Recent Discovery of Heterocyclic Alkaloids from Marine-Derived Aspergillus Species
Abstract
1. Introduction
2. Structural Diversity
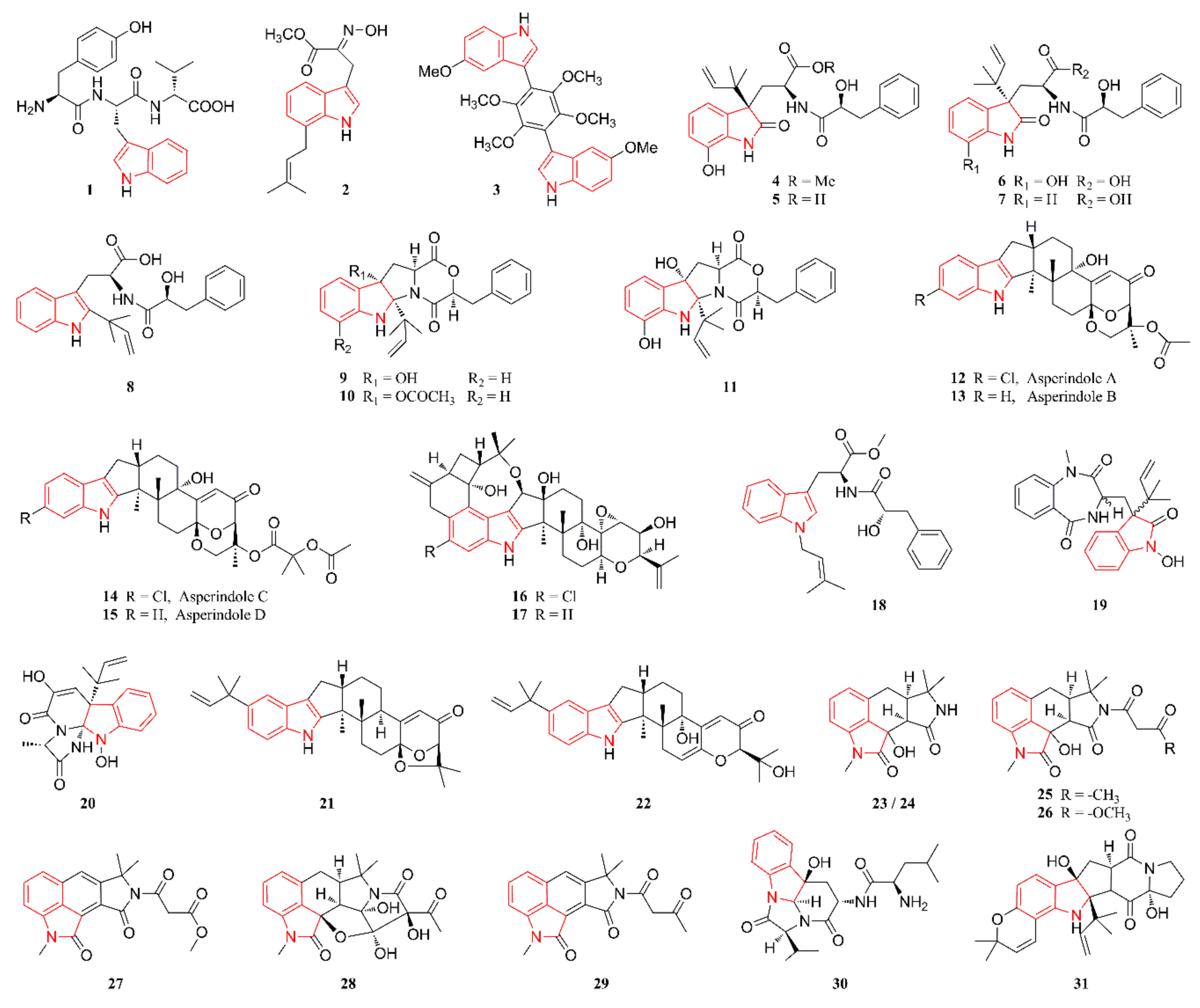
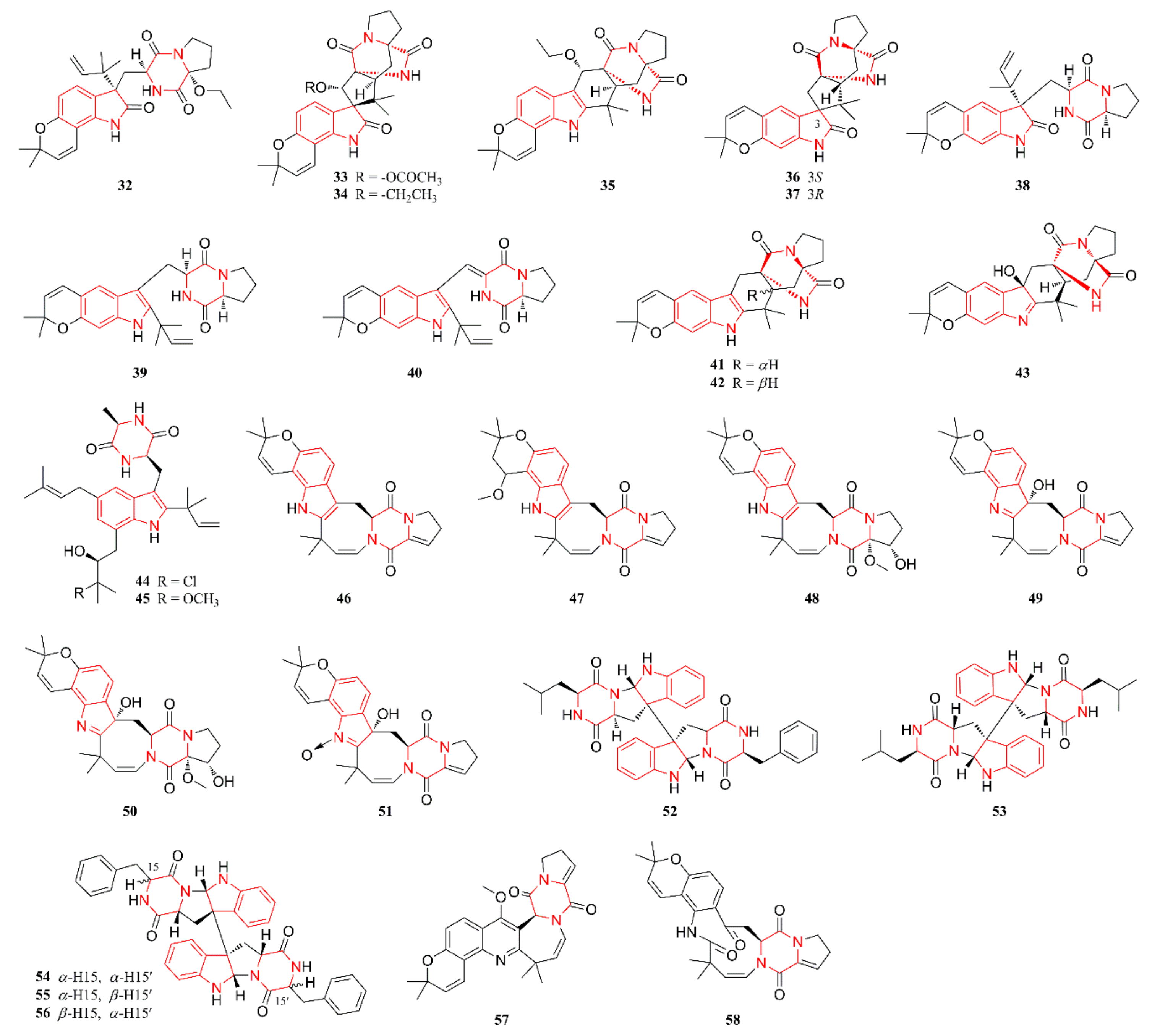
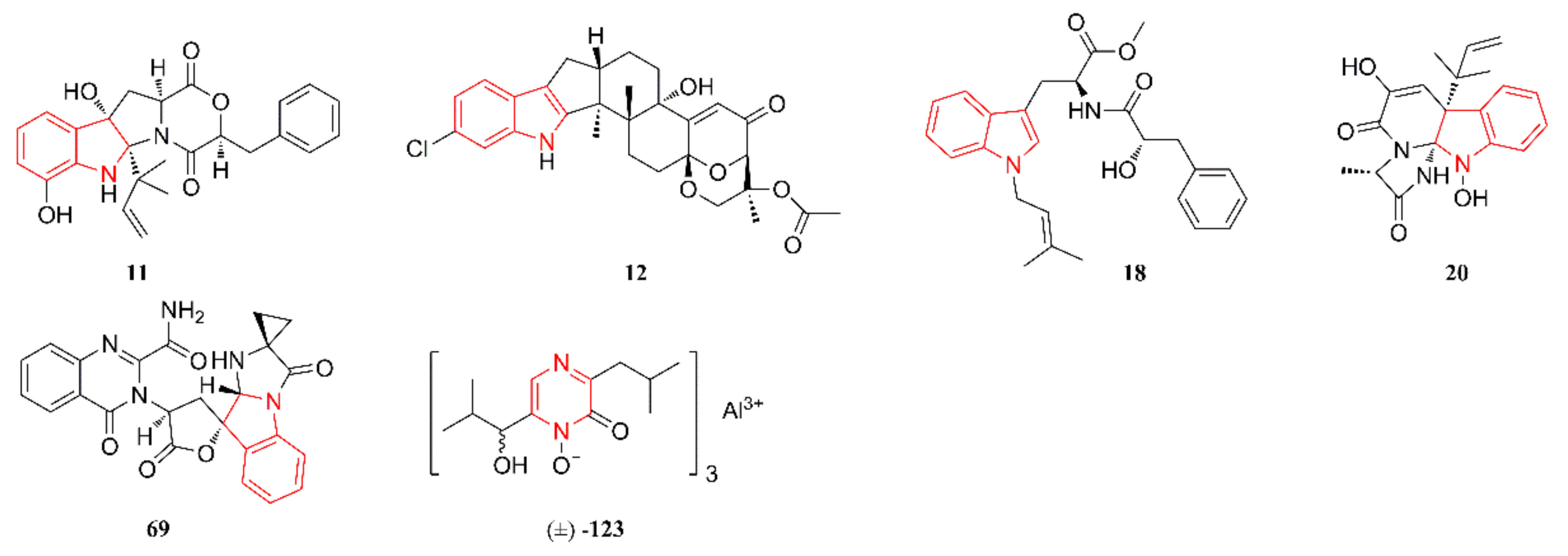
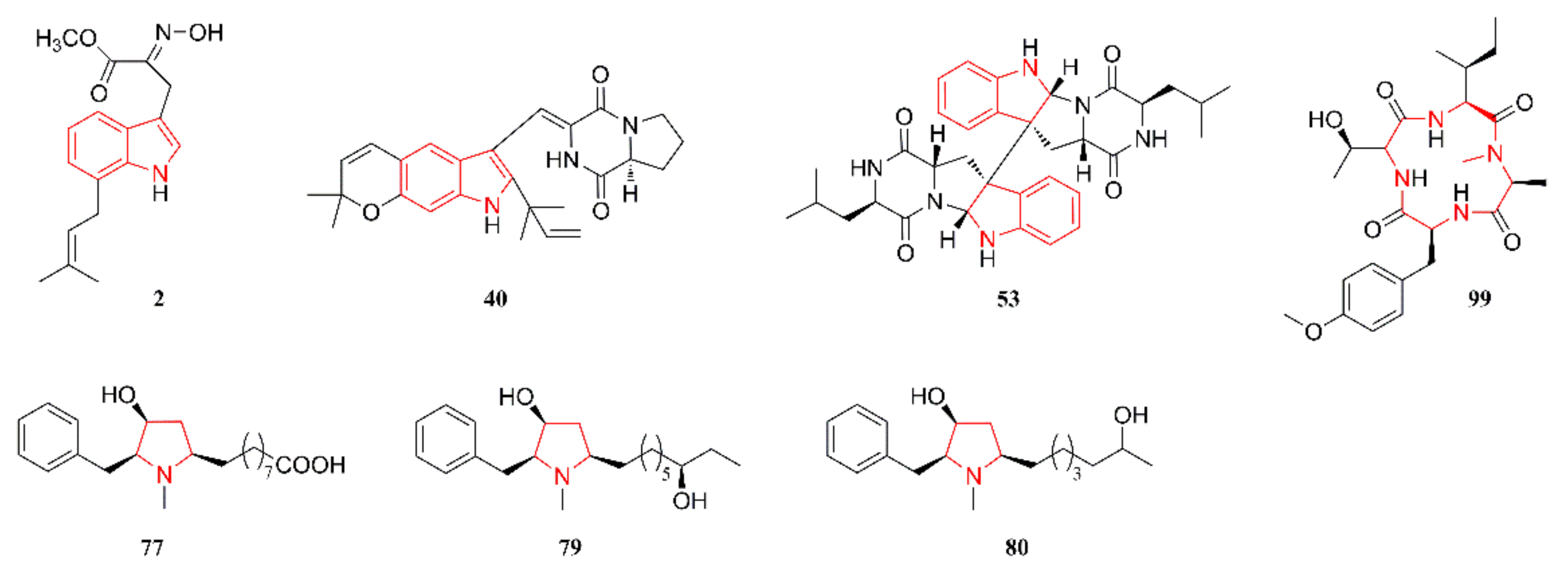
2.1. Indole Alkaloids
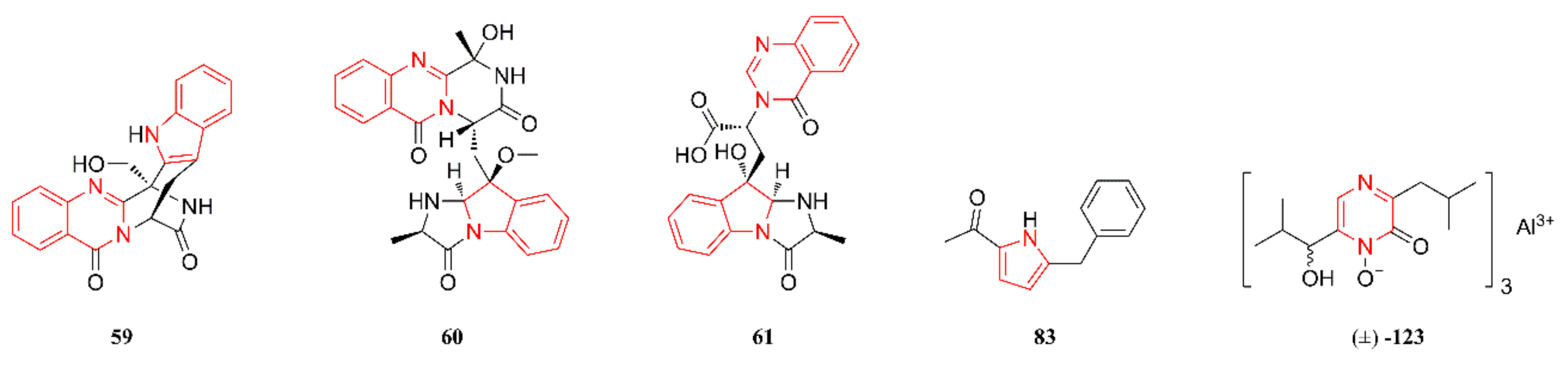
2.2. Diketopiperazine Alkaloids
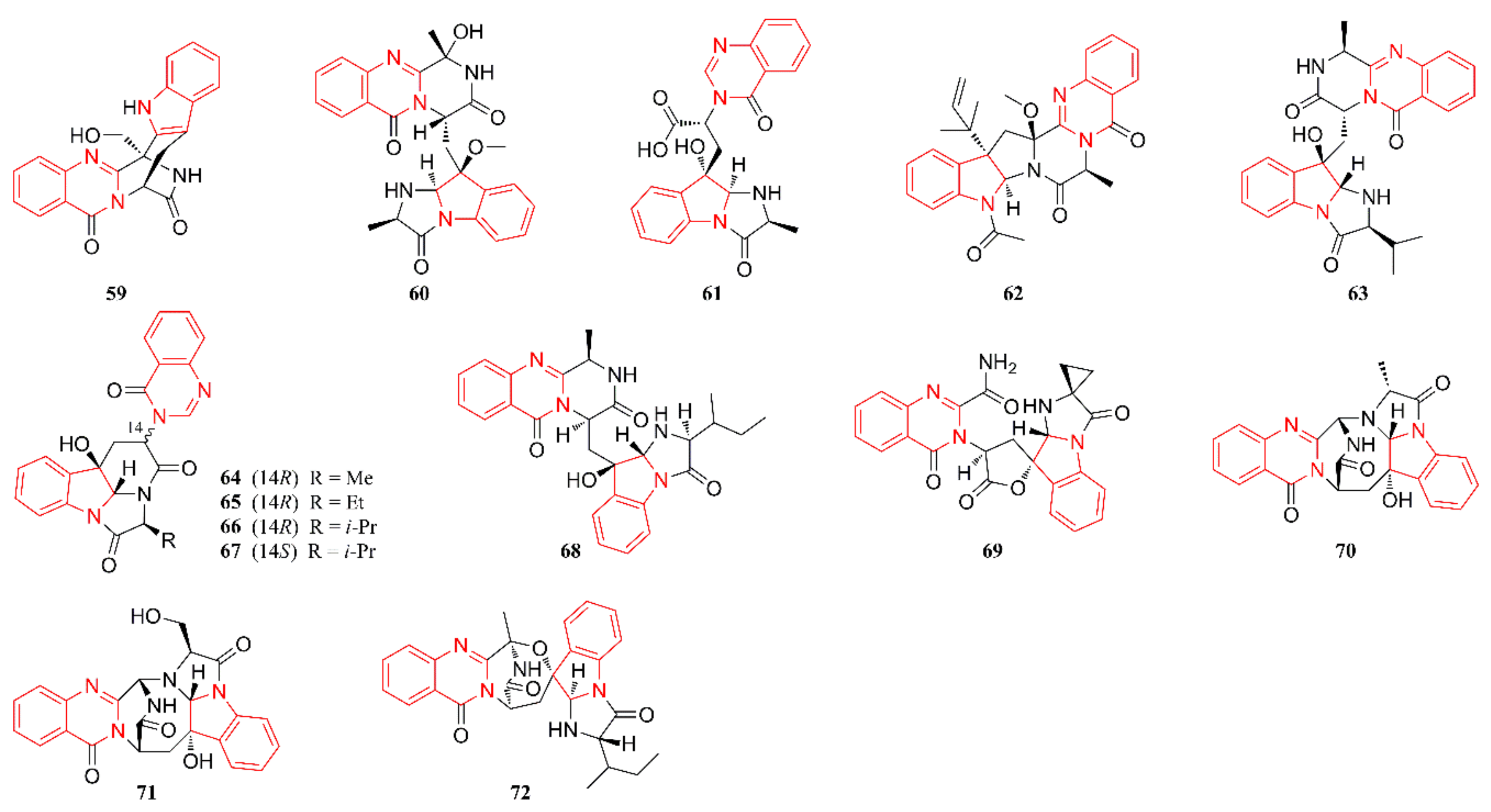
2.3. Quinazoline Alkaloids
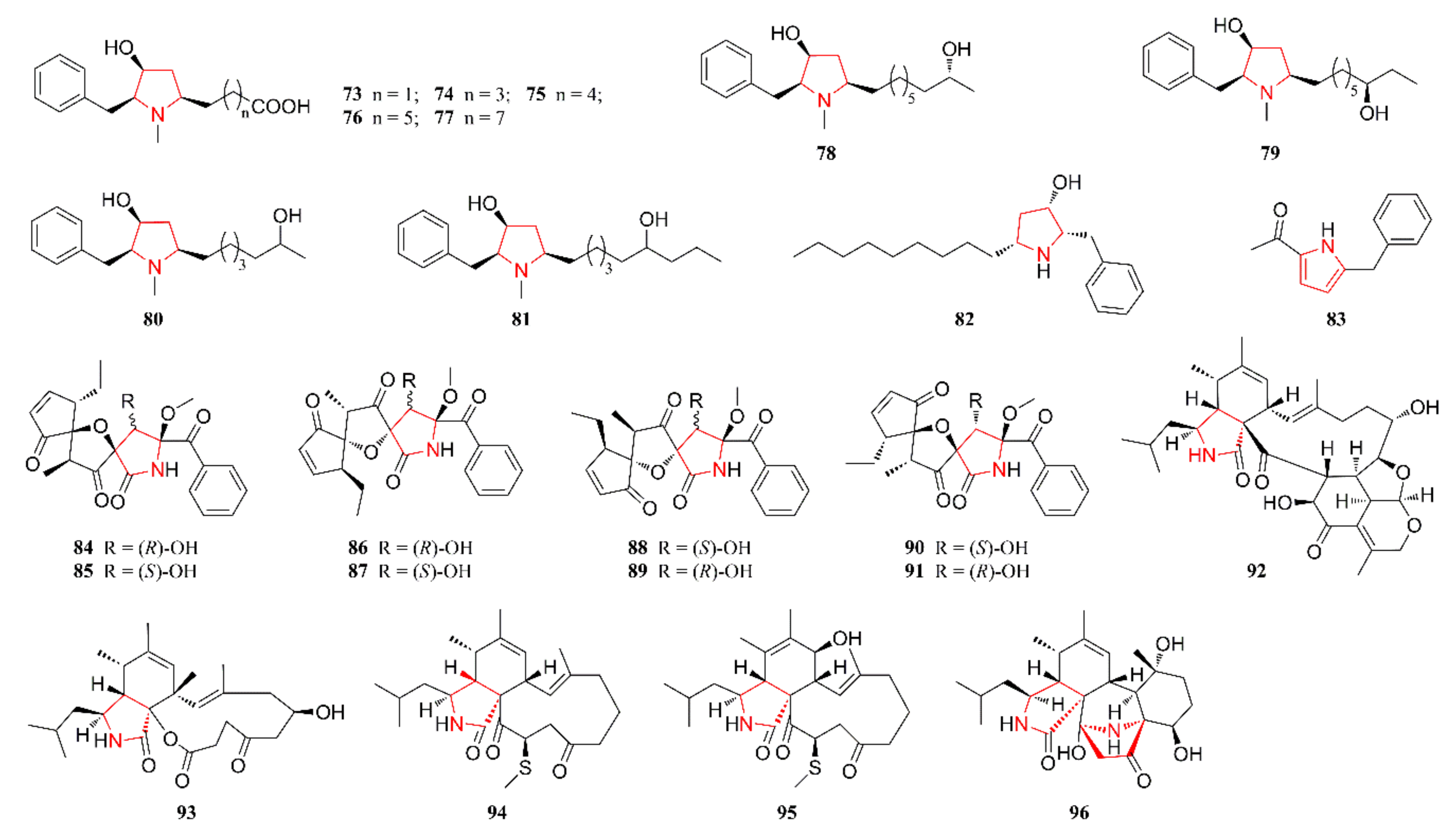
2.4. Pyrrolidine Alkaloids
2.5. Cyclopeptide Alkaloids
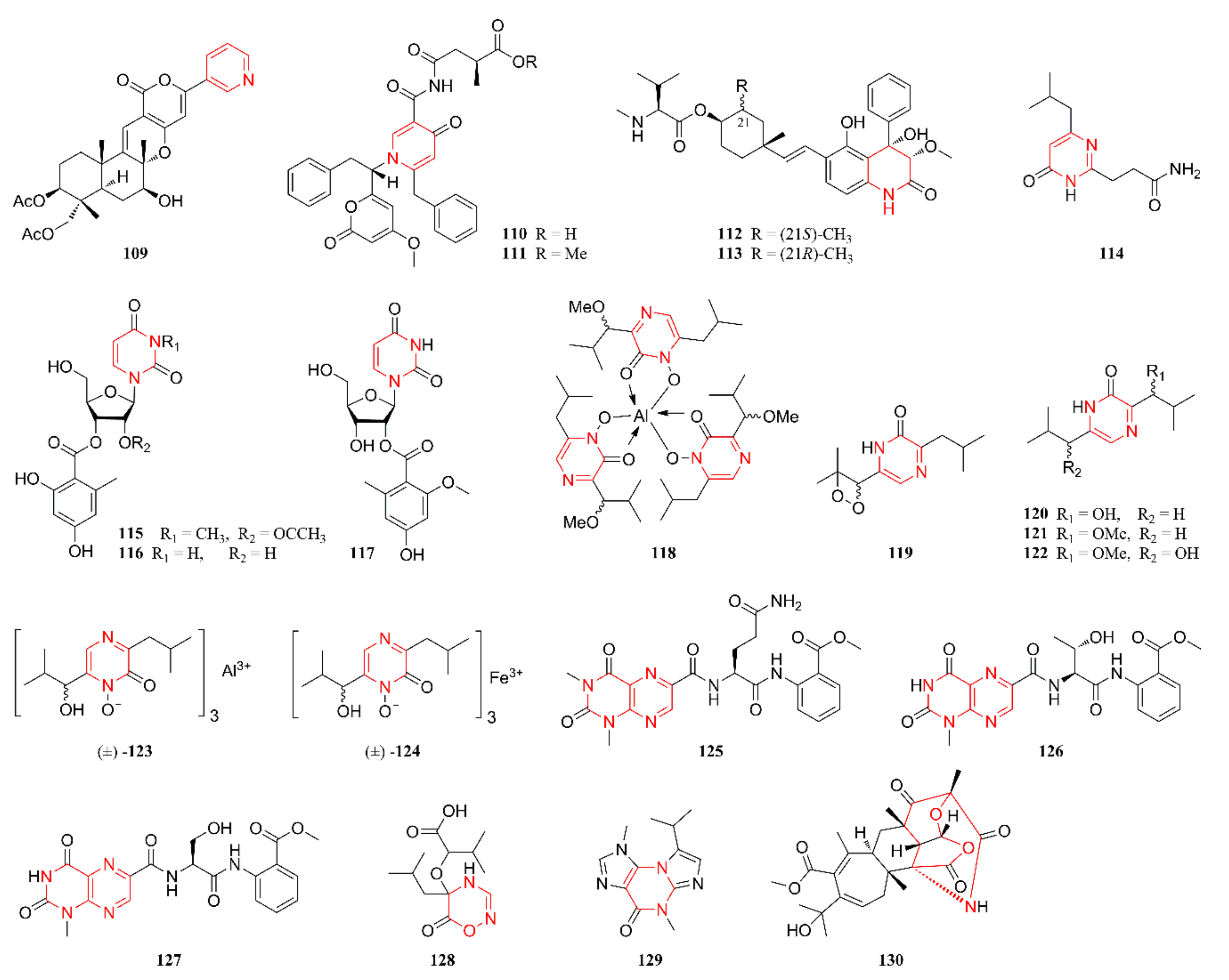
2.6. Other Heterocyclic Alkaloids
3. Production Environment
4. Biological Activities
4.1. Anticancer Activities
4.2. Antimicrobial Activities
4.3. Anti-Inflammatory Activities
4.4. Other Biological Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rida, P.C.; LiVecche, D.; Ogden, A.; Zhou, J.; Aneja, R. The noscapine chronicle: A pharmaco-historic biography of the opiate alkaloid family and its clinical applications. Med. Res. Rev. 2015, 35, 1072–1096. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.A.A.; de Oliveira Júnior, R.G.; Picot, L.; da Silva Almeida, J.R.G.; Nunes, X.P. Pre-clinical investigations of β-carboline alkaloids as antidepressant agents: A systematic review. Fitoterapia 2019, 137, 104196. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; Gloer, J.B.; Ji, N.Y.; Zhao, J.C. Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013, 113, 3632–3685. [Google Scholar] [CrossRef]
- Gogineni, V.; Schinazi, R.F.; Hamann, M.T. Role of marine natural products in the genesis of antiviral agents. Chem. Rev. 2015, 115, 9655–9706. [Google Scholar] [CrossRef]
- Bideau, F.L.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef]
- Soldatou, S.; Baker, B.J. Cold-water marine natural products, 2006 to 2016. Nat. Prod. Rep. 2017, 34, 585–626. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Guo, C.J.; Wang, C.C.C. Recent advances in genome mining of secondary metabolites in Aspergillus terreus. Front. Microbiol. 2014, 5, 717. [Google Scholar] [CrossRef]
- Yaegashi, J.; Oakley, B.R.; Wang, C.C.C. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans. J. Ind. Microbiol. Biotechnol. 2014, 41, 433–442. [Google Scholar] [CrossRef]
- Anyaogu, D.C.; Mortensen, U.H. Heterologous production of fungal secondary metabolites in Aspergilli. Front. Microbiol. 2015, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.W.; Ding, P. New bioactive metabolites from the marine-derived fungi Aspergillus. Mini-Rev. Med. Chem. 2018, 18, 1072–1094. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Moller, L.L.H.; Larsen, T.O.; Kumar, R.; Arnau, J. Safety of the fungal workhorses of industrial biotechnology: Update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reese. Appl. Microbiol. Biotechnol. 2018, 102, 9481–9515. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, B.; Chen, W.P.; Cox, R.J.; He, J.R.; Chen, F.S. Recent advances in reconstructing microbial secondary metabolites biosynthesis in Aspergillus spp. Biotechnol. Adv. 2018, 36, 739–783. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Nicoletti, R.; Salvatore, F.; Naviglio, D.; Andolfi, A. GC-MS approaches for the screening of metabolites produced by marine-derived Aspergillus. Mar. Chem. 2018, 206, 19–33. [Google Scholar] [CrossRef]
- Romsdahl, J.; Wang, C.C.C. Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012–2018). Medchemcomm 2019, 10, 840–866. [Google Scholar] [CrossRef]
- Xu, K.; Guo, C.; Meng, J.; Tian, H.; Guo, S.; Shi, D. Discovery of natural dimeric naphthopyrones as potential cytotoxic agents through ROS-mediated apoptotic pathway. Mar. Drugs 2019, 17, 207. [Google Scholar] [CrossRef]
- Soosaraei, M.; Khasseh, A.A.; Fakhar, M.; Hezarjaribi, H.Z. A decade bibliometric analysis of global research on leishmaniasis in Web of Science database. Ann. Med. Surg. (Lond.) 2018, 26, 30–37. [Google Scholar] [CrossRef]
- Chang, H.T.; Lin, M.H.; Hwang, I.H.; Chen, T.J.; Lin, H.C.; Hou, M.C.; Hwang, S.J. Scientific publications in gastroenterology and hepatology in Taiwan: An analysis of Web of Science from 1993 to 2013. J. Chin. Med. Assoc. 2017, 80, 80–85. [Google Scholar] [CrossRef][Green Version]
- Singh, T.P.; Singh, O.M. Recent progress in biological activities of indole and indole alkaloids. Mini-Rev. Med. Chem. 2018, 18, 9–25. [Google Scholar] [CrossRef]
- Li, S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010, 27, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Netz, N.; Opatz, T. Marine indole alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nong, X.H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Liu, M.T.; Sun, W.G.; Wang, J.P.; He, Y.; Zhang, J.W.; Li, F.L.; Qi, C.X.; Zhu, H.C.; Xue, Y.B.; Hu, Z.X.; et al. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bioorg. Chem. 2018, 80, 525–530. [Google Scholar] [CrossRef]
- Buttachon, S.; Ramos, A.A.; Inacio, A.; Dethoup, T.; Gales, L.; Lee, M.; Costa, P.M.; Silva, A.M.S.; Sekeroglu, N.; Rocha, E.; et al. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar. Drugs 2018, 16, 119. [Google Scholar] [CrossRef]
- Yang, S.Q.; Li, X.M.; Li, X.; Chi, L.P.; Wang, B.G. Two new diketomorpholine derivatives and a new highly conjugated ergostane-type steroid from the marine algal-derived endophytic fungus Aspergillus alabamensis EN-547. Mar. Drugs 2018, 16, 114. [Google Scholar] [CrossRef]
- Aparicio-Cuevas, M.A.; Rivero-Cruz, I.; Sanchez-Castellanos, M.; Menendez, D.; Raja, H.A.; Joseph-Nathan, P.; Gonzalez, M.D.; Figueroa, M. Dioxomorpholines and derivatives from a marine-facultative Aspergillus species. J. Nat. Prod. 2017, 80, 2311–2318. [Google Scholar] [CrossRef]
- Khalil, Z.G.; Huang, X.C.; Raju, R.; Piggott, A.M.; Capon, R.J. Shornephine A: Structure, chemical stability, and P-glycoprotein inhibitory properties of a rare diketomorpholine from an Australian marine-derived Aspergillus sp. J. Org. Chem. 2014, 79, 8700–8705. [Google Scholar] [CrossRef]
- Ivanets, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Rasin, A.B.; Zhuravleva, O.I.; Pivkin, M.V.; Popov, R.S.; von Amsberg, G.; Afiyatullov, S.S.; Dyshlovoy, S.A. Asperindoles A-D and a p-terphenyl derivative from the ascidian-derived fungus Aspergillus sp. KMM 4676. Mar. Drugs 2018, 16, 232. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.M.; Li, X.; Wang, B.G. New indole-diterpenoids from the algal-associated fungus Aspergillus nidulans. Phytochem. Lett. 2015, 12, 182–185. [Google Scholar] [CrossRef]
- Zhou, R.; Liao, X.J.; Li, H.B.; Li, J.; Peng, P.J.; Zhao, B.X.; Xu, S.H. Isolation and synthesis of misszrtine A: A novel indole alkaloid from marine sponge-associated Aspergillus sp. SCSIO XWS03F03. Front. Chem. 2018, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; Debbab, A.; Wray, V.; Lin, W.H.; Schulz, B.; Trepos, R.; Pile, C.; Hellio, C.; Proksch, P.; Aly, A.H. Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014, 55, 2789–2792. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Liu, D.; Cheng, W.; Proksch, P.; Lin, W.H. Versiquinazolines L-Q, new polycyclic alkaloids from the marine-derived fungus Aspergillus versicolor. RSC Adv. 2018, 8, 31427–31439. [Google Scholar] [CrossRef]
- Sun, K.L.; Li, Y.; Guo, L.; Wang, Y.; Liu, P.P.; Zhu, W.M. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs 2014, 12, 3970–3981. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Q.W.; Zhao, Y.Y.; Zheng, Q.H.; Liu, Q.Y.; Chen, L.; Zhang, Q.Q. Speradines B–E, four novel tetracyclic oxindole alkaloids from the marine-derived fungus Aspergillus oryzae. Heterocycles 2014, 89, 1662–1669. [Google Scholar]
- Hu, X.; Xia, Q.W.; Zhao, Y.Y.; Zheng, Q.H.; Liu, Q.Y.; Chen, L.; Zhang, Q.Q. Speradines F–H, three new oxindole alkaloids from the marine-derived fungus Aspergillus oryzae. Chem. Pharm. Bull. 2014, 62, 942–946. [Google Scholar] [CrossRef]
- Wang, P.M.; Zhao, S.Z.; Liu, Y.; Ding, W.J.; Qiu, F.; Xu, J.Z. Asperginine, an unprecedented alkaloid from the marine-derived fungus Aspergillus sp. Nat. Prod. Commun. 2015, 10, 1363–1364. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Berdyshev, D.V.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Gerasimenko, A.V.; von Amsberg, G.; Dyshlovoy, S.A.; et al. Prenylated indole alkaloids from co-culture of marine-derived fungi Aspergillus sulphureus and Isaria felina. J. Antibiot. 2018, 71, 846–853. [Google Scholar] [CrossRef]
- Ma, Y.M.; Liang, X.A.; Kong, Y.; Jia, B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef]
- Li, H.Q.; Sun, W.G.; Deng, M.Y.; Zhou, Q.; Wang, J.P.; Liu, J.J.; Chen, C.M.; Qi, C.X.; Luo, Z.W.; Xue, Y.B.; et al. Asperversiamides, linearly fused prenylated indole alkaloids from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2018, 83, 8483–8492. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, H.; Ko, W.; Kim, D.C.; Kim, K.W.; Kwon, H.C.; Guo, Y.Q.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; et al. Chemical constituents isolated from Antarctic marine-derived Aspergillus sp. SF-5976 and their anti-inflammatory effects in LPS-stimulated RAW 264.7 and BV2 cell. Tetrahedron 2017, 73, 3905–3912. [Google Scholar] [CrossRef]
- Peng, J.X.; Gao, H.Q.; Li, J.; Ai, J.; Geng, M.Y.; Zhang, G.J.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Prenylated indole diketopiperazines from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2014, 79, 7895–7904. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Sohn, J.H.; Oh, H. Isolation and structure determination of a new diketopiperazine dimer from marine-derived fungus Aspergillus sp. SF-5280. Nat. Prod. Res. 2018, 32, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Gu, B.B.; Yang, L.J.; Yang, F.; Lin, H.W. New anti-inflammatory cyclopeptides from a sponge-derived fungus Aspergillus violaceofuscus. Front. Chem. 2018, 6, 226. [Google Scholar] [CrossRef]
- Xu, J.Z.; Hu, Q.; Ding, W.J.; Wang, P.M.; Di, Y.N. New asymmetrical bispyrrolidinoindoline diketopiperazines from the marine fungus Aspergillus sp. DX4H. Nat. Prod. Res. 2018, 32, 815–820. [Google Scholar] [CrossRef]
- Limbadri, S.; Luo, X.W.; Lin, X.P.; Liao, S.R.; Wang, J.F.; Zhou, X.F.; Yang, B.; Liu, Y.H. Bioactive novel indole alkaloids and steroids from deep sea-derived fungus Aspergillus fumigatus SCSIO 41012. Molecules 2018, 23, 2379. [Google Scholar] [CrossRef]
- Liao, L.; You, M.; Chung, B.K.; Oh, D.C.; Oh, K.B.; Shin, J. Alkaloidal metabolites from a marine-derived Aspergillus sp. fungus. J. Nat. Prod. 2015, 78, 349–354. [Google Scholar] [CrossRef]
- Gu, B.B.; Jiao, F.R.; Wu, W.; Jiao, W.H.; Li, L.; Sun, F.; Wang, S.P.; Yang, F.; Lin, H.W. Preussins with inhibition of IL-6 expression from Aspergillus flocculosus 16D-1, a fungus isolated from the marine sponge Phakellia fusca. J. Nat. Prod. 2018, 81, 2275–2281. [Google Scholar] [CrossRef]
- Bao, J.; Wang, J.; Zhang, X.Y.; Nong, X.H.; Qi, S.H. New furanone derivatives and alkaloids from the co-culture of marine-derived fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017, 14, e1600327. [Google Scholar] [CrossRef]
- Yamada, T.; Kimura, H.; Arimitsu, K.; Kajimoto, T.; Kikuchi, T.; Tanaka, R. Absolute configuration of eight cephalimysins isolated from the marine-derived Aspergillus fumigatus. ChemistrySelect 2017, 2, 10936–10940. [Google Scholar] [CrossRef]
- Li, X.Y.; Ding, W.J.; Wang, P.M.; Xu, J.Z. Two novel aspochalasins from the gut fungus Aspergillus sp. Z4. Mar. Drugs 2018, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, S.Z.; Ding, W.J.; Wang, P.M.; Yang, X.W.; Xu, J.Z. Methylthio-aspochalasins from a marine-derived fungus Aspergillus sp. Mar. Drugs 2014, 12, 5124–5131. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zhao, Z.H.; Ding, W.J.; Ye, B.; Wang, P.M.; Xu, J.Z. Aspochalazine A, a novel polycyclic aspochalasin from the fungus Aspergillus sp. Z4. Tetrahedron Lett. 2017, 58, 2405–2408. [Google Scholar] [CrossRef]
- Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef]
- Lee, Y.; Phat, C.; Hong, S.C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 2017, 95, 94–105. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, P.Y.; Tang, J.Q.; Wu, Z.Q.; Li, X.F.; Li, S.M.; Wang, Y.; Liu, G.; He, Z.D.; Gou, D.M.; et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016, 30, 52–57. [Google Scholar] [CrossRef]
- Peng, J.X.; Gao, H.Q.; Zhang, X.M.; Wang, S.; Wu, C.M.; Gu, Q.Q.; Guo, P.; Zhu, T.J.; Li, D.H. Psychrophilins E-H and Versicotide C, cyclic peptides from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Nat. Prod. 2014, 77, 2218–2223. [Google Scholar] [CrossRef]
- Ebada, S.S.; Fischer, T.; Hamacher, A.; Du, F.Y.; Roth, Y.O.; Kassack, M.U.; Wang, B.G.; Roth, E.H. Psychrophilin E, a new cyclotripeptide, from co-fermentation of two marine alga-derived fungi of the genus Aspergillus. Nat. Prod. Res. 2014, 28, 776–781. [Google Scholar] [CrossRef]
- Nong, X.H.; Zhang, X.Y.; Xu, X.Y.; Qi, S.H. Antifouling compounds from the marine-derived fungus Aspergillus terreus SCSGAF0162. Nat. Prod. Commun. 2015, 10, 1033–1034. [Google Scholar] [CrossRef]
- Prompanya, C.; Fernandes, C.; Cravo, S.; Pinto, M.M.M.; Dethoup, T.; Silva, A.M.S.; Kijjoa, A. A new cyclic hexapeptide and a new isocoumarin derivative from the marine sponge-associated fungus Aspergillus similanensis KUFA 0013. Mar. Drugs 2015, 13, 1432–1450. [Google Scholar] [CrossRef]
- Hou, X.M.; Zhang, Y.H.; Hai, Y.; Zheng, J.Y.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Aspersymmetide A, a new centrosymmetric cyclohexapeptide from the marine-derived fungus Aspergillus versicolor. Mar. Drugs 2017, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.F.; Fang, W.; Tan, S.Y.; Lin, X.P.; Xun, T.R.; Yang, B.J.; Liu, S.W.; Liu, Y.H. Aspernigrins with anti-HIV-1 activities from the marine-derived fungus Aspergillus niger SCSIO Jcsw6F30. Bioorg. Med. Chem. Lett. 2016, 26, 361–365. [Google Scholar] [CrossRef]
- Chen, M.; Shao, C.L.; Meng, H.; She, Z.G.; Wang, C.Y. Anti-respiratory syncytial virus prenylated dihydroquinolone derivatives from the gorgonian-derived fungus Aspergillus sp. XS-20090B15. J. Nat. Prod. 2014, 77, 2720–2724. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Liu, D.; Xu, Y.; Chen, J.L.; Lin, W.H. Antioxidant xanthones and anthraquinones isolated from a marine-derived fungus Aspergillus versicolor. Chin. J. Nat. Med. 2018, 16, 219–224. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Pivkin, M.V.; Afiyatullov, S.S. New kipukasin from marine isolate of the fungus Aspergillus flavus. Chem. Nat. Compd. 2016, 52, 266–268. [Google Scholar] [CrossRef]
- Chen, M.; Fu, X.M.; Kong, C.J.; Wang, C.Y. Nucleoside derivatives from the marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 2014, 28, 895–900. [Google Scholar] [CrossRef]
- Peng, X.P.; Wang, Y.; Zhu, T.H.; Zhu, W.M. Pyrazinone derivatives from the coral-derived Aspergillus ochraceus LCJ11-102 under high iodide salt. Arch. Pharm. Res. 2018, 41, 184–191. [Google Scholar] [CrossRef]
- Zheng, C.J.; Wu, L.Y.; Li, X.B.; Song, X.M.; Niu, Z.G.; Song, X.P.; Chen, G.Y.; Wang, C.Y. Structure and absolute configuration of Aspergilumamide A, a novel lumazine peptide from the mangrove-derived fungus Aspergillus sp. Helv. Chim. Acta 2015, 98, 368–373. [Google Scholar] [CrossRef]
- You, M.; Liao, L.; Hong, S.H.; Park, W.; Kwon, D.I.; Lee, J.; Noh, M.; Oh, D.C.; Oh, K.B.; Shin, J. Lumazine peptides from the marine-derived fungus Aspergillus terreus. Mar. Drugs 2015, 13, 1290–1303. [Google Scholar] [CrossRef]
- Li, W.T.; Luo, D.; Huang, J.N.; Wang, L.L.; Zhang, F.G.; Xi, T.; Liao, J.M.; Lu, Y.Y. Antibacterial constituents from Antarctic fungus, Aspergillus sydowii SP-1. Nat. Prod. Res. 2018, 32, 662–667. [Google Scholar] [CrossRef]
- Ding, C.H.; Wu, X.D.; Auckloo, B.N.; Chen, C.T.; Ye, Y.; Wang, K.W.; Wu, B. An unusual stress metabolite from a hydrothermal vent fungus Aspergillus sp. WU 243 induced by cobalt. Molecules 2016, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Wang, B.G. Secondary metabolites from the marine algal-derived endophytic fungi: Chemical diversity and biological activity. Planta Med. 2016, 82, 832–842. [Google Scholar] [CrossRef]

| NO. | Producing Strain | Environmental Source | Biological Activities | Ref. |
|---|---|---|---|---|
| 1 | A. sp. SCSIO 41501 | the gorgonian Melitodes squamata collected from the South China Sea, Sanya, China | moderate antiviral activity against HSV-1 under non-cytotoxic concentrations against Vero cells | [23] |
| 2 | A. terreus | the coral Sarcophyton subviride collected from the coast of Xisha Island in the South China Sea | potent inhibition on LPS-induced NO production; nonsignificant inhibition on α-Glucosidase | [24] |
| 3 | A. candidus KUFA0062 | the marine sponge Epipolasis sp. collected at Similan Island National Park (15–20 m), Thailand | weak cytotoxic activity against eight cell lines; nonsignificant antibacterial activity | [25] |
| 4 | A. alabamensis EN-547 | the fresh inner tissue of marine alga Ceramium japonicum collected at Qingdao, China | moderate antimicrobial activities against E. coli, M. luteus, Ed. ictaluri and V. alginolyticus | [26] |
| 5 | A. alabamensis EN-547 | the fresh inner tissue of marine alga Ceramium japonicum collected at Qingdao, China | moderate antimicrobial activities against E. coli, M. luteus, Ed. ictaluri and V. alginolyticus | [26] |
| 6 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | nonsignificant cytotoxic activities | [27] |
| 7 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | no biological activity was tested | [27] |
| 8 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | nonsignificant cytotoxic activities | [27] |
| 9 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | no biological activity was tested | [27] |
| 10 | A. sp. MEXU 27854 | sandy soil collected in the intertidal zone located in Caleta Bay, Acapulco, Guerrero, Mexico | no biological activity was tested | [27] |
| 11 | A. sp. CMB-M081F | the marine sediment collected at an intertidal depth of 1 m near Shorncliffe, Queensland, Australia | nonsignificant cytotoxic activities; potent inhibition on P-glycoprotein-mediated drug efflux | [28] |
| 12 | A. sp. KMM 4676 | an unidentified colonial ascidian (Shikotan Island, Pacific Ocean) | potent cytotoxicity against 22Rv1, while moderate cytotoxicity against PC-3 and LNCaP | [29] |
| 13 | A. sp. KMM 4676 | an unidentified colonial ascidian (Shikotan Island, Pacific Ocean) | no biological activity was tested | [29] |
| 14 | A. sp. KMM 4676 | an unidentified colonial ascidian (Shikotan Island, Pacific Ocean) | nonsignificant cytotoxic activities against PC-3, LNCaP and 22Rv1 cell lines | [29] |
| 15 | A. sp. KMM 4676 | an unidentified colonial ascidian (Shikotan Island, Pacific Ocean) | no biological activity was tested | [29] |
| 16 | A. nidulans EN-330 | The marine red alga P. scopulorum var. villum collected from Yantai coastline of north China | moderate antimicrobial activities against four human- and aqua-pathogens | [30] |
| 17 | A. nidulans EN-330 | The marine red alga P. scopulorum var. villum collected from Yantai coastline of north China | weak antimicrobial activities against four human- and aqua-pathogens | [30] |
| 18 | A. sp. SCSIO XWS03F03 | a sponge collected from the sea area Xuwen County, Guangdong, China | potent cytotoxic activity against HL-60 and LNCap cell lines | [31] |
| 19 | A. sp. | the sponge Tethya aurantium (Pallas 1766) collected at the entrance of Limski kanal (a depth of 20 m) | moderate selective activity against marine bacteria; nonsignificant cytotoxicity against L5178Y cells | [32] |
| 20 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; potent inhibitory activity against TrxR | [33] |
| 21 | A. flavus OUCMDZ-2205 | the prawn Penaeus vannamei collected in Lianyungang sea area, Jiangsu Province of China | moderate antibacterial and cytotoxic activities, as well as PKC-beta inhibition | [34] |
| 22 | A. flavus OUCMDZ-2205 | the prawn Penaeus vannamei collected in Lianyungang sea area, Jiangsu Province of China | moderate cytotoxicity; nonsignificant antibacterial activity and PKC-beta inhibition | [34] |
| 23 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | weak cytotoxic activities against Hela and MGC803 cell lines | [35] |
| 24 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela, HL-60, and K562 cell lines | [36] |
| 25 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela and MGC803 cell lines | [35] |
| 26 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela and MGC803 cell lines | [35] |
| 27 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | weak cytotoxic activities against Hela and MGC803 cell lines | [35] |
| 28 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela, HL-60, and K562 cell lines | [36] |
| 29 | A. oryzae | the marine sediments collected from Langqi Island, Fujian, China | nonsignificant cytotoxic activities against Hela, HL-60, and K562 cell lines | [36] |
| 30 | A. sp Z-4 | the marine isopod Ligia oceanica collected in Zhoushan, Zhejiang, China | nonsignificant cytotoxicity against PC3 and HCT116 | [37] |
| 31 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 32 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 33 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 34 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 35 | A. sulphureus KMM 4640 and I. felina KMM 4639 | marine sediments (no detailed description) | nonsignificant cytotoxic activities against MRC-9, HEK 293T, 22Rv1, PC-3, and LNCaP | [38] |
| 36 | A. versicolor | the mud of the South China Sea | moderate inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 37 | A. versicolor | the mud of the South China Sea | moderate inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 38 | A. versicolor | the mud of the South China Sea | nonsignificant inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 39 | A. versicolor | the mud of the South China Sea | moderate inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 40 | A. versicolor | the mud of the South China Sea | potent inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 41 | A. versicolor | the mud of the South China Sea | nonsignificant inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 42 | A. versicolor | the mud of the South China Sea | nonsignificant inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 43 | A. versicolor | the mud of the South China Sea | nonsignificant inhibitory activities against LPS-induced NO production and iNOS enzyme | [40] |
| 44 | A. sp. SF-5976 | an unidentified marine organism collected in the Ross Sea | weak inhibitory activities against LPS-induced NO production in RAW 264.7 and BV2 cells | [41] |
| 45 | A. sp. SF-5976 | an unidentified marine organism collected in the Ross Sea | moderate inhibitory activities against LPS-induced NO production in RAW 264.7 and BV2 cells | [41] |
| 46 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 47 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 48 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 49 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 50 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 51 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HCT-116, HL-60 and K562 cell lines | [42] |
| 52 | A. sp. SF-5280 | an unidentified sponge collected at Cheju Island, Korea | moderate inhibitory effects against PTP1B activity | [43] |
| 53 | A. violaceofuscus | the inner part of marine sponge Reniochalina sp. collected from Xisha Islands in South China Sea | potent anti-inflammatory activity against IL-10 expression of the LPS-induced THP-1 cells | [44] |
| 54 | A. sp. DX4H | marine shrimp collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activities against PC3 cell line | [45] |
| 55 | A. sp. DX4H | marine shrimp collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activities against PC3 cell line | [45] |
| 56 | A. sp. DX4H | marine shrimp collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activities against PC3 cell line | [45] |
| 57 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | moderate cytotoxic activities and selective PTK inhibitory activities | [42] |
| 58 | A. versicolor HDN08-60 | the sediments (at a depth of 35 m) collected in the South China Sea, China | nonsignificant cytotoxic activities against HeLa, HL-60, and K562 cell lines | [42] |
| 59 | A. fumigatus SCSIO 41012 | the deep-sea sediments (3614 m) collected from the Indian Ocean | potent antifungal and antibacterial activities | [46] |
| 60 | A. fumigatus SCSIO 41012 | the deep-sea sediments (3614 m) collected from the Indian Ocean | potent antibacterial activities | [46] |
| 61 | A. fumigatus SCSIO 41012 | the deep-sea sediments (3614 m) collected from the Indian Ocean | potent antibacterial activities | [46] |
| 62 | A. sp. CMB-M081F | the marine sediment collected at an intertidal depth of 1 m near Shorncliffe, Queensland, Australia | nonsignificant cytotoxic activities and inhibition on P-glycoprotein-mediated drug efflux | [28] |
| 63 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 64 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 65 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 66 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 67 | A. sp. F452 | submerged decaying wood off the shore of Jeju Island, Korea | moderate cytotoxicity; nonsignificant antibacterial activity; weak inhibition against Na+/K+-ATPase | [47] |
| 68 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; nonsignificant inhibitory activity against TrxR | [33] |
| 69 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; potent inhibitory activity against TrxR | [33] |
| 70 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; nonsignificant inhibitory activity against TrxR | [33] |
| 71 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; nonsignificant inhibitory activity against TrxR | [33] |
| 72 | A. versicolor LZD-14-1 | the gorgonian Pseudopterogorgia sp. (LZD-14) collected from the South China Sea | weak cytotoxic activity against A549; nonsignificant inhibitory activity against TrxR | [33] |
| 73 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 74 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 75 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 76 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 77 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | potent inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 78 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 79 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | potent inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 80 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | potent inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 81 | A. flocculosus 16D-1 | the inner tissue of marine sponge Phakellia fusca collected from Yongxing Island, China | moderate inhibitory activity against IL-6 production in LPS-induced THP-1 cells | [48] |
| 82 | A. candidus KUFA0062 | the marine sponge Epipolasis sp. collected at Similan Island National Park (15–20 m), Thailand | weak cytotoxic activity against eight cell lines; nonsignificant antibacterial activity | [25] |
| 83 | A. sclerotiorum and P. citrinum | the gorgonian Muricella flexuosa collected from the South China Sea, Sanya, Hainan Province, China | moderate brine shrimp lethality; nonsignificant antibacterial and anti-biofilm activities | [49] |
| 84 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 85 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 86 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 87 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 88 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 89 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 90 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 91 | A. fumigatus | the marine fish Mugil cephalus | cytotoxic tests are in progress | [50] |
| 92 | A. sp. Z-4 | the marine isopod Ligia oceanica collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activity against PC3 cell line | [51] |
| 93 | A. sp. Z-4 | the marine isopod Ligia oceanica collected in seaside of Dinghai in Zhoushan, Zhejiang Province of China | weak cytotoxic activity against PC3 cell line | [51] |
| 94 | A. sp. Z-4 | the marine isopod Ligia oceanica collected in seaside of Dinghai, Zhejiang Province of China | moderate cytotoxic activities against PC3 and HCT116 cell lines | [52] |
| 95 | A. sp. Z-4 | the marine isopod Ligia oceanica collected in seaside of Dinghai, Zhejiang Province of China | no biological activity was tested | [52] |
| 96 | A. sp. Z-4 | the intestinal of the marine isopod Ligia oceanica | weak cytotoxic activities against PC3 cell line | [53] |
| 97 | A. sp. SCSIOW2 | the deep marine sediment (2439 m) collected in the South China Sea | weak inhibitory activity on NO production induced by lipopolysaccharide (LPS)/INF-γ | [56] |
| 98 | A. violaceofuscus | the inner part of marine sponge Reniochalina sp. collected from Xisha Islands in South China Sea | nonsignificant anti-inflammatory activity against IL-10 expression of the LPS-induced THP-1 cells | [44] |
| 99 | A. violaceofuscus | the inner part of marine sponge Reniochalina sp. collected from Xisha Islands in South China Sea | potent anti-inflammatory activity against IL-10 expression of the LPS-induced THP-1 cells | [44] |
| 100 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | nonsignificant cytotoxic activities and lipid-lowering effect | [57] |
| A. sp. BM-05 and BM-05ML | a brown algal species belonging to the genus Sargassum collected off Helgoland, North Sea, Germany | moderate cytotoxicities against K562, HCT116, A2780, and A2780CisR cell lines | [58] | |
| 101 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | nonsignificant cytotoxic activities and lipid-lowering effect | [57] |
| 102 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | potent lipid-lowering effect; nonsignificant cytotoxic activities | [57] |
| 103 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | nonsignificant cytotoxic activities and lipid-lowering effect | [57] |
| 104 | A. versicolor ZLN-60 | the mud (depth, 20 m) of the Yellow Sea, China | nonsignificant cytotoxic activities and lipid-lowering effect | [57] |
| 105 | A. terreus SCSGAF0162 | the gorgonian coral Echinogorgia aurantiaca in the South China Sea | nonsignificant antifouling activity towards larvae of the barnacle B. amphitrite | [59] |
| 106 | A. sp. SCSIO 41501 | the gorgonian Melitodes squamata collected from the South China Sea, Sanya, China | moderate antiviral activity against HSV-1 under non-cytotoxic concentrations against Vero cells | [23] |
| 107 | A. similanensis KUFA 0013 | the marine sponge Rhabdermia sp. collected in coral reef of Similan Islands, Phang Nga, Thailand | nonsignificant cytotoxic and antibacterial activities | [60] |
| 108 | A. versicolor TA01-14 | a gorgonian Carijoa sp. GX-WZ-2010001 collected in Weizhou coral reefs in the South China Sea | weak cytotoxic activity; nonsignificant brine shrimp lethality, antibacterial and antiviral activities, as well as AChE, Top I, and α-glucosacharase inhibition | [61] |
| 109 | A. similanensis KUFA 0013 | the marine sponge Rhabdermia sp. collected in coral reef of Similan Islands, Phang Nga, Thailand | weak cytotoxicity; nonsignificant antibacterial activities against four reference strains | [60] |
| 110 | A. niger SCSIO Jcsw6F30 | a marine alga Sargassum sp. collected in Yongxing Island, South China Sea | potent cytotoxic activity against TZM-bl cells; moderate anti-HIV-1 activity against HIV-1 SF162 | [62] |
| 111 | A. niger SCSIO Jcsw6F30 | a marine alga Sargassum sp. collected in Yongxing Island, South China Sea | no biological activity was tested | [62] |
| 112 | A. sp. XS20090B15 | the Muricella abnormaliz gorgonian collected from the Xisha Islands coral reef in South China Sea | nonsignificant antiviral activity against RSV virus-induced cytopathogenicity in Hep-2 cells | [63] |
| 113 | A. sp. XS20090B15 | the Muricella abnormaliz gorgonian collected from the Xisha Islands coral reef in South China Sea | potent antiviral activity against RSV virus-induced cytopathogenicity in Hep-2 cells | [63] |
| 114 | A. versicolor A-21-2-7 | the deep-sea sediment (3002 m) in South China Sea | no biological activity was tested | [64] |
| 115 | A. flavus KMM 4650 | Sakhalin Bay marine sediments (32 m, Sea of Okhotsk) | nonsignificant antimicrobial activity | [65] |
| 116 | A. versicolor | the inner part of gorgonian D. gemmacea collected from the Xisha Islands coral reef of the South China Sea | moderate antibacterial activities and brine shrimp lethality; nonsignificant cytotoxicities | [66] |
| 117 | A. versicolor | the inner part of gorgonian D. gemmacea collected from the Xisha Islands coral reef of the South China Sea | moderate antibacterial activities and brine shrimp lethality; nonsignificant cytotoxicities | [66] |
| 118 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | moderate antimicrobial activity against E. aerogenes; nonsignificant cytotoxic activities | [67] |
| 119 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | nonsignificant antimicrobial and cytotoxic activities | [67] |
| 120 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | nonsignificant antimicrobial and cytotoxic activities | [67] |
| 121 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | moderate antimicrobial activity against E. aerogenes; nonsignificant cytotoxic activities | [67] |
| 122 | A. ochraceus LCJ11-102 | the gorgonian Dichotella gemmacea (Valenciennes) collected in Lingao, Hainan province of China | nonsignificant antimicrobial and cytotoxic activities | [67] |
| 123 | A. sclerotiorum and P. citrinum | the gorgonian Muricella flexuosa collected from the South China Sea, Sanya, Hainan Province, China | potent brine shrimp lethality and cytotoxic activities; nonsignificant antibacterial and anti-biofilm activities | [49] |
| 124 | A. sclerotiorum and P. citrinum | the gorgonian Muricella flexuosa collected from the South China Sea, Sanya, Hainan Province, China | moderate brine shrimp lethality and cytotoxic activities; nonsignificant antibacterial and anti-biofilm activities | [49] |
| 125 | A. sp. (33241) | the mangrove Bruguiera sexangula var. rhynchopetala collected in the South China Sea | nonsignificant antibacterial and cytotoxic activities | [68] |
| 126 | A. terreus FA009 | the marine sediment collected in Jeju Island, Korea | moderate enhancement effect on insulin sensitivity | [69] |
| 127 | A. terreus FA009 | the marine sediment collected in Jeju Island, Korea | moderate enhancement effect on insulin sensitivity | [69] |
| 128 | A. sclerotiorum and P. citrinum | the gorgonian Muricella flexuosa collected from the South China Sea, Sanya, Hainan Province, China | weak brine shrimp lethality; nonsignificant cytotoxic, antibacterial and anti-biofilm activities | [49] |
| 129 | A. sydowii SP-1 | the marine sediment sample collected from site in the Antarctic Great Wall Station | weak antimicrobial activities against MRSA and MRSE | [70] |
| 130 | A. sp. WU 243 | the crab Xenograpsus testudinatus collected from a Kueishantao hydrothermal vent, Taiwan, China | no biological activity was tested | [71] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Yuan, X.-L.; Li, C.; Li, a.X.-D. Recent Discovery of Heterocyclic Alkaloids from Marine-Derived Aspergillus Species. Mar. Drugs 2020, 18, 54. https://doi.org/10.3390/md18010054
Xu K, Yuan X-L, Li C, Li aX-D. Recent Discovery of Heterocyclic Alkaloids from Marine-Derived Aspergillus Species. Marine Drugs. 2020; 18(1):54. https://doi.org/10.3390/md18010054
Chicago/Turabian StyleXu, Kuo, Xiao-Long Yuan, Chen Li, and and Xiao-Dong Li. 2020. "Recent Discovery of Heterocyclic Alkaloids from Marine-Derived Aspergillus Species" Marine Drugs 18, no. 1: 54. https://doi.org/10.3390/md18010054
APA StyleXu, K., Yuan, X.-L., Li, C., & Li, a. X.-D. (2020). Recent Discovery of Heterocyclic Alkaloids from Marine-Derived Aspergillus Species. Marine Drugs, 18(1), 54. https://doi.org/10.3390/md18010054





