Marine-Derived Penicillium purpurogenum Reduces Tumor Size and Ameliorates Inflammation in an Erlich Mice Model
Abstract
1. Introduction
2. Results
2.1. P. purpurogenum Extracellular Extract Contains Meroterpenoids
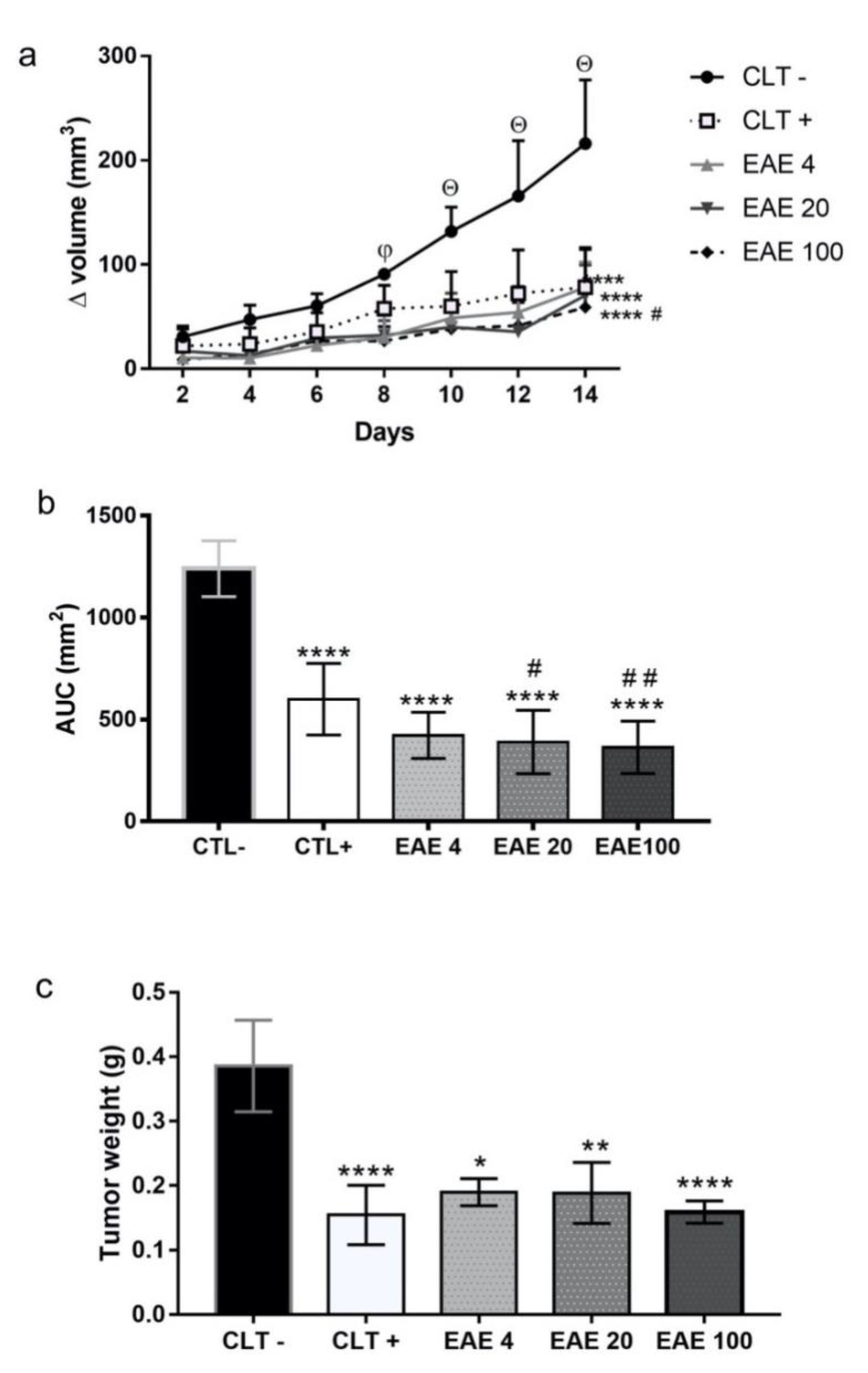
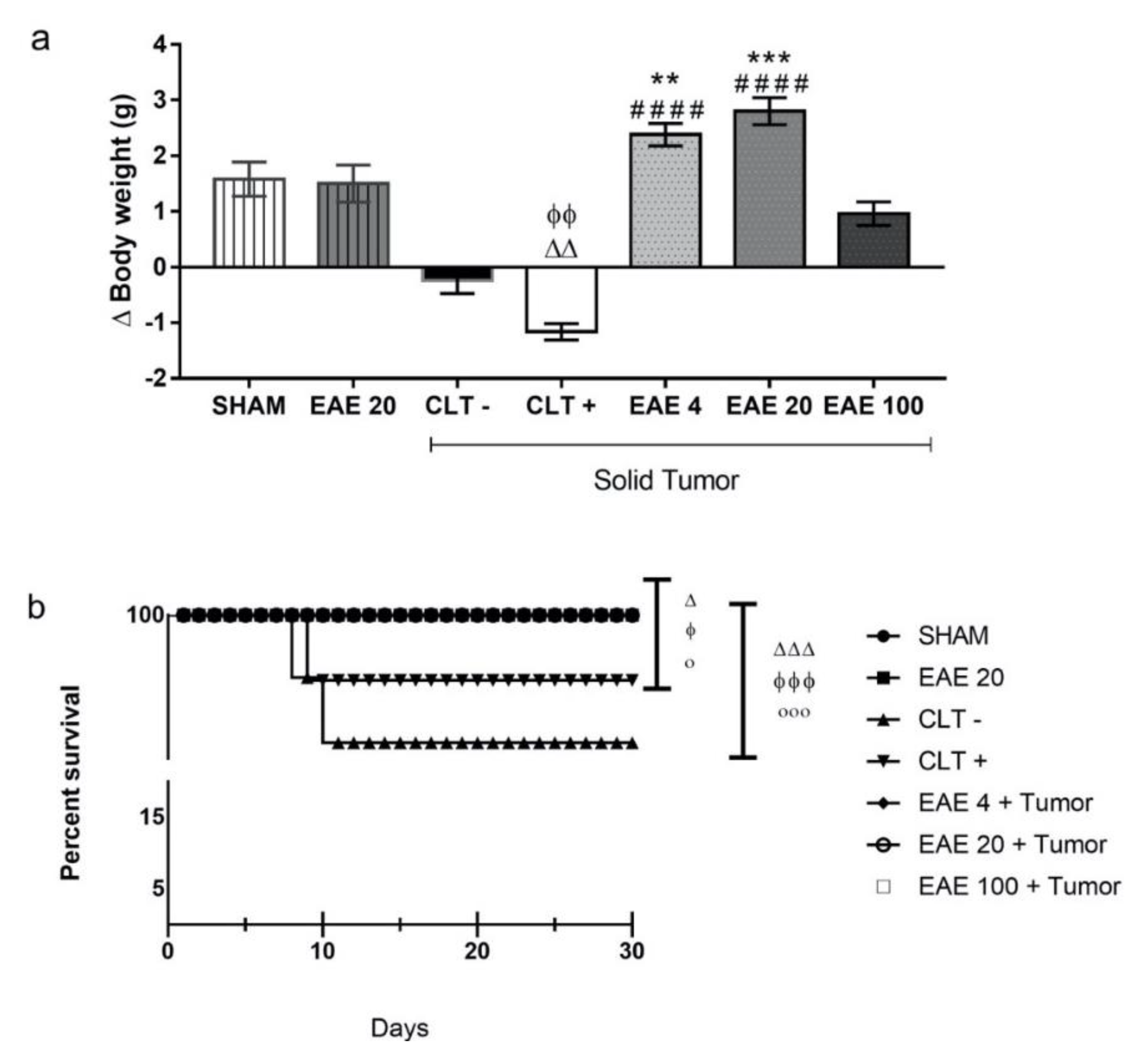
2.2. P. purpurogenum Extract Showed Activity Against Ehrlich’s Solid Tumor
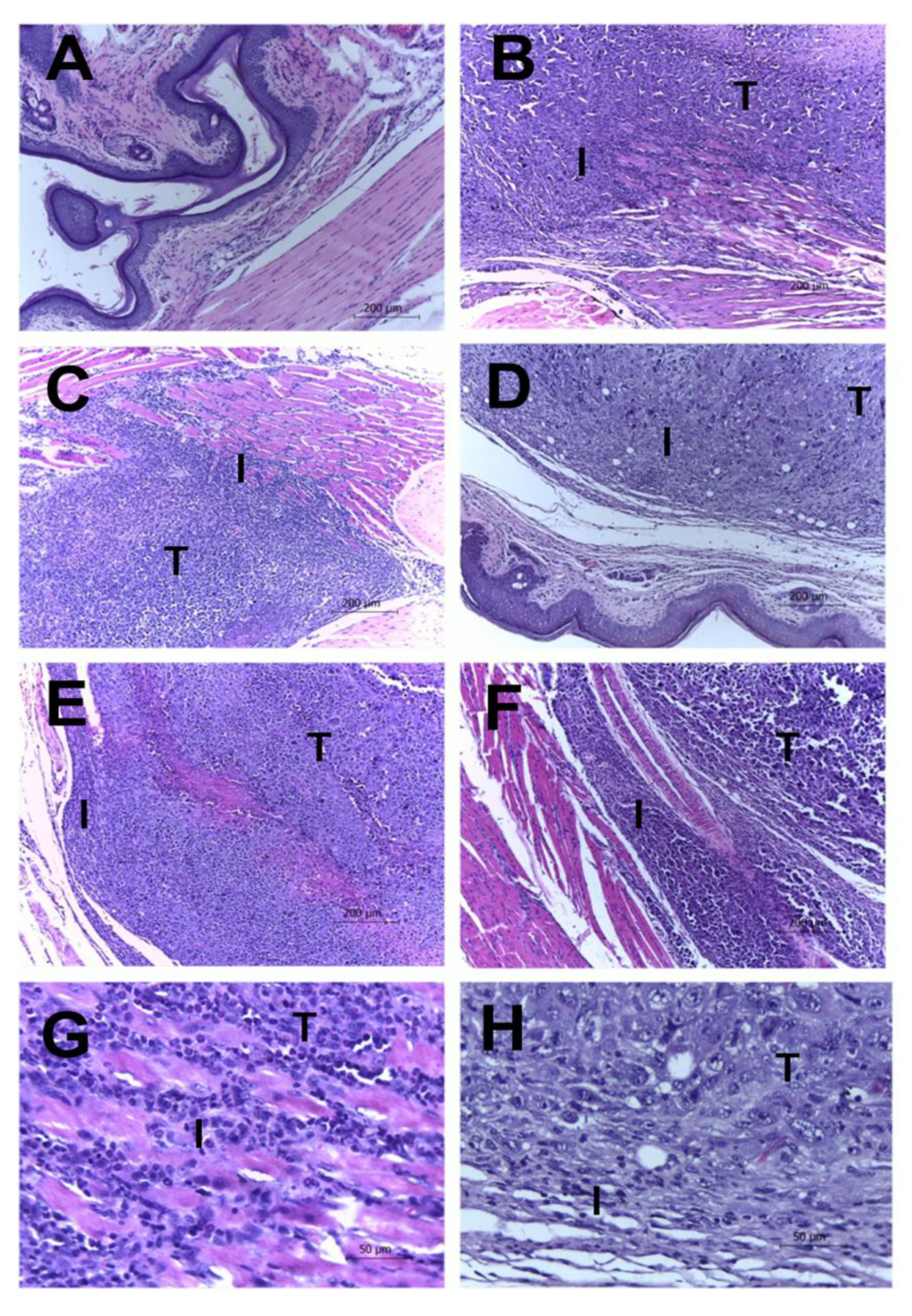
2.3. Histological Results
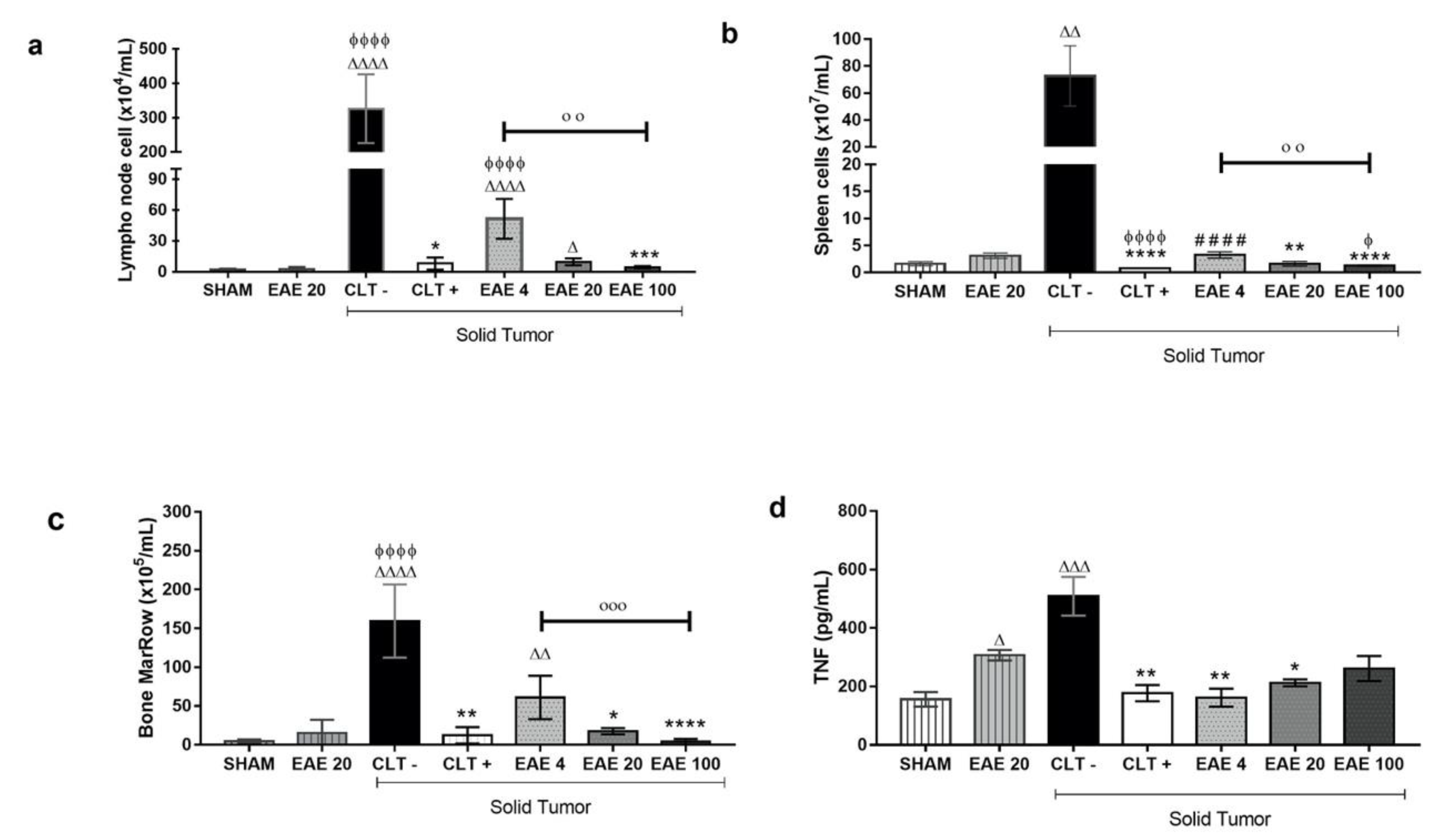
2.4. P. purpurogenum Extract Induced Immunomodulatory Effects
2.5. Toxicity Studies of P. purpurogenum EAE
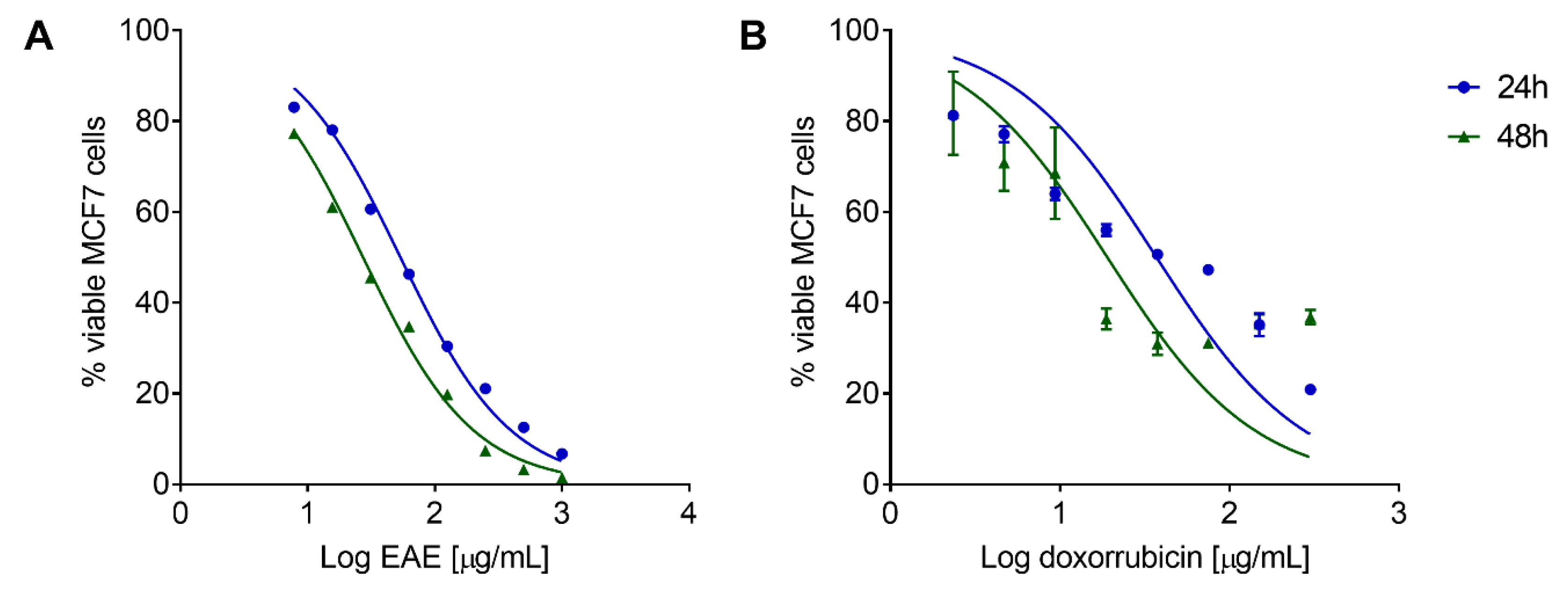
2.6. P. purpurogenum Extract Has a Cytotoxic Effect against MCF7 Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Fermentation and Preparation of Ethyl Acetate Extracellular Extract (EAE)
4.2. High-Performance Liquid Chromatograph Coupled to Diode-Array UV-Vis Detector (HPLC-DAD-UV)
4.3. Tandem Mass Spectrometry with Electrospray Ionization (ESI-MS/MS)
4.4. Animals
4.5. Ehrlich Solid Tumor Model
4.6. Treatment Groups
4.7. Tumor Development Assay
4.8. Histopathological Analyses
4.9. Lymphoid Organ Cellularity
4.10. Blood Samples
4.11. Cytokine Quantification
4.12. Extract Toxicity
4.13. In Vitro Cytoxicity against MCF7 Cells
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teixeira, T.R.; Santos, G.S.D.; Armstrong, L.; Colepicolo, P.; Debonsi, H.M. Antitumor Potential of Seaweed Derived-Endophytic Fungi. Antibiotics 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Jovanović, K.K.; Mojović, M.; Savić, A.G. New and highly potent antitumor natural products from marine-derived fungi: Covering the period from 2003 to 2012. Curr. Top. Med. Chem. 2013, 13, 2745–2766. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Kim, S.K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Cafêu, M.C.; Silva, G.H.; Teles, H.L.; Bolzani, V.S.; Araújo, A.R.; Young, M.C.M.; Pfenning, L.H. Substâncias antifúngicas de Xylaria sp., um fungo endofítico isolado de Palicourea marcgravii (Rubiaceae). Quim Nov. 2005, 28, 991–995. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004, 49, 201–241. [Google Scholar]
- Baker, C.J.O.; Shaban-Nejad, A.; Su, X.; Haarslev, V.; Butler, G. Semantic Web Infrastructure for Fungal Enzyme Biotechnologists. J. Web Semant. 2006, 4, 168–180. [Google Scholar] [CrossRef]
- Samson, R.A. Food and Indoor Fungi; Samson, R.A., Ed.; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010; Volume 2, 390p. [Google Scholar]
- Chai, Y.J.; Cui, C.B.; Li, C.W.; Wu, C.J.; Tian, C.K.; Hua, W. Activation of the dormant secondary metabolite production by introducing gentamicin-resistance in a marine-derived P. purpurogenum G59. Mar. Drugs 2012, 10, 559–582. [Google Scholar] [CrossRef]
- Wu, C.J.; Yi, L.; Cui, C.B.; Li, C.W.; Wang, N.; Han, X. Activation of the silent secondary metabolite production by introducing neomycin-resistance in a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2015, 13, 2465–2487. [Google Scholar] [CrossRef]
- Cutrim, M.V.J.; Ferreira, F.S.; dos Santos, A.K.D.; Cavalcanti, L.F.; de Oliveira Araújo, B.; Gomes de Azevedo-Cutrim, A.C.; Lima Oliveira, A.L. Trophic state of an urban coastal lagoon (northern Brazil), seasonal variation of the phytoplankton community and environmental variables. Estuar. Coast. Shelf Sci. 2019, 217, 98–109. [Google Scholar] [CrossRef]
- Santos, J.M.O.; Moreira-Pais, A.; Neto, T.; Peixoto da Silva, S.; Oliveira, P.A.; Ferreira, R.; Mendes, J.; Bastos, M.M.S.M.; Lopes, C.; Casaca, F.; et al. Dimethylaminoparthenolide reduces the incidence of dysplasia and ameliorates a wasting syndrome in HPV16-transgenic mice. Drug Dev. Res. 2019, 80, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Fonseca, B.; Mestre, V.F.; Colaço, B.; Pires, M.J.; Martins, T.; Gil da Costa, R.M.; Neuparth, M.J.; Medeiros, R.; Moutinho, M.S.S.; Dias, M.I.; et al. Laurus nobilis (laurel) aqueous leaf extract’s toxicological and anti-tumor activities in HPV16-transgenic mice. Food Funct. 2018, 9, 4419–4428. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Ferreira, T.; Almeida, J.; Pires, M.J.; Colaço, A.; Lemos, S.; Gil da Costa, R.M.; Medeiros, R.; Bastos, M.M.S.M.; Neuparth, M.J.; et al. Dietary supplementation with the red seaweed Porphyra umbilicalis protects against DNA damage and pre-malignant dysplastic skin lesions in HPV-transgenic mice. Mar. Drugs 2019, 17, 615. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 coutries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.I.; Silva, A.; Gabriel, J.; Gil da Costa, R.; Gama, A.; Ferreira, R.; Oliveira, P.A.; Ginja, M. Ultrasonographic, thermographic and histologic evaluation of MNU-induced mammary tumors in female Sprague-Dawley rats. Biomed. Pharmacother. 2013, 67, 771–776. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.I.; Colaço, B.; Oliveira, P.A. Experimental mammarycarcinogenesis―Rat models. Life Sci. 2017, 173, 116–134. [Google Scholar]
- Segura, J.A.; Barbero, L.G.; Márquez, J. Ehrlich ascites tumour unbalances splenic cell populations and reduces responsiveness of T cells to Staphylococcus aureus enterotoxin B stimulation. Immunol. Lett. 2000, 74, 111–115. [Google Scholar] [CrossRef]
- Ali, A.D.; Badr El-Din, K.N.; Abou-El-Magd, F.R. Antioxidant and hepatoprotective activities of grape seeds and skin against Ehrlich solid tumor induced oxidative stress in mice. Egypt. J. Basic Appl. Sci. 2015, 2, 98–109. [Google Scholar] [CrossRef]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef]
- Fang, S.M.; Cui, C.B.; Li, C.W.; Wu, C.J.; Zhang, Z.J.; Li, L.; Huang, X.J.; Ye, W.C. Purpurogemutantin and purpurogemutantidin, new drimenyl cyclohexenone derivatives produced by a mutant obtained by diethyl sulfate mutagenesis of a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2012, 10, 1266–1287. [Google Scholar] [CrossRef] [PubMed]
- Natori, S.; Sakaki, S.; Kurata, H.; Udagawa, S.I.; Ichinoe, M. Production of rubratoxin B by Penicillium purpurogenum Stoll. Appl Microbiol. 1970, 19, 613–617. [Google Scholar] [CrossRef]
- Li, S.S.; Li, J.; Sun, J.; Guo, R.; Yu, L.Z.; Zhao, Y.F.; Zhu, Z.X.; Tu, P.F. Berkeleyacetal C, a meroterpenoid isolated from the fungus Penicillium purpurogenum MHZ 111, exerts anti-inflammatory effects via inhibiting NF-κB, ERK1/2 and IRF3 signaling pathways. Eur. J. Pharmacol. 2017, 814, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.C.; Cui, X.; He, X.; Ding, Z.; Zhu, T.; Tang, Y.; Li, D. Late-stage terpene cyclization by an integral membrane cyclase in the biosynthesis of isoprenoid epoxycyclohexenone natural products. Org. Lett. 2017, 19, 5376–5379. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Zhou, X.J.; Qin, X.C.; Mai, Y.X.; Lin, X.P.; Liao, S.R.; Yang, B.; Zhang, T.; Tu, Z.C.; Wang, J.F.; et al. Quinone/hydroquinone meroterpenoids with antitubercular and cytotoxic activities produced by the sponge-derived fungus Gliomastix sp. ZSDS1-F7. Nat. Prod. Res. 2017, 31, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Stierle, D.B.; Stierle, A.A.; Patacini, B. The berkeleyacetals, three meroterpenes from a deep water acid mine waste Penicillium. J. Nat. Prod. 2007, 70, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Etoh, T.; Kin, Y.P.; Tanaka, H.; Hayashi, M. Anti-inflammatory effect of berkeleyacetal C through the inhibition of interleukin-1 receptor-associated kinase-4 activity. Eur. J. Pharmacol. 2013, 698, 435–443. [Google Scholar] [CrossRef]
- Nagashima, H.; Goto, T. Calcium channel blockers verapamil and diltiazem impaired rubratoxin B-caused toxicity in HL60 cells. Toxicol. Lett. 2000, 118, 47–51. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Wang, Y.; Pei, Y. Anti-tumor effects of Rubratoxin B on cell toxicity, inhibition of cell proliferation, cytotoxic activity and matrix metalloproteinase-2,9. Toxicol. In Vitro 2007, 21, 646–650. [Google Scholar] [CrossRef]
- Nagashima, H. Rubratoxin-B-induced secretion of chemokine ligands of cysteine–cysteine motif chemokine receptor 5 (CCR5) and its dependence on heat shock protein 90 in HL60 cells. Environm. Toxicol. Pharmacol. 2015, 40, 997–1000. [Google Scholar] [CrossRef]
- Qin, X.J.; Yu, Q.; Yan, H.; Khan, A.; Feng, M.Y.; Li, P.P.; Hao, X.J.; An, L.K.; Liu, H.Y. Meroterpenoids with Antitumor Activities from Guava (Psidium guajava). J. Agric. Food Chem. 2017, 65, 4993–4999. [Google Scholar] [CrossRef]
- Sharma, S.H.; Thulasingam, S.; Nagarajan, S. Terpenoids as anti-colon cancer agents―A comprehensive review on its mechanistic perspectives. Eur. J. Pharmacol. 2017, 795, 169–178. [Google Scholar] [CrossRef]
- Qin, F.Y.; Yan, Y.M.; Tu, Z.C.; Cheng, Y.X. Meroterpenoid dimers from Ganoderma cochlear and their cytotoxic and COX-2 inhibitory activities. Fitoterapia 2018, 129, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.P.; Brissow, E.; Kellner Filho, L.C.; Senabio, J.; Siqueira, K.A.; Vandresen Filho, S.; Damasceno, J.L.; Mendes, S.A.; Tavares, D.C.; Magalhaes, L.G.; et al. Bioactive compounds of Aspergillus terreus—F7, an endophytic fungus from Hyptis suaveolens (L.) Poit. World J. Microbiol. Biotechnol. 2017, 33, 62. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.J.; Sauaia Filho, E.N.; Nascimento, F.R.F.; Silva Júnior, F.C.; Fialho, E.D.S.; Santos, R.H.P.; Santos, R.A.P.; Bogea Serra, I.C.P. Use of raw Euphorbia tirucalli extract for inhibition of ascitic Ehrlich tumor. Rev. Col. Bras. Cir. 2016, 43, 18–21. [Google Scholar] [CrossRef]
- Bianchi-Frias, D.; Damodarasamy, M.; Hernandez, S.A.; Gil da Costa, R.M.; Vakar-Lopez, F.; Coleman, I.; Reed, M.J.; Nelson, P.S. The aged microenvironment influences the tumorigenic potential of malignant prostate epithelial cells. Mol. Cancer Res. 2019, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.M.G.; Bastos, M.M.S.M.; Medeiros, R.; Oliveira, P.A. The NFkB signalling pathway in papillomavirus-induced lesions: Friend or foe? Anticancer Res. 2016, 36, 2073–2083. [Google Scholar]
- Gupta, I.; Burney, I.; Al-Moundhri, M.S.; Tamimi, Y. Molecular genetics complexity impeding research progress in breast and ovarian cancers. Mol. Clin. Onc. 2017, 7, 3–14. [Google Scholar] [CrossRef]
- Zbakh, H.; Zubía, E.; de Los Reyes, C.; Calderón-Montaño, J.M.; López-Lázaro, M.; Motilva, V. Meroterpenoids from the Brown Alga Cystoseira usneoides as Potential Anti-Inflammatory and Lung Anticancer Agents. Mar. Drugs 2020, 18, 207. [Google Scholar] [CrossRef]
- Darsih, C.; Prachyawarakorn, V.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cytotoxic metabolites from the endophytic fungus Penicillium chermesinum: Discovery of a cysteine-targeted Michael acceptor as a pharmacophore for fragment-based drug discovery, bioconjugation and click reactions. RSC Adv. 2015, 5, 70595–70603. [Google Scholar] [CrossRef]
- Wan, H.; Li, J.; Zhang, K.; Zou, X.; Ge, L.; Zhu, F.; Zhou, H.; Gong, M.; Wang, T.; Chen, D.; et al. A new meroterpenoid functions as an anti-tumor agent in hepatoma cells by downregulating mTOR activation and inhibiting EMT. Sci. Rep. 2018, 8, 13152. [Google Scholar] [CrossRef]
- McCoy, M.K.; Ruhn, K.A.; Blesch, A.; Tansey, M.G. TNF: A Key Neuroinflammatory Mediator of Neurotoxicity and Neurodegeneration in Models of Parkinson’s Disease. Adv. Exp. Med. Biol. 2011, 691, 539–540. [Google Scholar]
- Yoon, W.J.; Ham, Y.M.; Kim, S.S.; Yoo, B.S.; Moon, J.Y.; Baik, J.S.; Lee, N.H.; Hyun, C.G. Supressão de citocinas pró-inflamatórias, iNOS e expressão de COX-2 por algas marrons Sargassum micracanthum em macrófagos RAW264.7. Eurásia J. Biosci. 2009, 3, 130–143. [Google Scholar] [CrossRef]
- Schefer, F.A.; Ricardo, S.; Blind, C.L.Z.; de Oliveira Souza, B.L.; Filippin, M.F.B.; Weber, B.M.; Orofino, K.M.R. Antitumoral activity of sesquiterpene lactone diacethylpiptocarphol in mice. J. Ethnopharmacol. 2017, 198, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Calixto-Campos, C.; Corrêa, M.P.; Carvalho, T.T.; Zarpelon, A.C.; Hohmann, M.S.N.; Rossaneis, A.C.; Ceolho-Silva, L.; Pavanelli, W.R.; Pinge-Filho, P.; Crespigio, J.; et al. Quercetin reduces Ehrlich tumor-induced cancer pain in mice. Anal. Cell. Pathol. 2015, 2015, 285708. [Google Scholar] [CrossRef]
- Aldubayan, M.A.; Elgharabawy, R.M.; Ahmed, A.S.; Tousson, E. Antineoplastic Activity and Curative Role of Avenanthramides against the Growth of Ehrlich Solid Tumors in Mice. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Harun, A.; Vidyadaran, S.; Meng, L.S.; Cole, A.L.J.; Ramasamy, K. Malaysian endophytic fungal extracts-induced anti-inflammation in lipopolysaccharide-activated BV-2 microglia is associated with attenuation of NO production and, IL-6 and TNF-α expression. BMC Compl. Alt. Med. 2015, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.C.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011, 117, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Hacker, U. Gewichtsverlust aus Onkologischer Sicht. Dtsch. Med. Wochenschr 2016, 141, 247–252. [Google Scholar] [CrossRef]
- Lau, S.K.M.; Iyengar, P. Implications of weight loss for cancer patients receiving radiotherapy. Curr. Opin. Support. Palliat. Care 2017, 11, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Hess, L.M.; Barakat, R.; Tian, C.; Ozols, R.F.; Alberts, D.S. Weight change during chemotherapy as a potential prognostic factor for stage III epithelial ovarian carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2007, 107, 260–265. [Google Scholar] [CrossRef]
- Moreira-Pais, A.; Ferreira, R.; da Costa, R.M.G. Platinum-induced muscle wasting in cancer chemotherapy: Mechanisms and potential targets for therapeutic intervention. Life Sci. 2018, 208, 1–9. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa e Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Dagli, M.L.Z.; Guerra, J.L.; Saldiva, P.H.N. An experimental study on the lymphatic dissemination of the solid Ehrlich tumor in mice. Braz. J. Vet. Res. An. Sci. 1992, 29, 97–103. [Google Scholar] [CrossRef]
- Fortes, T.S.; Fialho, S.E.M.; Reis, A.S.; Assuncão, A.K.M.; Azevedo, A.P.S.; Barroqueiro, E.S.B.; Guerra, R.N.M.; Nascimento, F.R.F. Desenvolvimento do tumor de Ehrlich em camundongos após tratamento in vitro com mesocarpo de babaçu Mart. Rev. Ciências Saúde 2009, 11, 101–105. [Google Scholar]
- Machado, J.L.; Assunção, A.K.; da Silva, M.C.; Dos Reis, A.S.; Costa, G.C.; de Sousa Arruda, D.; Rocha, B.A.; de Oliveira Lima Leite Vaz, M.M.; de Andrade Paes, A.M.; Guerra, R.N.M.; et al. Brazilian Green Propolis: Anti-Inflammatory Property by an Immunomodulatory Activity. Evid. Based Complement. Alternat. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef]
- Nascimento, F.R.; Cruz, G.V.; Pereira, P.V.; Maciel, M.C.; Silva, L.A.; Azevedo, A.P.S.; Barroqueiro, E.S.B.; Guerra, R.N.M. Ascitic and solid Ehrlich tumor inhibition by Chenopodium ambrosioides L. treatment. Life Sci. 2006, 78, 2650–2653. [Google Scholar] [CrossRef]
- Fialho, S.E.M.; Maciel, M.C.G.; Silva, A.C.B.; Reis, A.A.; Assuncao, A.K.M.; Fortes, T.S.; Silva, L.A.; Guerra, R.N.M.; Kwasniewski, F.H.; Nascimento, F.R.F. Immune cells recruitment and activation by Tityus serrulatus scorpion venom. Toxicon 2011, 1, 1–6. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Cook, H.C. Manual of Histological Techniques and Their Diagnostic Application; Churchill Livingstone: Edinburgh, UK, 1994; Volume 2, 457p. [Google Scholar]
- Conselho Nacional de Controle de Experimentação Animal. Available online: http://www.sbcal.org.br/conteudo/view?ID_CONTEUDO=41 (accessed on 18 August 2020).
- Teles, A.M.; Rosa, T.D.D.S.; Mouchrek, A.N.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Almeida-Souza, F. Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa Essential Oils: Chemical Composition, Antimicrobial and Antileishmanial Activity. Evid. Based Complement. Alternat. Med. 2019. [Google Scholar] [CrossRef]
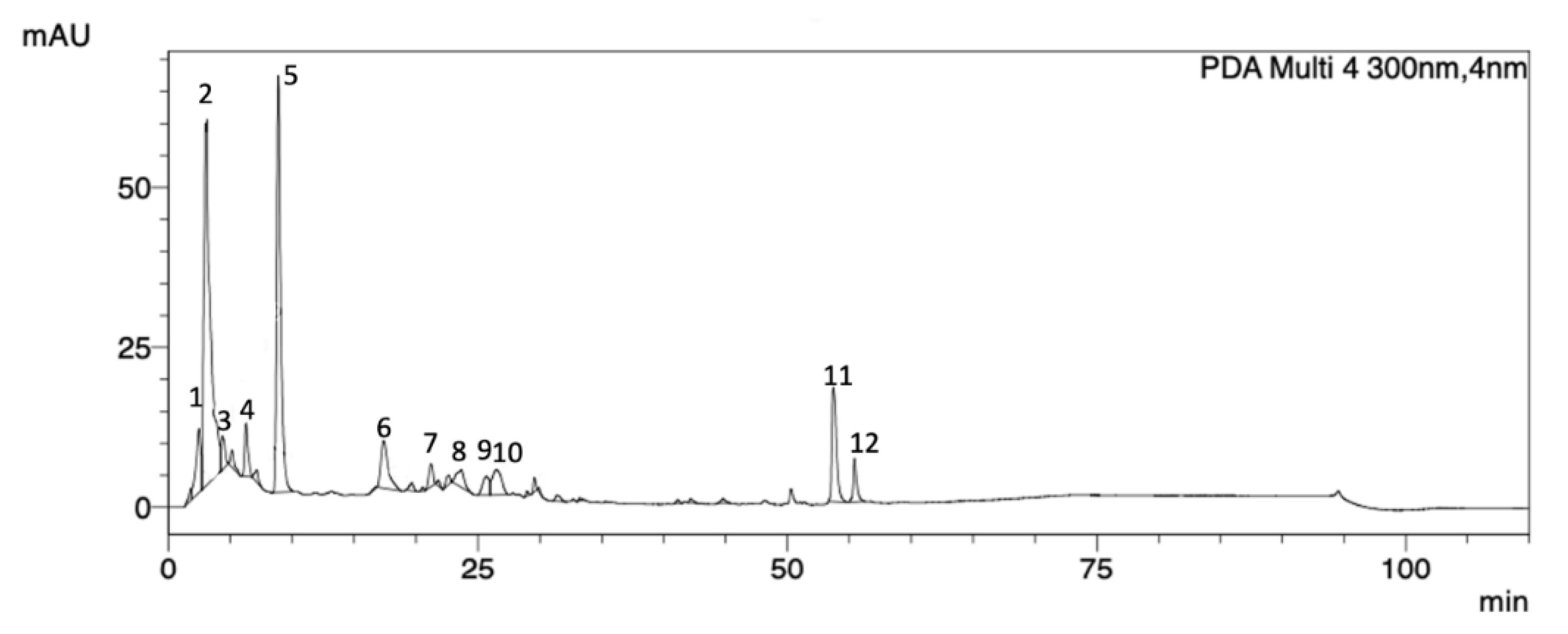
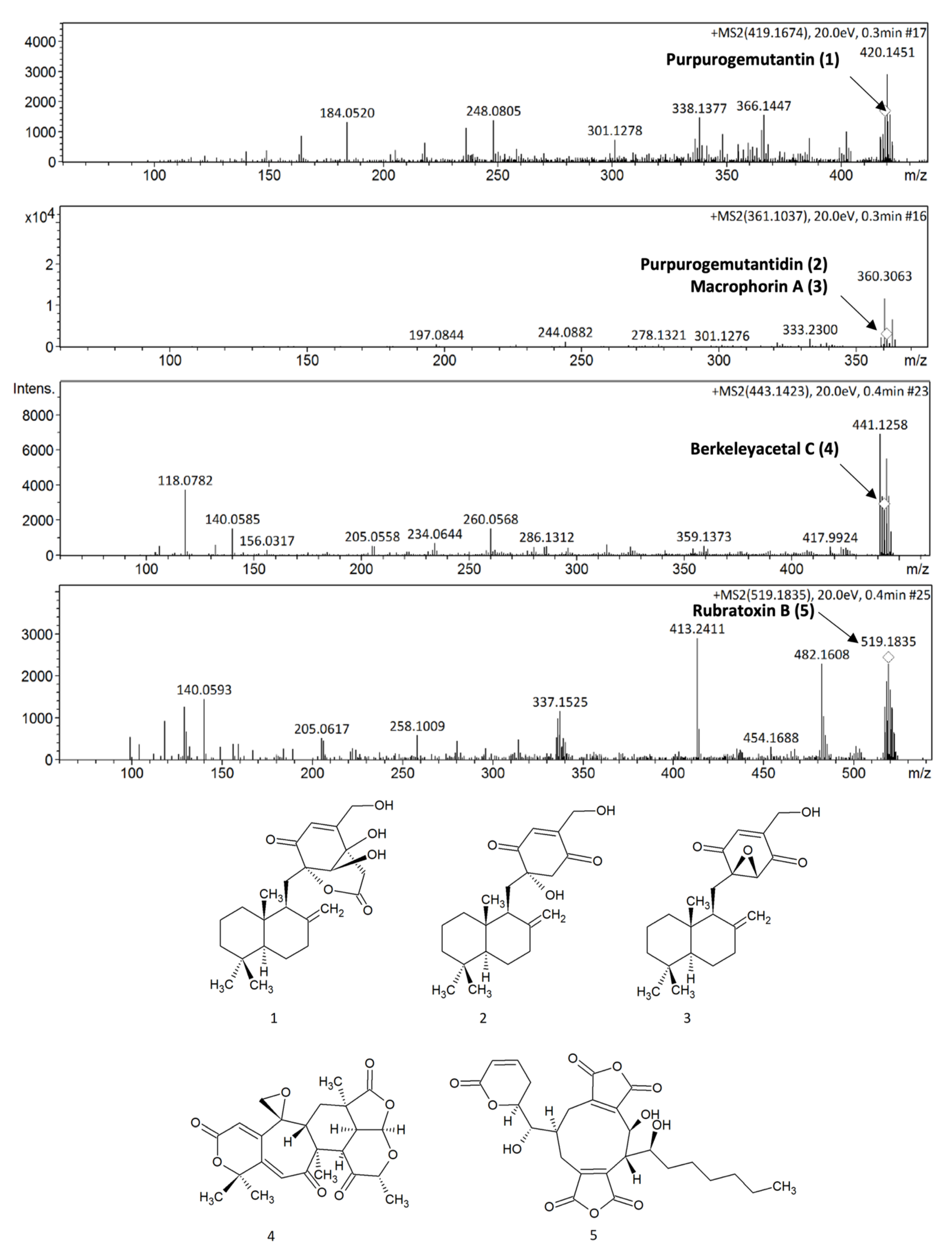
| Peak | Rt (min) | Area % | UV (λmax, nm) |
|---|---|---|---|
| 1 | 2.46 | 4.89 | 258 |
| 2 | 3.37 | 37.30 | 259 |
| 3 | 4.36 | 2.00 | 264 |
| 4 | 6.27 | 2.90 | 283 |
| 5 | 8.87 | 26.96 | 272 |
| 6 | 17.38 | 5.63 | 282 |
| 7 | 21.15 | 1.52 | 265 |
| 8 | 23.65 | 2.13 | 271 |
| 9 | 26.27 | 2.02 | 276 |
| 10 | 26.48 | 3.82 | 277 |
| 11 | 53.81 | 8.31 | 278 |
| 12 | 55.41 | 2.48 | 278 |
| Pleomorphism | Necrosis | Mitosis Figures | Inflammation | Invasion | |
|---|---|---|---|---|---|
| CLT− | 2.4 ± 0.511 | 2.4 ± 0.843 | 1.0 ± 1.155 | 2.8 ± 0.421 | 2.0 ± 0.666 |
| CLT+ | 0.2 ± 0.421 * | 0.2 ± 0.421 * | 0.0 ± 0.000 * | 1.0 ± 1.333 * | 0.6 ± 0.843 * |
| EAE4 | 2.2 ± 1.122 | 1.2 ± 1.033 | 1.0 ± 1.155 | 1.4 ± 1.075 | 1.0 ± 0.667 |
| EAE20 | 2.0 ± 1.155 | 0.6 ± 1.265 * | 0.2 ± 0.421 | 0.8 ± 0.788 * | 1.0 ± 0.667 |
| EAE100 | 0.8 ± 1.033 * | 0.2 ± 0.421 * | 0.0 ± 0.000 * | 0.6 ± 0.843 * | 0.4 ± 0.843 * |
| ALT (U/L) | AST (U/L) | |
|---|---|---|
| Sham | 125.2 ± 103.5 | 125.2 ± 103.5 |
| EAE20 * | 122.5 ± 101.5 | 295.3 ± 236.1 |
| CLT− | 207.9 ± 181.8 | 438.3 ± 328.5 |
| CLT+ | 438.3 ± 328.5 | 293 ± 131.8 |
| EAE4 + Tumor | 50.12 ± 33.38 | 178.6 ± 95.15 |
| EAE20 + Tumor | 68.3 ± 57.5 | 185.1 ± 111.2 |
| EAE100 + Tumor | 48.88 ± 21.68 | 179.1 ± 61.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teles, A.M.; Pontes, L.P.P.; Guimarães, S.J.A.; Butarelli, A.L.; Silva, G.X.; do Nascimento, F.R.F.; de Barros Bezerra, G.F.; Moragas-Tellis, C.J.; Costa, R.M.G.d.; da Silva, M.A.C.N.; et al. Marine-Derived Penicillium purpurogenum Reduces Tumor Size and Ameliorates Inflammation in an Erlich Mice Model. Mar. Drugs 2020, 18, 541. https://doi.org/10.3390/md18110541
Teles AM, Pontes LPP, Guimarães SJA, Butarelli AL, Silva GX, do Nascimento FRF, de Barros Bezerra GF, Moragas-Tellis CJ, Costa RMGd, da Silva MACN, et al. Marine-Derived Penicillium purpurogenum Reduces Tumor Size and Ameliorates Inflammation in an Erlich Mice Model. Marine Drugs. 2020; 18(11):541. https://doi.org/10.3390/md18110541
Chicago/Turabian StyleTeles, Amanda Mara, Leticia Prince Pereira Pontes, Sulayne Janayna Araújo Guimarães, Ana Luiza Butarelli, Gabriel Xavier Silva, Flavia Raquel Fernandes do Nascimento, Geusa Felipa de Barros Bezerra, Carla Junqueira Moragas-Tellis, Rui Miguel Gil da Costa, Marcos Antonio Custódio Neto da Silva, and et al. 2020. "Marine-Derived Penicillium purpurogenum Reduces Tumor Size and Ameliorates Inflammation in an Erlich Mice Model" Marine Drugs 18, no. 11: 541. https://doi.org/10.3390/md18110541
APA StyleTeles, A. M., Pontes, L. P. P., Guimarães, S. J. A., Butarelli, A. L., Silva, G. X., do Nascimento, F. R. F., de Barros Bezerra, G. F., Moragas-Tellis, C. J., Costa, R. M. G. d., da Silva, M. A. C. N., Almeida-Souza, F., Silva Calabrese, K. d., Azevedo-Santos, A. P. S., & Nascimento, M. d. D. S. B. (2020). Marine-Derived Penicillium purpurogenum Reduces Tumor Size and Ameliorates Inflammation in an Erlich Mice Model. Marine Drugs, 18(11), 541. https://doi.org/10.3390/md18110541








