Stereochemistry of Astaxanthin Biosynthesis in the Marine Harpacticoid Copepod Tigriopus Californicus
Abstract
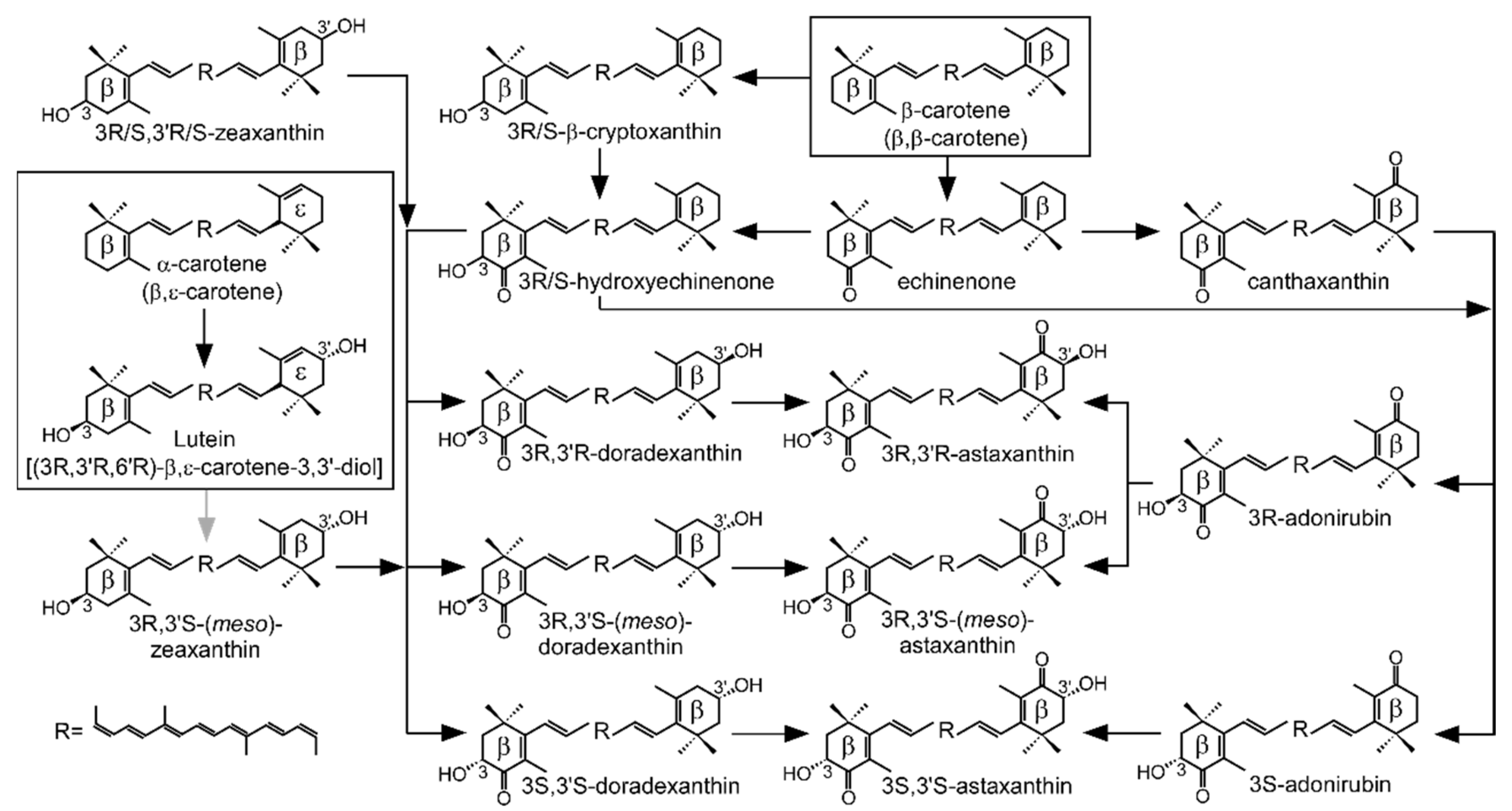
1. Introduction
2. Results
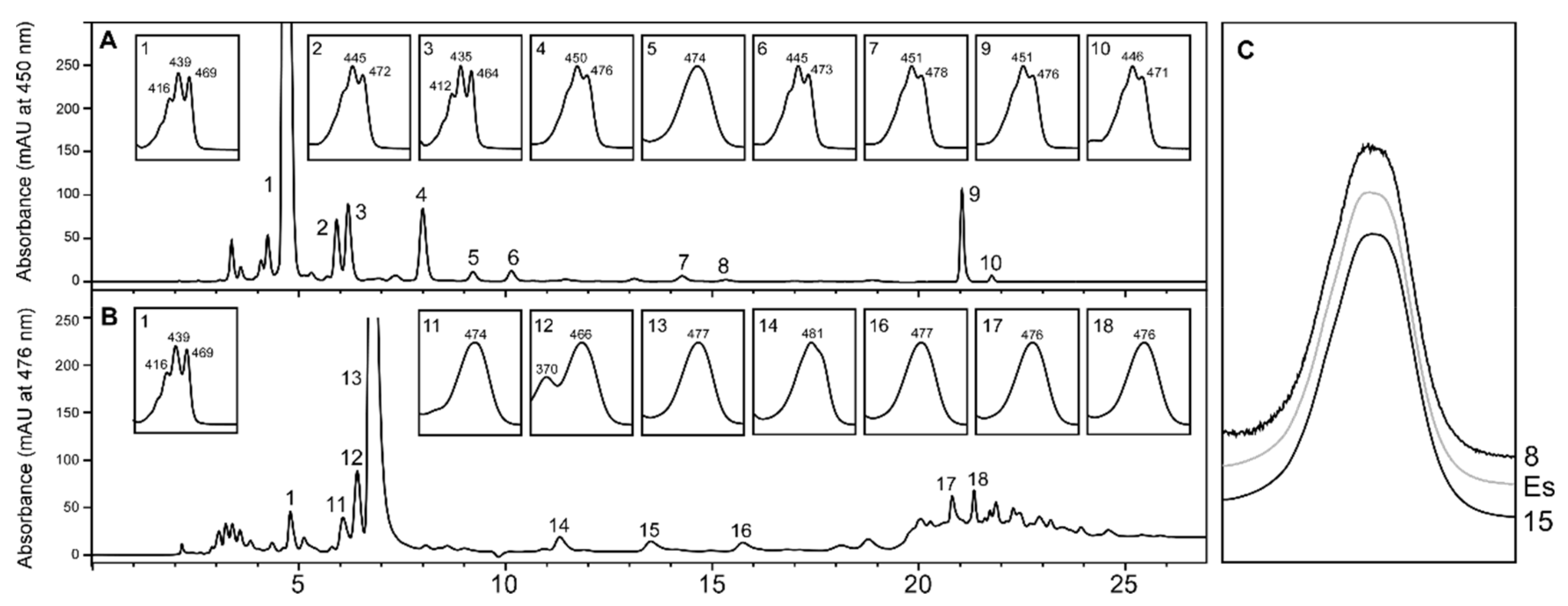
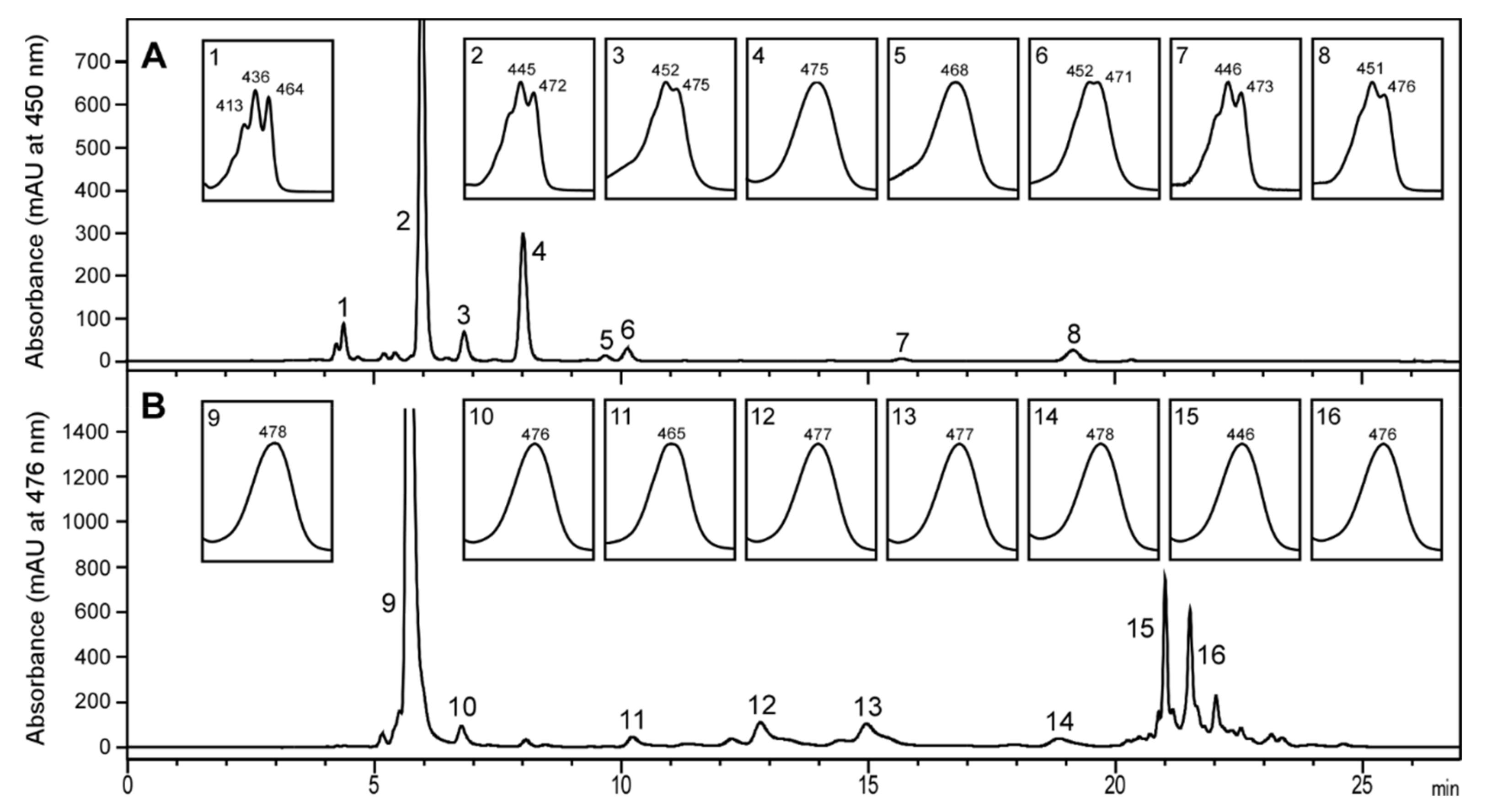
2.1. Carotenoid Profile of Co-Cultured Microalgae and Tigriopus Californicus
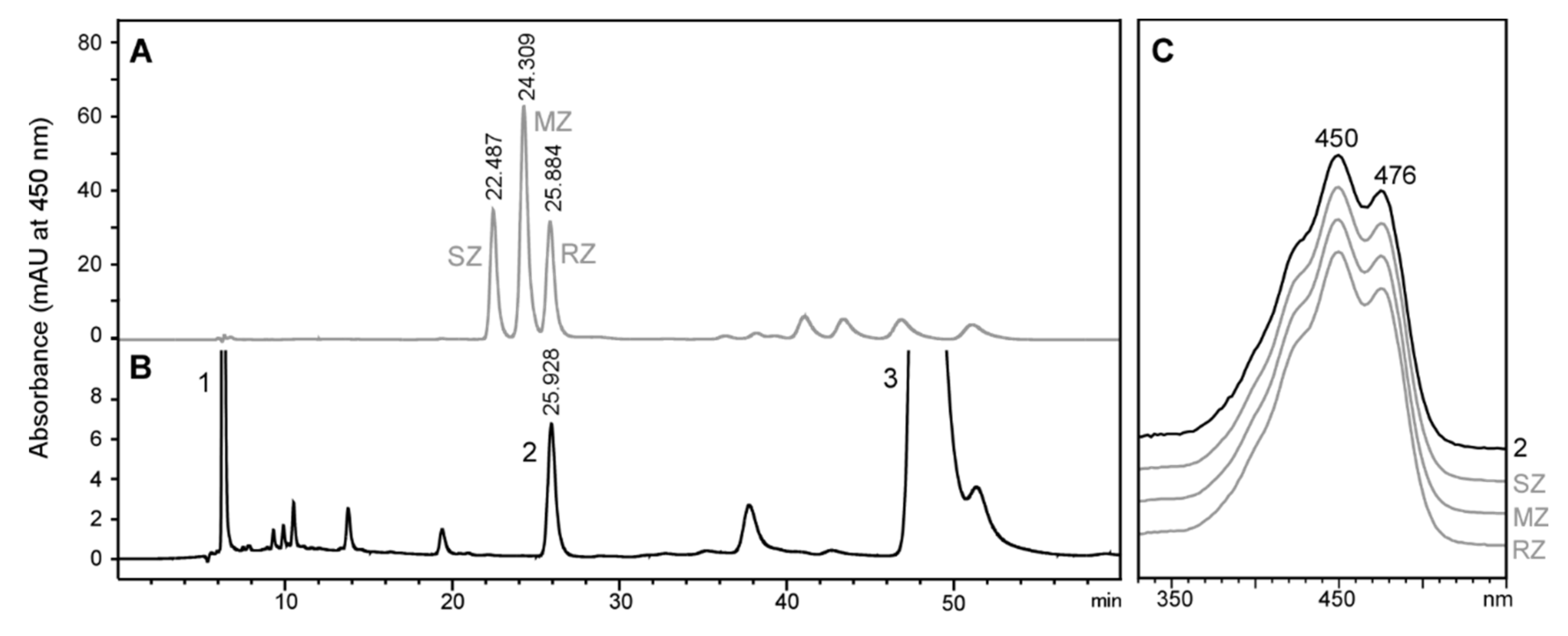
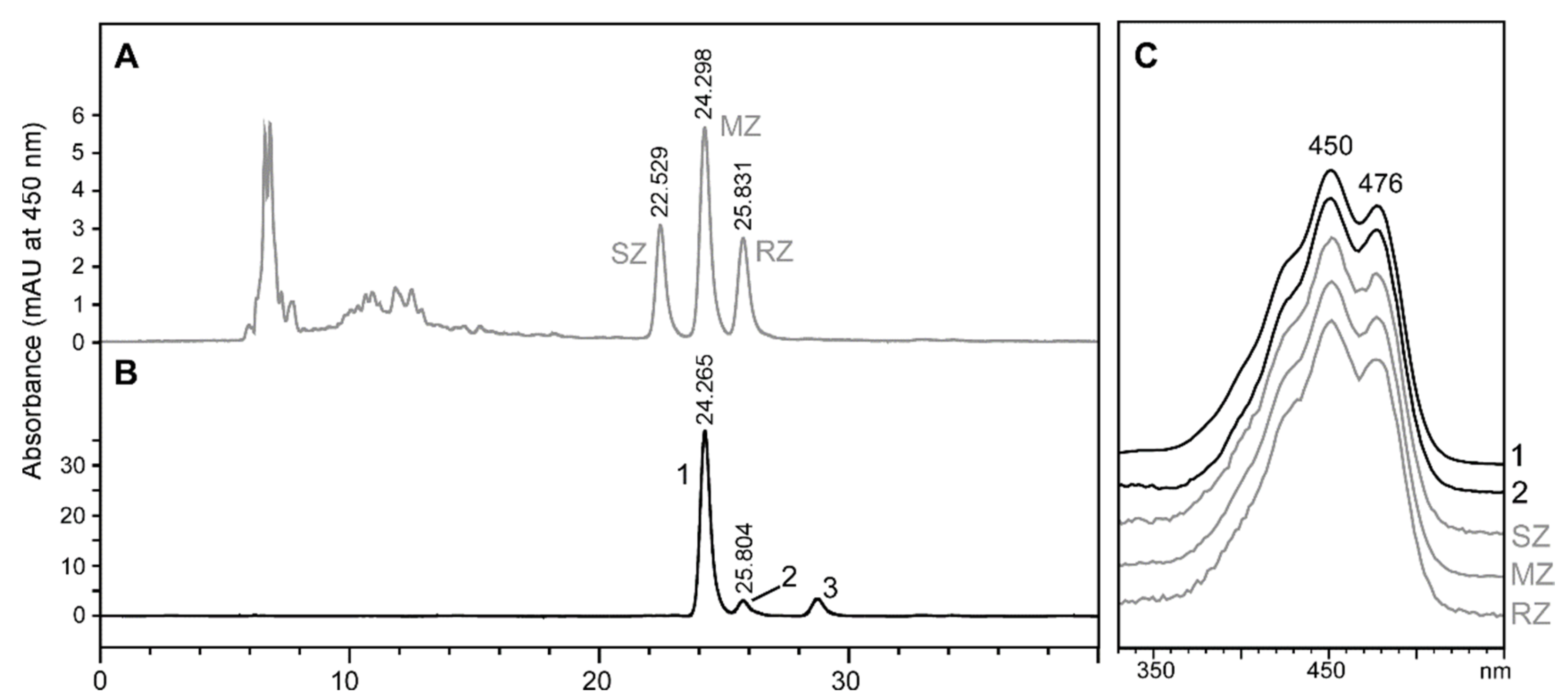
2.2. Stereochemistry of Zeaxanthin Produced by the Microalgae Culture
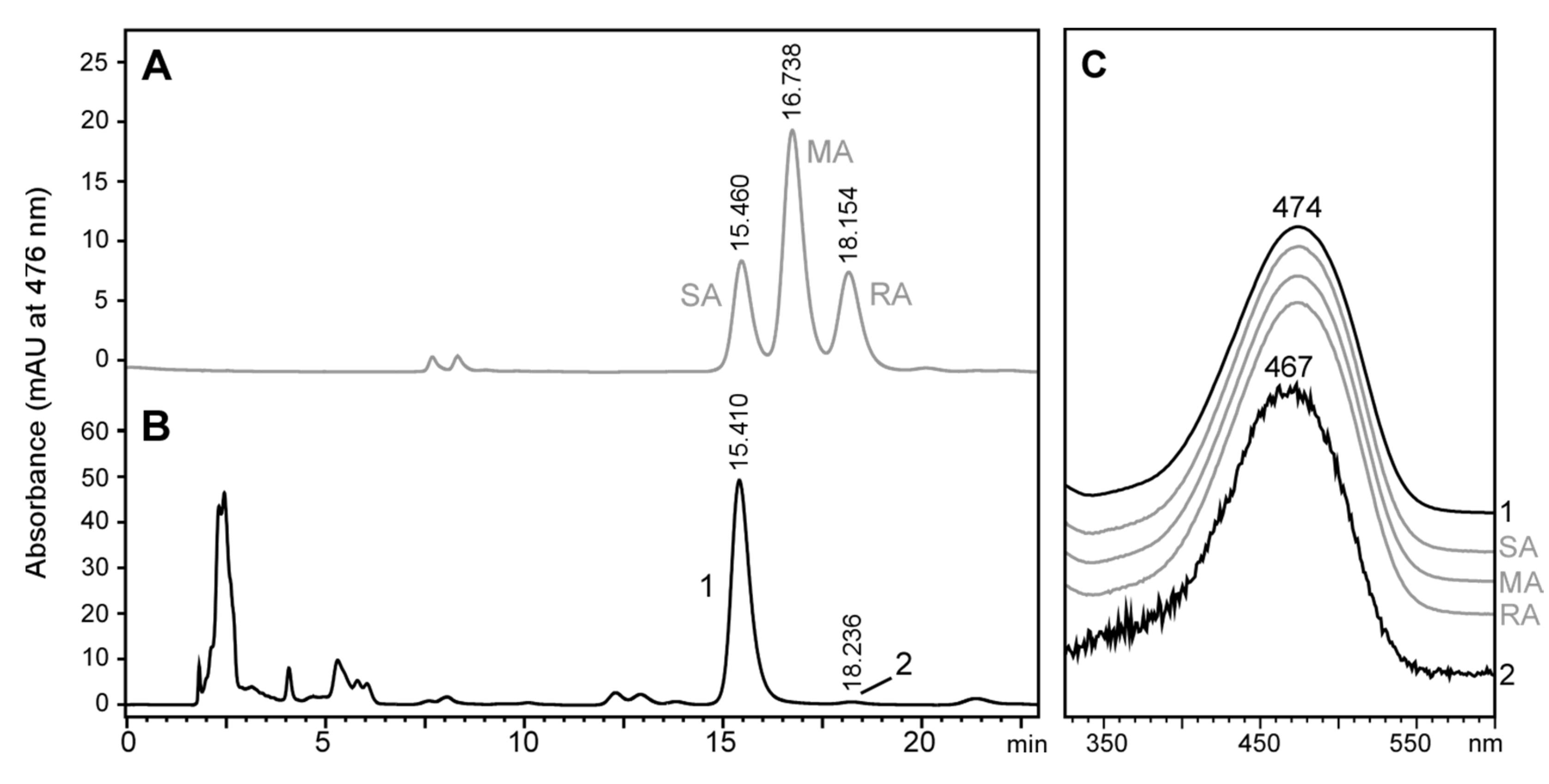
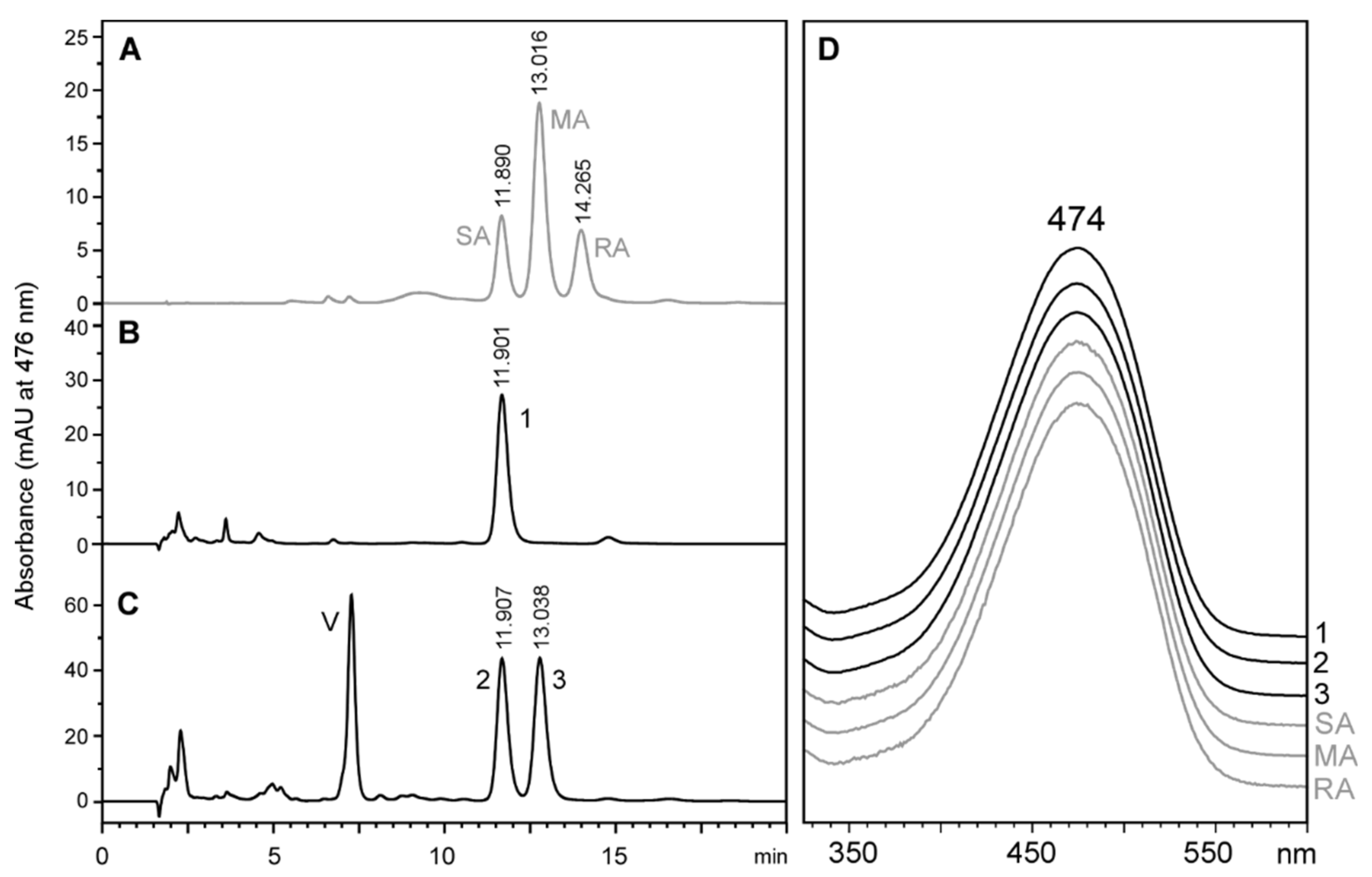
2.3. Stereochemistry of Astaxanthin Produced by Tigriopus Californicus
2.4. Astaxanthin Biosynthesis in Tigriopus Californicus Using Meso-Zeaxanthin as Precursor
2.5. Testing Lutein as a Precursor for Astaxanthin Biosynthesis in Tigriopus Californicus
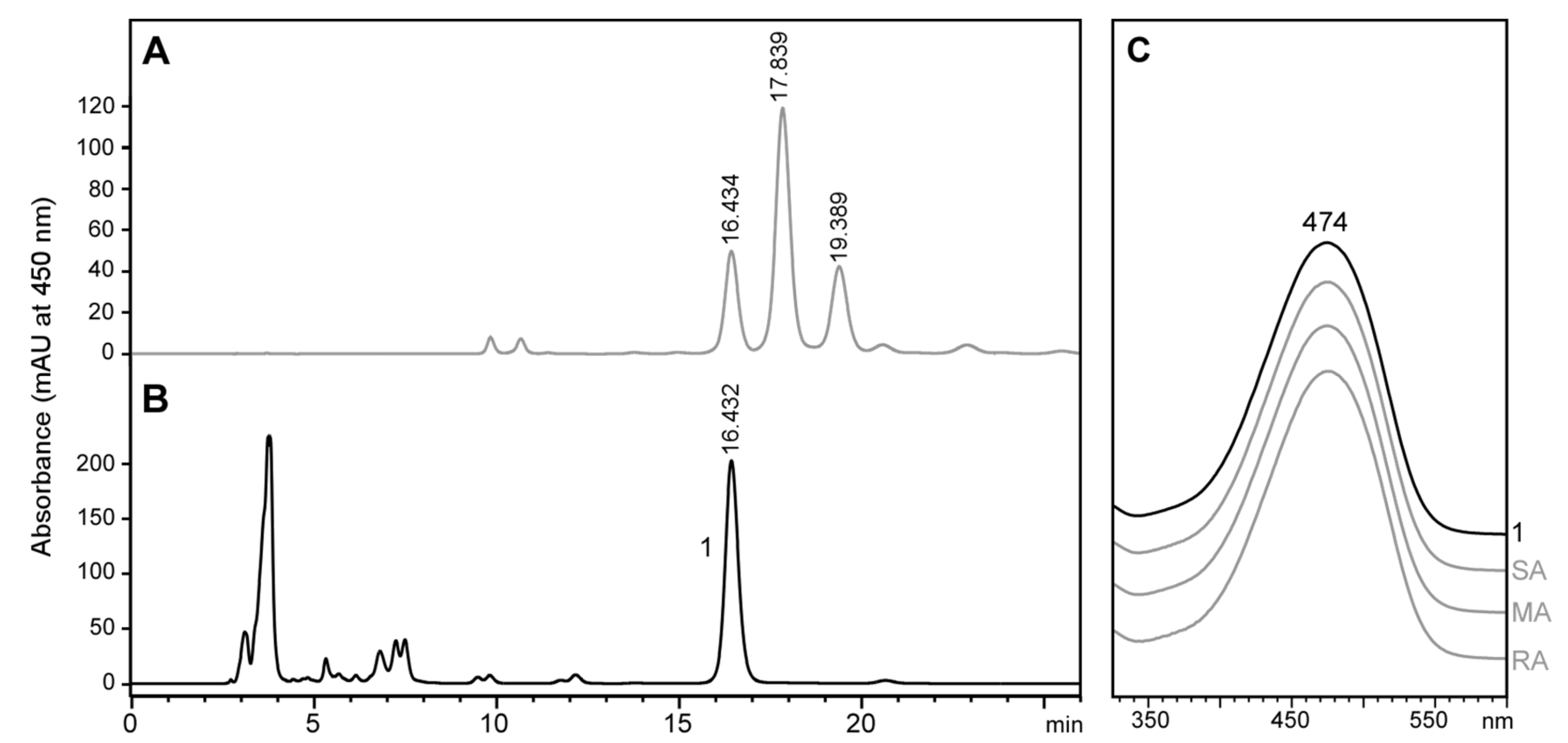
2.6. Stereochemistry of Astaxanthin in Tigriopus Californicus Feeding on Lutein-Rich Microalgae
3. Discussion
4. Materials and Methods
4.1. Organisms and Chemicals
4.2. Zooplankton Culture
4.2.1. Tigriopus Culture with Microalgae
4.2.2. Meso-Zeaxanthin Feeding Experiment
4.3. Carotenoid Extraction
4.3.1. Tigriopus
4.3.2. Microalgae
4.4. HPLC Analysis
4.4.1. System 1, Reverse Phase Carotenoid Analysis
4.4.2. System 2, Chiral Analysis of Zeaxanthin
4.4.3. System 3, Chiral Analysis of Astaxanthin
Author Contributions
Funding
Conflicts of Interest
References
- Chullasorn, S.; Dahms, H.U.; Klangsin, P. A new species of Tigriopus (Copepoda: Harpacticoida: Harpacticidae) from Thailand with a key to the species of the genus. J. Nat. Hist. 2013, 47, 427–447. [Google Scholar] [CrossRef]
- Raisuddin, S.; Kwok, K.W.; Leung, K.M.; Schlenk, D.; Lee, J.S. The copepod Tigriopus: A promising marine model organism for ecotoxicology and environmental genomics. Aquat. Toxicol. 2007, 83, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Fukusho, K. Mass production of a copepod, Tigriopus japonicus in combination culture with a rotifer Brachionus plicatilis, fed omega -yeast as a food source. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 625–629. [Google Scholar] [CrossRef]
- Hagiwara, A.; Kim, H.-J.; Matsumoto, H.; Ohta, Y.; Morita, T.; Hatanaka, A.; Ishizuka, R.; Sakakura, Y. Production and use of two marine zooplanktons, Tigriopus japonicus and Diaphanosoma celebensis, as live food for red sea bream Pagrus major larvae. Fish. Sci. 2016, 82, 799–809. [Google Scholar] [CrossRef]
- Kreeger, K.E.; Langdon, C.J.; Lowry, R.R. The nutritional value of Artemia and Tigriopus californicus (Baker) for two Pacific mysid species, Metamysidopsis elongata (Holmes) and Mysidopsis intii (Holmquist). J. Exp. Mar. Biol. Ecol. 1991, 148, 147–158. [Google Scholar] [CrossRef]
- Goodwin, T.W.; Srisukh, S. Some observations on astaxanthin distribution in marine crustacea. Biochem. J. 1949, 45, 268–270. [Google Scholar] [CrossRef]
- Wade, N.M.; Gabaudan, J.; Glencross, B.D. A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquacult. 2017, 9, 141–156. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquacult. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res.-Thessalon. 2014, 21, 6–16. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Yook, J.S.; Rakwal, R.; Shibato, J.; Takahashi, K.; Koizumi, H.; Shima, T.; Ikemoto, M.J.; Oharomari, L.K.; McEwen, B.S.; Soya, H. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA 2019, 116, 10988–10993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.-z.; Zhao, S.-h.; Ji, X.; Qiu, J.; Wang, J.; Zhou, Y.; Cai, Q.; Zhang, J.; Gao, H.-Q. Astaxanthin attenuated pressure overload-induced cardiac dysfunction and myocardial fibrosis: Partially by activating SIRT1. Biochim. Biophys. Actagen. Subj. 2017, 1861, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, Y.; Wu, Q.; Dai, H.; Li, W.; Lv, S.; Zhou, X.; Zhang, X.; Hang, C.; Wang, J. Astaxanthin mitigates subarachnoid hemorrhage injury primarily by increasing sirtuin 1 and inhibiting the Toll-like receptor 4 signaling pathway. Faseb J. 2019, 33, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, H.; Chen, J.; Zhang, L. Effects of Astaxanthin Supplementation on Oxidative Stress. Int. J. Vitam. Nutr. Res. 2020, 90, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ricardo, J.; Johnson, J.D.; Toomey, M.B.; Ferreira, M.S.; Araujo, P.M.; Melo-Ferreira, J.; Andersson, L.; Hill, G.E.; Corbo, J.C.; Carneiro, M. Genetic Basis for Red Coloration in Birds. Curr. Biol. 2016, 26, 1427–1434. [Google Scholar]
- Mundy, N.I.; Stapley, J.; Bennison, C.; Tucker, R.; Twyman, H.; Kim, K.-W.; Burke, T.; Birkhead, T.R.; Andersson, S.; Slate, J. Red Carotenoid Coloration in the Zebra Finch Is Controlled by a Cytochrome P450 Gene Cluster. Curr. Biol. 2016, 26, 1435–1440. [Google Scholar] [CrossRef]
- Weaver, R.J.; Gonzalez, B.K.; Santos, S.R.; Havird, J.C. Red Coloration in an Anchialine Shrimp: Carotenoids, Genetic Variation, and Candidate Genes. Biol. Bull. 2020, 238, 119–130. [Google Scholar] [CrossRef]
- Weaver, R.J.; Cobine, P.A.; Hill, G.E. On the bioconversion of dietary carotenoids to astaxanthin in the marine copepod, Tigriopus californicus. J. Plankton Res. 2018, 40, 142–150. [Google Scholar] [CrossRef]
- Caramujo, M.-J.; de Carvalho, C.C.C.R.; Silva, S.J.; Carman, K.R. Dietary carotenoids regulate astaxanthin content of copepods and modulate their susceptibility to UV light and copper toxicity. Mar. Drugs 2012, 10, 998–1018. [Google Scholar] [CrossRef]
- Mojib, N.; Amad, M.; Thimma, M.; Aldanondo, N.; Kumaran, M.; Irigoien, X. Carotenoid metabolic profiling and transcriptome-genome mining reveal functional equivalence among blue-pigmented copepods and appendicularia. Mol. Ecol. 2014, 23, 2740–2756. [Google Scholar] [CrossRef]
- Moretti, V.M.; Mentasti, T.; Bellagamba, F.; Luzzana, U.; Caprino, F.; Turchini, G.M.; Giani, I.; Valfrè, F. Determination of astaxanthin stereoisomers and colour attributes in flesh of rainbow trout (Oncorhynchus mykiss) as a tool to distinguish the dietary pigmentation source. Food Addit. Contam. 2006, 23, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Katsuyama, M.; Kaneko, N.; Matsuno, T. Stereochemical Investigation of Carotenoids in the Antarctic Krill Euphausia superba. Bull. Jap. Soc. Sci. Fish 1985, 51, 1671–1673. [Google Scholar] [CrossRef]
- Foss, P.; Renstrøm, B.; Liaaen-Jensen, S. Natural occurrence of enantiomeric and Meso-astaxanthin 7∗-crustaceans including zooplankton. Comp. Biochem. Physiol. B: Comp. Biochem. 1987, 86, 313–314. [Google Scholar] [CrossRef]
- Megdal, P.A.; Craft, N.A.; Handelman, G.J. A Simplified Method to Distinguish Farmed (Salmo salar) from Wild Salmon: Fatty Acid Ratios Versus Astaxanthin Chiral Isomers. Lipids 2009, 44, 569–576. [Google Scholar] [CrossRef]
- Turujman, S.A.; Wamer, W.G.; Wei, R.R.; Albert, R.H. Rapid Liquid Chromatographic Method to Distinguish Wild Salmon from Aquacultured Salmon Fed Synthetic Astaxanthin. J. Aoac Int. 2020, 80, 622–632. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Q.; Rakariyatham, K.; Cao, Y.; Goulette, T.; Liu, X.; Xiao, H. Antioxidation and anti-ageing activities of different stereoisomeric astaxanthin in vitro and in vivo. J. Funct. Foods 2016, 25, 50–61. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Weesepoel, Y.; Lamers, P.P.; Vincken, J.-P.; Martens, D.E.; Wijffels, R.H. Growth and pigment accumulation in nutrient-depleted Isochrysis aff. galbana T-ISO. J. Appl. Phycol. 2013, 25, 1421–1430. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Handbook; Birkhauuser Verlag: Basel, Switzerland, 2004. [Google Scholar]
- Bartlett, L.; Klyne, W.; Mose, W.P.; Scopes, P.M.; Galasko, G.; Mallams, A.K.; Weedon, B.C.L.; Szabolcs, J.; Tóth, G. Optical rotatory dispersion of carotenoids. J. Chem. Soc. C. Org. 1969, 19, 2527–2544. [Google Scholar] [CrossRef]
- Maoka, T.; Arai, A.; Shimizu, M.; Matsuno, T. The first isolation of enantiomeric and Meso-zeaxanthin in nature. Comp. Biochem. Physiol. B: Comp. Biochem. 1986, 83, 121–124. [Google Scholar] [CrossRef]
- Aasen, A.J.; Liaaen-Jensen, S.; Borch, G. Carotenoids of Flexibacteria V. The Chirality of Zeaxanthin from Different Natural Sources. Acta Chem. Scand. 1972, 26, 404–405. [Google Scholar] [CrossRef]
- Rhodes, A. Dietary Effects on Carotenoid Composition in the Marine Harpacticoid Copepod Nitokra Lacustris. J. Plankton Res. 2006, 29. [Google Scholar] [CrossRef]
- Hill, G.E.; Hood, W.R.; Ge, Z.; Grinter, R.; Greening, C.; Johnson, J.D.; Park, N.R.; Taylor, H.A.; Andreasen, V.A.; Powers, M.J.; et al. Plumage redness signals mitochondrial function in the house finch. Proc. R. Soc. B 2019, 286, 1354. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado-Cabrero, A.; Saefurahman, G.; Nolan, J.M. Stereochemistry of Astaxanthin Biosynthesis in the Marine Harpacticoid Copepod Tigriopus Californicus. Mar. Drugs 2020, 18, 506. https://doi.org/10.3390/md18100506
Prado-Cabrero A, Saefurahman G, Nolan JM. Stereochemistry of Astaxanthin Biosynthesis in the Marine Harpacticoid Copepod Tigriopus Californicus. Marine Drugs. 2020; 18(10):506. https://doi.org/10.3390/md18100506
Chicago/Turabian StylePrado-Cabrero, Alfonso, Ganjar Saefurahman, and John M. Nolan. 2020. "Stereochemistry of Astaxanthin Biosynthesis in the Marine Harpacticoid Copepod Tigriopus Californicus" Marine Drugs 18, no. 10: 506. https://doi.org/10.3390/md18100506
APA StylePrado-Cabrero, A., Saefurahman, G., & Nolan, J. M. (2020). Stereochemistry of Astaxanthin Biosynthesis in the Marine Harpacticoid Copepod Tigriopus Californicus. Marine Drugs, 18(10), 506. https://doi.org/10.3390/md18100506







