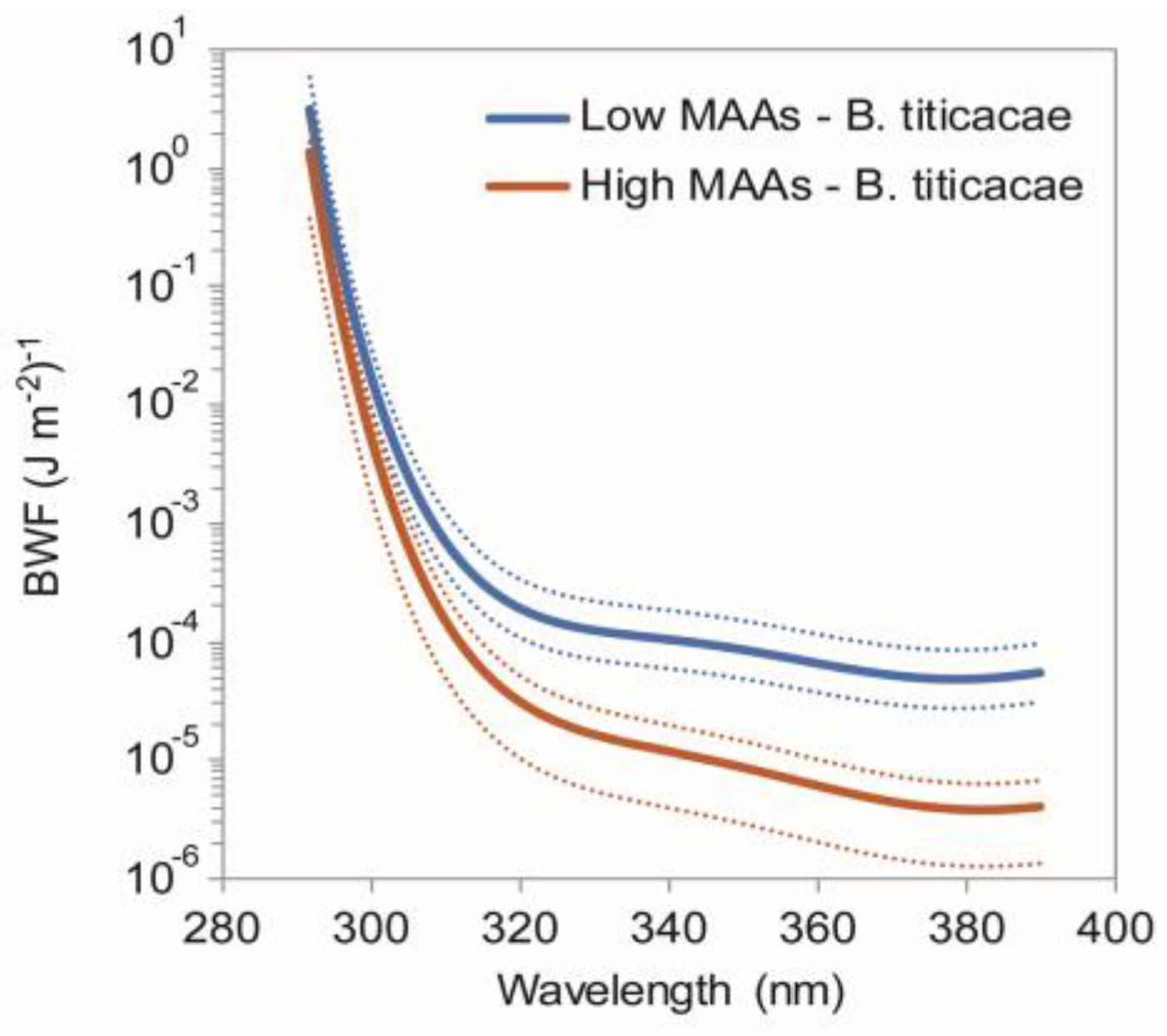
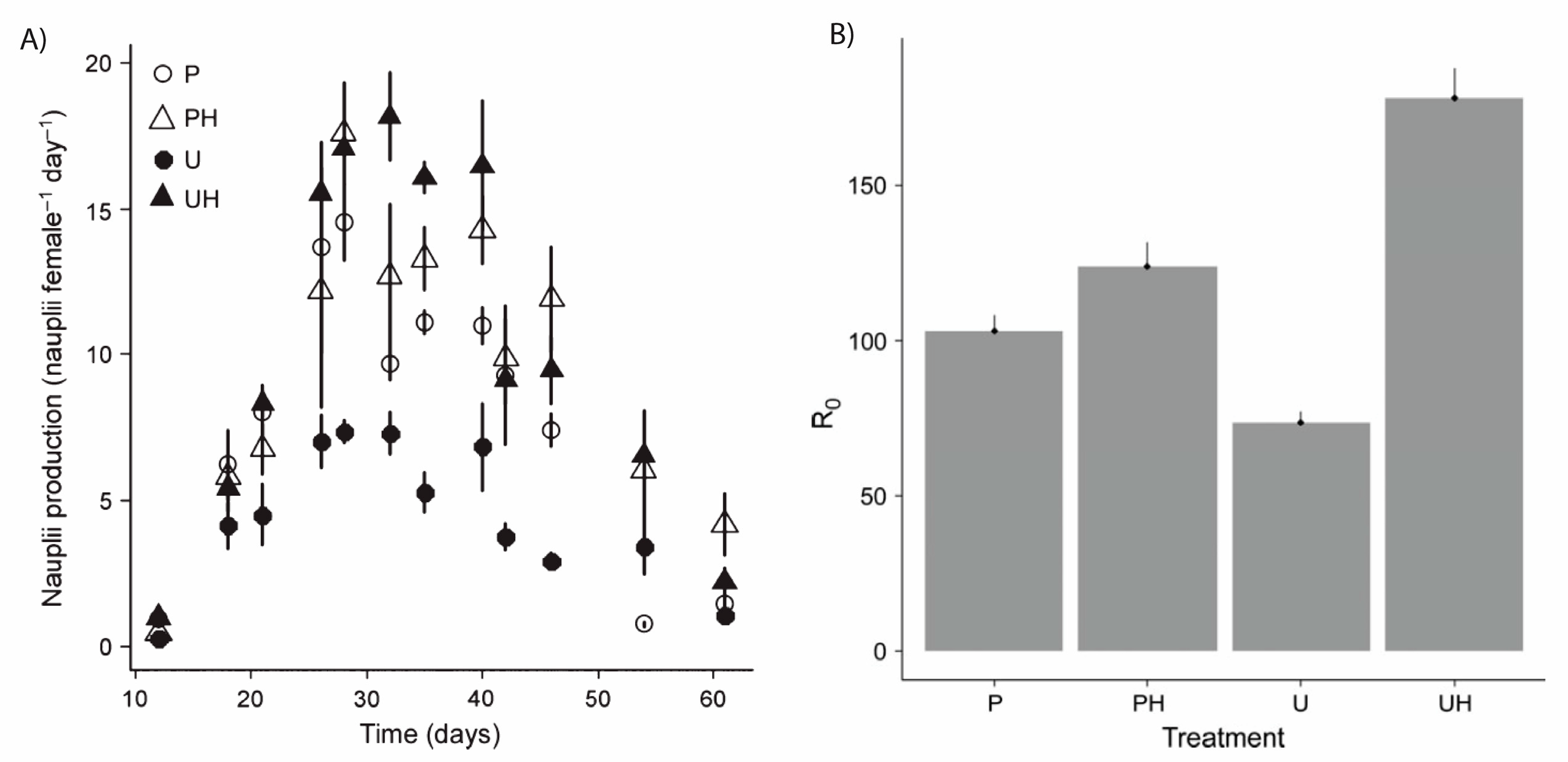
2.5. Function of MAAs in Zooplankton
3. Gaps of Knowledge
4. Materials and Methods
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, P.W.; Williamson, C.E.; Lucas, R.M.; Robinson, S.A.; Madronich, S.; Paul, N.D.; Bornman, J.F.; Bais, A.F.; Sulzberger, B.; Wilson, S.R.; et al. Ozone depletion, ultraviolet radiation, climate change and prospects for a sustainable future. Nat. Sustain. 2019, 2, 569–579. [Google Scholar] [CrossRef]
- Williamson, C.E.; Neale, P.J.; Hylander, S.; Rose, K.C.; Figueroa, F.L.; Robinson, S.A.; Häder, D.-P.; Wängberg, S.-Å.; Worrest, R.C. The interactive effects of stratospheric ozone depletion, UV radiation, and climate change on aquatic ecosystems. Photochem. Photobiol. Sci. 2019, 18, 717–746. [Google Scholar] [CrossRef] [PubMed]
- Hessen, D.O. Solar Radiation and the Evolution of Life; Bjertness, E., Ed.; The Norwegian Academy of Science and Letters: Oslo, Norway, 2008. [Google Scholar]
- Hansson, L.A.; Hylander, S. Effects of ultraviolet radiation on pigmentation, photoenzymatic repair, behavior, and community ecology of zooplankton. Photochem. Photobiol. Sci. 2009, 8, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Rautio, M.; Tartarotti, B. UV Radiation and Freshwater Zooplankton: Damage, Protection and Recovery. Freshw. Rev. 2010, 3, 105–131. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related Gadusols: Biosynthesis, acumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Sommaruga, R. The role of solar UV radiation in the ecology of alpine lakes. J. Photochem. Photobiol. B 2001, 62, 35–42. [Google Scholar] [CrossRef]
- Karentz, D.; Mceuen, F.S.; Land, M.C.; Dunlap, W.C. Survey of Mycosporine-Like Amino-Acid Compounds in Antarctic Marine Organisms—Potential Protection from Ultraviolet Exposure. Mar. Biol. 1991, 108, 157–166. [Google Scholar] [CrossRef]
- Sommaruga, R.; Garcia-Pichel, F. UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake. Arch. Hydrobiol. 1999, 144, 255–269. [Google Scholar] [CrossRef]
- Villafane, V.E.; Andrade, M.; Lairana, V.; Zaratti, F.; Helbling, E.W. Inhibition of phytoplankton photosynthesis by solar ultraviolet radiation: Studies in Lake Titicaca, Bolivia. Freshw. Biol. 1999, 42, 215–224. [Google Scholar] [CrossRef]
- Tartarotti, B.; Sommaruga, R. The effect of different methanol concentrations and temperatures on the extraction of mycosporine-like amino acids (MAAs) in algae and zooplankton. Arch. Hydrobiol. 2002, 154, 691–703. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Defaye, D. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia 2007, 595, 195–207. [Google Scholar] [CrossRef]
- Fileman, E.S.; White, D.A.; Harmer, R.A.; Aytan, Ü.; Tarran, G.A.; Smyth, T.; Atkinson, A. Stress of life at the ocean’s surface: Latitudinal patterns of UV sunscreens in plankton across the Atlantic. Prog. Oceanogr. 2017, 158, 171–184. [Google Scholar] [CrossRef]
- Garcia, P.E.; Dieguez, M.C.; Ferraro, M.A.; Zagarese, H.E.; Perez, A.P. Mycosporine-like Amino Acids in Freshwater Copepods: Potential Sources and Some Factors That Affect Their Bioaccumulation. Photochem. Photobiol. 2010, 86, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.E.; Ferraro, M.A.; Perez, A.P.; Zagarese, H.E.; Dieguez, M.C. Contrasting patterns of MAAs accumulation in two populations of the copepod Boeckella gracilipes. Photochem. Photobiol. Sci. 2014, 13, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.A.; Hylander, S.; Sommaruga, R. Escape from UV threats in zooplankton: A cocktail of behavior and protective pigmentation. Ecology 2007, 88, 1932–1939. [Google Scholar] [CrossRef]
- Hylander, S.; Jephson, T. UV protective compounds transferred from a marine dinoflagellate to its copepod predator. J. Exp. Mar. Biol. Ecol. 2010, 389, 38–44. [Google Scholar] [CrossRef]
- Hylander, S.; Kiørboe, T.; Snoeijs, P.; Sommaruga, R.; Nielsen, T.G. Concentrations of sunscreens and antioxidant pigments in ArcticCalanusspp. in relation to ice cover, ultraviolet radiation, and the phytoplankton spring bloom. Limnol. Oceanogr. 2015, 60, 2197–2206. [Google Scholar] [CrossRef]
- Hylander, S.; Larsson, N.; Hansson, L.A. Zooplankton vertical migration and plasticity of pigmentation arising from simultaneous UV and predation threats. Limnol. Oceanogr. 2009, 54, 483–491. [Google Scholar] [CrossRef]
- Moeller, R.E.; Gilroy, S.; Williamson, C.E.; Grad, G.; Sommaruga, R. Dietary acquisition of photoprotective compounds (mycosporine-like amino acids, carotenoids) and acclimation to ultraviolet radiation in a freshwater copepod. Limnol. Oceanogr. 2005, 50, 427–439. [Google Scholar] [CrossRef]
- Obertegger, U.; Flaim, G.; Sommaruga, R. Multifactorial nature of rotifer water layer preferences in an oligotrophic lake. J. Plankton Res. 2008, 30, 633–643. [Google Scholar] [CrossRef]
- Perez, A.P.; Ferraro, M.A.; Zagarese, H.E. The relative contributions of diet and associated microbiota to the accumulation of UV-absorbing mycosporine-like amino acids in the freshwater copepod Boeckella antiqua. Freshw. Biol. 2012, 57, 993–1004. [Google Scholar] [CrossRef]
- Persaud, A.D.; Moeller, R.E.; Williamson, C.E.; Burns, C.W. Photoprotective compounds in weakly and strongly pigmented copepods and co-occurring cladocerans. Freshw. Biol. 2007, 52, 2121–2133. [Google Scholar] [CrossRef]
- Rautio, M.; Bonilla, S.; Vincent, W.F. UV photoprotectants in arctic zooplankton. Aquat. Biol. 2009, 7, 93–105. [Google Scholar] [CrossRef]
- Sommaruga, R. Preferential accumulation of carotenoids rather than of mycosporine-like amino acids in copepods from high altitude Himalayan lakes. Hydrobiologia 2010, 648, 143–156. [Google Scholar] [CrossRef]
- Tartarotti, B.; Alfreider, A.; Egg, M.; Saul, N.; Schneider, T.; Sommaruga, R.; Tischler, A.; Vetter, J. Seasonal plasticity in photoprotection modulates UV-induced hsp gene expression in copepods from a clear lake. Limnol. Oceanogr. 2018, 63, 1579–1592. [Google Scholar] [CrossRef]
- Tartarotti, B.; Baffico, G.; Temporetti, P.; Zagarese, H.E. Mycosporine-like amino acids in planktonic organisms living under different UV exposure conditions in Patagonian lakes. J. Plankton Res. 2004, 26, 753–762. [Google Scholar] [CrossRef]
- Tartarotti, B.; Saul, N.; Chakrabarti, S.; Trattner, F.; Steinberg, C.E.W.; Sommaruga, R. UV-induced DNA damage in Cyclops abyssorum tatricus populations from clear and turbid alpine lakes. J. Plankton Res. 2014, 36, 557–566. [Google Scholar] [CrossRef]
- Tartarotti, B.; Sommaruga, R. Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnol. Oceanogr. 2006, 51, 1530–1541. [Google Scholar] [CrossRef]
- Tartarotti, B.; Trattner, F.; Remias, D.; Saul, N.; Steinberg, C.E.W.; Sommaruga, R. Distribution and UV protection strategies of zooplankton in clear and glacier-fed alpine lakes. Sci. Rep. 2017, 7, 4487. [Google Scholar] [CrossRef]
- Tartarotti, B.; Laurion, I.; Sommaruga, R. Large variability in the concentration of mycosporine-like amino acids among zooplankton from lakes located across an altitude gradient. Limnol. Oceanogr. 2001, 46, 1546–1552. [Google Scholar] [CrossRef]
- Newman, S.J.; Dunlap, W.C.; Nicol, S.; Ritz, D. Antarctic krill (Euphausia superba) acquire a UV-absorbing mycosporine-like amino acid from dietary algae. J. Exp. Mar. Biol. Ecol. 2000, 255, 93–110. [Google Scholar] [CrossRef]
- Riemer, U.; Lamare, M.D.; Peake, B.M. Temporal concentrations of sunscreen compounds (Mycosporine-like Amino Acids) in phytoplankton and in the New Zealand krill, Nyctiphanes australis G.O. Sars. J. Plankton Res. 2007, 29, 1077–1086. [Google Scholar] [CrossRef]
- Osborn, A.R.; Almabruk, K.H.; Holzwarth, G.; Asamizu, S.; LaDu, J.; Kean, K.M.; Karplus, P.A.; Tanguay, R.L.; Bakalinsky, A.T.; Mahmud, T. De novo synthesis of a sunscreen compound in vertebrates. Elife 2015, 4, e05919. [Google Scholar] [CrossRef]
- Rosic, N.N.; Dove, S. Mycosporine-like amino acids from coral dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478–8486. [Google Scholar] [CrossRef]
- Starcevic, A.; Akthar, S.; Dunlap, W.; Shick, J.; Hranueli, D.; Cullum, J.; Long, P. Enzymes of the shikimic acid pathway encoded in the genome of a basal metazoan, Nematostella vectensis, have microbial origins. Proc. Natl. Acad. Sci. USA 2008, 105, 2533–2537. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; MacTavish, H.S.; Dunlap, W.C.; Vesk, M.; Groenewoud, K. Occurrence of UVA- and UVB-absorbing compounds in 152 species (206 strains) of marine microalgae. Mar. Ecol. Prog. Ser. 1999, 189, 35–51. [Google Scholar] [CrossRef]
- Laurion, I.; Lami, A.; Sommaruga, R. Distribution of mycosporine-like amino acids and photoprotective carotenoids among freshwater phytoplankton assemblages. Aquat. Microb. Ecol. 2002, 26, 283–294. [Google Scholar] [CrossRef]
- Hylander, S.; Boeing, W.J.; Graneli, W.; Karlsson, J.; von Einem, J.; Gutseit, K.; Hansson, L.A. Complementary UV protective compounds in zooplankton. Limnol. Oceanogr. 2009, 54, 1883–1893. [Google Scholar] [CrossRef]
- Hylander, S.; Grenvald, J.C.; Kiørboe, T.; Pfrender, M. Fitness costs and benefits of ultraviolet radiation exposure in marine pelagic copepods. Funct. Ecol. 2014, 28, 149–158. [Google Scholar] [CrossRef]
- Hylander, S.; Hansson, L.-A. Vertical distribution and pigmentation of Antarctic zooplankton determined by a blend of UV radiation, predation and food availability. Aquat. Ecol. 2013, 47, 467–480. [Google Scholar] [CrossRef]
- Hylander, S.; Jephson, T.; Lebret, K.; Von Einem, J.; Fagerberg, T.; Balseiro, E.; Modenutti, B.; Souza, M.S.; Laspoumaderes, C.; Jonsson, M.; et al. Climate-induced input of turbid glacial meltwater affects vertical distribution and community composition of phyto- and zooplankton. J. Plankton Res. 2011, 33, 1239–1248. [Google Scholar] [CrossRef]
- Garcia, P.E.; Perez, A.P.; Dieguez, M.D.C.; Ferraro, M.A.; Zagarese, H.E. Dual control of the levels of photoprotective compounds by ultraviolet radiation and temperature in the freshwater copepod Boeckella antiqua. J. Plankton Res. 2008, 30, 817–827. [Google Scholar] [CrossRef]
- Perez, P.; Libkind, D.; Dieguez, M.D.; Summerer, M.; Sonntag, B.; Sommaruga, R.; van Broock, M.; Zagarese, H.E. Mycosporines from freshwater yeasts: A trophic cul-de-sac? Photochem. Photobiol. Sci. 2006, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Shoguchi, E.; Beedessee, G.; Tada, I.; Hisata, K.; Kawashima, T.; Takeuchi, T.; Arakaki, N.; Fujie, M.; Koyanagi, R.; Roy, M.; et al. Two divergent Symbiodinium genomes reveal conservation of a gene cluster for sunscreen biosynthesis and recently lost genes. BMC Genom. 2018, 19, 458. [Google Scholar] [CrossRef] [PubMed]
- Orfeo, M.; Ventura, M.; Tartarotti, B.; Sommaruga, R. Body distribution and source of mycosporine-like amino acids in the cyclopoid copepod Cyclops abyssorum tatricus. J. Plankton Res. 2011, 33, 1430–1444. [Google Scholar] [CrossRef]
- Schneider, T.; Herzig, A.; Koinig, K.A.; Sommaruga, R. Copepods in turbid shallow soda lakes accumulate unexpected high levels of carotenoids. PLoS ONE 2012, 7, e43063. [Google Scholar] [CrossRef][Green Version]
- Johnsen, S.; Widder, E.A. Ultraviolet absorption in transparent zooplankton and its implications for depth distribution and visual predation. Mar. Biol. 2001, 138, 717–730. [Google Scholar] [CrossRef]
- Leech, D.M.; Boeing, W.J.; Cooke, S.L.; Williamson, C.E.; Torres, L. UV-enhanced fish predation and the differential migration of zooplankton to UV radiation and fish. Limnol. Oceanogr. 2009, 54, 1152–1161. [Google Scholar] [CrossRef]
- Helbling, E.W.; Zaratti, F.; Sala, L.O.; Palenque, E.R.; Menchi, C.F.; Villafane, V.E. Mycosporine-like amino acids protect the copepod Boeckella titicacae (Harding) against high levels of solar UVR. J. Plankton Res. 2002, 24, 225–234. [Google Scholar] [CrossRef][Green Version]
- Rocco, V.E.; Oppezzo, O.; Pizarro, R.; Sommaruga, R.; Ferraro, M.; Zagarese, H.E. Ultraviolet damage and counteracting mechanisms in the freshwater copepod Boeckella poppei from the Antarctic Peninsula. Limnol. Oceanogr. 2002, 47, 829–836. [Google Scholar] [CrossRef]
- Goncalves, R.J.; Villafane, V.E.; Helblingh, E.W. Photorepair activity and protective compounds in two freshwater zooplankton species (Daphnia menucoensis and Metacyclops mendocinus) from Patagonia, Argentina. Photochem. Photobiol. Sci. 2002, 1, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Setlow, R.B. The Wavelengths in Sunlight Effective in Producing Skin Cancer: A Theoretical Analysis. Proc. Natl. Acad. Sci. USA 1974, 71, 3363–3366. [Google Scholar] [CrossRef]
- Laspoumaderes, C.; Bastidas Navarro, M.; Souza, M.S.; Modenutti, B.; Balseiro, E. Effect of ultraviolet radiation on clearance rate of planktonic copepods with different photoprotective strategies. Int. Rev. Hydrobiol. 2019, 104, 34–44. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Hylander, S.; Souza, M.S.; Balseiro, E.; Modenutti, B.; Hansson, L.-A. Fish-mediated trait compensation in zooplankton. Funct. Ecol. 2012, 26, 608–615. [Google Scholar] [CrossRef]
- Souza, M.S.; Balseiro, E.; Laspoumaderes, C.; Modenutti, B. Effect of Ultraviolet Radiation on Acetylcholinesterase Activity in Freshwater Copepods. Photochem. Photobiol. 2010, 86, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.S.; Modenutti, B.E.; Balseiro, E.G. Antioxidant Defences in Planktonic Crustaceans Exposed to Different Underwater Light Irradiances in Andean Lakes. Water Air Soil Pollut. 2007, 183, 49–57. [Google Scholar] [CrossRef]
- Rhode, S.C.; Pawlowski, M.; Tollrian, R. The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature 2001, 412, 69–72. [Google Scholar] [CrossRef]
- Ekvall, M.T.; Hylander, S.; Walles, T.; Yang, X.; Hansson, L.-A. Diel vertical migration, size distribution and photoprotection in zooplankton as response to UV-A radiation. Limnol. Oceanogr. 2015, 60, 2048–2058. [Google Scholar] [CrossRef]
- Ma, Z.; Li, W.; Gao, K. Horizontal migration of Acartia pacifica Steuer (copepoda) in response to UV-radiation. J. Photochem. Photobiol. B 2010, 101, 233–237. [Google Scholar] [PubMed]
- Overholt, E.P.; Rose, K.C.; Williamson, C.E.; Fischer, J.M.; Cabrol, N.A. Behavioral responses of freshwater calanoid copepods to the presence of ultraviolet radiation: Avoidance and attraction. J. Plankton Res. 2016, 38, 16–26. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Bianco, G.; Ekvall, M.; Heuschele, J.; Hylander, S.; Yang, X. Instantaneous threat escape and differentiated refuge demand among zooplankton taxa. Ecology 2016, 97, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, W.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Suh, H.-J.; Lee, H.-W.; Jung, J. Mycosporine Glycine Protects Biological Systems Against Photodynamic Damage by Quenching Singlet Oxygen with a High Efficiency. Photochem. Photobiol. 2003, 78, 109–113. [Google Scholar] [CrossRef]
- Yakovleva, I.; Bhagooli, R.; Takemura, A.; Hidaka, M. Differential susceptibility to oxidative stress of two scleractinian corals: Antioxidant functioning of mycosporine-glycine. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 721–730. [Google Scholar] [CrossRef]


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hylander, S. Mycosporine-Like Amino Acids (MAAs) in Zooplankton. Mar. Drugs 2020, 18, 72. https://doi.org/10.3390/md18020072
Hylander S. Mycosporine-Like Amino Acids (MAAs) in Zooplankton. Marine Drugs. 2020; 18(2):72. https://doi.org/10.3390/md18020072
Chicago/Turabian StyleHylander, Samuel. 2020. "Mycosporine-Like Amino Acids (MAAs) in Zooplankton" Marine Drugs 18, no. 2: 72. https://doi.org/10.3390/md18020072
APA StyleHylander, S. (2020). Mycosporine-Like Amino Acids (MAAs) in Zooplankton. Marine Drugs, 18(2), 72. https://doi.org/10.3390/md18020072




