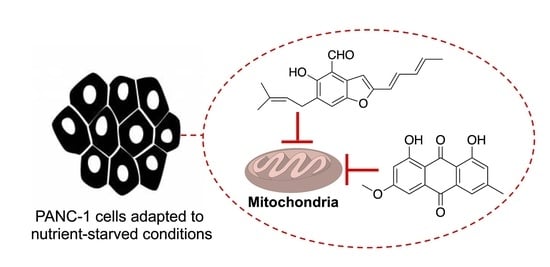
Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Analysis of Active Compounds 1 and 2
2.2. Cytotoxic Effect of Compounds 1 and 2 on PANC-1 Cells under Glucose-Deficient and General Culture Conditions
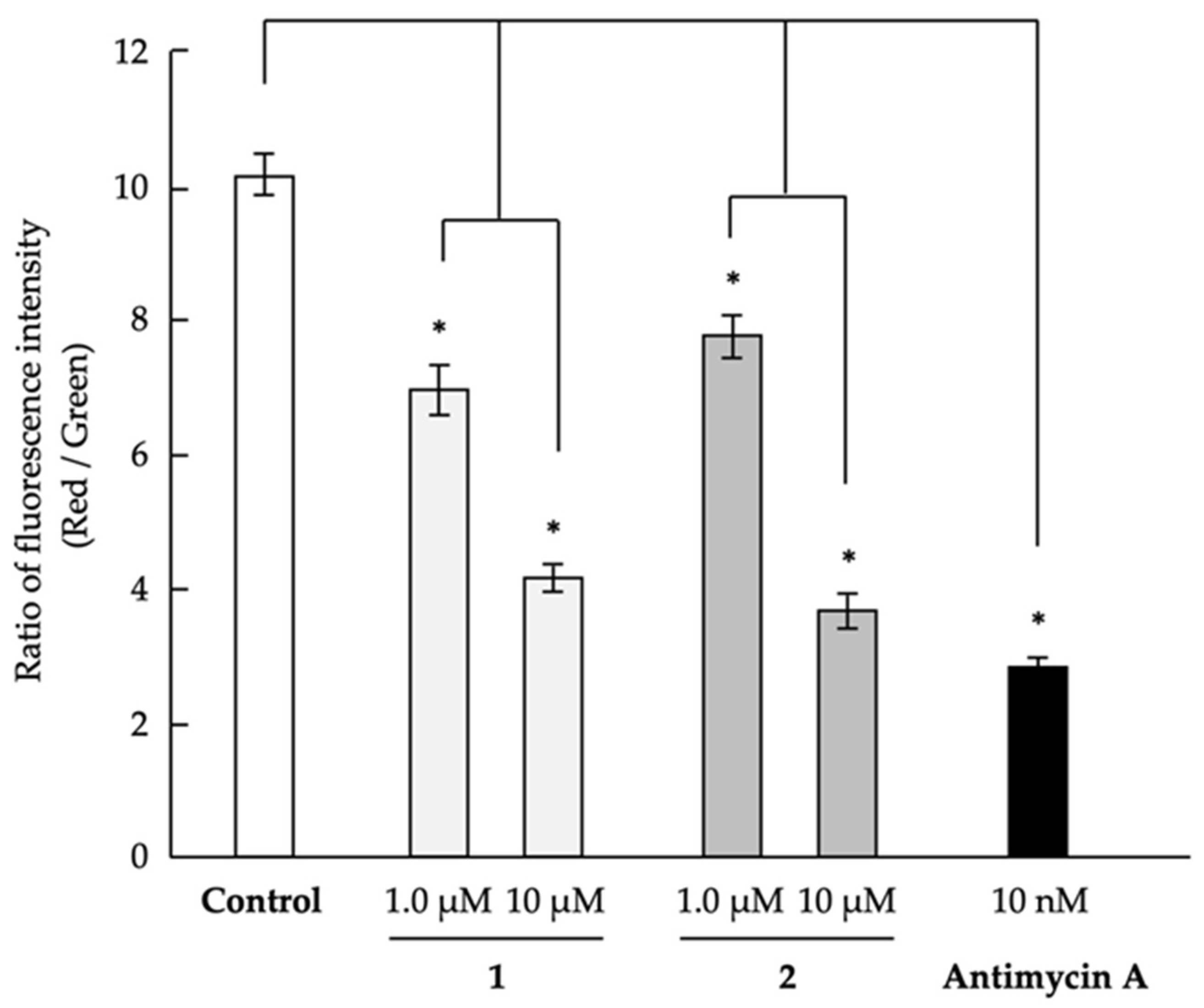
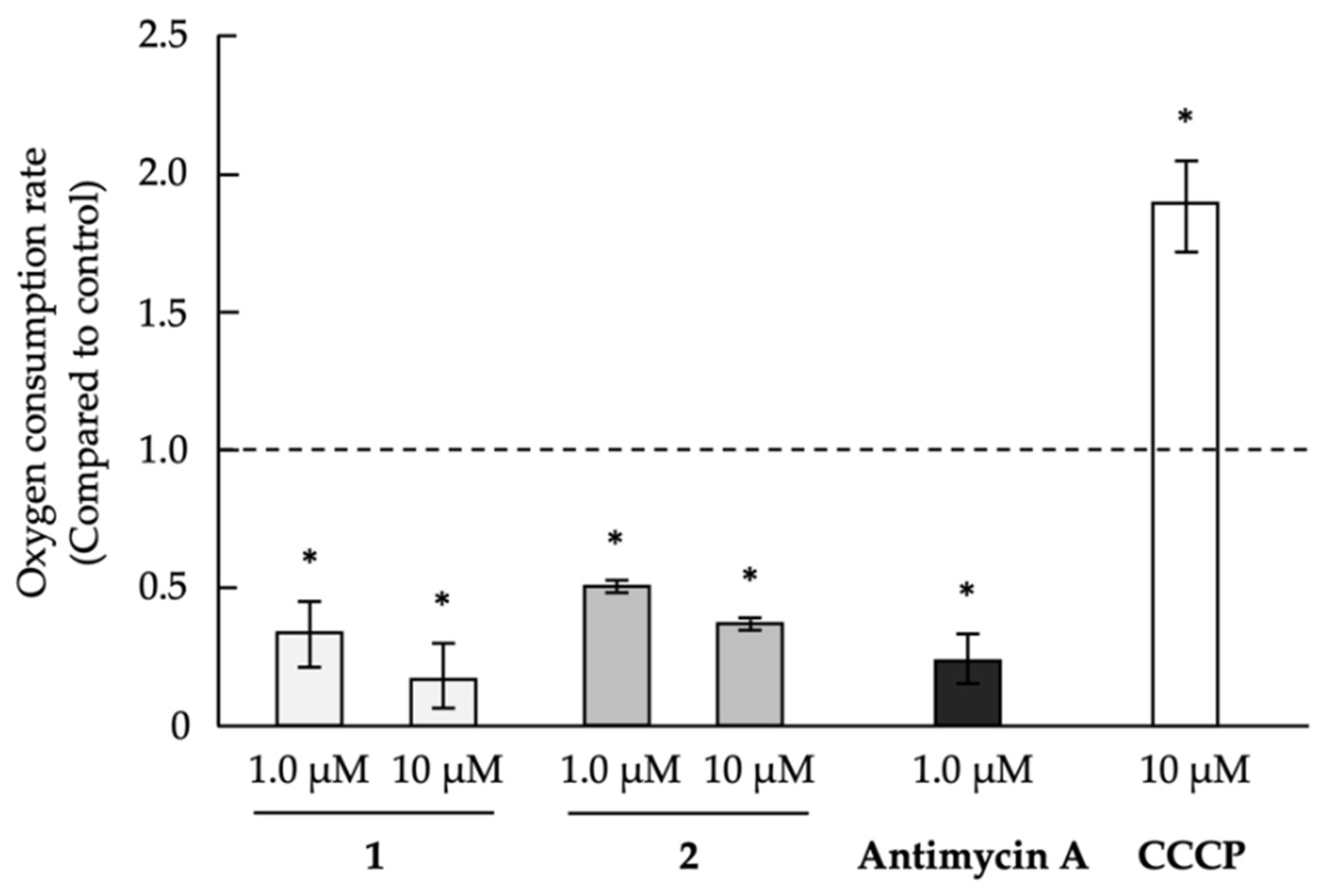
2.3. Analysis of Mechanism of Action as Anti-Austerity Agents
3. Materials and Methods
3.1. General
3.2. Materials
3.3. Cell Culture and Bioassay
3.4. Evaluation of Mitochondrial Membrane Potential
3.5. Evaluation of Oxygen Consumption
3.6. Measurement of Mitochondrial Complex Activity
3.7. Isolation of Active Compounds 1 and 2
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood-flow, oxygen and nutrient supply, and metabolic microenvironment of human-tumors—A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updates 2011, 14, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rehman, S.K.; Zhang, W.; Wen, A.; Yao, L.; Zhang, J. Autophagy is a therapeutic target in anticancer drug resistance. Biochim. Biophys. Acta 2010, 1806, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Lee, J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Izuishi, K.; Kato, K.; Ogura, T.; Kinoshita, T.; Esumi, H. Remarkable tolerance of tumor cells to nutrient deprivation: Possible new biochemical target for cancer therapy. Cancer Res. 2000, 60, 6201. [Google Scholar] [PubMed]
- Ueda, J.Y.; Athikomkulchai, S.; Miyatake, R.; Saiki, I.; Esumi, H.; Awale, S. (+)-Grandifloracin, an antiausterity agent, induces autophagic PANC-1 pancreatic cancer cell death. Drug Des. Dev. Ther. 2014, 8, 39–47. [Google Scholar]
- Lu, J.; Kunimoto, S.; Yamazaki, Y.; Kaminishi, M.; Esumi, H. Kigamicin D, a novel anticancer agent based on a new anti-austerity strategy targeting cancer cells’ tolerance to nutrient starvation. Cancer Sci. 2004, 95, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, S.; Lu, J.; Esumi, H.; Yamazaki, Y.; Kinoshita, N.; Honma, Y.; Hamada, M.; Ohsono, M.; Ishizuka, M.; Takeuchi, T. Kigamicins, novel antitumor antibiotics. I. Taxonomy, isolation, physico-chemical properties and biological activities. J. Antibiot. (Tokyo) 2003, 56, 1004–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awale, S.; Dibwe, D.F.; Balachandran, C.; Fayez, S.; Feineis, D.; Lombe, B.K.; Bringmann, G. Ancistrolikokine E3, a 5,8′-Coupled Naphthylisoquinoline Alkaloid, Eliminates the Tolerance of Cancer Cells to Nutrition Starvation by Inhibition of the Akt/mTOR/Autophagy Signaling Pathway. J. Nat. Prod. 2018, 81, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Casertano, M.; Menna, M.; Imperatore, C. The ascidian-derived metabolites with antimicrobial properties. Antibiotics 2020, 9, 510. [Google Scholar] [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Kimishima, A.; Ishida, R.; Setiawan, A.; Arai, M. Selective cytotoxicity of epidithiodiketopiperazine DC1149B, produced by marine-derived Trichoderma lixii on the cancer cells adapted to glucose starvation. J. Nat. Med. 2020, 74, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Kamiya, K.; Shin, D.; Matsumoto, H.; Hisa, T.; Setiawan, A.; Kotoku, N.; Kobayashi, M. N-Methylniphatyne A, a New 3-Alkylpyridine Alkaloid as an Inhibitor of the Cancer Cells Adapted to Nutrient Starvation, from an Indonesian Marine Sponge of Xestospongia sp. Chem. Pharm. Bull. 2016, 64, 766–771. [Google Scholar] [CrossRef] [Green Version]
- Kotoku, N.; Ishida, R.; Matsumoto, H.; Arai, M.; Toda, K.; Setiawan, A.; Muraoka, O.; Kobayashi, M. Biakamides A-D, Unique Polyketides from a Marine Sponge, Act as Selective Growth Inhibitors of Tumor Cells Adapted to Nutrient Starvation. J. Org. Chem. 2017, 82, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Ohba, S.; Tatsuda, D.; Kawada, M.; Masuda, T.; Tsujiuchi, G.; Yamori, T.; Esumi, H.; Ikeda, D. Mitochondrial inhibitors show preferential cytotoxicity to human pancreatic cancer PANC-1 cells under glucose-deprived conditions. Biochem. Biophys. Res. Commun. 2010, 392, 460–466. [Google Scholar] [CrossRef]
- Tang, R.; Kimishima, A.; Setiawan, A.; Arai, M. Secalonic acid D as a selective cytotoxic substance on the cancer cells adapted to nutrient starvation. J. Nat. Med. 2020, 74, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Sun, Y.; Protopopova, M.; Gera, S.; Bandi, M.; Bristow, C.; McAfoos, T.; Morlacchi, P.; Ackroyd, J.; Agip, A.A.; et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 2018, 24, 1036–1046. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, R.; Mochizuki, N.; Ikeda, M.; Sato, A.; Nomura, S.; Owada, S.; Yomoda, S.; Tsuchihara, K.; Kishino, S.; Esumi, H. Change in plasma lactate concentration during arctigenin administration in a phase I clinical trial in patients with gemcitabine-refractory pancreatic cancer. PLoS ONE 2018, 13, e0198219. [Google Scholar] [CrossRef] [Green Version]
- Jose, C.; Bellance, N.; Rossignol, R. Choosing between glycolysis and oxidative phosphorylation: A tumor’s dilemma? BBA-Bioenergetics 2011, 1807, 552–561. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birsoy, K.; Possemato, R.; Lorbeer, F.K.; Bayraktar, E.C.; Thiru, P.; Yucel, B.; Wang, T.; Chen, W.W.; Clish, C.B.; Sabatini, D.M. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 2014, 508, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sungkeun, C.; Yonghyun, P.; Seuk, C.; Inkyu, K.; Youngwan, S.; Kiwoong, C.; Jongheon, S. Anthraquinones and Sterols from the Korean Marine Echiura Urechis unicintus. J. Korean Chem. Soc. 1998, 42, 64–68. [Google Scholar]
- Minh, T.N.; Van, T.M.; Andriana, Y.; Vinh, L.T.; Hau, D.V.; Duyen, D.H.; Guzman-Gelani, C. Antioxidant, Xanthine Oxidase, α-Amylase and α-Glucosidase Inhibitory Activities of Bioactive Compounds from Rumex crispus L. Root. Molecules 2019, 24, 3899. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.W.; Ji, C.Z.; Gu, Q.Q.; Li, D.H.; Zhu, T.J. Three new polyketides from marine-derived fungus Aspergillus glaucus HB1-19. J. Asian Nat. Prod. Res. 2013, 9, 956–961. [Google Scholar] [CrossRef]
- Inoue, S.; Hashizume, K.; Takamatsu, N.; Nagano, H.; Kishi, Y. Synthetic studies on Echinulin and related natural products. IV. Isolation, structure and synthesis of Flavoglaucin-Auroglaucin type natural products isolated from Aspergillus amstelodami. Yakugaku Zasshi 1977, 97, 569–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuete, V.; Wabo, H.K.; Eyong, K.O.; Feussi, M.T.; Wiench, B.; Krusche, B.; Tane, P.; Folefoc, G.N.; Efferth, T. Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS ONE 2011, 6, e21762. [Google Scholar] [CrossRef] [PubMed]
- King, N.M. Isolation of physcion from Ditremmexa occidentialis, L. J. Am. Pharm. Assoc. 1957, 46, 271–272. [Google Scholar] [CrossRef]
- Kang, L.; Si, L.; Rao, J.; Li, D.; Wu, Y.; Wu, S.; Wu, M.; He, S.; Zhu, W.; Wu, Y.; et al. Polygoni multiflora radix derived anthraquinones alter bile acid disposition in sandiwich-cultured hepatocytes. Toxicol. In Vitro 2017, 40, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.C.; Lee, C.M.; Choung, E.S.; Bak, J.P.; Bae, J.J.; Yoo, H.S.; Kwak, J.H.; Zee, O.P. Anti-proliferative effects of estrogen receptor-modulating compounds isolated from Rheum pamatum. Arch. Pharm. Res. 2008, 31, 722–726. [Google Scholar] [CrossRef]
- Feng, T.S.; Yuan, Z.Y.; Yang, R.Q.; Zhao, S.; Lei, F.; Xiao, X.Y.; Xing, D.M.; Wang, W.H.; Ding, Y.; Du, L.J. Purgative components in rhubarb: Adrenergic receptor inhibitors linked with glucose carriers. Fitoterapia 2013, 91, 236–246. [Google Scholar] [CrossRef]
- Hu, L.; Chen, N.N.; Hu, Q.; Yang, C.; Yang, Q.S.; Wang, F.F. An unusual piaceatannol dimer from Rheum austral D.Don with antioxidant activity. Molecules 2014, 19, 11453–11464. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Guo, J.; Xu, F. Optimized extraction process and identification of antibacterial substances from rhubarb aquatic pathogenic Vibrio harveyi. 3 Biotech 2017, 7, 377. [Google Scholar] [CrossRef]
- Bachmann, M.; Luthy, J.; Schlatter, C. Toxicity and mutagenicity of molds of the Aspergillus glaucus group. Identification of physcion and three related anthraquinones as main toxic constituents from Aspergillus chevalieri. J. Agric. Food Chem. 1979, 27, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Sadorn, k.; Saepua, S.; Boonyuen, N.; Boonruangprapa, T.; Rachtawee, P.; Pittayakhajonwut, P. Antimicrobial activity and cytotoxicity of xanthoquinodine analogs from the fungus Cytospora eugeniae BCC42696. Phytochemistry 2018, 151, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, R.; Yi, W.; Chai, W.; Zhang, Z.; Lian, X.Y. Peniciphenalenins A-F from the culture of marine-associated fungus Penicillium sp. ZZ901. Phytochemistry 2018, 152, 53–60. [Google Scholar] [CrossRef]
- Li, F.; Xue, F.; Yu, X. GC-MS, FTIR and Raman analysis of antioxidant components of red pigments from Stemphylium lycopersici. Curr. Microbiol. 2017, 74, 532–539. [Google Scholar] [CrossRef]
- Wijesekara, I.; Zhang, C.; Ta, Q.V.; Vo, T.S.; Li, Y.X.; Kim, S.K. Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma Hela cells. Microbiol. Res. 2014, 169, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Rigano, D.; Loppi, S.; Santi, A.D.; Nebbioso, A.; Sorbo, S.; Conte, B.; Paoli, L.; Ruberto, F.D.; Molinari, A.M.; et al. Antiproliferative, antibacterial and antifungal activity of the Lichen Xanthoria parietina and its secondary metabolite parietin. Int. J. Mol. Sci. 2015, 16, 7861–7875. [Google Scholar] [CrossRef]
- Comini, L.R.; Vieyra, F.E.M.; Mignone, R.A.; Páez, P.L.; Mugas, M.L.; Konigheim, B.S.; Cabrera, J.L.; Montoya, S.C.N.; Borsarelli, C.D. Parietin: An efficient photo-screening pigment in vivo with good photosensitizing and photodynamic antibacterial effects in vitro. Photochem. Photobiol. Sci. 2017, 16, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.T.; Adler, M.T.; Bertoni, M.D.; Sepúlveda, C.S.; Damonte, E.B.; Maier, M.S. Lichen secondary metabolites from the cultured lichen mycobionts of Teloschistes chrysophthalmus and Ramalina celastri and their antiviral activities. Z. Naturforsch. C J. Biosci. 2007, 62, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosale, I.; Kremer, D.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Randić, M.; Koncic, M.Z. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem. 2013, 136, 335–341. [Google Scholar] [CrossRef]
- Wei, Q.; Ji, X.Y.; Long, X.S.; Li, Q.R.; Yin, H. Chemical Constituents from Leaves of “Chuju” Chrysanthemum morifolium and their antioxidant activities in vitro. Zhong Yao Cai 2015, 38, 305–310. [Google Scholar] [PubMed]
- Kwon, K.S.; Lee, J.H.; So, K.S.; Park, B.K.; Lim, H.; Choi, J.S.; Kim, H.P. Aurantio-obtusin, an anthraquinone from cassiae semen, ameliorates lung inflammatory responses. Phytother. Res. 2018, 32, 1537–1545. [Google Scholar] [CrossRef]
- Ghosh, S.; Das Sarma, M.; Patra, A.; Hazra, B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. J. Pharm. Pharmacol. 2010, 62, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, W.; Guo, Q.; Bai, Y.; Yang, H.; Chen, H. Physcion blocks cell cycle and induces apoptosis in human B cell precursor acute lymphoblastic leukemia cells by downregulating HOXA5. Biomed. Pharmacother. 2017, 94, 850–857. [Google Scholar] [CrossRef]
- Lin, R.; Elf, S.; Shan, C.; Kang, H.B.; Ji, Q.; Zhou, L.; Hitosugi, T.; Zhang, L.; Zhang, S.; Seo, J.H.; et al. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat. Cell Biol. 2015, 17, 1484–1496. [Google Scholar] [CrossRef] [Green Version]
- Özenver, N.; Saeed, M.; Demirezer, L.Ö.; Efferth, T. Aloe-emodin as drug candidate for cancer therapy. Oncotarget 2018, 9, 17770–17796. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.Y.; Chung, H.J.; Bae, S.Y.; Trung, T.N.; Bae, K.; Lee, S.K. Induction of cell cycle arrest and apoptosis by physcion, an anthraquinone isolated from rhubarb (rhizomes of Rheum tanguticum), in MDA-MB-231 human breast cancer cells. J. Cancer Prev. 2014, 19, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.T.; Chen, X.H.; Gao, H.; Ye, J.L.; Wang, C.B. Physcion inhibits the metastatic potential of human colorectal cancer SW620 cells in vitro by suppressing the transcripntion factor SOX2. Acta Pharmacol. Sin. 2016, 37, 264–275. [Google Scholar] [CrossRef]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef]
- Rayanil, K.O.; Bunchornmaspan, P.; Tuntiwachwuttikul, P. A new phenolic compound with anticancer activity from the wood of Millettia leucantha. Arch. Pharm. Res. 2011, 34, 881–886. [Google Scholar] [CrossRef]
- Pang, M.J.; Yang, Z.; Zhang, X.L.; Liu, Z.F.; Fan, J.; Zhang, H.Y. Physcion, a naturally occurring anthraquinone derivative, induces apoptosis and autophagy in human nasopharyngeal carcinoma. Acta Pharmacol. Sin. 2016, 37, 1623–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Wang, H.; Tong, D.; Wang, C.; Sun, L.; Zhao, C.; Li, Y.; Zhu, L.; Wu, D. Physcion induces apoptosis in hepatocellular carcinoma by modulating miR-370. Am. J. Cancer Res. 2016, 6, 2919–2931. [Google Scholar]
- Pan, X.P.; Wang, C.; Li, Y.; Huang, L.H. Physcion induces apoptosis through triggering endoplasmic reticulum stress in hepatocellular carcinoma. Biomed. Pharmacother. 2018, 99, 894–903. [Google Scholar] [CrossRef]
- Shi, J.L.; Kang, D.; Huang, Y.; Kong, W.; Xiang, Y.; Zhu, X.; Duan, Y.; Huang, Y. Isolation and characterization of benzaldehyde derivatives with anti-inflammatory activities from Eurotium cristatum, the dominant fungi species in fuzhuan brick tea. ACS Omega 2019, 4, 6630–6636. [Google Scholar] [CrossRef]
- Wu, M.D.; Cheng, M.J.; Hsieh, S.Y.; Yuan, G.F. Chemical constituents of the fungus of Eurotium chevalieri BCRC 07F0022. Chem. Nat. Comp. 2014, 49, 1175–1176. [Google Scholar] [CrossRef]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Proksch, P.; Wang, B.G. Benzaldehyde derivatives from Eurotium rubrum, an endophytic fungus derived from the Mangrove plant Hibiscus tiliaceus. Chem. Pharm. Bull. 2008, 56, 1282–1285. [Google Scholar] [CrossRef] [Green Version]
- Xiaoti, P.; Faliang, L.; Dongli, L.; Yuchan, C.; Meihua, T.; Weimin, Z.; Yunlin, Z. Secondary metabolites of Eurotium cristatum from Fu Brick Tea and their biological activities. Zhong Cao Yao 2013, 44, 1881–1886. [Google Scholar]
- Xia, D.; Yu, C.A.; Kim, H.; Xia, J.Z.; Kachurin, A.M.; Zhang, L.; Deisenhofer, J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science 1997, 277, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weatherly, L.M.; Shim, J.; Hashmi, H.N.; Kennedy, R.H.; Hess, S.T.; Gosse, J.A. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J. Appl. Toxicol. 2016, 36, 777–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Mathew, R.; White, E. Metabolic catastrophe as a means to cancer cell death. J. Cell Sci. 2007, 120, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007, 67, 3496–3499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, M.; Shin, D.; Kamiya, K.; Ishida, R.; Setiawan, A.; Kotoku, N.; Kobayashi, M. Marine spongean polybrominated diphenyl ethers, selective growth inhibitors against the cancer cells adapted to glucose starvation, inhibits mitochondrial complex II. J. Nat. Med. 2017, 71, 44–49. [Google Scholar] [CrossRef]
- Hajmousa, G.; Vogelaar, P.; Brouwer, L.A.; van der Graaf, A.C.; Henning, R.H.; Krenning, G. The 6-chromanol derivate SUL-109 enables prolonged hypothermic storage of adipose tissue-derived stem cells. Biomaterials 2017, 119, 43–52. [Google Scholar] [CrossRef] [PubMed]

| Compounds | IC50 (µM) | ||
|---|---|---|---|
| Glucose − 1 | Glucose + 2 | S.I. 3 | |
| 1 | 6.0 ± 0.1 | 1017 ± 0.05 | 169.5 |
| 2 | 1.7 ± 0.05 | 859 ± 1 | 505.3 |
| Antimycin A 4 | 0.0003 ± 0.001 | 288 ± 0.5 | 960,000 |
| IC50 (µM) | |||||
|---|---|---|---|---|---|
| Complex I | Complex II | Complex III | Complex IV | Complex V | |
| 1 | 100 ± 0.92 | 90 ± 0.11 | >100 ± 4.43 | 34 ± 0.3 | 100 ± 0.92 |
| 2 | >100 ± 1.60 | >100 ± 0.92 | >100 ± 3.36 | 20 ± 0.3 | >100 ± 1.60 |
| Positive control 1 | 0.19 ± 0.02 | 68 ± 0.01 | 0.03 ± 0.01 | 21 ± 0.0 | 0.19 µg/mL ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Naime, W.A.; Kimishima, A.; Setiawan, A.; Fahim, J.R.; Fouad, M.A.; Kamel, M.S.; Arai, M. Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp. Mar. Drugs 2020, 18, 555. https://doi.org/10.3390/md18110555
Abdel-Naime WA, Kimishima A, Setiawan A, Fahim JR, Fouad MA, Kamel MS, Arai M. Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp. Marine Drugs. 2020; 18(11):555. https://doi.org/10.3390/md18110555
Chicago/Turabian StyleAbdel-Naime, Waleed A, Atsushi Kimishima, Andi Setiawan, John Refaat Fahim, Mostafa A. Fouad, Mohamed Salah Kamel, and Masayoshi Arai. 2020. "Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp." Marine Drugs 18, no. 11: 555. https://doi.org/10.3390/md18110555
APA StyleAbdel-Naime, W. A., Kimishima, A., Setiawan, A., Fahim, J. R., Fouad, M. A., Kamel, M. S., & Arai, M. (2020). Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp. Marine Drugs, 18(11), 555. https://doi.org/10.3390/md18110555