Sterol Composition of Sponges, Cnidarians, Arthropods, Mollusks, and Echinoderms from the Deep Northwest Atlantic: A Comparison with Shallow Coastal Gulf of Mexico
Abstract
1. Introduction
2. Results
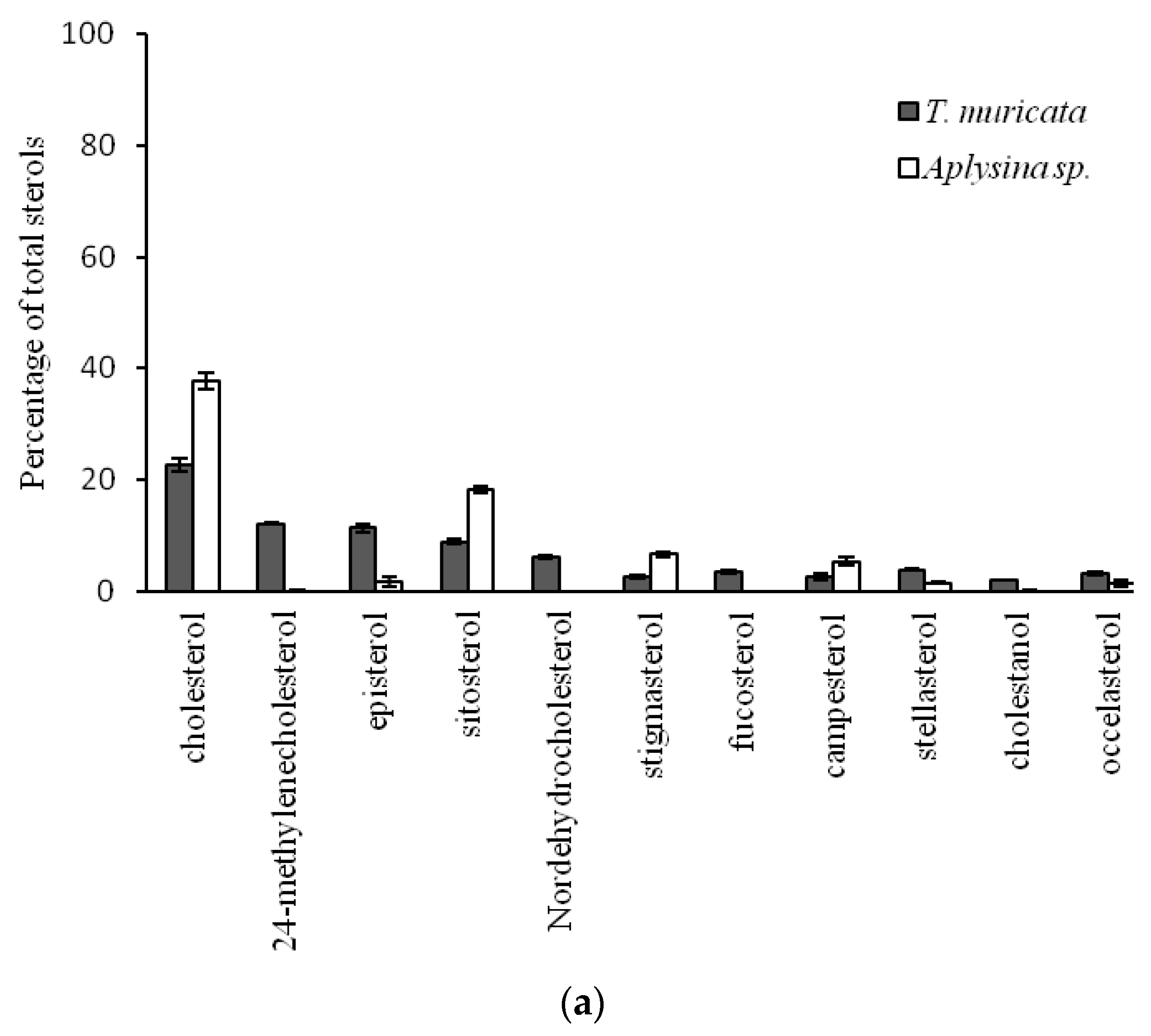
2.1. Sterol Composition of Five Benthic Phyla, Porifera, Cnidaria, Mollusca, Arthropoda, and Echinodermata, from the Cold and Deep Northeast Atlantic
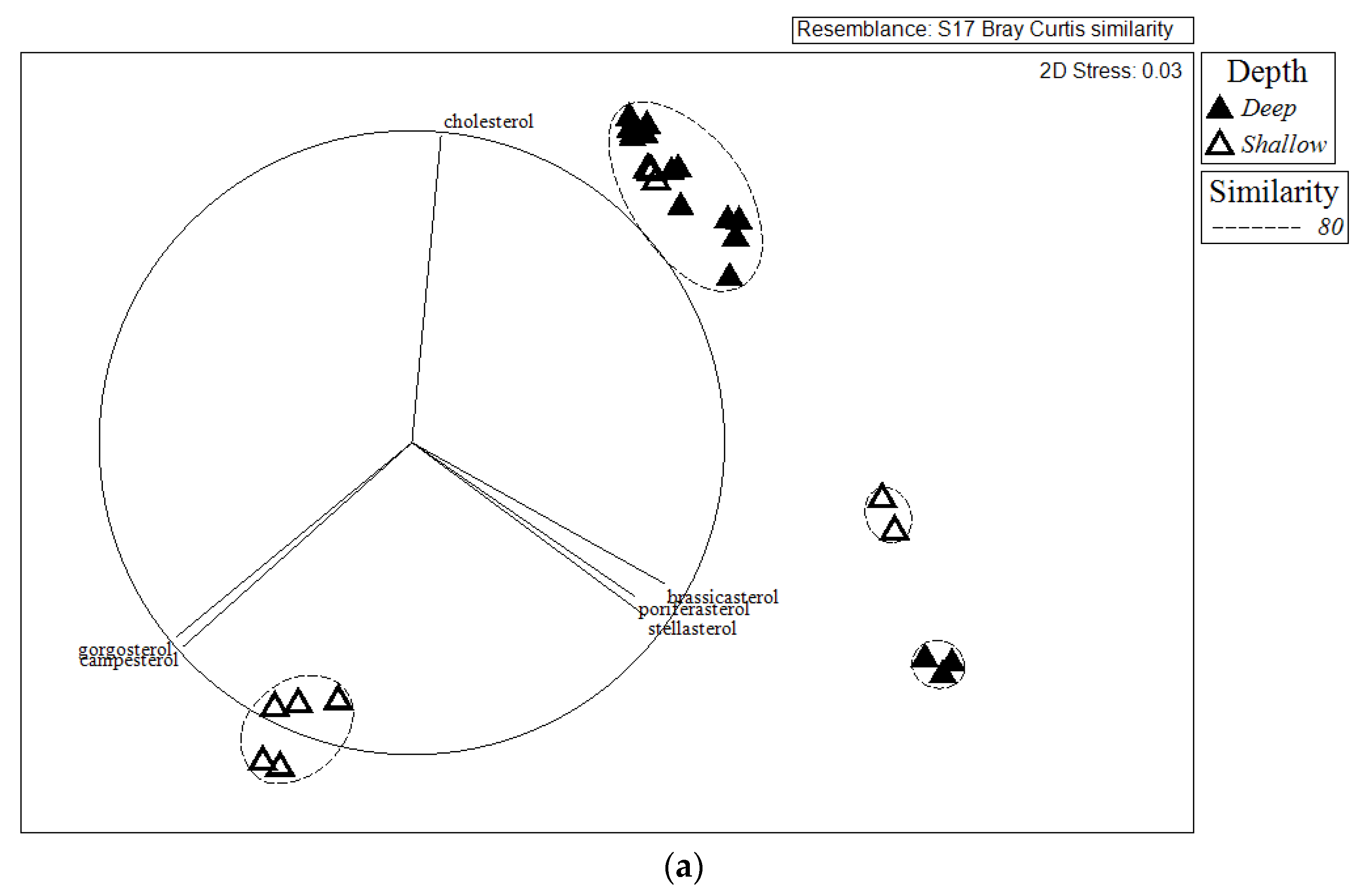
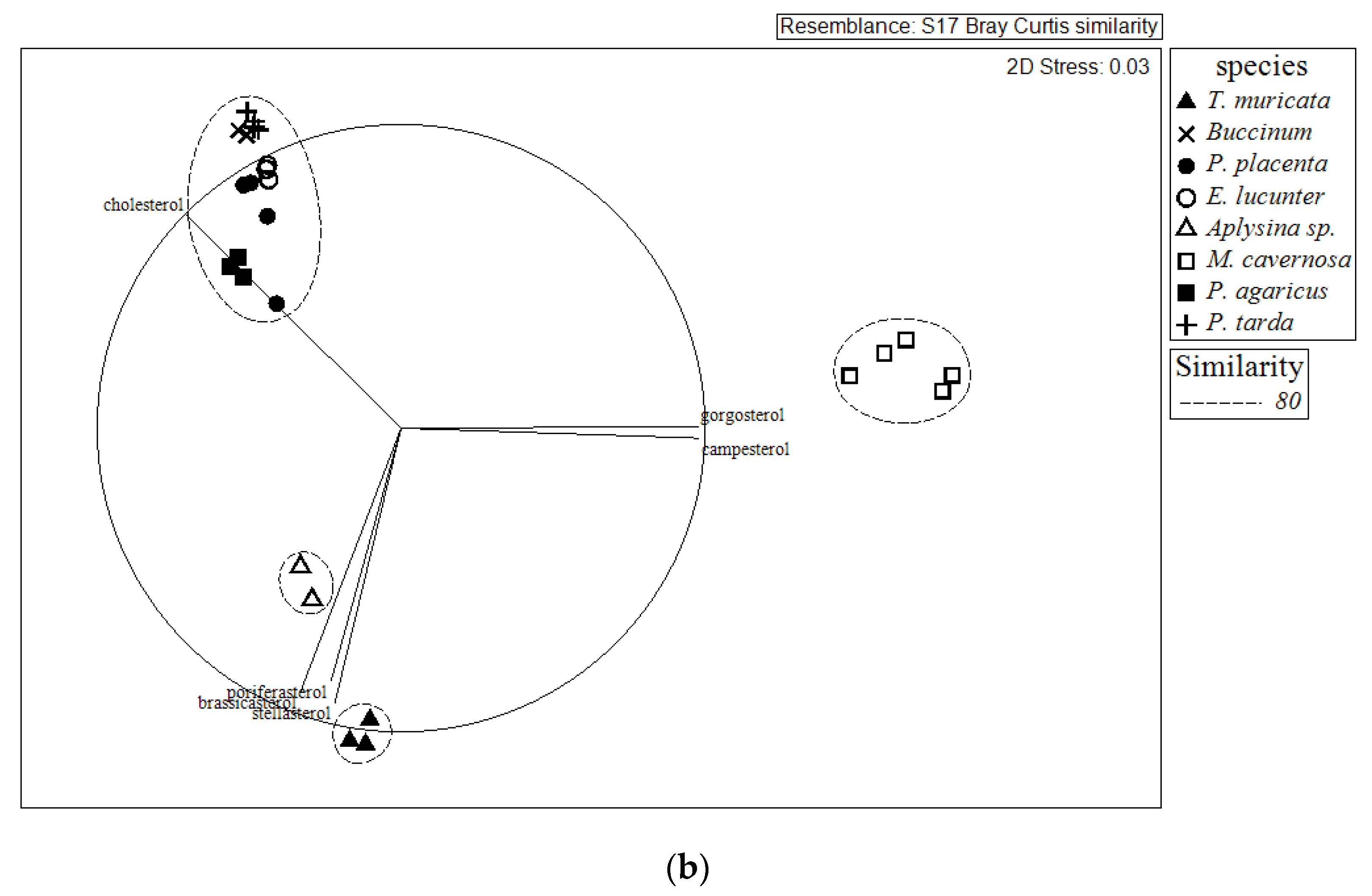
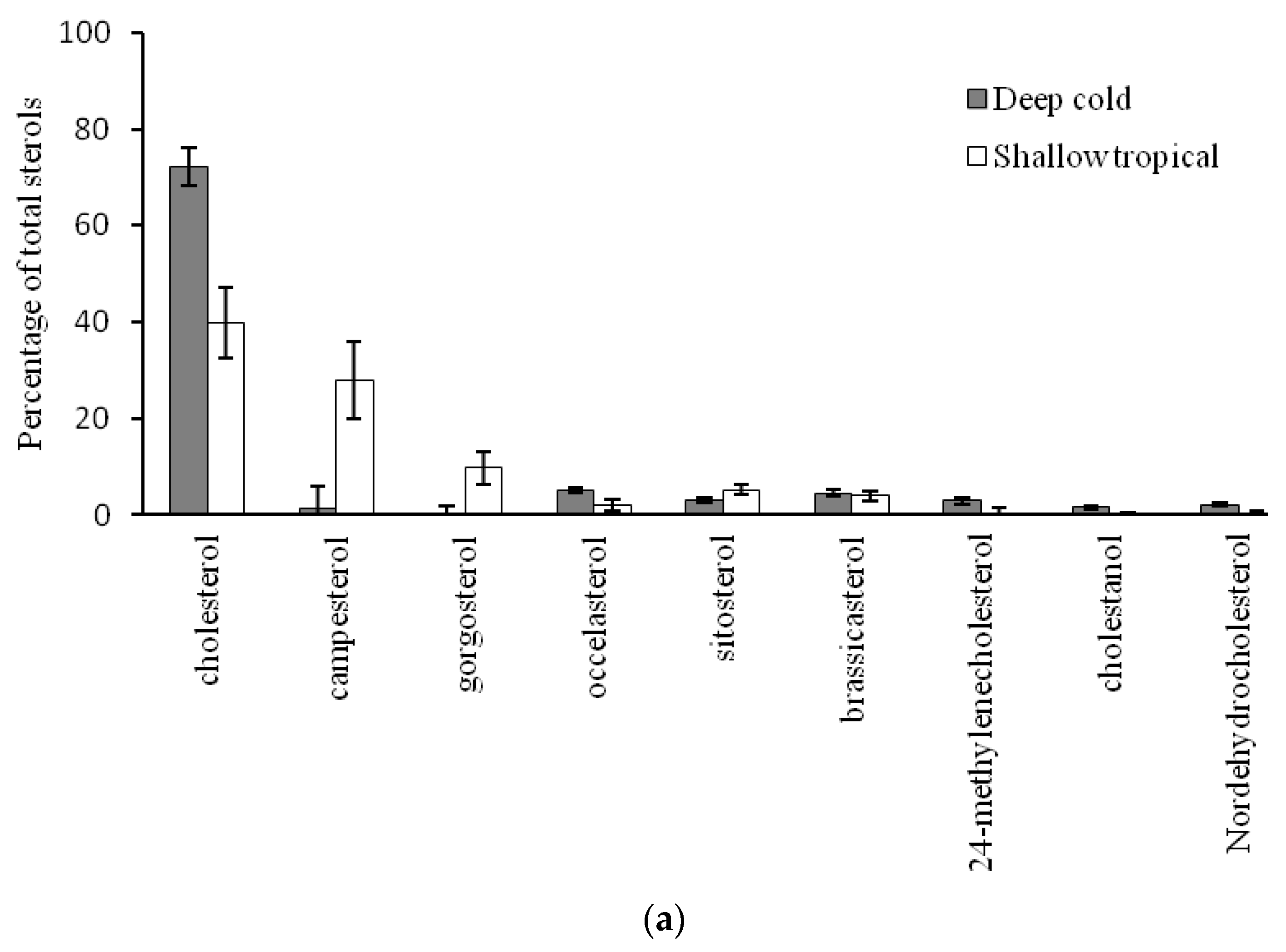
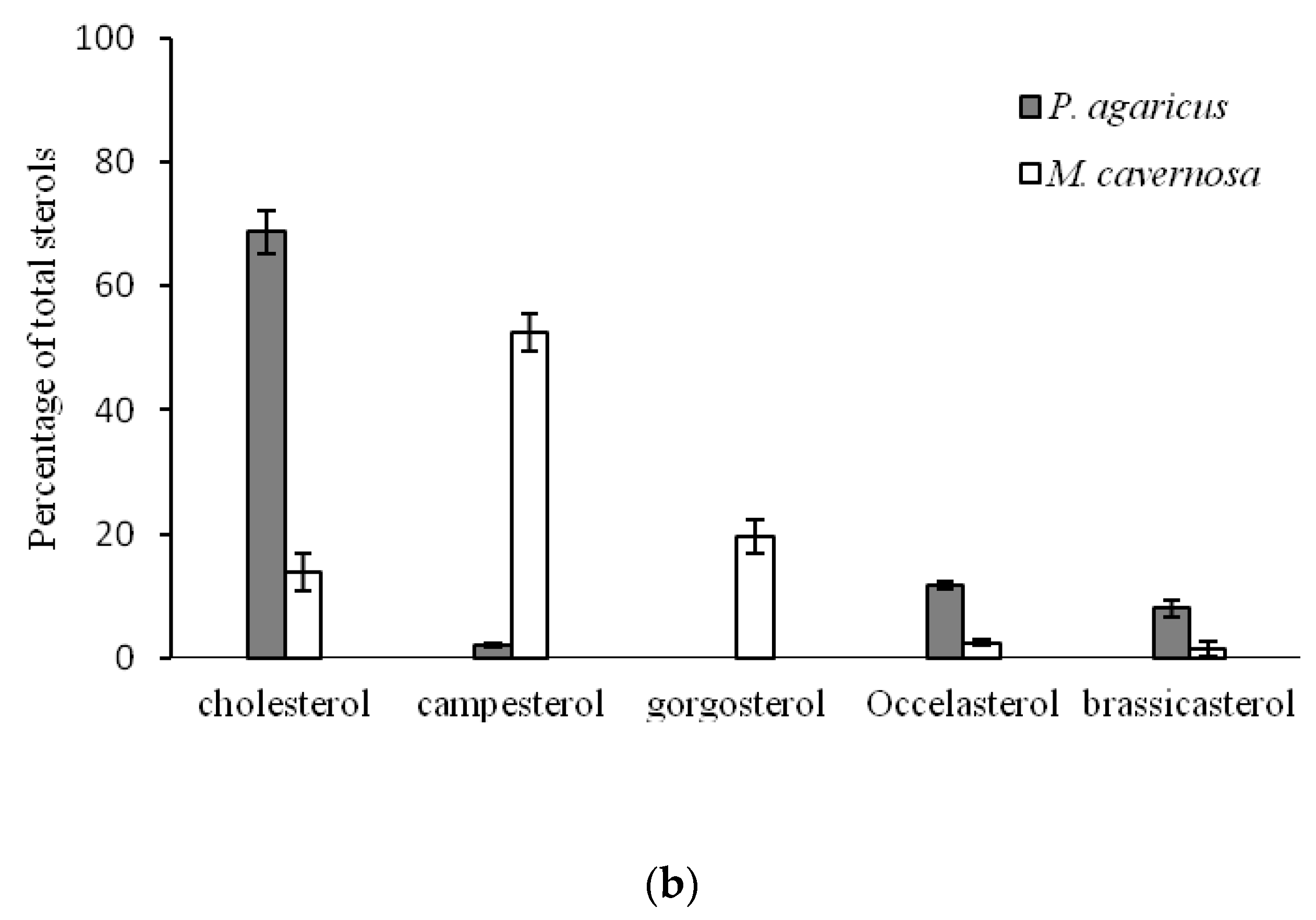
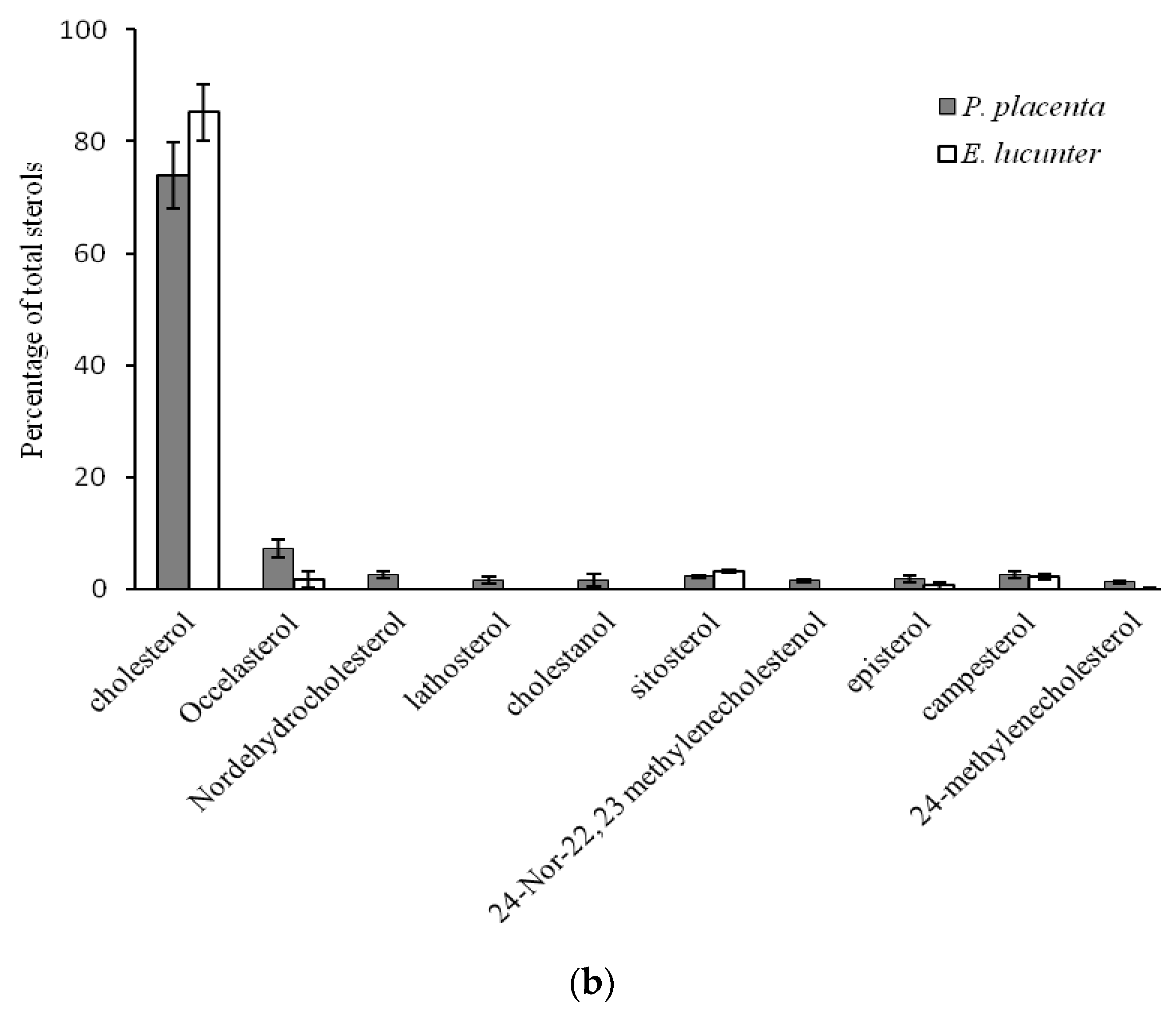
2.2. Comparison of Sterol Composition of Benthic Invertebrates from the Deep, Cold Sea Northwest Atlantic and Shallow, Tropical Coastal Gulf of Mexico
3. Discussion
3.1. Food Supply to Deep Cold Ocean Invertebrates Using Sterols, C:N Ratios, and Stable Isotopes of Carbon and Nitrogen
3.2. Comparison of Sterol Composition of Benthic Invertebrates from the Cold, Deep Northwest Atlantic and Tropical, Shallow Coastal Gulf of Mexico
4. Materials and Methods
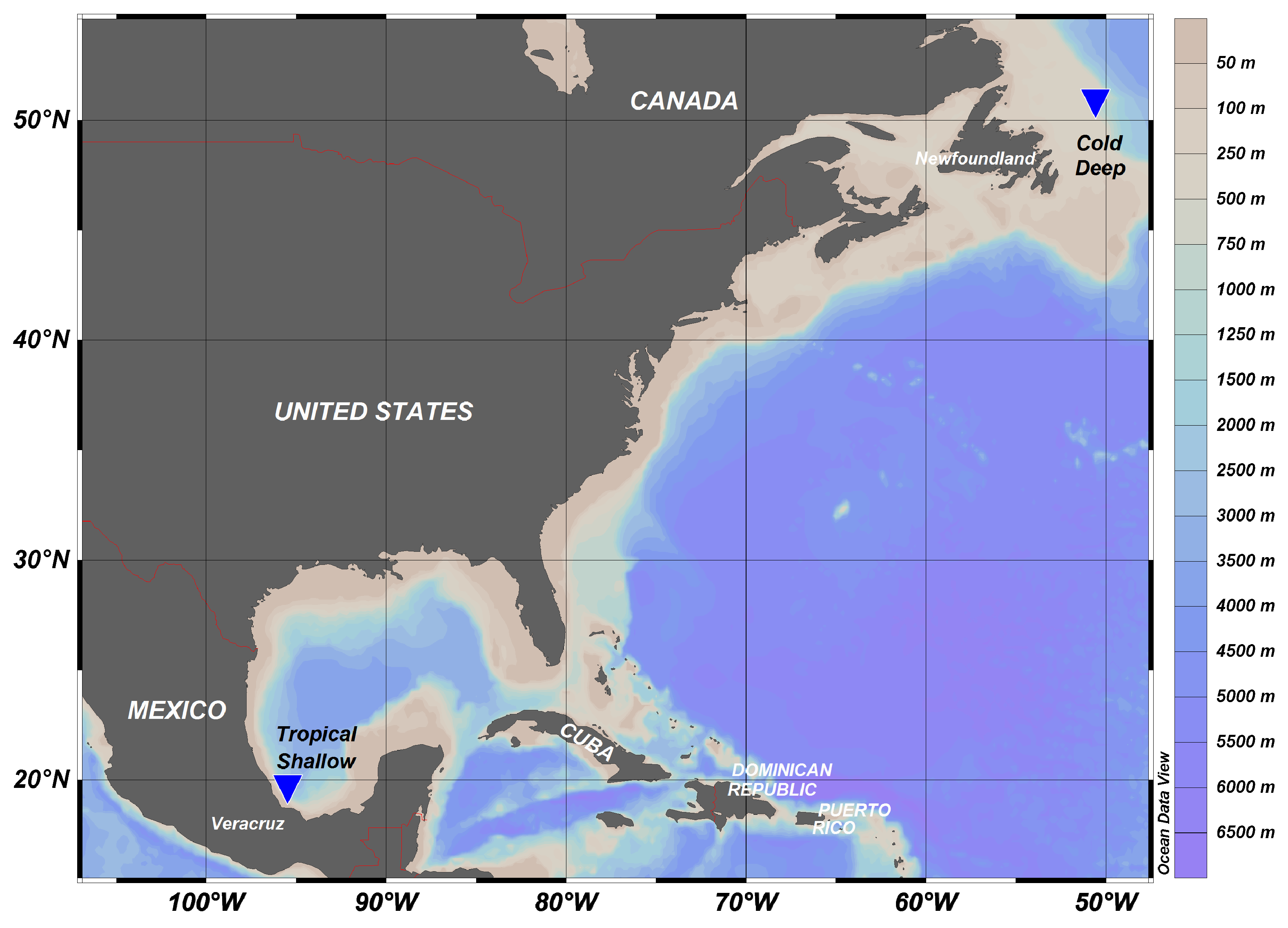
4.1. Study Area
4.2. Sampling
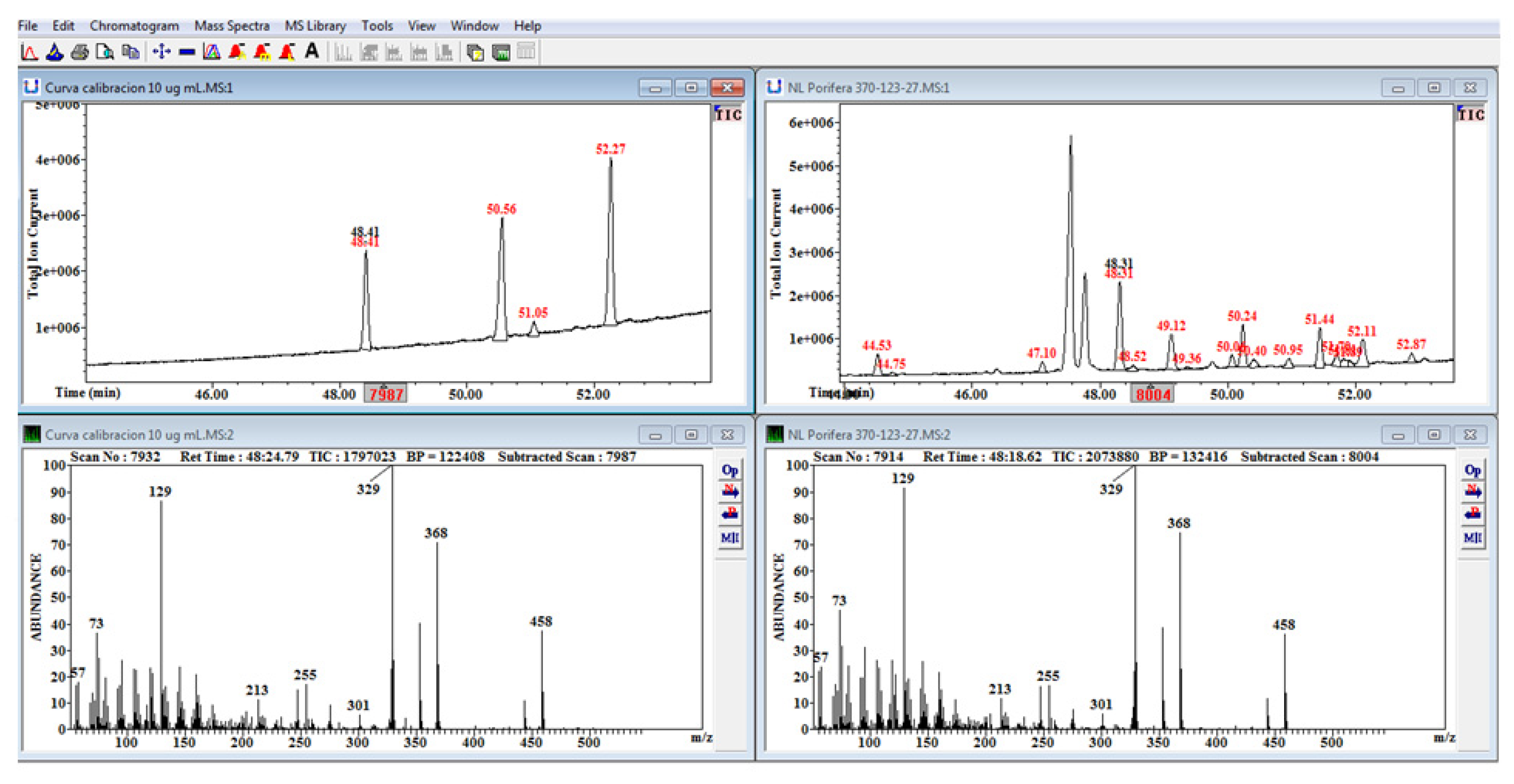
4.3. Laboratory Analysis
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thistle, D. The deep-sea floor: An overview. In Ecosystems of the Deep Oceans; Tyler, P.A., Ed.; Elsevier Science Publisher B.V.: Amsterdam, The Netherlands, 2003; pp. 5–38. [Google Scholar]
- Campanyà-Llovet, N.; Snelgrove, P.V.; Parrish, C.C. Rethinking the importance of food quality in marine benthic food webs. Prog. Oceanogr. 2017, 156, 240–251. [Google Scholar] [CrossRef]
- Drazen, J.C.; Phleger, C.F.; Guest, M.A.; Nichols, P.D. Lipid, sterols and fatty acid composition of abyssal holothurians and ophiuroids from the North-East Pacific Ocean: Food web implications. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 79–87. [Google Scholar] [CrossRef]
- Govenar, B. Energy transfer through food webs at hydrothermal vents: Linking the lithosphere to the biosphere. Oceanography 2012, 25, 246–255. [Google Scholar] [CrossRef]
- Boschker, H.T.S.; Middelburg, J.J. Stable isotopes and biomarkers in microbial ecology. FEMS Microbiol. Ecol. 2002, 40, 85–95. [Google Scholar] [CrossRef]
- Nichols, D. Biological Markers in the Marine Environment. Ph.D. Thesis, University of Melbourne, Melbourne, Australia, 1983. [Google Scholar]
- Sargent, J.R.; Parkes, R.J.; Mueller-Harvey, I.; Henderson, R.J. Lipid biomarkers in marine ecology. In Microbes in the Sea; Sleigh, M.A., Ed.; Ellis Horwood: Chichester, UK, 1987; pp. 119–138. [Google Scholar]
- Parrish, C.C. Dissolved and particulate marine lipid classes: A review. Mar. Chem. 1988, 23, 17–40. [Google Scholar] [CrossRef]
- Budge, S.M.; Iverson, S.J.; Koopman, H.N. Studying trophic ecology in marine ecosystems using fatty acids: A primer on analysis and interpretation. Mar. Mamm. Sci. 2006, 22, 759–801. [Google Scholar] [CrossRef]
- Litzow, M.A.; Bailey, K.M.; Prahl, F.G.; Heintz, R. Climate regime shifts and reorganization of fish communities: The essential fatty acid limitation hypothesis. Mar. Ecol. Prog. Ser. 2006, 315, 1–11. [Google Scholar] [CrossRef]
- Laureillard, J.; Saliot, A. Biomarkers in organic matter produced in estuaries: A case study of the Krka Estuary (adriatic Sea) using the sterol marker series. Mar. Chem. 1993, 43, 247–261. [Google Scholar] [CrossRef]
- Li, W.; Dagaut, J.; Saliot, A. The application of sterol biomarkers to the study of the sources of particulate organic matter in the Solo River system and serayu River, Java, Indonesia. Biochemistry 1995, 31, 139–154. [Google Scholar] [CrossRef]
- Crockett, E.L. Cholesterol function in plasma membranes from ectotherms: Membrane-specific roles in adaptation to temperature. Amer. Zool. 1998, 38, 291–304. [Google Scholar] [CrossRef]
- Nes, W.R. Role of sterols in membranes. Lipids 1974, 9, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Culkin, F. Fish. In Marine Biogenic Lipids, Fats and Oils; Ackman, R.G., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1989; pp. 146–178. [Google Scholar]
- Lehninger, A. Biosynthesis of Lipids. In Biochemistry; Worth Publishers Inc.: New York, NY, USA, 1975; pp. 583–614. [Google Scholar]
- Napolitano, G.E.; Ackman, R.G.; Silva-Serra, M.A. Incorporation of dietary sterols by the sea scallop Placopecten magellanicus (Gmelin) fed on microalgae. Mar. Biol. 1993, 117, 647–654. [Google Scholar] [CrossRef]
- Paibulkichakul, C.; Piyatiratitivorakul, S.; Kittakoop, P.; Viyakarn, V.; Fast, A.W.; Menasveta, P. Optimal dietary levels of lecithin and cholesterol for black tiger prawn Penaeus monodon larvae and postlarvae. Aquaculture 1998, 167, 273–281. [Google Scholar] [CrossRef]
- Parrish, C.C. Lipids in marine ecosystems. ISRN Oceanogr. 2013. [Google Scholar] [CrossRef]
- Jones, G.J.; Nichols, P.D.; Shaw, P.M. Analysis of microbial sterols and hopanoids. In Chemical Methods in Prokaryotic Systematic; Goodfellow, M., O’Donnell, A.G., Eds.; John Wiley: Chichester, UK, 1994; pp. 163–195. [Google Scholar]
- Wei, J.H.; Yin, X.; Welander, P.V. Sterol Synthesis in Diverse Bacteria. Front. Microbiol. 2016, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Desmond, E.; Gribaldo, S. Diversity of a key eukaryotic feature genome. Biol. Evol. 2009, 364–381. [Google Scholar] [CrossRef]
- Shmoop University Inc. 2020. Available online: https://www.shmoop.com/study-guides/biology/animal-evolution-diversity/animal-family-tree (accessed on 11 April 2020).
- Zhang, T.; Yuan, D.; Xie, J.; Lei, Y.; Li, J.; Fang, G.; Tian, L.; Liu, J.; Cui, Y.; Zhang, M.; et al. Evolution of the cholesterol biosynthesis pathway in animals. Mol. Biol. Evol. 2019, 36, 2548–2556. [Google Scholar] [CrossRef]
- Ruess, L.; Müller-Navarra, D.C. Essential Biomolecules in Food Webs. Front. Ecol. Evol. 2019, 7, 269. [Google Scholar] [CrossRef]
- Kanazawa, A. Sterols in marine invertebrates. Fish. Sci. 2001, 67, 997–1007. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Oexle, S.; Wacker, A.J. Thresholds for sterol-limited growth of Daphnia magna: A comparative approach using 10 different sterols. J. Chem. Ecol. 2014, 40, 1039–1050. [Google Scholar] [CrossRef]
- Carreón-Palau, L.; Parrish, C.C.; Del Angel-Rodríguez, J.A.; Pérez-España, H. Seasonal Shifts in Fatty Acids and Sterols in Sponges, Corals, and Bivalves, in a southern Gulf of Mexico coral reef under river influence. Coral Reefs, (accepted).
- Del Angel-Rodríguez, J.A.; Carreón-Palau, L.; Parrish, C.C. Identification and quantification of sterols in coral reef food webs. In Sterols: Types, Classification and Structure, 1st ed.; Jimenez, S., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2020; pp. 1–42. [Google Scholar]
- Parzanini, C.; Parrish, C.C.; Hamel, J.-F.; Mercier, A. Functional diversity and nutritional content in a deep-sea faunal assemblage through total lipid, lipid class, and fatty acid analyses. PLoS ONE 2018, 13, e0207395. [Google Scholar] [CrossRef] [PubMed]
- Carreón-Palau, L.; Parrish, C.C.; Del Angel-Rodríguez, J.A.; Pérez-España, H.; Aguiñiga-García, S. Revealing organic carbon sources fueling a coral reef food web in the Gulf of Mexico using stable isotopes and fatty acids. Limnol. Oceanogr. 2013, 58, 593–612. [Google Scholar] [CrossRef]
- Henrichs, S.M. Organic matter in coastal marine sediments. In The Global Coastal Ocean: Multiscale Interdisciplinary Processes, The Sea; Robinson, A.R., Brink, K.H., Eds.; Harvard University Press: Boston, MA, USA, 2005; Volume 13, pp. 129–162. [Google Scholar]
- Reed, J.K. Deepest distribution of Atlantic hermatypic corals discovered in the Bahamas. In Proceedings of the Fifth International Coral Reef Symposium Congress, Tahiti, French Polynesia, 27 May–1 June 1985; Volume 6, pp. 249–254. [Google Scholar]
- Fricke, H.W.; Vareschi, E.; Schlichter, D. Photoecology of the coral Leptoseris fragilis in the Red Sea twilight zone (an experimental study by submersible). Oecologia 1987, 73, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Maragos, J.E.; Jokiel, P. Reef corals of Johnston Atoll: One of the worlds’s most isolated reefs. Coral Reefs 1986, 4, 141–150. [Google Scholar] [CrossRef]
- Gold, D.A.; Grabenstatter, J.; Mendoza, A.; Riesgo, A.; Ruiz-Trillo, I.; Summons, R.E. Sponge sterol biomarker hypothesis. Proc. Nat. Acad. Sci. Mar. 2016, 113, 2684–2689. [Google Scholar] [CrossRef]
- Weete, J.D.; Abril, M.; Blackwell, M. Phylogenetic Distribution of Fungal Sterols. PLoS ONE 2010, 5, e10899. [Google Scholar] [CrossRef]
- Zakharenko, A.; Romanchenko, D.; Thinh, P.D.; Pikula, K.; Hang, C.T.T.; Yuan, W.; Xia, X.; Chaika, V.; Chernyshev, V.; Zakharenko, S.; et al. Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica Semper, 1868. Molecules 2020, 25, 4088. [Google Scholar] [CrossRef]
- Giraldo, C.; Mayzaud, P.; Tavernier, E.; Irisson, J.O.; Penot, F.; Becciu, J.; Chartier, A.; Boutoute, M.; Koubbi, P. Lipid components as a measure of nutritional condition in fish larvae (Pleuragramma antarcticum) in East Antarctica. Mar. Biol. 2013, 160, 877–887. [Google Scholar] [CrossRef]
- Carreón-Palau, L.; Parrish, C.C.; Pérez-España, H.; Aguiñiga-García, S. Elemental ratios and lipid classes in a coral reef food web under river influence. Prog. Oceanogr. 2018, 164, 1–11. [Google Scholar] [CrossRef]
- Maillet, G.; Casault, B.; Pepin, P.; Johnson, C.; Plourde, S.; Starr, M.; Caverhill, C.; Maass, H.; Spry, J.; Fraser, S.; et al. Ocean Productivity Trends in the Northwest Atlantic During 2013. NAFO SCR Doc. 2014, 14, 1–30. [Google Scholar]
- Salas-Monreal, D.; Marin-Hernandez, M.; Salas-Perez, J.J.; Salas-de-Leon, D.A.; Monreal-Gomez, M.A.; Pérez-España, H. Coral reef connectivity within the Western Gulf of Mexico. J. Mar. Syst. 2018, 179, 88–99. [Google Scholar] [CrossRef]
- The Geographical Location and Depth Profile of the Northwest Atlantic and Southwest Gulf of Mexico Sites. Available online: https://www.nafo.int/About-us/Maps (accessed on 30 September 2019).
- Snelgrove, P.; Haedrich, R. Structure of the deep demersal fish fauna off Newfoundland. Mar. Ecol. Prog. Ser. 1985, 27, 99–107. [Google Scholar] [CrossRef]
- Williams, R.G.; Follows, M.J. The Ekman transfer of nutrients and maintenance of new production over the North Atlantic. Deep Sea Res. Part. I Oceanogr. Res. Pap. 1998, 45, 461–489. [Google Scholar] [CrossRef]
- Afanasyev, Y.D.; Nezlin, N.P.; Kostianoy, A.G. Patterns of seasonal dynamics of remotely sensed chlorophyll and physical environment in the Newfoundland region. Remote Sens. Environ. 2001, 76, 268–282. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Aké-Castillo, J.A.; Gutiérrez-Quevedo, M.G.; Pérez-España, H.; Salas-Monreal, D. Annual cycle of the plankton biomass in the National Park Sistema Arrecifal Veracruzano, southwestern Gulf of Mexico. In Zooplankton and Phytoplankton; Kattel, G., Ed.; Nova Science: New York, NY, USA, 2011; pp. 1–26. [Google Scholar]
- Parrish, C.C. Separation of aquatic lipid classes by Chromarodthin-layer chromatography with measurement by Iatroscan flame ionization detection. Can. J. Fish. Aquat. Sci. 1987, 44, 722–731. [Google Scholar] [CrossRef]
- Copeman, L.A.; Parrish, C.C. Lipids classes, fatty acids, and sterols in seafood from Gilbert Bay, southern Labrador. J. Agric. Food Chem. 2004, 52, 4872–4881. [Google Scholar] [CrossRef]
- McLafferty, F.W.; Turecek, F. Interpretation of Mass Spectra, 4th ed.; University Sciences Books: Sausalito, CA, USA, 1993; p. 360. Available online: https://www.nafo.int/About-us/Maps (accessed on 30 September 2019).
- Salas, S.; Chakraborty, K. First report of bioactive sterols from the muricid gastropod Chicoreus ramosus. Steroids 2018, 137, 57–63. [Google Scholar] [CrossRef]
- Díaz de Vivar, M.E.D.; Zárate, E.V.; Rubilar, T.; Epherra, L.; Avaro, M.G.; Sewell, M.A. Lipid and fatty acid profiles of gametes and spawned gonads of Arbacia dufresnii (Echinodermata: Echinoidea): Sexual differences in the lipids of nutritive phagocytes. Mar. Biol. 2019, 166, 96. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Alexander, N.; Shikov, A.N.; Laakso, I.; Seppänen-Laakso, T.; Makarenko, I.E.; Faustova, N.M.; Makarova, M.N.; Makarov, V.G. Bioactivity and chemical characterization of gonads of green sea urchin Strongylocentrotus droebachiensis from Barents Sea. J. Funct. Foods 2015, 17, 227–234. [Google Scholar] [CrossRef]
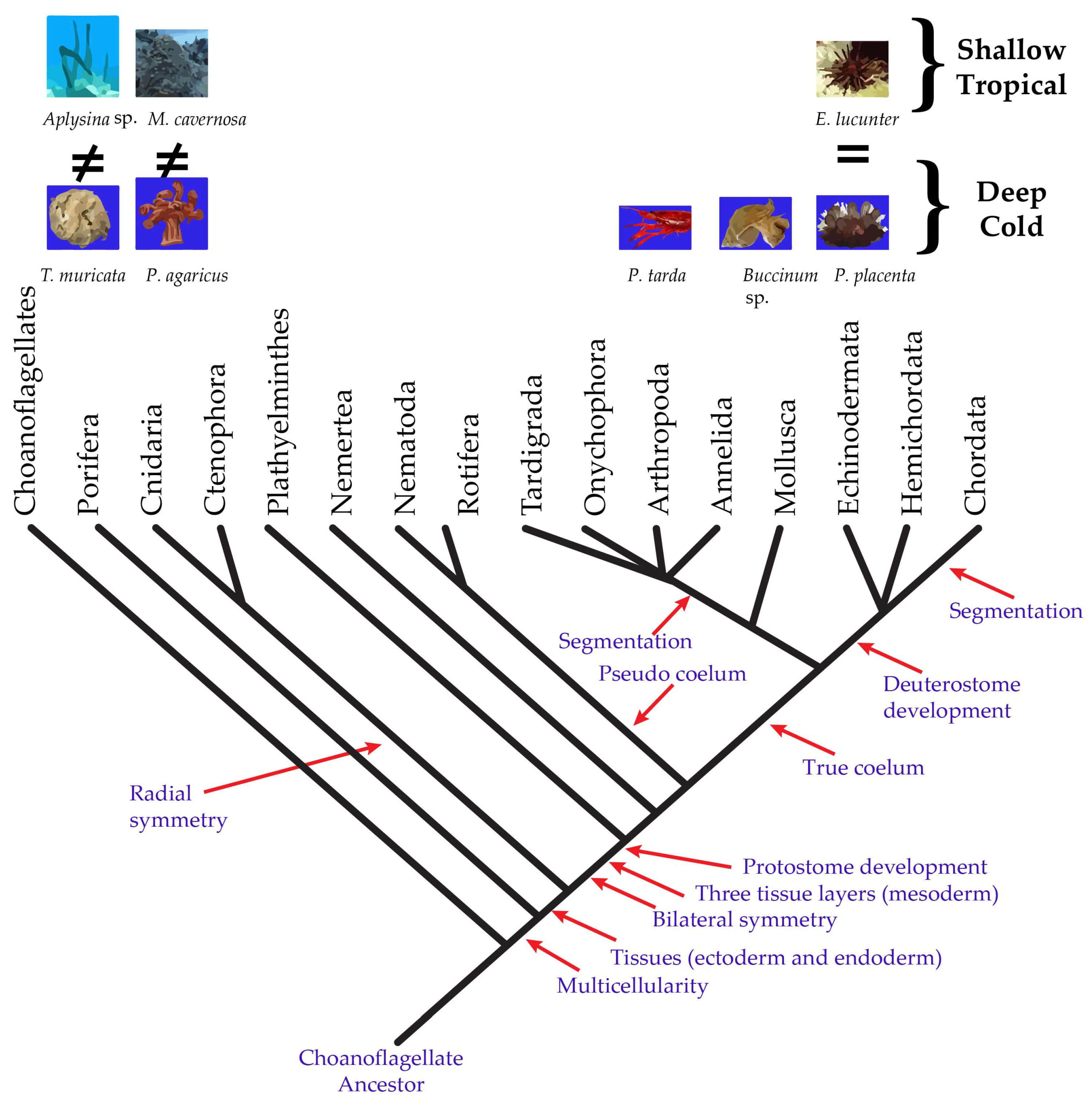
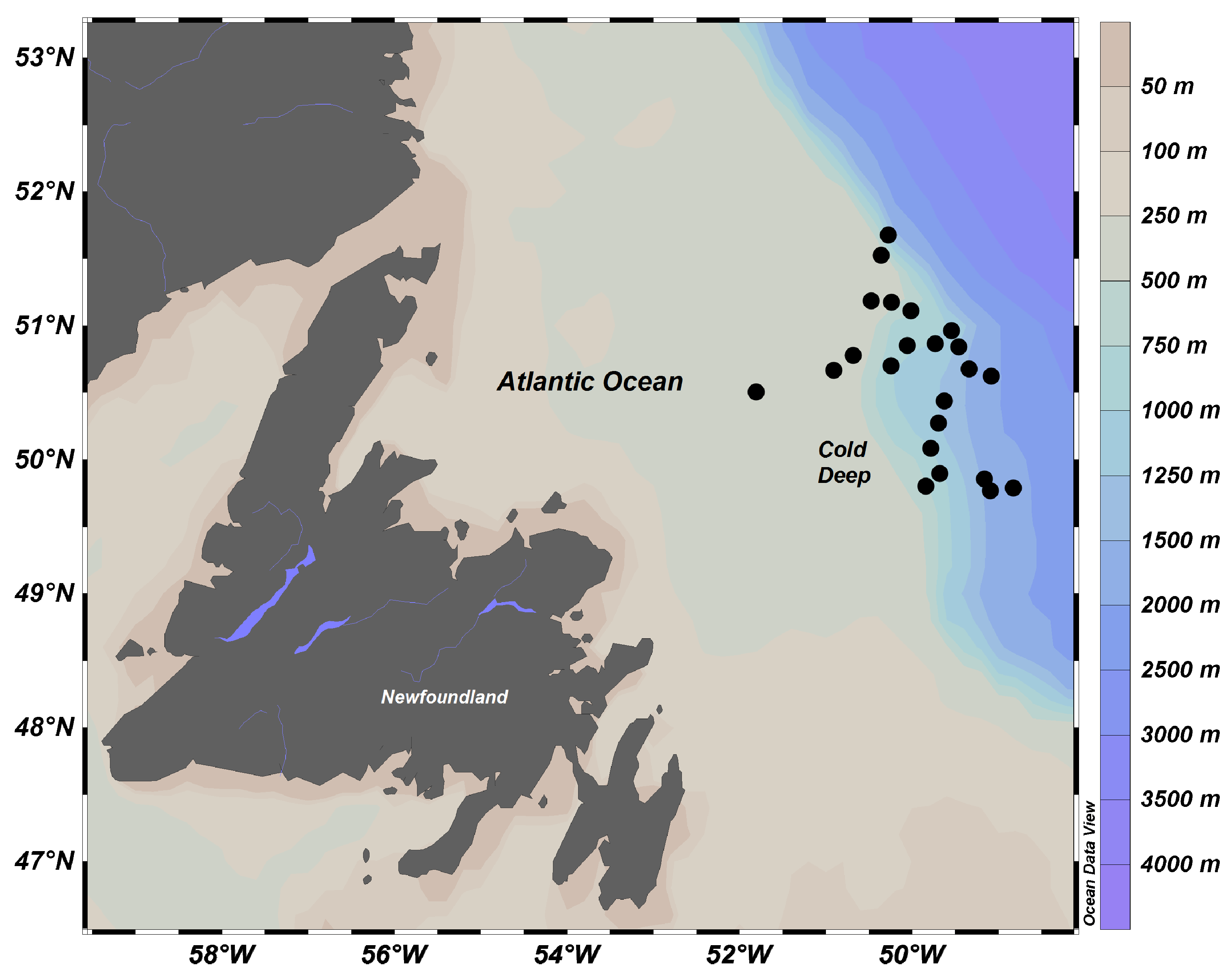
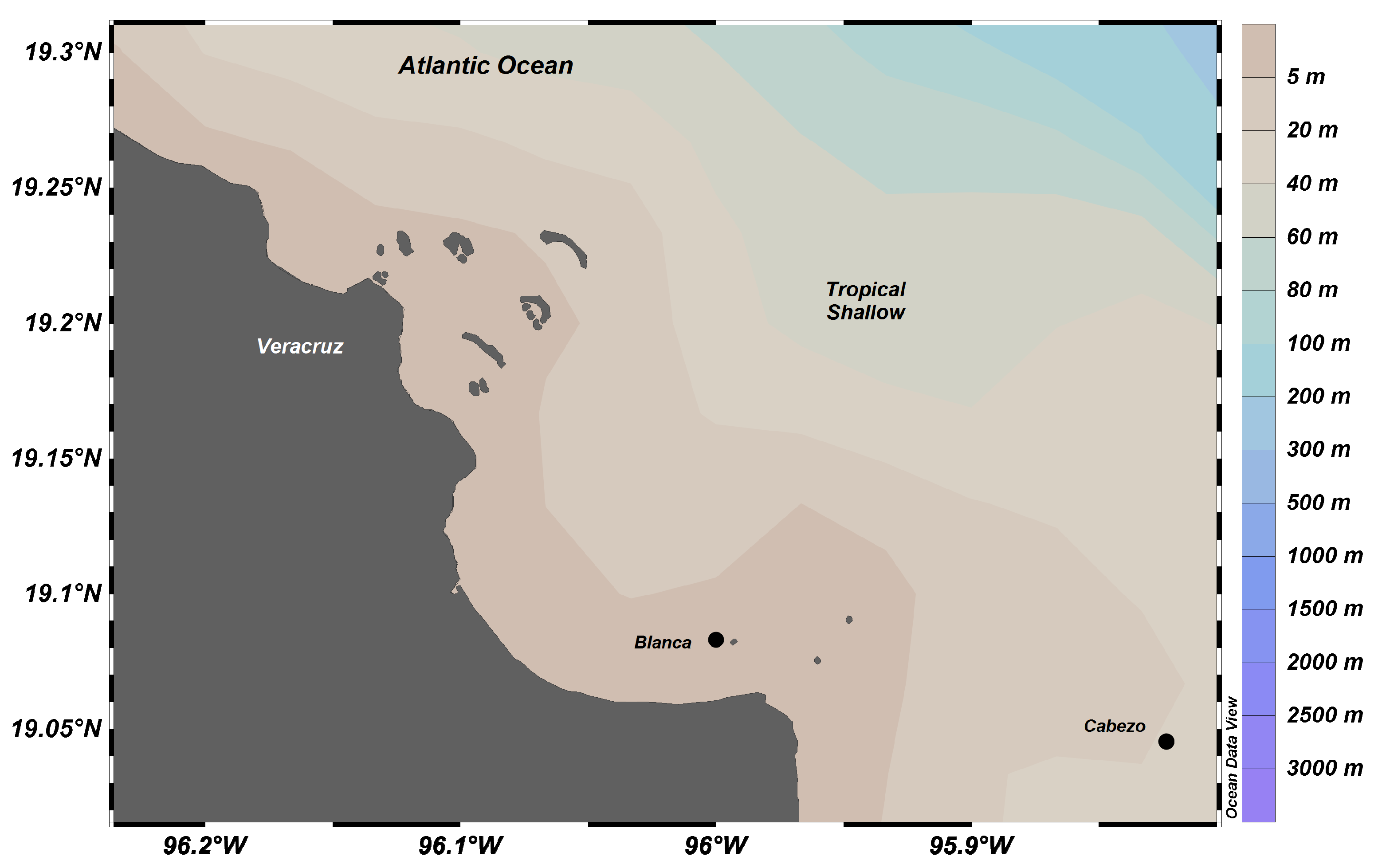
| Scientific Name | Phyllum | Class | Order | Family | n | Depth (m) | Temperature (°C) |
|---|---|---|---|---|---|---|---|
| Thenea muricata | Porifera | Desmospongiae | Astrophorida | Pachastrellidae | 4 | 353 | 4.0 ± 0.3 |
| Aplysina sp. | Verongida | Aplysinidae | 2 | 20 | 25.3 ± 0.9 | ||
| Pseudoanthomastus agaricus | Cnidaria | Anthozoa | Alcyonacea | Alcyoniidae | 3 | 1027 | 4.0 ± 0.3 |
| Montastraea cavernosa | Scleractinia | Montastreidae | 4 | 20 | 25.3 ± 0.4 | ||
| Buccinum sp. | Mollusca | Gastropoda | Neogastropoda | Buccinidae | 3 | 759 | 4.0 ± 0.3 |
| Pasiphaea tarda | Arthropoda | Malacostraca | Decapoda | Pasiphaeidae | 21 | 1321 | 4.0 ± 0.3 |
| Phormosoma placenta | Echinodermata | Echinoidea | Echinothurioida | Phormosomatidae | 3 | 889 | 4.0 ± 0.3 |
| Echinometra lucunter | Echinoida | Echinometridae | 4 | 20 | 25.3 ± 0.4 |
| Sterol Composition (%) | Sponge Thenea muricata | Cnidaria Pseudoanthomastus agaricus | Mollusk Buccinum sp. | Arthropod Pasiphaea terda | Echinoderm Phormosoma placenta | F | p |
|---|---|---|---|---|---|---|---|
| 24-Nordehydrocholesterol | 6.1 ± 0.8 a | 0.9 ± 0.7 b | 0.9 ± 0.1 b | 0.3 ± 0.3 b | 2.5 ± 1.3 b | 23.23 | <0.001 |
| 24-Nordehydrocholestanol | 1.3 ± 0.3 | - | - | - | - | ||
| 24-Nor-22, 23 methylenecholest-5-en-3-β-ol | - | - | - | - | 1.4 ± 0.3 | ||
| Occelasterol | 3.2 ± 0.5 a | 11.6 ± 0.7 b | 1.9 ± 0.9 a | 2.2 ± 0.9 a | 7.2 ± 3.1 a,b | 18.77 | <0.001 |
| Cholesterol | 22.8 ± 1.8 a | 68.8 ± 0.3 b | 91.7 ± 1.0 c | 95.3 ± 2.0 c | 73.9 ± 11.0 b,c | 49.82 | <0.001 |
| Cholestanol | 2.0 ± 0.3 a | 3.6 ± 2.6 a | 1.5 ± 0.5 a | - | 1.5 ± 1.0 a | 2.92 | 0.067 |
| 9-10 Secocholesta-5(10), 6, 8 trien-3-βol (analogue of vitamin D) | - | - | - | - | 1.0 ± 0.2 | ||
| Brassicasterol | 12.1 ± 0.5 a | 8.0 ± 2.6 a,b | 0.8 ± 0.4 c | 0.7 ± 0.9 c | 2.9 ± 0.9 b,c | 57.18 | <0.001 |
| Brassicastanol | 1.5 ± 0.6 | - | - | - | - | ||
| Stellasterol | 3.9 ± 0.4 a | - | - | - | 0.3 ± 0.04 b | 41.13 | <0.001 |
| 24-Methylenecholesterol | 12.2 ± 0.4 a | 1.3 ± 0.3 b | 1.7 ± 0.8 b | - | 1.1 ± 0.3 b | 65.67 | <0.001 |
| Campesterol | 2.6 ± 1.4 a | 2.0 ± 0.4 a | - | - | 2.5 ± 1.6 a | 0.24 | 0.827 |
| Lathosterol | - | - | - | - | 1.5 ± 0.9 | ||
| Stigmasterol | 2.6 ± 0.3 a | - | 0.6 ± 0.2 b | 1.5 ± 0.6 a,b | 0.4 ± 0.1 b | 22.56 | <0.001 |
| Episterol | 11.4 ± 1.6 a | - | - | - | 1.8 ± 1.4 b | 79.29 | <0.001 |
| 4-24 Dimethyl 5, 7-dien-3-β-ol | 2.9 ± 1.9 | - | - | - | - | ||
| Poriferasterol | 2.0 ± 0.6 | - | - | - | - | ||
| Spinasterol | 1.2 ± 0.4 | - | - | - | - | ||
| β-Sitosterol | 8.9 ± 0.8 a | 3.8 ± 0.7 b | 0.9 ± 0.4 c | - | 2.1 ± 1.3 b,c | 69.47 | <0.001 |
| Fucosterol | 3.5 ± 0.7 | - | - | - | - | ||
| Total sterols (GC–MS) Concentration (mg·g−1 wet weight) 1 | 0.6 ± 0.5 a | 0.59 ± 0.56 a | 0.5 ± 0.4 a | 1.6 ± 0.5 b | 3.8 ± 1.2 c | 15.39 | <0.001 |
| Total free sterols (Iatroscan) concentration (mg·g−1 wet weight) | 0.6 ± 0.2 | 0.5 ± 0.2 | 1.4 ± 0.4 | 1.5 ± 1.2 | 0.9 ± 0.2 | 17.53 | <0.001 |
| Organic carbon source using sterols | Phytoplankton macroalgae, and higher plants (?) | Phytoplankton and zooplankton | Zooplankton | Zooplankton and detritus | Phytodetritus |
| Scientific Name | Wet Weight (g) | Lipid Content (mg·g−1 WW) | TAG:ST Ratio | δ13C | Organic Carbon Source | δ15N | C:N Molar Ratio | Functional Group |
|---|---|---|---|---|---|---|---|---|
| Thenea muricata | 16.2 ± 2.1 b | 2.6 ± 1.2 a | 0.3 | −17.4 ± 0.4 b | Pelagic | 14.2 ± 0.3 a | 5.4 ± 0.3 b | Filter feeder zooplanktivore |
| Pseudoanthomastus agaricus | 12.2 ± 8.0 a,b | 4.1 ± 2.2 a | 0.4 | −13.8 ± 4.9 b | Bentho- pelagic | 11.0 ± 1.4 b,c | 6.5 ± 3.1 a,b,c | Omnivore detritivore |
| Buccinum sp. | 5.8 ± 2.9 c | 6.9 ± 0.3 b | 0.02 | −17.0 ± 1.0 b | Pelagic | 12.6 ± 0.6 b | 4.2 ± 0.1 a | Carnivore scavenger |
| Pasiphaea tarda | 29.2 ± 6.6 a | 8.7 ± 2.3 b | 0.5 | −19.2 ± 0.1 a | Pelagic | 11.4 ± 0.1 c | 3.8 ± 0.04 a | Zooplanktivore |
| Phormosoma placenta | 19.6 ± 8.4 a,b | 6.0 ± 3.1 a,b | 0.5 | −14.3 ± 0.9 c | Bentho- pelagic | 12.3 ± 0.3 b | 5.3 ± 0.5 b | Omnivore carnivore |
| Aplysina sp. | 28 ± 11 a | 15.4 ± 5.4 b,c | 0.4 ± 0.1 | Pelagic | Filter feeder | |||
| Montastraea cavernosa | 9.8 ± 3.8 c | 7.2 ± 3.6 b,c | 1.4 ± 0.5 | −11.1 ± 2.5 c,d | Symbiont | 4.0 ± 1.9 d | 16.9 ± 9.5 c,1 | Zooxanthellae and zooplankton |
| Echinometra. lucunter | 6.9 ± 1.6 c | 12.6 ± 1.2 c | 25 ± 14 | −10 ± 0.1 d | Macroalgae | 3.3 ± 0.3 d | 8.5 ± 0.4 c | Herbivore |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreón-Palau, L.; Özdemir, N.Ş.; Parrish, C.C.; Parzanini, C. Sterol Composition of Sponges, Cnidarians, Arthropods, Mollusks, and Echinoderms from the Deep Northwest Atlantic: A Comparison with Shallow Coastal Gulf of Mexico. Mar. Drugs 2020, 18, 598. https://doi.org/10.3390/md18120598
Carreón-Palau L, Özdemir NŞ, Parrish CC, Parzanini C. Sterol Composition of Sponges, Cnidarians, Arthropods, Mollusks, and Echinoderms from the Deep Northwest Atlantic: A Comparison with Shallow Coastal Gulf of Mexico. Marine Drugs. 2020; 18(12):598. https://doi.org/10.3390/md18120598
Chicago/Turabian StyleCarreón-Palau, Laura, Nurgül Şen Özdemir, Christopher C. Parrish, and Camilla Parzanini. 2020. "Sterol Composition of Sponges, Cnidarians, Arthropods, Mollusks, and Echinoderms from the Deep Northwest Atlantic: A Comparison with Shallow Coastal Gulf of Mexico" Marine Drugs 18, no. 12: 598. https://doi.org/10.3390/md18120598
APA StyleCarreón-Palau, L., Özdemir, N. Ş., Parrish, C. C., & Parzanini, C. (2020). Sterol Composition of Sponges, Cnidarians, Arthropods, Mollusks, and Echinoderms from the Deep Northwest Atlantic: A Comparison with Shallow Coastal Gulf of Mexico. Marine Drugs, 18(12), 598. https://doi.org/10.3390/md18120598





