Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action
Abstract
1. Introduction
2. Marine Compounds with Antibacterial, Antifungal, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities
2.1. Antibacterial Activity
2.2. Antifungal Activity
2.3. Antiprotozoal and Antituberculosis Activity
2.4. Antiviral Activity
2.5. Anthelmintic Activity
3. Marine Compounds with Antidiabetic and Anti-Inflammatory Activity, and Affecting the Immune and Nervous System
3.1. Antidiabetic Activity
3.2. Anti-Inflammatory Activity
3.3. Marine Compounds with Activity on the Immune System
3.4. Marine Compounds Affecting the Nervous System
4. Marine Compounds with Miscellaneous Mechanisms of Action
5. Reviews on Marine Pharmacology and Pharmaceuticals
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mayer, A.M.S.; Lehmann, V.K.B. Marine pharmacology in 1998: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist 2000, 42, 62–69. [Google Scholar]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 1999: Compounds with antibacterial, anticoagulant, antifungal, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities affecting the cardiovascular, endocrine, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2002, 132, 315–339. [Google Scholar]
- Mayer, A.M.S.; Hamann, M.T. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. 2004, 6, 37–52. [Google Scholar] [PubMed]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2001-2: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 2005, 140, 265–286. [Google Scholar]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2003-4: Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2007, 145, 553–581. [Google Scholar]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005-6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 283–308. [Google Scholar]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007-8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2011, 153, 191–222. [Google Scholar]
- Mayer, A.M.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2009-2011: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and other Miscellaneous Mechanisms of Action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar]
- Mayer, A.M.S.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2012-2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2017, 15, 273. [Google Scholar]
- Schmitz, F.J.; Bowden, B.F.; Toth, S.I. Antitumor and Cytotoxic Compounds from Marine Organisms. In Marine Biotechnology, Pharmaceutical and Bioactive Natural Products; Attaway, D.H., Zaborsky, O.R., Eds.; Plenum Press: New York, NY, USA; London, UK, 1993; Volume 1, pp. 197–308. [Google Scholar]
- Ochoa, J.L.; Bray, W.M.; Lokey, R.S.; Linington, R.G. Phenotype-Guided Natural Products Discovery Using Cytological Profiling. J. Nat. Prod. 2015, 78, 2242–2248. [Google Scholar] [CrossRef]
- Quintana, J.; Brango-Vanegas, J.; Costa, G.M.; Castellanos, L.; Arevalo, C.; Duque, C. Marine organisms as source of extracts to disrupt bacterial communication: Bioguided isolation and identification of quorum sensing inhibitors from Ircinia felix. Rev. Brasil. Farmacogn. 2015, 25, 199–207. [Google Scholar] [CrossRef]
- Henriquez, M.; Vergara, K.; Norambuena, J.; Beiza, A.; Maza, F.; Ubilla, P.; Araya, I.; Chavez, R.; San-Martin, A.; Darias, J.; et al. Diversity of cultivable fungi associated with Antarctic marine sponges and screening for their antimicrobial, antitumoral and antioxidant potential. World J. Microbiol. Biotechnol. 2014, 30, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rahelivao, M.P.; Gruner, M.; Andriamanantoanina, H.; Andriamihaja, B.; Bauer, I.; Knölker, H.J. Red algae (Rhodophyta) from the coast of Madagascar: Preliminary bioactivity studies and isolation of natural products. Mar. Drugs 2015, 13, 4197–4216. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Polyketide family of novel antibacterial 7-O-methyl-5′-hydroxy-3′-heptenoate-macrolactin from seaweed-associated Bacillus subtilis MTCC 10403. J. Agric. Food Chem. 2014, 62, 12194–12208. [Google Scholar] [CrossRef] [PubMed]
- Shanthi, J.; Senthil, A.; Gopikrishnan, V.; Balagurunathan, R. Characterization of a potential beta-lactamase inhibitory metabolite from a marine Streptomyces sp. PM49 active against multidrug-resistant pathogens. Appl. Biochem. Biotechnol. 2015, 175, 3696–3708. [Google Scholar] [CrossRef]
- Pereira, D.M.; Correia-da-Silva, G.; Valentao, P.; Teixeira, N.; Andrade, P.B. Anti-inflammatory effect of unsaturated fatty acids and ergosta-7,22-dien-3-ol from Marthasterias glacialis: Prevention of CHOP-mediated ER-stress and NF-kappaB activation. PLoS ONE 2014, 9, e88341. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Ju, B.; Chen, B.; Zhang, X.; Han, C.; Jiang, A. Purification and characterization of bioactive compounds from Styela clava. J. Chem. 2014, 2014, 525141. [Google Scholar] [CrossRef]
- Abou-Hussein, D.R.; Badr, J.M.; Youssef, D.T. Dragmacidoside: A new nucleoside from the Red sea sponge Dragmacidon coccinea. Nat. Prod. Res. 2014, 28, 1134–1141. [Google Scholar] [CrossRef]
- Deghrigue, M.; Festa, C.; Ghribi, L.; D’Auria, M.V.; De, M.S.; Ben, J.H.; Bouraoui, A. Anti-inflammatory and analgesic activities with gastroprotective effect of semi-purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis. Asian Pac. J. Trop. Med. 2015, 8, 606–611. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Lobo-da-Cunha, A.; Taveira, M.; Ferreira, M.; Valentao, P.; Andrade, P.B. Digestive gland from Aplysia depilans Gmelin: Leads for inflammation treatment. Molecules 2015, 20, 15766–15780. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, H.; Seo, Y. Edible brown alga Ecklonia cava derived phlorotannin-induced anti-adipogenic activity in vitro. J. Food Biochem. 2015, 39, 1–10. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Barrios, S.D.; Kawamata, Y.; Su, S.; Smith, P.A.; Steed, T.C.; Romesberg, F.E.; Baran, P.S. Axinellamines as broad-spectrum antibacterial agents: Scalable synthesis and biology. J. Am. Chem. Soc. 2014, 136, 15403–15413. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Chung, B.; Shin, Y.; Rheingold, A.L.; Moore, C.E.; Park, S.J.; Park, S.; Lee, S.K.; Oh, K.B.; Shin, J.; et al. Pentacyclic antibiotics from a tidal mud flat-derived actinomycete. J. Nat. Prod. 2015, 78, 524–529. [Google Scholar] [CrossRef]
- Wei, L.; Gao, J.; Zhang, S.; Wu, S.; Xie, Z.; Ling, G.; Kuang, Y.Q.; Yang, Y.; Yu, H.; Wang, Y. Identification and characterization of the first cathelicidin from sea snakes with potent antimicrobial and anti-inflammatory activity and special mechanism. J. Biol. Chem. 2015, 290, 16633–16652. [Google Scholar] [CrossRef]
- Silva, O.N.; Fensterseifer, I.C.; Rodrigues, E.A.; Holanda, H.H.; Novaes, N.R.; Cunha, J.P.; Rezende, T.M.; Magalhaes, K.G.; Moreno, S.E.; Jeronimo, M.S.; et al. Clavanin A improves outcome of complications from different bacterial infections. Antimicrob. Agents Chemother. 2015, 59, 1620–1626. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Cheng, C.; Reimer, A.; Kozjak-Pavlovic, V.; Ibrahim, A.K.; Rudel, T.; Hentschel, U.; Edrada-Ebel, R.; Ahmed, S.A. Antichlamydial sterol from the Red sea sponge Callyspongia aff. implexa. Planta Med. 2015, 81, 382–387. [Google Scholar] [CrossRef]
- Pieri, C.; Borselli, D.; Di, G.C.; De, M.M.; Bolla, J.M.; Vidal, N.; Combes, S.; Brunel, J.M. New Ianthelliformisamine derivatives as antibiotic enhancers against resistant Gram-negative bacteria. J. Med. Chem. 2014, 57, 4263–4272. [Google Scholar] [CrossRef]
- Huang, H.N.; Pan, C.Y.; Chan, Y.L.; Chen, J.Y.; Wu, C.J. Use of the antimicrobial peptide pardaxin (GE33) to protect against methicillin-resistant Staphylococcus aureus infection in mice with skin injuries. Antimicrob. Agents Chemother. 2014, 58, 1538–1545. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, D.S.; Jung, Y.J.; Park, J.H.; Choi, J.I.; Yim, M.J.; Jeon, J.M.; Kim, H.W.; Son, K.T.; Je, J.Y.; et al. The mechanism of antibacterial activity of phlorofucofuroeckol-A against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9795–9804. [Google Scholar] [CrossRef]
- Hassan, H.M.; Degen, D.; Jang, K.H.; Ebright, R.H.; Fenical, W. Salinamide F, new depsipeptide antibiotic and inhibitor of bacterial RNA polymerase from a marine-derived Streptomyces sp. J. Antibiot. 2015, 68, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Chen, J.C.; Chen, T.L.; Wu, J.L.; Hui, C.F.; Chen, J.Y. Piscidin is highly active against carbapenem-resistant Acinetobacter baumannii and NDM-1-producing Klebsiella pneumonia in a systemic septicaemia infection mouse model. Mar. Drugs 2015, 13, 2287–2305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.M.; Meng, L.H.; Jiang, W.L.; Xu, G.M.; Huang, C.G.; Wang, B.G. Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J. Nat. Prod. 2015, 78, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Kusama, T.; Tanaka, N.; Sakai, K.; Gonoi, T.; Fromont, J.; Kashiwada, Y.; Kobayashi, J. Agelamadins A and B, dimeric bromopyrrole alkaloids from a marine sponge Agelas sp. Org. Lett. 2014, 16, 3916–3918. [Google Scholar] [CrossRef]
- Chen, M.; Wang, K.L.; Liu, M.; She, Z.G.; Wang, C.Y. Bioactive steroid derivatives and butyrolactone derivatives from a gorgonian-derived Aspergillus sp. fungus. Chem. Biodivers. 2015, 12, 1398–1406. [Google Scholar] [CrossRef]
- Gomes, N.M.; Bessa, L.J.; Buttachon, S.; Costa, P.M.; Buaruang, J.; Dethoup, T.; Silva, A.M.; Kijjoa, A. Antibacterial and antibiofilm activities of tryptoquivalines and meroditerpenes isolated from the marine-derived fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the soil fungi N. fischeri and N. siamensis. Mar. Drugs 2014, 12, 822–839. [Google Scholar] [CrossRef]
- Zhou, Y.; Debbab, A.; Wray, V.; Lin, W.H.; Schulz, B.; Trepos, R.; Pile, C.; Hellio, C.; Proksch, P.; Aly, A.H. Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014, 55, 2789–2792. [Google Scholar] [CrossRef]
- Meng, L.H.; Liu, Y.; Li, X.M.; Xu, G.M.; Ji, N.Y.; Wang, B.G. Citrifelins A and B, citrinin adducts with a tetracyclic framework from cocultures of marine-derived isolates of Penicillium citrinum and Beauveria felina. J. Nat. Prod. 2015, 78, 2301–2305. [Google Scholar] [CrossRef]
- Du, F.Y.; Zhang, P.; Li, X.M.; Li, C.S.; Cui, C.M.; Wang, B.G. Cyclohexadepsipeptides of the isaridin class from the marine-derived fungus Beauveria felina EN-135. J. Nat. Prod. 2014, 77, 1164–1169. [Google Scholar] [CrossRef]
- Sun, S.; Canning, C.B.; Bhargava, K.; Sun, X.; Zhu, W.; Zhou, N.; Zhang, Y.; Zhou, K. Polybrominated diphenyl ethers with potent and broad spectrum antimicrobial activity from the marine sponge Dysidea. Bioorg. Med. Chem. Lett. 2015, 25, 2181–2183. [Google Scholar] [CrossRef]
- Khanthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. An antibacterial cytochalasin derivative from the marine-derived fungus Diaporthaceae sp. PSU-SP2/4. Phytochemistry 2014, 10, 5–9. [Google Scholar] [CrossRef]
- Pereira, U.A.; Barbosa, L.C.; Maltha, C.R.; Demuner, A.J.; Masood, M.A.; Pimenta, A.L. gamma-alkylidene-gamma-lactones and isobutylpyrrol-2(5H)-ones analogues to rubrolides as inhibitors of biofilm formation by gram-positive and gram-negative bacteria. Bioorg. Med. Chem. Lett. 2014, 24, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Woo, J.K.; Kim, S.H.; Cho, E.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Meroterpenoids from a tropical Dysidea sp. sponge. J. Nat. Prod. 2015, 78, 2814–2821. [Google Scholar] [CrossRef]
- Jiao, W.H.; Li, J.; Liu, Q.; Xu, T.T.; Shi, G.H.; Yu, H.B.; Yang, F.; Han, B.N.; Li, M.; Lin, H.W. Dysidinoid A, an unusual meroterpenoid with anti-MRSA activity from the South China sea sponge Dysidea sp. Molecules 2014, 19, 18025–18032. [Google Scholar] [CrossRef]
- Martinez-Diaz, Y.; Vaneges Laverde, G.; Reina Gamba, L.; Mayorga Wandurraga, H.; Arevalo-Ferro, C.; Ramos Rodriguez, F.; Duque, C.; Hernandez, L.M.C. Biofilm inhibition activity of compounds isolated from two Eunicea species collected at the Caribbean sea. Rev. Brasil. Farmacogn. 2015, 25, 605–611. [Google Scholar] [CrossRef][Green Version]
- Bai, Z.Q.; Lin, X.; Wang, Y.; Wang, J.; Zhou, X.; Yang, B.; Liu, J.; Yang, X.; Wang, Y.; Liu, Y. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia 2014, 95, 194–202. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Non-cytotoxic antifungal agents: Isolation and structures of gageopeptides A-D from a Bacillus strain 109GGC020. J. Agric. Food Chem. 2014, 62, 5565–5572. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Gageotetrins A-C, noncytotoxic antimicrobial linear lipopeptides from a marine bacterium Bacillus subtilis. Org. Lett. 2014, 16, 928–931. [Google Scholar] [CrossRef]
- Bae, M.; Chung, B.; Oh, K.B.; Shin, J.; Oh, D.C. Hormaomycins B and C: New antibiotic cyclic depsipeptides from a marine mudflat-derived Streptomyces sp. Mar. Drugs 2015, 13, 5187–5200. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Shin, H.J. Ieodoglucomide C and Ieodoglycolipid, new glycolipids from a marine-derived bacterium Bacillus licheniformis 09IDYM23. Lipids 2015, 50, 513–519. [Google Scholar] [CrossRef]
- Lacret, R.; Oves-Costales, D.; Gomez, C.; Diaz, C.; de la Cruz, M.; Perez-Victoria, I.; Vicente, F.; Genilloud, O.; Reyes, F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar. Drugs 2015, 13, 128–140. [Google Scholar] [CrossRef]
- Kusama, T.; Tanaka, N.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Bromopyrrole alkaloids from a marine sponge Agelas sp. Chem. Pharm. Bull. 2014, 62, 499–503. [Google Scholar] [CrossRef]
- Yang, I.; Choi, H.; Won, D.H.; Nam, S.J.; Kang, H. An antibacterial 9.11-secosterol from a marine sponge Ircinia sp. Bull. Korean Chem. Soc. 2014, 35, 3360–3362. [Google Scholar] [CrossRef]
- Yang, I.; Choi, H.; Nam, S.J.; Kang, H. A new 9,11-secosterol with a 1,4-quinone from a Korean marine sponge Ircinia sp. Arch. Pharm. Res. 2015, 38, 1970–1974. [Google Scholar] [CrossRef]
- Radwan, M.M.; Wanas, A.S.; Fronczek, F.R.; Jacob, M.R.; Ross, S.A. Polybrominated diphenyl ethers from the marine organisms Lendenfeldia dendyi and Sinularia dura with anti-MRSa activity. Med. Chem. Res. 2015, 24, 3398–3404. [Google Scholar] [CrossRef]
- Wu, B.; Wiese, J.; Labes, A.; Kramer, A.; Schmaljohann, R.; Imhoff, J.F. Lindgomycin, an unusual antibiotic polyketide from a marine fungus of the Lindgomycetaceae. Mar. Drugs 2015, 13, 4617–4632. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, H.; Lie, J.; Ju, J. New anti-infective cycloheptadepsipeptide congeners and absolute stereochemistry from the deep sea-derived Streptomyces drozdowiczii SCSIO 10141. Tetrahedron 2014, 70, 7795–7901. [Google Scholar] [CrossRef]
- Raju, R.; Khalil, Z.G.; Piggott, A.M.; Blumenthal, A.; Gardiner, D.L.; Skinner-Adams, T.S.; Capon, R.J. Mollemycin A: An antimalarial and antibacterial glyco-hexadepsipeptide-polyketide from an Australian marine-derived Streptomyces sp. (CMB-M0244). Org. Lett. 2014, 16, 1716–1719. [Google Scholar] [CrossRef]
- Kamada, T.; Vairappan, C.S. New laurene-type sesquiterpene from Bornean Laurencia nangii. Nat. Prod. Commun. 2015, 10, 843–844. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Zhu, T.; Gu, Q.; Li, D. Penicyclones A-E, antibacterial polyketides from the deep-sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 2015, 78, 2699–2703. [Google Scholar] [CrossRef]
- Li, X.; Li, X.M.; Zhang, P.; Wang, B.G. A new phenolic enamide and a new meroterpenoid from marine alga-derived endophytic fungus Penicillium oxalicum EN-290. J Asian Nat. Prod. Res. 2015, 17, 1204–1212. [Google Scholar] [CrossRef]
- Hagiwara, K.; Garcia Hernandez, J.E.; Harper, M.K.; Carroll, A.; Motti, C.A.; Awaya, J.; Nguyen, H.Y.; Wright, A.D. Puupehenol, a potent antioxidant antimicrobial meroterpenoid from a Hawaiian deep-water Dactylospongia sp. sponge. J. Nat. Prod. 2015, 78, 325–329. [Google Scholar] [CrossRef]
- Hassan, M.H.; Rateb, M.E.; Hetta, M.; Abdelaziz, T.A.; Sleim, M.A.; Jaspars, M.; Mohammed, R. Scalarane sesterterpenes from the Egyptian Red sea sponge Phyllospongia lamellosa. Tetrahedron 2015, 71, 577–583. [Google Scholar] [CrossRef]
- Al-Footy, K.O.; Alarif, W.M.; Asiri, F.; Aly, M.M.; Ayyad, S.E. Rare pyrane-based cembranoids from the Red sea soft coral Sarcophyton trocheliophorum as potential antimicrobial–antitumor agents. Med. Chem. Res. 2015, 24, 505–512. [Google Scholar] [CrossRef]
- Niu, S.; Liu, D.; Hu, X.; Proksch, P.; Shao, Z.; Lin, W. Spiromastixones A-O, antibacterial chlorodepsidones from a deep-sea-derived Spiromastix sp. fungus. J. Nat. Prod. 2014, 77, 1021–1030. [Google Scholar] [CrossRef]
- Wu, B.; Oesker, V.; Wiese, J.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Spirocyclic drimanes from the marine fungus Stachybotrys sp. strain MF347. Mar. Drugs 2014, 12, 1924–1938. [Google Scholar] [CrossRef]
- Reimer, A.; Blohm, A.; Quack, T.; Grevelding, C.G.; Kozjak-Pavlovic, V.; Rudel, T.; Hentschel, U.; Abdelmohsen, U.R. Inhibitory activities of the marine streptomycete-derived compound SF2446A2 against Chlamydia trachomatis and Schistosoma mansoni. J. Antibiot. 2015, 68, 674–679. [Google Scholar] [CrossRef]
- Sun, X.P.; Cao, F.; Shao, C.L.; Wang, M.; Zhang, X.L.; Wang, C.Y. Antibacterial delta(1) -3-ketosteroids from the South China sea gorgonian coral Subergorgia rubra. Chem. Biodivers. 2015, 12, 1068–1074. [Google Scholar] [CrossRef]
- Liaw, C.C.; Chen, P.C.; Shih, C.J.; Tseng, S.P.; Lai, Y.M.; Hsu, C.H.; Dorrestein, P.C.; Yang, Y.L. Vitroprocines, new antibiotics against Acinetobacter baumannii, discovered from marine Vibrio sp. QWI-06 using mass-spectrometry-based metabolomics approach. Sci. Rep. 2015, 5, 12856. [Google Scholar] [CrossRef]
- Ayyad, S.E.; Katoua, D.F.; Alarif, W.M.; Sobahi, T.R.; Aly, M.M.; Shaala, L.A.; Ghandourah, M.A. Two new polyacetylene derivatives from the Red sea sponge Xestospongia sp. Z. Naturforsch. C 2015, 70, 297–303. [Google Scholar] [CrossRef]
- Lee, S.H.; Moon, K.; Kim, H.; Shin, J.; Oh, D.C.; Oh, K.B. Bahamaolide A from the marine-derived Streptomyces sp. CNQ343 inhibits isocitrate lyase in Candida albicans. Bioorg. Med. Chem. Lett. 2014, 24, 4291–4293. [Google Scholar] [CrossRef]
- Sugiyama, R.; Nishimura, S.; Matsumori, N.; Tsunematsu, Y.; Hattori, A.; Kakeya, H. Structure and biological activity of 8-deoxyheronamide C from a marine-derived Streptomyces sp.: Heronamides target saturated hydrocarbon chains in lipid membranes. J. Am. Chem. Soc. 2014, 136, 5209–5212. [Google Scholar] [CrossRef]
- Wyche, T.P.; Piotrowski, J.S.; Hou, Y.; Braun, D.; Deshpande, R.; McIlwain, S.; Ong, I.M.; Myers, C.L.; Guzei, I.A.; Westler, W.M.; et al. Forazoline A: Marine-derived polyketide with antifungal in vivo efficacy. Angew. Chem. Int. Ed. Engl. 2014, 53, 11583–11586. [Google Scholar] [CrossRef]
- Yu, H.B.; Yang, F.; Sun, F.; Li, J.; Jiao, W.H.; Gan, J.H.; Hu, W.Z.; Lin, H.W. Aaptamine derivatives with antifungal and anti-HIV-1 activities from the South China sea sponge Aaptos aaptos. Mar. Drugs 2014, 12, 6003–6013. [Google Scholar] [CrossRef]
- Kubota, T.; Iwai, T.; Ishiyama, H.; Sakai, K.; Gonoi, T.; Kobayashi, J. Amphidinin G, a putative biosynthetic precursor of amphidinin A from marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2015, 56, 990–993. [Google Scholar] [CrossRef]
- Nuzzo, G.; Cutignano, A.; Sardo, A.; Fontana, A. Antifungal amphidinol 18 and its 7-sulfate derivative from the marine dinoflagellate Amphidinium carterae. J. Nat. Prod. 2014, 77, 1524–1527. [Google Scholar] [CrossRef]
- Jamison, M.T.; Molinski, T.F. Antipodal crambescin A2 homologues from the marine sponge Pseudaxinella reticulata. Antifungal structure-activity relationships. J. Nat. Prod. 2015, 78, 557–561. [Google Scholar] [CrossRef]
- Elbandy, M.; Rho, J.R.; Afifi, R. Analysis of saponins as bioactive zoochemicals from the marine functional food sea cucumber Bohadschia cousteaui. Eur. Food Res. Technol. 2014, 238, 937–955. [Google Scholar] [CrossRef]
- Yu, X.Q.; He, W.F.; Liu, D.Q.; Feng, M.T.; Fang, Y.; Wang, B.; Feng, L.H.; Guo, Y.W.; Mao, S.C. A seco-laurane sesquiterpene and related laurane derivatives from the red alga Laurencia okamurai Yamada. Phytochemistry 2014, 103, 162–170. [Google Scholar] [CrossRef]
- Feng, M.T.; Yu, X.Q.; Yang, P.; Yang, H.; Lin, K.; Mao, S.C. Two new antifungal polyunsaturated fatty acid ethyl esters from the red alga Laurencia okamurai. Chem. Nat. Compd. 2015, 51, 418–422. [Google Scholar] [CrossRef]
- Bae, M.; Kim, H.; Moon, K.; Nam, S.J.; Shin, J.; Oh, K.B.; Oh, D.C. Mohangamides A and B, new dilactone-tethered pseudo-dimeric peptides inhibiting Candida albicans isocitrate lyase. Org. Lett. 2015, 17, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Zhou, Y.; Liu, X.; Zhang, W.J.; Hu, S.; Lin, L.P.; HUO, G.M.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Antimicrobial and anti-inflammatory compounds from a marine fungus Pleosporales sp. Tetrahedron Lett. 2015, 56, 6183–6189. [Google Scholar] [CrossRef]
- Lin, K.; Yang, P.; Yang, H.; Liu, A.H.; Yao, L.G.; Guo, Y.W.; Mao, S.C. Lysophospholipids from the Guangxi sponge Spirastrella purpurea. Lipids 2015, 50, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Suzuki, H.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J. Taurospongins B and C, new acetylenic fatty acid derivatives possessing a taurine amide residu from a marine sponge of the family Spongiidae. RSC Adv. 2014, 4, 11073–11079. [Google Scholar] [CrossRef]
- Wang, X.H.; Zou, Z.R.; Yi, Y.H.; Han, H.; Li, L.; Pan, M.X. Variegatusides: New non-sulphated triterpene glycosides from the sea cucumber Stichopus variegates semper. Mar. Drugs 2014, 12, 2004–2018. [Google Scholar] [CrossRef] [PubMed]
- Young, R.M.; Adendorff, M.R.; Wright, A.D.; Davies-Coleman, M.T. Antiplasmodial activity: The first proof of inhibition of heme crystallization by marine isonitriles. Eur. J. Med. Chem. 2015, 93, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Cao, S.; Raveh, A.; MacArthur, R.; Dranchak, P.; Chlipala, G.; Okoneski, M.T.; Guha, R.; Eastman, R.T.; Yuan, J.; et al. Actinoramide A identified as a potent antimalarial from titration-based screening of marine natural product extracts. J. Nat. Prod. 2015, 78, 2411–2422. [Google Scholar] [CrossRef]
- Yang, F.; Zou, Y.; Wang, R.P.; Hamann, M.T.; Zhang, H.J.; Jiao, W.H.; Han, B.N.; Song, S.J.; Lin, H.W. Relative and absolute stereochemistry of diacarperoxides: Antimalarial norditerpene endoperoxides from marine sponge Diacarnus megaspinorhabdosa. Mar. Drugs 2014, 12, 4399–4416. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Brun, R.; Kaiser, M.; Van, K.P.; Van, M.C.; Schmidt, T.J.; Kang, J.S.; Kim, Y.H. Anti-protozoal activities of cembrane-type diterpenes from Vietnamese soft corals. Molecules 2015, 20, 12459–12468. [Google Scholar] [CrossRef]
- Aviles, E.; Prudhomme, J.; Le Roch, K.G.; Rodriguez, A.D. Structures, semisyntheses, and absolute configurations of the antiplasmodial alpha-substituted beta-lactam monamphilectines B and C from the sponge Svenzea flava. Tetrahedron 2015, 71, 487–494. [Google Scholar] [CrossRef][Green Version]
- Gros, E.; Al-Mourabit, A.; Martin, M.T.; Sorres, J.; Vacelet, J.; Frederich, M.; Aknin, M.; Kashman, Y.; Gauvin-Bialecki, A. Netamines H-N, tricyclic alkaloids from the marine sponge Biemna laboutei and their antimalarial activity. J. Nat. Prod. 2014, 77, 818–823. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Pierens, G.K.; Skinner-Adams, T.; Andrews, K.T.; Bernhardt, P.V.; Krenske, E.H.; Mollo, E.; Garson, M.J. Antimalarial isocyano and isothiocyanato sesquiterpenes with tri- and bicyclic skeletons from the nudibranch Phyllidia ocellata. J. Nat. Prod. 2015, 78, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Persico, M.; Yang, F.; Lin, H.W.; Guo, Y.W.; Basilico, N.; Parapini, S.; Taramelli, D.; Taglialatela-Scafati, O.; Fattorusso, C. Endoperoxide polyketides from a Chinese Plakortis simplex: Further evidence of the impact of stereochemistry on antimalarial activity of simple 1,2-dioxanes. Bioorg. Med. Chem. 2014, 22, 4572–4580. [Google Scholar] [CrossRef] [PubMed]
- Oli, S.; Abdelmohsen, U.R.; Hentschel, U.; Schirmeister, T. Identification of plakortide E from the Caribbean sponge Plakortis halichondroides as a trypanocidal protease inhibitor using bioactivity-guided fractionation. Mar. Drugs 2014, 12, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.; Harper, P.M.; Williams, D.E.; Mesquita, J.T.; Pinto, E.G.; da Costa-Silva, T.A.; Hajdu, E.; Ferreira, A.G.; Santos, R.A.; Murphy, P.J.; et al. Anti-parasitic guanidine and pyrimidine alkaloids from the marine sponge Monanchora arbuscula. J. Nat. Prod. 2015, 78, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef]
- Thao, N.P.; No, J.H.; Luyen, B.T.; Yang, G.; Byun, S.Y.; Goo, J.; Kim, K.T.; Cuong, N.X.; Nam, N.H.; Van, M.C.; et al. Secondary metabolites from Vietnamese marine invertebrates with activity against Trypanosoma brucei and T. cruzi. Molecules 2014, 19, 7869–7880. [Google Scholar] [CrossRef]
- Viegelmann, C.; Parker, J.; Ooi, T.; Clements, C.; Abbott, G.; Young, L.; Kennedy, J.; Dobson, A.D.; Edrada-Ebel, R. Isolation and identification of antitrypanosomal and antimycobacterial active steroids from the sponge Haliclona simulans. Mar. Drugs 2014, 12, 2937–2952. [Google Scholar] [CrossRef]
- Schulze, C.J.; Donia, M.S.; Siqueira-Neto, J.L.; Ray, D.; Raskatov, J.A.; Green, R.E.; McKerrow, J.H.; Fischbach, M.A.; Linington, R.G. Genome-directed lead discovery: Biosynthesis, structure elucidation, and biological evaluation of two families of polyene macrolactams against Trypanosoma brucei. ACS Chem. Biol. 2015, 10, 2373–2381. [Google Scholar] [CrossRef]
- Nakashima, T.; Iwatsuki, M.; Ochiai, J.; Kamiya, Y.; Nagai, K.; Matsumoto, A.; Ishiyama, A.; Otoguro, K.; Shiomi, K.; Takahashi, Y.; et al. Mangromicins A and B: Structure and antitrypanosomal activity of two new cyclopentadecane compounds from Lechevalieria aerocolonigenes K10-0216. J. Antibiot. 2014, 67, 253–260. [Google Scholar] [CrossRef]
- Yang, F.; Gan, J.H.; Liu, X.Y.; Lin, H.W. Scalarane sesterterpenes from the Paracel islands marine sponge Hyrtios sp. Nat. Prod. Commun. 2014, 9, 763–764. [Google Scholar] [CrossRef] [PubMed]
- von Salm, J.L.; Wilson, N.G.; Vesely, B.A.; Kyle, D.E.; Cuce, J.; Baker, B.J. Shagenes A and B, new tricyclic sesquiterpenes produced by an undescribed Antarctic octocoral. Org. Lett. 2014, 16, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Han, C.; Yamano, Y.; Setiawan, A.; Kobayashi, M. Aaptamines, marine spongean alkaloids, as anti-dormant mycobacterial substances. J. Nat. Med. 2014, 68, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; de Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the marine sponge Callyspongia aerizusa: Cyclic peptides with antitubercular activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef]
- Kumar, M.M.K.; Naik, J.D.; Satyavathi, K.; Ramana, H.; Varma, P.R.; Nagasree, K.P. Denigrins A-C: New antitubercular 3,4-diarylpyrrole alkaloids from Dendrilla nigra. Nat. Prod. Res. 2014, 28, 888–894. [Google Scholar] [CrossRef]
- Lin, Z.; Koch, M.; Abdel Aziz, M.H.; Galindo-Murillo, R.; Tianero, M.D.; Cheatham, T.E.; Barrows, L.R.; Reilly, C.A.; Schmidt, E.W. Oxazinin A, a pseudodimeric natural product of mixed biosynthetic origin from a filamentous fungus. Org. Lett. 2014, 16, 4774–4777. [Google Scholar] [CrossRef]
- Gonzalez-Almela, E.; Sanz, M.A.; Garcia-Moreno, M.; Northcote, P.; Pelletier, J.; Carrasco, L. Differential action of pateamine A on translation of genomic and subgenomic mRNAs from Sindbis virus. Virology 2015, 484, 41–50. [Google Scholar] [CrossRef]
- Leon, B.; Navarro, G.; Dickey, B.J.; Stepan, G.; Tsai, A.; Jones, G.S.; Morales, M.E.; Barnes, T.; Ahmadyar, S.; Tsiang, M.; et al. Abyssomicin 2 reactivates latent HIV-1 by a PKC- and HDAC-independent mechanism. Org. Lett. 2015, 17, 262–265. [Google Scholar] [CrossRef]
- Karadeniz, F.; Kang, K.H.; Park, J.W.; Park, S.J.; Kim, S.K. Anti-HIV-1 activity of phlorotannin derivative 8,4’’’-dieckol from Korean brown alga Ecklonia cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef]
- Zhao, Y.; Si, L.; Liu, D.; Proksch, P.; Zhou, D.; Lin, W. Truncateols A–N, new isoprenylated cyclohexanols from the sponge-associated fungus Truncatella angustata with anti-H1N1 virus activities. Tetrahedron 2015, 71, 2708–2718. [Google Scholar] [CrossRef]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, Z.; Shen, L.; Pedpradab, P.; Bruhn, T.; Wu, J.; Bringmann, G. Antiviral limonoids including Khayanolides from the Trang mangrove plant Xylocarpus moluccensis LI2015. J. Nat. Prod. 2015, 78, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shao, C.L.; Meng, H.; She, Z.G.; Wang, C.Y. Anti-respiratory syncytial virus prenylated dihydroquinolone derivatives from the gorgonian-derived fungus Aspergillus sp. XS-20090B15. J. Nat. Prod. 2014, 77, 2720–2724. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.H.; Wang, Y.F.; Zhang, X.Y.; Zhou, M.P.; Xu, X.Y.; Qi, S.H. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus. Mar. Drugs 2014, 12, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Cen, S.; Proksch, P.; Lin, W. Isoindolinone-type alkaloids from the sponge-derived fungus Stachybotrys chartarum. Tetrahedron 2014, 70, 7010–7015. [Google Scholar] [CrossRef]
- Gupta, D.K.; Kaur, P.; Leong, S.T.; Tan, L.T.; Prinsep, M.R.; Chu, J.J. Anti-Chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar. Drugs 2014, 12, 115–127. [Google Scholar] [CrossRef]
- Pardo-Vargas, A.; de Barcelos Oliveira, I.; Stephens, P.R.; Cirne-Santos, C.C.; de Palmer Paixao, I.C.; Ramos, F.A.; Jimenez, C.; Rodriguez, J.; Resende, J.A.; Teixeira, V.L.; et al. Dolabelladienols A-C, new diterpenes isolated from Brazilian brown alga Dictyota pfaffii. Mar. Drugs 2014, 12, 4247–4259. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, M.; Sun, Z.; Yuan, W.; Zhang, S.; Xiang, Z.; Cai, Y.; Dong, J.; Huang, K.; Yan, P. Diterpenes from a Chinese collection of the brown alga Dictyota plectens. J. Nat. Prod. 2014, 77, 2685–2693. [Google Scholar] [CrossRef]
- Yamashita, A.; Fujimoto, Y.; Tamaki, M.; Setiawan, A.; Tanaka, T.; Okuyama-Dobashi, K.; Kasai, H.; Watashi, K.; Wakita, T.; Toyama, M.; et al. Identification of antiviral agents targeting Hepatitis B virus promoter from extracts of Indonesian marine organisms by a novel cell-based screening assay. Mar. Drugs 2015, 13, 6759–6773. [Google Scholar] [CrossRef]
- Cao, F.; Shao, C.L.; Chen, M.; Zhang, M.Q.; Xu, K.X.; Meng, H.; Wang, C.Y. Antiviral C-25 epimers of 26-acetoxy steroids from the South China sea gorgonian Echinogorgia rebekka. J. Nat. Prod. 2014, 77, 1488–1493. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wei, M.Y.; Chen, H.Y.; Guan, F.F.; Wang, C.Y.; Shao, C.L. (+)- and (-)-Pestaloxazine A, a pair of antiviral enantiomeric alkaloid dimers with a symmetric spiro[oxazinane-piperazinedione] skeleton from Pestalotiopsis sp. Org. Lett. 2015, 17, 4216–4219. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Moon, S.Y.; Lee, D.S.; Kim, H.J.; Park, K.; Lee, E.W.; Kim, T.H.; Chung, Y.H.; Lee, M.S.; Kim, Y.M. In vitro antiviral activity of dieckol and phlorofucofuroeckol-A isolated from edible brown alga Eisenia bicyclis against murine norovirus. Algae 2015, 30, 241–246. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.K.; Duh, C.Y. Secocrassumol, a seco-cembranoid from the Dongsha atoll soft coral Lobophytum crassum. Mar. Drugs 2014, 12, 6028–6037. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, K.; Aparna, V.; Madhuri, K.Z.; Hopper, W. Biological activity of sporolides A and B from Salinispora tropica: In silico target prediction using ligand-based pharmacophore mapping and in vitro activity validation on HIV-1 reverse transcriptase. Chem. Biol. Drug Des. 2014, 83, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelleta sp. Tetrahedron Lett. 2015, 56, 4215–4219. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Wang, J.; Wang, Y.F.; Zhang, X.Y.; Nong, X.H.; Chen, M.; Xu, X.Y. Cytotoxic and antiviral tetramic acid derivatives from the deep-sea-derived fungus Trichobotrys effuse DFFSCS021. Tetrahedron 2015, 71, 9328–9332. [Google Scholar] [CrossRef]
- Farrugia, M.; Trotter, N.; Vijayasarathy, S.; Salim, A.A.; Khalil, Z.G.; Lacey, E.; Capon, R.J. Isolation and synthesis of N-acyladenine and adenosine alkaloids from a southern Australian marine sponge, Phoriospongia sp. Tetrahedron Lett. 2014, 55, 5902–5904. [Google Scholar] [CrossRef]
- Woo, J.K.; Kim, C.K.; Kim, S.H.; Kim, H.; Oh, D.C.; Oh, K.B.; Shin, J. Gombaspiroketals A-C, sesterterpenes from the sponge Clathria gombawuiensis. Org. Lett. 2014, 16, 2826–2829. [Google Scholar] [CrossRef]
- Cantisani, M.; Finamore, E.; Mignogna, E.; Falanga, A.; Nicoletti, G.F.; Pedone, C.; Morelli, G.; Leone, M.; Galdiero, M.; Galdiero, S. Structural insights into and activity analysis of the antimicrobial peptide myxinidin. Antimicrob. Agents Chemother. 2014, 58, 5280–5290. [Google Scholar] [CrossRef]
- Kusama, T.; Tanaka, N.; Sakai, K.; Gonoi, T.; Fromont, J.; Kashiwada, Y.; Kobayashi, J. Agelamadins C-E, bromopyrrole alkaloids comprising oroidin and 3-hydroxykynurenine from a marine sponge Agelas sp. Org. Lett. 2014, 16, 5176–5179. [Google Scholar] [CrossRef]
- Sun, K.; Li, Y.; Guo, L.; Wang, Y.; Liu, P.; Zhu, W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs 2014, 12, 3970–3981. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kusama, T.; Tanaka, N.; Gonoi, T.; Fromont, J.; Kobayashi, J. 2-Debromonagelamide U, 2-Debromomukanadin G, and 2-Debromonagelamide P from marine sponge Agelas sp. Heterocycles 2015, 90, 425–431. [Google Scholar]
- Huang, Q.; Cheng, W.; Long, H.; Liu, H.; van Ofwegen, L.; Lin, W. Subergane-type sesquiterpenes from Gorgonian coral Subergorgia suberosa with antibacterial activities. Helv. Chim. Acta 2015, 98, 1202–1209. [Google Scholar] [CrossRef]
- Gotsbacher, M.P.; Karuso, P. New antimicrobial bromotyrosine analogues from the sponge Pseudoceratina purpurea and its predator Tylodina corticalis. Mar. Drugs 2015, 13, 1389–1409. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Desouky, S.; El-Kassem, A.; Elkhateeb, W. Alternariol derivatives from Alternaria alternata, an endophytic fungus residing in red sea soft coral, inhibit HCV NS3/4A protease. Appl. Biochem. Microbiol. 2015, 51, 579–584. [Google Scholar] [CrossRef]
- Kubota, T.; Iwai, T.; Sakai, K.; Gonoi, T.; Kobayashi, J. Amphidinins C-F, amphidinolide Q analogues from marine dinoflagellate Amphidinium sp. Org. Lett. 2014, 16, 5624–5627. [Google Scholar] [CrossRef]
- Dasyam, N.; Munkacsi, A.B.; Fadzilah, N.H.; Senanayake, D.S.; O’Toole, R.F.; Keyzers, R.A. Identification and bioactivity of 3-epi-xestoaminol C isolated from the New Zealand brown alga Xiphophora chondrophylla. J. Nat. Prod. 2014, 77, 1519–1523. [Google Scholar] [CrossRef]
- Chen, M.; Shao, C.L.; Fu, X.M.; Kong, C.J.; She, Z.G.; Wang, C.Y. Lumazine peptides penilumamides B-D and the cyclic pentapeptide asperpeptide A from a gorgonian-derived Aspergillus sp. fungus. J. Nat. Prod. 2014, 77, 1601–1606. [Google Scholar] [CrossRef]
- Chen, M.; Fu, X.M.; Kong, C.J.; Wang, C.Y. Nucleoside derivatives from the marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 2014, 28, 895–900. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Zhang, P.; Li, C.S.; Wang, B.G. Cyclodepsipeptides and other O-containing heterocyclic metabolites from Beauveria felina EN-135, a marine-derived entomopathogenic fungus. Mar. Drugs 2014, 12, 2816–2826. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Abdelmohsen, U.R.; Ibrahim, A.K.; Hassanean, H.A.; Hentschel, U.; Ahmed, S.A. New antibacterial xanthone from the marine sponge-derived Micrococcus sp. EG45. Bioorg. Med. Chem. Lett. 2014, 24, 4939–4942. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Q.; Liu, X.; Chen, Y.; Zhang, Y.; Sun, A.; Zhang, W.; Zhang, J.; Ju, J. Cyclic Hexapeptides from the deep South China sea-derived Streptomyces scopuliridis SCSIO ZJ46 active against pathogenic Gram-positive bacteria. J. Nat. Prod. 2014, 77, 1937–1941. [Google Scholar] [CrossRef] [PubMed]
- Tareq, F.S.; Hasan, C.M.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Surovy, M.Z.; Islam, M.T.; Shin, H.J. Gageopeptins A and B, new inhibitors of zoospore motility of the phytopathogen Phytophthora capsici from a marine-derived bacterium Bacillus sp. 109GGC020. Bioorg. Med. Chem. Lett. 2015, 25, 3325–3329. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.L.; Labes, A.; Wiese, J.; Bruhn, T.; Bringmann, G.; Imhoff, J.F. Nature’s lab for derivatization: New and revised structures of a variety of streptophenazines produced by a sponge-derived Streptomyces strain. Mar. Drugs 2014, 12, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Reyes, L.A.; Sneed, J.; Paul, V.J.; Luesch, H. Amantelides A and B, Polyhydroxylated macrolides with differential broad-spectrum cytotoxicity from a Guamanian marine cyanobacterium. J. Nat. Prod. 2015, 78, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Gong, T.; Liu, F.; Zhang, P.C.; Zhou, W.Q.; Li, Y.; Zhu, P. A new analogue of echinomycin and a new cyclic dipeptide from a marine-derived Streptomyces sp. LS298. Mar. Drugs 2015, 13, 6947–6961. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Badr, J.M.; Shaala, L.A.; Mohamed, G.A.; Baer, R.J.; Bamanie, F.H. Ehrenasterol and biemnic acid; new bioactive compounds from the Red sea sponge Biemna ehrenbergi. Phytochem. Lett. 2015, 12, 296–301. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Rae, M.; Soldatou, S.; Ding, Y.; Wolff, C.W.; McCormack, G.; Coleman, C.M.; Ferreira, D.; Tasdemir, D. Sulfated steroid-amino acid conjugates from the Irish marine sponge Polymastia boletiformis. Mar. Drugs 2015, 13, 1632–1646. [Google Scholar] [CrossRef]
- Kubota, T.; Watase, S.; Sakai, K.; Fromont, J.; Gonoi, T.; Kobayashi, J. Tyrokeradines G and H, new bromotyrosine alkaloids from an Okinawan Verongid sponge. Bioorg. Med. Chem. Lett. 2015, 25, 5221–5223. [Google Scholar] [CrossRef]
- Xu, X.; Yin, L.; Gao, J.; Gao, L.; Song, F. Antifungal bromophenols from marine red alga Symphyocladia latiuscula. Chem. Biodivers. 2014, 11, 807–811. [Google Scholar] [CrossRef]
- Koch, L.; Lodin, A.; Herold, I.; Ilan, M.; Carmeli, S.; Yarden, O. Sensitivity of Neurospora crassa to a marine-derived Aspergillus tubingensis anhydride exhibiting antifungal activity that is mediated by the MAS1 protein. Mar. Drugs 2014, 12, 4713–4731. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T. Identification and bioactivity of compounds from the fungus Penicillium sp. CYE-87 isolated from a marine tunicate. Mar. Drugs 2015, 13, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, metabolite of fucoxanthin, improves obesity-induced inflammation in adipocyte cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Lee, S.H.; Lee, W.W.; Kang, N.; Kim, E.A.; Kim, S.Y.; Lee, D.H.; Kim, D.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Ishige okamurae against high-glucose induced oxidative stress in human umbilical vein endothelial cells and zebrafish model. J. Funct. Foods 2014, 11, 304–312. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, S.M.; Ko, S.C.; Moon, S.H.; Jeon, B.T.; Lee, D.H.; Jeon, Y.J. Octaphlorethol A: A potent alpha-glucosidase inhibitor isolated from Ishige foliacea shows an anti-hyperglycemic effect in mice with streptozotocin-induced diabetes. Food Funct. 2014, 5, 2602–2608. [Google Scholar] [CrossRef]
- You, H.N.; Lee, H.A.; Park, M.H.; Lee, J.H.; Han, J.S. Phlorofucofuroeckol A isolated from Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2015, 752, 92–96. [Google Scholar] [CrossRef]
- Safavi-Hemami, H.; Gajewiak, J.; Karanth, S.; Robinson, S.D.; Ueberheide, B.; Douglass, A.D.; Schlegel, A.; Imperial, J.S.; Watkins, M.; Bandyopadhyay, P.K.; et al. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc. Natl. Acad. Sci. USA 2015, 112, 1743–1748. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takahashi, O.; Kanno, S.; Nakazawa, T.; Takahashi, S.; Ukai, K.; Sumilat, D.A.; Ishikawa, M.; Namikoshi, M. Absolute structures and bioactivities of euryspongins and eurydiene obtained from the marine sponge Euryspongia sp. collected at Iriomote island. Bioorg. Med. Chem. 2015, 23, 797–802. [Google Scholar] [CrossRef]
- Xia, X.; Qi, J.; Liu, Y.; Jia, A.; Zhang, Y.; Liu, C.; Gao, C.; She, Z. Bioactive isopimarane diterpenes from the fungus, Epicoccum sp. HS-1, associated with Apostichopus japonicus. Mar. Drugs 2015, 13, 1124–1132. [Google Scholar] [CrossRef]
- Shin, B.; Ahn, S.; Noh, M.; Shin, J.; Oh, D.C. Suncheonosides A-D, benzothioate glycosides from a marine-derived Streptomyces sp. J. Nat. Prod. 2015, 78, 1390–1396. [Google Scholar] [CrossRef]
- You, M.; Liao, L.; Hong, S.H.; Park, W.; Kwon, D.I.; Lee, J.; Noh, M.; Oh, D.C.; Oh, K.B.; Shin, J. Lumazine peptides from the marine-derived fungus Aspergillus terreus. Mar. Drugs 2015, 13, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- He, W.F.; Liang, L.F.; Cai, Y.S.; Gao, L.X.; Li, Y.F.; Li, J.; Liu, H.L.; Guo, Y.W. Brominated polyunsaturated lipids with protein tyrosine phosphatase-1B inhibitory activity from Chinese marine sponge Xestospongia testudinaria. J. Asian Nat. Prod. Res. 2015, 17, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Tsuchida, E.; Uehara, M.; Kinjyo, Y.; Roy, P.K.; Ueda, K. Dual biological functions of the apoptotic activity and anti-inflammatory effect by alcyonolide congeners from the Okinawan soft coral, Cespitularia sp. Bioorg. Med. Chem. Lett. 2015, 25, 4496–4499. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; Zhou, H.L.; Huang, C.L.; You, C.G.; Fang, Q.; Wu, P.; Wang, X.G.; Han, C.M. Astaxanthin attenuates early acute kidney injury following severe burns in rats by ameliorating oxidative stress and mitochondrial-related apoptosis. Mar. Drugs 2015, 13, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Jung, S.H.; Lee, K.T.; Choi, J.H. 8,8′-Bieckol, isolated from edible brown algae, exerts its anti-inflammatory effects through inhibition of NF-kappaB signaling and ROS production in LPS-stimulated macrophages. Int. Immunopharmacol. 2014, 23, 460–468. [Google Scholar] [CrossRef]
- Fernandes, P.D.; Zardo, R.S.; Figueiredo, G.S.; Silva, B.V.; Pinto, A.C. Anti-inflammatory properties of convolutamydine A and two structural analogues. Life Sci. 2014, 116, 16–24. [Google Scholar] [CrossRef]
- Phan, C.S.; Ng, S.Y.; Kim, E.A.; Jeon, Y.J.; Palaniveloo, K.; Vairappan, C.S. Capgermacrenes A and B, bioactive secondary metabolites from a Bornean soft coral, Capnella sp. Mar. Drugs 2015, 13, 3103–3115. [Google Scholar] [CrossRef]
- Jimenez-Romero, C.; Mayer, A.M.; Rodriguez, A.D. Dactyloditerpenol acetate, a new prenylbisabolane-type diterpene from Aplysia dactylomela with significant in vitro anti-neuroinflammatory activity. Bioorg. Med. Chem. Lett. 2014, 24, 344–348. [Google Scholar] [CrossRef]
- Choi, H.J.; Park, J.H.; Lee, B.H.; Chee, H.Y.; Lee, K.B.; Oh, S.M. Suppression of NF-kappaB by dieckol extracted from Ecklonia cava negatively regulates LPS induction of inducible nitric oxide synthase gene. Appl. Biochem. Biotechnol. 2014, 173, 957–967. [Google Scholar] [CrossRef]
- Kang, N.J.; Koo, D.H.; Kang, G.J.; Han, S.C.; Lee, B.W.; Koh, Y.S.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; Kang, H.K.; et al. Dieckol, a component of Ecklonia cava, suppresses the production of MDC/CCL22 via down-regulating STAT1 pathway in interferon-gamma stimulated HaCaT human keratinocytes. Biomol. Ther. 2015, 23, 238–244. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Lin, S.C.; Feng, C.W.; Chen, P.C.; Su, Y.D.; Li, C.M.; Yang, S.N.; Jean, Y.H.; Sung, P.J.; Duh, C.Y.; et al. Anti-inflammatory and analgesic effects of the marine-derived compound excavatolide B isolated from the culture-type Formosan gorgonian Briareum excavatum. Mar. Drugs 2015, 13, 2559–2579. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.F.; Huang, S.Y.; Lu, C.H.; Chen, C.L.; Feng, C.W.; Chen, C.H.; Hung, H.C.; Lin, Y.Y.; Sung, P.J.; Sung, C.S.; et al. Flexibilide obtained from cultured soft coral has anti-neuroinflammatory and analgesic effects through the upregulation of spinal transforming growth factor-beta1 in neuropathic rats. Mar. Drugs 2014, 12, 3792–3817. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, H.H.; Jeong, J.W.; Seo, M.J.; Kang, B.W.; Park, J.U.; Kim, K.S.; Cho, Y.S.; Seo, K.I.; Kim, G.Y.; et al. Anti-inflammatory effects of 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone via NF-kappaB inactivation in lipopolysaccharide-stimulated RAW 264.7 macrophage. Mol. Med. Rep. 2014, 9, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.; Kang, M.C.; Lee, W.W.; Lee, H.S.; Kamada, T.; Vairappan, C.S.; Jeon, Y.J. 5ß-Hydroxypalisadin B isolated from red alga Laurencia snackeyi attenuates inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Algae 2014, 29, 333–341. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef]
- Yu, D.K.; Lee, B.; Kwon, M.; Yoon, N.; Shin, T.; Kim, N.G.; Choi, J.S.; Kim, H.R. Phlorofucofuroeckol B suppresses inflammatory responses by down-regulating nuclear factor kappaB activation via Akt, ERK, and JNK in LPS-stimulated microglial cells YU2015. Int. Immunopharmacol. 2015, 28, 1068–1075. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Itoh, T.; Koketsu, M.; Yokota, N.; Touho, S.; Ando, M.; Tsukamasa, Y. Reduced scytonemin isolated from Nostoc commune suppresses LPS/IFNgamma-induced NO production in murine macrophage RAW264 cells by inducing hemeoxygenase-1 expression via the Nrf2/ARE pathway. Food Chem. Toxicol. 2014, 69, 330–338. [Google Scholar] [CrossRef]
- Lillsunde, K.E.; Festa, C.; Adel, H.; De, M.S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D’Souza, L.; D’Auria, M.V.; et al. Bioactive cembrane derivatives from the Indian ocean soft coral, Sinularia kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Sun, Y.N.; Song, S.B.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Kim, Y.H.; Minh, C.V. NF-kappaB inhibitory activity of polyoxygenated steroids from the Vietnamese soft coral Sarcophyton pauciplicatum. Bioorg. Med. Chem. Lett. 2014, 24, 2834–2838. [Google Scholar] [CrossRef]
- Thao, N.P.; Nam, N.H.; Cuong, N.X.; Luyen, B.T.; Tai, B.H.; Kim, J.E.; Song, S.B.; Kiem, P.V.; Minh, C.V.; Kim, Y.H. Inhibition of NF-kappaB transcriptional activation in HepG2 cells by diterpenoids from the soft coral Sinularia maxima. Arch. Pharm. Res. 2014, 37, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, N.T.T.; Ko, W.; Kim, D.C.; Yoon, C.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Tanzawaic acid derivatives from a marine isolate of Penicillium sp. (SF-6013) with anti-inflammatory and PTP1B inhibitory activities. Bioorg. Med. Chem. Lett. 2014, 24, 5787–5791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, S.; Zhan, Y.; Zhang, N.; Wu, A.A.; Gui, F.; Guo, K.; Yang, Y.; Cao, S.; Hu, Z.; et al. Aspertetranones A-D, putative meroterpenoids from the marine algal-associated fungus Aspergillus sp. ZL0-1b14. J. Nat. Prod. 2015, 78, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.D.; Cheng, C.H.; Chen, W.F.; Chang, Y.C.; Chen, Y.H.; Hwang, T.L.; Wen, Z.H.; Wang, W.H.; Fang, L.S.; Chen, J.J.; et al. Briarenolide J, the first 12-chlorobriarane diterpenoid from an octoral Briareum sp. (Briareidae). Tetrahedron 2014, 55, 6065–6067. [Google Scholar] [CrossRef]
- Su, Y.D.; Su, T.R.; Wen, Z.H.; Hwang, T.L.; Fang, L.S.; Chen, J.J.; Wu, Y.C.; Sheu, J.H.; Sung, P.J. Briarenolides K and L, new anti-inflammatory briarane diterpenoids from an octocoral Briareum sp. (Briareidae). Mar. Drugs 2015, 13, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.D.; Wu, T.Y.; Wen, Z.H.; Su, C.C.; Chen, Y.H.; Chang, Y.C.; Wu, Y.C.; Sheu, J.H.; Sung, P.J. Briarenolides U-Y, New anti-inflammatory briarane diterpenoids from an octocoral Briareum sp. (Briareidae). Mar. Drugs 2015, 13, 7138–7149. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.C.; Cheng, Y.B.; Lin, Y.S.; Kuo, Y.H.; Hwang, T.L.; Shen, Y.C. New briarane diterpenoids from Taiwanese soft coral Briareum violacea. Mar. Drugs 2014, 12, 4677–4692. [Google Scholar] [CrossRef]
- Wagner, M.; Abdel-Mageed, W.M.; Ebel, R.; Bull, A.T.; Goodfellow, M.; Fiedler, H.P.; Jaspars, M. Dermacozines H-J isolated from a deep-sea strain of Dermacoccus abyssi from Mariana trench sediments. J. Nat. Prod. 2014, 77, 416–420. [Google Scholar] [CrossRef]
- Jiao, W.H.; Xu, T.T.; Zhao, F.; Gao, H.; Shi, G.H.; Wang, J.; Hong, L.L.; Yu, H.B.; Li, Y.S.; Yang, F.; et al. Dysifragilones A–C, unusual sesquiterpene aminoquinones and inhibitors of NO production from the South China sea sponge Dysidea fragilis. Eur. J. Org. Chem. 2015, 2015, 960–966. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, S.; Yuan, W.; Dong, J.; Huang, K.; Sun, Z.; Yan, P. Further new xenicanes from a Chinese collection of the brown alga Dictyota plectens. Chem. Pharm. Bull. 2015, 63, 1081–1086. [Google Scholar] [CrossRef]
- Chen, L.C.; Lin, Y.Y.; Jean, Y.H.; Lu, Y.; Chen, W.F.; Yang, S.N.; Wang, H.M.; Jang, I.Y.; Chen, I.M.; Su, J.H.; et al. Anti-inflammatory and analgesic effects of the marine-derived compound comaparvin isolated from the crinoid Comanthus bennetti. Molecules 2014, 19, 14667–14686. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Z.; Chen, B.W.; Huang, C.Y.; Hwang, T.L.; Uvarani, C.; Dai, C.F.; Sung, P.J.; Su, J.H.; Sheu, J.H. Eunicellin-based diterpenoids, hirsutalins S-V, from the Formosan soft coral Cladiella hirsuta. Mar. Drugs 2015, 13, 2757–2769. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Z.; Chen, B.W.; Huang, C.Y.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Eunicellin-based diterpenoids, hirsutalins N-R, from the formosan soft coral Cladiella hirsuta. Mar. Drugs 2014, 12, 2446–2457. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.W.; Uvarani, C.; Huang, C.Y.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. New anti-inflammatory tocopherol-derived metabolites from the Taiwanese soft coral Cladiella hirsuta. Bioorg. Med. Chem. Lett. 2015, 25, 92–95. [Google Scholar] [CrossRef]
- Tsai, T.C.; Chen, H.Y.; Sheu, J.H.; Chiang, M.Y.; Wen, Z.H.; Dai, C.F.; Su, J.H. Structural elucidation and structure-anti-inflammatory activity relationships of cembranoids from cultured soft corals Sinularia sandensis and Sinularia flexibilis. J. Agric. Food Chem. 2015, 63, 7211–7218. [Google Scholar] [CrossRef]
- TSAI, C.R.; Huang, C.Y.; Chen, B.W.; Tsai, Y.Y.; Shih, S.P.; Hwang, T.L.; Dai, C.F.; Wang, S.Y.; Sheu, J.H. New bioactive steroids from the soft coral Klyxum flaccidum. RSC Adv. 2015, 5, 12546–12554. [Google Scholar] [CrossRef]
- Lee, Y.N.; Tai, C.J.; Hwang, T.L.; Sheu, J.H. Krempfielins N-P, new anti-inflammatory eunicellins from a Taiwanese soft coral Cladiella krempfi. Mar. Drugs 2014, 12, 1148–1156. [Google Scholar] [CrossRef]
- Tai, C.J.; Chokkalingam, U.; Cheng, Y.; Shih, S.P.; Lu, M.C.; Su, J.H.; Hwang, T.L.; Sheu, J.H. Krempfielins Q and R, two new eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Int. J. Mol. Sci. 2014, 15, 21865–21874. [Google Scholar] [CrossRef]
- Fang, H.Y.; Chokkalingam, U.; Chiou, S.F.; Hwang, T.L.; Chen, S.L.; Wang, W.L.; Sheu, J.H. Bioactive chemical constituents from the brown alga Homoeostrichus formosana. Int. J. Mol. Sci. 2014, 16, 736–746. [Google Scholar] [CrossRef]
- Wang, W.; Lee, T.G.; Patil, R.S.; Mun, B.; Yang, I.; Kim, H.; Hahn, D.; Won, D.H.; Lee, J.; Lee, Y.; et al. Monanchosterols A and B, bioactive bicyclosteroids from a Korean sponge Monanchora sp. J. Nat. Prod. 2015, 78, 368–373. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Koo, J.E.; Kim, S.; Koh, Y.S.; Cuong, N.X.; Nam, N.H.; Van, K.P.; Kim, Y.H.; Van, M.C. Anti-inflammatory components of the Vietnamese starfish Protoreaster nodosus. Biol. Res. 2015, 48, 12. [Google Scholar] [CrossRef] [PubMed]
- Pudhom, K.; Teerawatananond, T. Rhytidenones A-F, Spirobisnaphthalenes from Rhytidhysteron sp. AS21B, an Endophytic Fungus. J. Nat. Prod. 2014, 77, 1962–1966. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Chen, B.W.; Huang, C.Y.; Wen, Z.H.; Sung, P.J.; Su, J.H.; Dai, C.F.; Sheu, J.H. Bioactive cembranoids, sarcocrassocolides P-R, from the Dongsha atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2014, 12, 840–850. [Google Scholar] [CrossRef]
- Lin, W.J.; Wu, T.Y.; Su, T.R.; Wen, Z.H.; Chen, J.J.; Fang, L.S.; Wu, Y.C.; Sung, P.J. Terpenoids from the octocoral Sinularia gaweli. Int. J. Mol. Sci. 2015, 16, 19508–19517. [Google Scholar] [CrossRef] [PubMed]
- Afiyatullov, S.S.; Leshchenko, E.V.; Sobolevskaya, M.P.; Denisenko, V.A.; Kirichuk, N.N.; Khudyakova, Y.V.; Hoai, T.; Dmitrenok, P.S.; Menchinskaya, E.S.; Pislyagin, E.A.; et al. New eudesmane sesquiterpenes from the marine-derived fungus Penicillium thomii. Phytochem. Lett. 2015, 14, 209–214. [Google Scholar] [CrossRef]
- Lin, K.H.; Tseng, Y.J.; Chen, B.W.; Hwang, T.L.; Chen, H.Y.; Dai, C.F.; Sheu, J.H. Tortuosenes A and B, new diterpenoid metabolites from the Formosan soft coral Sarcophyton tortuosum. Org. Lett. 2014, 16, 1314–1317. [Google Scholar] [CrossRef]
- Kwan, J.C.; Liu, Y.; Ratnayake, R.; Hatano, R.; Kuribara, A.; Morimoto, C.; Ohnuma, K.; Paul, V.J.; Ye, T.; Luesch, H. Grassypeptolides as natural inhibitors of dipeptidyl peptidase 8 and T-cell activation. Chembiochem 2014, 15, 799–804. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.; Luo, X.; de Voogd, N.J.; Li, P.; Li, G. (+)- and (-)-Spiroreticulatine, A Pair of Unusual spiro bisheterocyclic quinoline-imidazole alkaloids from the South China sea sponge Fascaplysinopsis reticulata. Org. Lett. 2015, 17, 3458–3461. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Ivanchina, N.V.; Dmitrenok, P.S.; Menchinskaya, E.S.; Yurchenko, E.A.; Pislyagin, E.A.; Aminin, D.L.; Huong, T.T.; et al. Cyclic steroid glycosides from the starfish Echinaster luzonicus: Structures and immunomodulatory activities. J. Nat. Prod. 2015, 78, 1397–1405. [Google Scholar] [CrossRef]
- Pislyagin, E.A.; Aminin, D.L.; Silchenko, A.S.; Avilov, S.A.; Andryjashchenko, P.V.; Kalinin, V.I.; Padmakumar, K. Immunomodulatory action of triterpene glycosides isolated from the sea cucumber Actinocucumis typica. Structure-activity relationships. Nat. Prod. Commun. 2014, 9, 771–772. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Qiao, F.; Xu, G.W.; Zhao, J.; Teng, J.F.; Li, C.; Deng, W.J. Neuroprotective metabolites from the endophytic fungus Penicillium citrinum of the mangrove Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 12, 148–152. [Google Scholar] [CrossRef]
- Hjornevik, L.V.; Froyset, A.K.; Gronset, T.A.; Rungruangsak-Torrissen, K.; Fladmark, K.E. Algal toxin azaspiracid-1 induces early neuronal differentiation and alters peripherin isoform stoichiometry. Mar. Drugs 2015, 13, 7390–7402. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Silva, L.H.; Falcao, M.A.; Vieira, A.C.; Viana, M.D.; de Araujo-Junior, J.X.; Sousa, J.C.; da Silva, T.M.; Barbosa-Filho, J.M.; Noel, F.; de Miranda, G.E.; et al. Assessment of mechanisms involved in antinociception produced by the alkaloid caulerpine. Molecules 2014, 19, 14699–14709. [Google Scholar] [CrossRef] [PubMed]
- Kasheverov, I.E.; Shelukhina, I.V.; Kudryavtsev, D.S.; Makarieva, T.N.; Spirova, E.N.; Guzii, A.G.; Stonik, V.A.; Tsetlin, V.I. 6-bromohypaphorine from marine nudibranch mollusk Hermissenda crassicornis is an agonist of human alpha7 nicotinic acetylcholine receptor. Mar. Drugs 2015, 13, 1255–1266. [Google Scholar] [CrossRef]
- Chen, W.F.; Huang, S.Y.; Liao, C.Y.; Sung, C.S.; Chen, J.Y.; Wen, Z.H. The use of the antimicrobial peptide piscidin (PCD)-1 as a novel anti-nociceptive agent. Biomaterials 2015, 53, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Liu, Z.; Wang, X.; Liu, N.; Du, W.; Dai, Q. Structural and Functional Characterization of a novel alpha-conotoxin Mr1.7 from Conus marmoreus targeting neuronal nAChR alpha3beta2, alpha9alpha10 and alpha6/alpha3beta2beta3 subtypes. Mar. Drugs 2015, 13, 3259–3275. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, L.; Wu, Y.; Liu, J.; Sun, D.; Zhu, X.; Feng, Y.; Qin, M.; Chen, S.; Xu, A. Soluble expression and sodium channel activity of lt16a, a novel framework XVI conotoxin from the M-superfamily. Toxicon 2015, 98, 5–11. [Google Scholar] [CrossRef]
- Li, M.; Chang, S.; Yang, L.; Shi, J.; McFarland, K.; Yang, X.; Moller, A.; Wang, C.; Zou, X.; Chi, C.; et al. Conopeptide Vt3.1 preferentially inhibits BK potassium channels containing beta4 subunits via electrostatic interactions. J. Biol. Chem. 2014, 289, 4735–4742. [Google Scholar] [CrossRef]
- Lee, S.R.; Pronto, J.R.; Sarankhuu, B.E.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Acetylcholinesterase inhibitory activity of pigment echinochrome A from sea urchin Scaphechinus mirabilis. Mar. Drugs 2014, 12, 3560–3573. [Google Scholar] [CrossRef]
- Yamagishi, M.; Hosoda-Yabe, R.; Tamai, H.; Konishi, M.; Imamura, A.; Ishida, H.; Yabe, T.; Ando, H.; Kiso, M. Structure-activity relationship study of the neuritogenic potential of the glycan of starfish ganglioside LLG-3 (double dagger). Mar. Drugs 2015, 13, 7250–7274. [Google Scholar] [CrossRef]
- Cassiano, C.; Esposito, R.; Tosco, A.; Zampella, A.; D’Auria, M.V.; Riccio, R.; Casapullo, A.; Monti, M.C. Heteronemin, a marine sponge terpenoid, targets TDP-43, a key factor in several neurodegenerative disorders. Chem. Commun. 2014, 50, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Sulzenbacher, G.; Radic, Z.; Araoz, R.; Reynaud, M.; Benoit, E.; Zakarian, A.; Servent, D.; Molgo, J.; Taylor, P.; et al. Marine macrocyclic imines, pinnatoxins A and G: Structural determinants and functional properties to distinguish neuronal alpha7 from muscle alpha1(2)betagammadelta nAChRs. Structure 2015, 23, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.A.; Salceda, E.; Garateix, A.G.; Zaharenko, A.J.; Peigneur, S.; Lopez, O.; Pons, T.; Richardson, M.; Diaz, M.; Hernandez, Y.; et al. A novel sea anemone peptide that inhibits acid-sensing ion channels. Peptides 2014, 53, 3–12. [Google Scholar] [CrossRef]
- Choi, B.W.; Lee, H.S.; Shin, H.C.; Lee, B.H. Multifunctional activity of polyphenolic compounds associated with a potential for Alzheimer’s disease therapy from Ecklonia cava. Phytother. Res. 2015, 29, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, N.A.; Ibrahim, A.K.; Radwan, M.M.; Zaitone, S.A.; Gomaa, M.; ElSohly, M.A.; Hassanean, H.A.; Ahmed, S.A. Mechanism of action of antiepileptic ceramide from Red sea soft coral Sarcophyton auritum. Bioorg. Med. Chem. Lett. 2015, 25, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Araoz, R.; Ouanounou, G.; Iorga, B.I.; Goudet, A.; Alili, D.; Amar, M.; Benoit, E.; Molgo, J.; Servent, D. The neurotoxic effect of 13,19-didesmethyl and 13-desmethyl spirolide C phycotoxins is mainly mediated by nicotinic rather than muscarinic acetylcholine receptors. Toxicol. Sci. 2015, 147, 156–167. [Google Scholar] [CrossRef]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef]
- Tian, L.W.; Feng, Y.; Shimizu, Y.; Pfeifer, T.; Wellington, C.; Hooper, J.N.; Quinn, R.J. Aplysinellamides A-C, bromotyrosine-derived metabolites from an Australian Aplysinella sp. marine sponge. J. Nat. Prod. 2014, 77, 1210–1214. [Google Scholar] [CrossRef]
- Franklin, J.B.; Rajesh, R.P. A sleep-inducing peptide from the venom of the Indian cone snail Conus araneosus. Toxicon 2015, 103, 39–47. [Google Scholar] [CrossRef]
- Harms, H.; Kehraus, S.; Nesaei-Mosaferan, D.; Hufendieck, P.; Meijer, L.; König, G.M. A beta-42 lowering agents from the marine-derived fungus Dichotomomyces cejpii. Steroids 2015, 104, 182–188. [Google Scholar] [CrossRef]
- Neves, J.L.; Lin, Z.; Imperial, J.S.; Antunes, A.; Vasconcelos, V.; Olivera, B.M.; Schmidt, E.W. Small molecules in the cone snail arsenal. Org. Lett. 2015, 17, 4933–4935. [Google Scholar] [CrossRef] [PubMed]
- Sirimangkalakitti, N.; Olatunji, O.J.; Changwichit, K.; Saesong, T.; Chamni, S.; Chanvorachote, P.; Ingkaninan, K.; Plubrukarn, A.; Suwanborirux, K. Bromotyrosine alkaloids with acetylcholinesterase inhibitory activity from the Thai sponge Acanthodendrilla sp. Nat. Prod. Commun. 2015, 10, 1945–1949. [Google Scholar] [CrossRef] [PubMed]
- Mevers, E.; Matainaho, T.; Allara’, M.; Di Marzo, V.; Gerwick, W.H. Mooreamide A: A cannabinomimetic lipid from the marine cyanobacterium Moorea bouillonii. Lipids 2014, 49, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Fedele, E.; Pittaluga, A.; Olivero, G.; Grilli, M.; Chen, J.; Mele, G.; Malafronte, N.; De, T.N.; Leddae, F.; et al. Isolation of hydroxyoctaprenyl-1′,4′-hydroquinone, a new octaprenylhydroquinone from the marine sponge Sarcotragus spinosulus and evaluation of its pharmacological activity on acetylcholine and glutamate release in the rat central nervous system. Nat. Prod. Commun. 2014, 9, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Nesher, N.; Zlotkin, E.; Hochner, B. The sea anemone toxin AdE-1 modifies both sodium and potassium currents of rat cardiomyocytes. Biochem. J. 2014, 461, 51–59. [Google Scholar] [CrossRef]
- Kim, M.J.; Woo, S.W.; Kim, M.S.; Park, J.E.; Hwang, J.K. Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. J. Asian Nat. Prod. Res. 2014, 16, 1139–1147. [Google Scholar] [CrossRef]
- Minamida, M.; Kumagai, K.; Ulanova, D.; Akakabe, M.; Konishi, Y.; Tominaga, A.; Tanaka, H.; Tsuda, M.; Fukushi, E.; Kawabata, J.; et al. Amphirionin-4 with potent proliferation-promoting activity on bone marrow stromal cells from a marine dinoflagellate Amphidinium species. Org. Lett. 2014, 16, 4858–4861. [Google Scholar] [CrossRef]
- Wang, J.Y.; Lee, Y.J.; Chou, M.C.; Chang, R.; Chiu, C.H.; Liang, Y.J.; Wu, L.S. Astaxanthin protects steroidogenesis from hydrogen peroxide-induced oxidative stress in mouse Leydig cells. Mar. Drugs 2015, 13, 1375–1388. [Google Scholar] [CrossRef]
- Eguchi, K.; Kato, H.; Fujiwara, Y.; Losung, F.; Mangindaan, R.E.; de Voogd, N.J.; Takeya, M.; Tsukamoto, S. Bastadins, brominated-tyrosine derivatives, suppress accumulation of cholesterol ester in macrophages. Bioorg. Med. Chem. Lett. 2015, 25, 5389–5392. [Google Scholar] [CrossRef]
- Kwon, T.H.; Wu, Y.X.; Kim, J.S.; Woo, J.H.; Park, K.T.; Kwon, O.J.; Seo, H.J.; Kim, T.; Park, N.H. 6,6’-Bieckol inhibits adipocyte differentiation through downregulation of adipogenesis and lipogenesis in 3T3-L1 cells. J. Sci. Food Agric. 2015, 95, 1830–1837. [Google Scholar] [CrossRef]
- Alvarez-Mico, X.; Rocha, D.D.; Guimaraes, L.A.; Ambrose, A.; Chapman, E.; Costa-Lotufo, L.V.; La Clair, J.J.; Fenical, W. The hybrid pyrroloisoindolone-dehydropyrrolizine alkaloid (-)-chlorizidine A targets proteins within the glycolytic pathway. Chembiochem 2015, 16, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wen, Z.H.; Lee, Y.H.; Chen, C.L.; Hung, H.C.; Chen, C.H.; Chen, W.F.; Tsai, M.C. Dihydroaustrasulfone alcohol inhibits PDGF-induced proliferation and migration of human aortic smooth muscle cells through inhibition of the cell cycle. Mar. Drugs 2015, 13, 2390–2406. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Huang, S.C.; Kuo, H.M.; Chen, C.H.; Ma, Y.L.; Chu, T.H.; Bee, Y.S.; Wang, E.M.; Wu, C.Y.; Sung, P.J.; et al. Coral-derived compound WA-25 inhibits angiogenesis by attenuating the VEGF/VEGFR2 signaling pathway. Mar. Drugs 2015, 13, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Hewage, S.R.; Han, X.; Kang, K.A.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Protective Effect of diphlorethohydroxycarmalol against ultraviolet B radiation-induced DNA damage by inducing the nucleotide excision repair system in HaCaT human keratinocytes. Mar. Drugs 2015, 13, 5629–5641. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Youm, J.B.; Jeong, S.H.; Lee, S.R.; Song, I.S.; Ko, T.H.; Pronto, J.R.; Ko, K.S.; Rhee, B.D.; Kim, N.; et al. Echinochrome A regulates phosphorylation of phospholamban Ser16 and Thr17 suppressing cardiac SERCA2A Ca(2)(+) reuptake. Pfluegers Arch. 2015, 467, 2151–2163. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Noh, S.J.; Marquez, J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; et al. Echinochrome a increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar. Drugs 2014, 12, 4602–4615. [Google Scholar] [CrossRef]
- Jun, Y.J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.H.; Kim, H.R. Eckol enhances heme oxygenase-1 expression through activation of Nrf2/JNK pathway in HepG2 cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef]
- Harms, H.; Rempel, V.; Kehraus, S.; Kaiser, M.; Hufendiek, P.; Müller, C.E.; König, G.M. Indoloditerpenes from a marine-derived fungal strain of Dichotomomyces cejpii with antagonistic activity at GPR18 and cannabinoid receptors. J. Nat. Prod. 2014, 77, 673–677. [Google Scholar] [CrossRef]
- Liu, D.; Yang, A.; Wu, C.; Guo, P.; Proksch, P.; Lin, W. Lipid-lowering effects of farnesylquinone and related analogues from the marine-derived Streptomyces nitrosporeus. Bioorg. Med. Chem. Lett. 2014, 24, 5288–5293. [Google Scholar] [CrossRef]
- Yan, T.; Wu, W.; Su, T.; Chen, J.; Zhu, Q.; Zhang, C.; Wang, X.; Bao, B. Effects of a novel marine natural product: Pyrano indolone alkaloid fibrinolytic compound on thrombolysis and hemorrhagic activities in vitro and in vivo. Arch. Pharm. Res. 2015, 38, 1530–1540. [Google Scholar] [CrossRef]
- Mizushina, Y.; Suzuki-Fukudome, H.; Takeuchi, T.; Takemoto, K.; Kuriyama, I.; Yoshida, H.; Kamisuki, S.; Sugawara, F. Formosusin A, a novel specific inhibitor of mammalian DNA polymerase beta from the fungus Paecilomyces formosus. Bioorg. Med. Chem. 2014, 22, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Piao, M.J.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kumara, M.H.; Han, X.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Fucodiphlorethol G purified from Ecklonia cava suppresses ultraviolet B radiation-induced oxidative stress and cellular damage. Biomol. Ther. 2014, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Lee, J.H.; Lee, W.; Ko, J.Y.; Kim, E.A.; Kim, J.S.; Heu, M.S.; Kim, G.H.; Jeon, Y.J. Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ. Toxicol. Pharmacol. 2015, 39, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Friedman, A.J.; Choi, H.; Hogan, J.; McCammon, J.A.; Hook, V.; Gerwick, W.H. The marine cyanobacterial metabolite gallinamide A is a potent and selective inhibitor of human cathepsin L. J. Nat. Prod. 2014, 77, 92–99. [Google Scholar] [CrossRef]
- Fung, S.Y.; Sofiyev, V.; Schneiderman, J.; Hirschfeld, A.F.; Victor, R.E.; Woods, K.; Piotrowski, J.S.; Deshpande, R.; Li, S.C.; de Voogd, N.J.; et al. Unbiased screening of marine sponge extracts for anti-inflammatory agents combined with chemical genomics identifies girolline as an inhibitor of protein synthesis. ACS Chem. Biol. 2014, 9, 247–257. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Alfonso, A.; Leiros, M.; Alonso, E.; Rateb, M.E.; Jaspars, M.; Houssen, W.E.; Ebel, R.; Botana, L.M. Spongionella secondary metabolites regulate store operated calcium entry modulating mitochondrial functioning in SH-SY5Y neuroblastoma cells. Cell. Physiol. Biochem. 2015, 37, 779–792. [Google Scholar] [CrossRef]
- Gladkikh, I.; Monastyrnaya, M.; Zelepuga, E.; Sintsova, O.; Tabakmakher, V.; Gnedenko, O.; Ivanov, A.; Hua, K.F.; Kozlovskaya, E. New Kunitz-type HCRG polypeptides from the sea anemone Heteractis crispa. Mar. Drugs 2015, 13, 6038–6063. [Google Scholar] [CrossRef]
- Festa, C.; Cassiano, C.; D’Auria, M.V.; Debitus, C.; Monti, M.C.; De, M.S. Scalarane sesterterpenes from Thorectidae sponges as inhibitors of TDP-43 nuclear factor. Org. Biomol. Chem. 2014, 12, 8646–8655. [Google Scholar] [CrossRef]
- Quach, H.T.; Hirano, S.; Fukuhara, S.; Watanabe, T.; Kanoh, N.; Iwabuchi, Y.; Usui, T.; Kataoka, T. Irciniastatin A induces potent and sustained activation of extracellular signal-regulated kinase and thereby promotes ectodomain shedding of tumor necrosis factor receptor 1 in human lung carcinoma A549 cells. Biol. Pharm. Bull. 2015, 38, 941–946. [Google Scholar] [CrossRef]
- Kazami, S.; Takaine, M.; Itoh, H.; Kubota, T.; Kobayashi, J.; Usui, T. Iejimalide C is a potent V-ATPase inhibitor, and induces actin disorganization. Biol. Pharm. Bull. 2014, 37, 1944–1947. [Google Scholar] [CrossRef]
- DeGroot, D.E.; Franks, D.G.; Higa, T.; Tanaka, J.; Hahn, M.E.; Denison, M.S. Naturally occurring marine brominated indoles are aryl hydrocarbon receptor ligands/agonists. Chem. Res. Toxicol. 2015, 28, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Shubina, L.K.; Makarieva, T.N.; Yashunsky, D.V.; Nifantiev, N.E.; Denisenko, V.A.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Fedorov, S.N.; Krasokhin, V.B.; Jeong, S.H.; et al. Pyridine nucleosides neopetrosides A and B from a marine Neopetrosia sp. sponge. Synthesis of neopetroside A and its beta-riboside analogue. J. Nat. Prod. 2015, 78, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPalpha and PPARgamma. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Matsunaga, S.; Oiki, S. Paradoxical one-ion pore behavior of the long beta-helical peptide of marine cytotoxic polytheonamide B. Sci. Rep. 2014, 4, 3636. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The green algal carotenoid siphonaxanthin inhibits adipogenesis in 3T3-L1 preadipocytes and the accumulation of lipids in white adipose tissue of KK-Ay mice. J. Nutr. 2015, 145, 490–498. [Google Scholar] [CrossRef]
- Wu, C.; Chen, R.; Liu, M.; Liu, D.; Li, X.; Wang, S.; Niu, S.; Guo, P.; Lin, W. Spiromastixones inhibit foam cell formation via regulation of cholesterol efflux and uptake in RAW264.7 macrophages. Mar. Drugs 2015, 13, 6352–6365. [Google Scholar] [CrossRef]
- Lu, L.; Meehan, M.J.; Gu, S.; Chen, Z.; Zhang, W.; Zhang, G.; Liu, L.; Huang, X.; Dorrestein, P.C.; Xu, Y.; et al. Mechanism of action of thalassospiramides, a new class of calpain inhibitors. Sci. Rep. 2015, 5, 8783. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Li, J.; Yuan, F.; Li, M.; Zhang, Q.; Huang, Y.Y.; Pang, J.Y.; Zhang, B.; Sun, F.Y.; Sun, H.S.; et al. Xyloketal B attenuates atherosclerotic plaque formation and endothelial dysfunction in apolipoprotein e deficient mice. Mar. Drugs 2015, 13, 2306–2326. [Google Scholar] [CrossRef]
- Su, J.; Chang, C.; Xiang, Q.; Zhou, Z.W.; Luo, R.; Yang, L.; He, Z.X.; Yang, H.; Li, J.; Bei, Y.; et al. Xyloketal B, a marine compound, acts on a network of molecular proteins and regulates the activity and expression of rat cytochrome P450 3a: A bioinformatic and animal study. Drug. Des. Devel. Ther. 2014, 8, 2555–2602. [Google Scholar]
- Liang, L.F.; Wang, T.; Cai, Y.S.; He, W.F.; Sun, P.; Li, Y.F.; Huang, Q.; Taglialatela-Scafati, O.; Wang, H.Y.; Guo, Y.W. Brominated polyunsaturated lipids from the Chinese sponge Xestospongia testudinaria as a new class of pancreatic lipase inhibitors. Eur. J. Med. Chem. 2014, 79, 290–297. [Google Scholar] [CrossRef]
- Hwang, B.S.; Kim, H.S.; Yih, W.; Jeong, E.J.; Rho, J.R. Acuminolide A: Structure and bioactivity of a new polyether macrolide from dinoflagellate Dinophysis acuminata. Org. Lett. 2014, 16, 5362–5365. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Takada, K.; Nogi, Y.; Okada, S.; Matsunaga, S. Lower homologues of ahpatinin, aspartic protease inhibitors, from a marine Streptomyces sp. J. Nat. Prod. 2014, 77, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Won, T.H.; Kim, C.K.; Lee, S.H.; Rho, B.J.; Lee, S.K.; Oh, D.C.; Oh, K.B.; Shin, J. Amino Acid-derived metabolites from the ascidian Aplidium sp. Mar. Drugs 2015, 13, 3836–3848. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Sobolevskaya, M.P.; Leshchenko, E.V.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Zakharenko, A.M.; Kim, N.Y.; Afiyatullov, S.S. Meroterpenoids from the alga-derived fungi Penicillium thomii Maire and Penicillium lividum Westling. J. Nat. Prod. 2014, 77, 1390–1395. [Google Scholar] [CrossRef]
- Ai, W.; Lin, X.P.; Tu, Z.; Tian, X.P.; Lu, X.; Mangaladoss, F.; Zhong, Z.L.; Liu, Y. Axinelline A, a new COX-2 inhibitor from Streptomyces axinellae SCSIO02208. Nat. Prod. Res. 2014, 28, 1219–1224. [Google Scholar] [CrossRef]
- Suzuki, R.; Irie, R.; Harntaweesup, Y.; Tachibana, K.; Holland, P.T.; Harwood, D.T.; Shi, F.; Beuzenberg, V.; Itoh, Y.; Pascal, S.; et al. Brevisulcatic acids, marine ladder-frame polyethers from the red tide dinoflagellate Karenia brevisulcata in New Zealand. Org. Lett. 2014, 16, 5850–5853. [Google Scholar] [CrossRef]
- Yang, P.; Liu, D.Q.; Liang, T.J.; Li, J.; Zhang, H.Y.; Liu, A.H.; Guo, Y.W.; Mao, S.C. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg. Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef]
- Daletos, G.; de Voogd, N.J.; Müller, W.E.; Wray, V.; Lin, W.; Feger, D.; Kubbutat, M.; Aly, A.H.; Proksch, P. Cytotoxic and protein kinase inhibiting nakijiquinones and nakijiquinols from the sponge Dactylospongia metachromia. J. Nat. Prod. 2014, 77, 218–226. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Badr, J.M.; Youssef, D.T.A. Didemnaketals D and E, bioactive terpenoids from a Red Sea ascidian Didemnum species. Tetrahedron 2014, 70, 35–40. [Google Scholar] [CrossRef]
- Jiao, W.H.; Xu, T.T.; Gu, B.B.; Shi, G.H.; Zhu, Y.; Yang, F.; Han, B.N.; Wang, S.P.; Li, Y.S.; Zhang, W.; et al. Bioactive sesquiterpene quinols and quinones from the marine sponge Dysidea avara. RSC Adv. 2015, 5, 87730–87738. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, H.; Xie, Q.; Sun, J.; Liu, R.; Hong, Z.; Yi, R.; Wu, H. Comparative evaluation of the radical-scavenging activities of fucoxanthin and its stereoisomers. Molecules 2014, 19, 2100–2113. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mai, L.H.; Longeon, A.; Copp, B.R.; Loaec, N.; Bescond, A.; Meijer, L.; Bourguet-Kondracki, M.L. Novel adociaquinone derivatives from the Indonesian sponge Xestospongia sp. Mar. Drugs 2015, 13, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Yamakuma, M.; Kato, H.; Matsuo, K.; El-Desoky, A.H.; Kawabata, T.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; Tsukamoto, S. 1-Hydroxyethylhalenaquinone: A new proteasome inhibitor from the marine sponge Xestospongia sp. Heterocycles 2014, 89, 2605–2610. [Google Scholar]
- Ebada, S.S.; Linh, M.H.; Longeon, A.; de Voogd, N.J.; Durieu, E.; Meijer, L.; Bourguet-Kondracki, M.L.; Singab, A.N.; Müller, W.E.; Proksch, P. Dispacamide E and other bioactive bromopyrrole alkaloids from two Indonesian marine sponges of the genus Stylissa. Nat. Prod. Res. 2015, 29, 231–238. [Google Scholar] [CrossRef]
- Plisson, F.; Prasad, P.; Xiao, X.; Piggott, A.M.; Huang, X.C.; Khalil, Z.; Capon, R.J. Callyspongisines A-D: Bromopyrrole alkaloids from an Australian marine sponge, Callyspongia sp. Org. Biomol. Chem. 2014, 12, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Abdjul, D.B.; Yamazaki, H.; Takahashi, O.; Kirikoshi, R.; Mangindaan, R.E.; Namikoshi, M. Two new protein tyrosine phosphatase 1B inhibitors, hyattellactones A and B, from the Indonesian marine sponge Hyattella sp. Bioorg. Med. Chem. Lett. 2015, 25, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.J.; Jiao, W.H.; Yang, F.; Yi, Y.H.; Di, Y.T.; Han, B.N.; Lin, H.W. New hippolide derivatives with protein tyrosine phosphatase 1B inhibitory activity from the marine sponge Hippospongia lachne. Mar. Drugs 2014, 12, 4096–4109. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Vullo, D.; Supuran, C.T.; Poulsen, S.A. Natural product polyamines that inhibit human carbonic anhydrases. Biomed. Res. Int. 2014, 2014, 374079. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Sepe, V.; Limongelli, V.; Renga, B.; D’Amore, C.; Zampella, A.; Taglialatela-Scafati, O.; Fiorucci, S. Incisterols, highly degraded marine sterols, are a new chemotype of PXR agonists. Steroids 2014, 83, 80–85. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Ngan, N.T.; Dat, L.D.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Song, S.B.; Minh, C.V.; Kim, Y.H. Peroxisome proliferator-activated receptor transactivational effects in HepG2 cells of cembranoids from the soft coral Lobophytum crassum Von Marenzeller. Arch. Pharm. Res. 2015, 38, 769–775. [Google Scholar] [CrossRef]
- Li, X.L.; He, W.F.; Li, J.; Lan, L.F.; Li, X.W.; Guo, Y.W. New laurane-type sesquiterpenoids from the Chinese red alga Laurencia okamurai Yamada. J. Asian Nat. Prod. Res. 2015, 17, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Cai, Y.H.; Fan, C.Q.; Tang, G.H.; Luo, H.B.; Yin, S. Six new tetraprenylated alkaloids from the South China sea gorgonian Echinogorgia pseudossapo. Mar. Drugs 2014, 12, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Punctaporonins H-M: Caryophyllene-type sesquiterpenoids from the sponge-associated fungus Hansfordia sinuosae. Mar. Drugs 2014, 12, 3904–3916. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, H.; Zhang, X.; Wang, S.; Wu, C.; Gu, Q.; Guo, P.; Zhu, T.; Li, D. Psychrophilins E-H and versicotide C, cyclic peptides from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Nat. Prod. 2014, 77, 2218–2223. [Google Scholar] [CrossRef]
- Yang, H.; Liu, D.Q.; Liang, T.J.; Li, J.; Liu, A.H.; Yang, P.; Lin, K.; Yu, X.Q.; Guo, Y.W.; Mao, S.C.; et al. Racemosin C, a novel minor bisindole alkaloid with protein tyrosine phosphatase-1B inhibitory activity from the green alga Caulerpa racemosa. J. Asian Nat. Prod. Res. 2014, 16, 1158–1165. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Deng, Y.L.; Fan, C.Q.; Han, Q.H.; Lin, S.L.; Tang, G.H.; Luo, H.B.; Yin, S. Prostaglandin Derivatives: Nonaromatic phosphodiesterase-4 inhibitors from the soft coral Sarcophyton ehrenbergi. J. Nat. Prod. 2014, 77, 1928–1936. [Google Scholar] [CrossRef]
- Liang, L.F.; Kurtan, T.; Mandi, A.; Gao, L.X.; Li, J.; Zhang, W.; Guo, Y.W. Sarsolenane and capnosane diterpenes from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller as PTP1B inhibitors. Eur. J. Org. Chem. 2014, 2014, 1841–1847. [Google Scholar] [CrossRef]
- Qin, C.; Lin, X.; Lu, X.; Wan, J.; Zhou, X.; Liao, S.; Tu, Z.; Xu, S.; Liu, Y. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2. J. Antibiot. 2015, 68, 121–125. [Google Scholar] [CrossRef]
- Kurihara, H.; Kagawa, Y.; Konno, R.; Kim, S.M.; Takahashi, K. Lipoxygenase inhibitors derived from marine macroalgae. Bioorg. Med. Chem. Lett. 2014, 24, 1383–1385. [Google Scholar] [CrossRef]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Med. 2015, 81, 813–820. [Google Scholar] [CrossRef]
- Yang, B.; Wei, X.; Huang, J.; Lin, X.; Liu, J.; Liao, S.; Wang, J.; Zhou, X.; Wang, L.; Liu, Y. Sinulolides A-H, new cyclopentenone and butenolide derivatives from soft coral Sinularia sp. Mar. Drugs 2014, 12, 5316–5327. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, C.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Chartarlactams A-P, phenylspirodrimanes from the sponge-associated fungus Stachybotrys chartarum with antihyperlipidemic activities. J. Nat. Prod. 2014, 77, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Noda, A.; Sakai, E.; Kato, H.; Losung, F.; Mangindaan, R.E.; de Voogd, N.J.; Yokosawa, H.; Tsukamoto, S. Strongylophorines, meroditerpenoids from the marine sponge Petrosia corticata, function as proteasome inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 2650–2653. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Sun, P.; Jiang, N.; Riccio, R.; Lauro, G.; Bifulco, G.; Li, T.J.; Gerwick, W.H.; Zhang, W. New steroids with a rearranged skeleton as (h)P300 inhibitors from the sponge Theonella swinhoei. Org. Lett. 2014, 16, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- He, W.F.; Yao, L.G.; Liu, H.L.; Guo, Y.W. Thunberol, a new sterol from the Chinese brown alga Sargassum thunbergii. J. Asian Nat. Prod. Res. 2014, 16, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Centko, R.M.; Steino, A.; Rosell, F.I.; Patrick, B.O.; de Voogd, N.; Mauk, A.G.; Andersen, R.J. Indoleamine 2,3-dioxygenase inhibitors isolated from the sponge Xestospongia vansoesti: Structure elucidation, analogue synthesis, and biological activity. Org. Lett. 2014, 16, 6480–6483. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Ogurtsova, E.K.; Denisenko, V.A.; Dmitrenok, P.S.; Tabakmakher, K.M.; Guzii, A.G.; Pislyagin, E.A.; Es’kov, A.A.; Kozhemyako, V.B.; Aminin, D.L.; et al. Urupocidin A: A new, inducing iNOS expression bicyclic guanidine alkaloid from the marine sponge Monanchora pulchra. Org. Lett. 2014, 16, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Sakai, E.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.; de Voogd, N.J.; Yokosawa, H.; Tsukamoto, S. Variabines A and B: New beta-carboline alkaloids from the marine sponge Luffariella variabilis. J. Nat. Med. 2014, 68, 215–219. [Google Scholar] [CrossRef]
- Inuzuka, T.; Yamamoto, K.; Iwasaki, A.; Ohno, O.; Suenaga, K.; Kawazoe, Y.; Uemura, D. An inhibitor of the adipogenic differentiation of 3T3-L1 cells, yoshinone A. and its analogs isolated from the marine cyonobacterium Leptolyngbya sp. Tetrahedron Lett. 2014, 55, 6711–6714. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Kang, K.H.; Sivakumar, K.; Li-Chan, E.C.; Oh, H.M.; Kim, S.K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Marzec, H.; Blaszczyk, A.; Felczykowska, A.; Hohlfeld, N.; Kobos, J. Baltic cyanobacteria--a source of biologically active compounds. Eur. J. Phycol. 2015, 50, 343–360. [Google Scholar] [CrossRef]
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar] [CrossRef]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef]
- Derby, C.D. Cephalopod ink: Production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700–2730. [Google Scholar] [CrossRef]
- Almeida, M.T.; Moritz, M.I.; Capel, K.C.; Perez, C.D.; Schenkel, E.P. Chemical and biological aspects of octocorals from the Brazilian coast. Rev. Brasil. Farmacogn. 2014, 24, 446–467. [Google Scholar] [CrossRef]
- Ioca, L.P.; Allard, P.M.; Berlinck, R.G. Thinking big about small beings--the (yet) underdeveloped microbial natural products chemistry in Brazil. Nat. Prod. Rep. 2014, 31, 646–675. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Trindade, M.; van Zyl, L.J.; Navarro-Fernandez, J.; Abd, E.A. Targeted metagenomics as a tool to tap into marine natural product diversity for the discovery and production of drug candidates. Front. Microbiol. 2015, 6, 890. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New horizons for old drugs and drug leads. J. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Seo, C.H.; Park, Y. The role of biophysical parameters in the antilipopolysaccharide activities of antimicrobial peptides from marine fish. Mar. Drugs 2014, 12, 1471–1494. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef]
- Ponnappan, N.; Budagavi, D.P.; Yadav, B.K.; Chugh, A. Membrane-active peptides from marine organisms--antimicrobials, cell-penetrating peptides and peptide toxins: Applications and prospects. Probiotics Antimicrob. Proteins 2015, 7, 75–89. [Google Scholar] [CrossRef]
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and antifungal compounds from marine fungi. Mar. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef]
- Gogineni, V.; Schinazi, R.F.; Hamann, M.T. Role of marine natural products in the genesis of antiviral agents. Chem. Rev. 2015, 115, 9655–9706. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Nikzad, S.; Kadir, H.A.; AbuBakar, S.; Zandi, K. Potential antiviral agents from marine fungi: An overview. Mar. Drugs 2015, 13, 4520–4538. [Google Scholar] [CrossRef]
- Torres, F.A.E.; Passalacqua, T.G.; Velasquez, A.M.A.; de Souza, R.A.; Colepicolo, P.; Graminha, M.A.S. New drugs with antiprozoal activity from marine algae: A review. Rev. Bras. Farmacogn. 2014, 24, 265–276. [Google Scholar] [CrossRef]
- Franca, P.H.; Barbosa, D.P.; da Silva, D.L.; Ribeiro, E.A.; Santana, A.E.; Santos, B.V.; Barbosa-Filho, J.M.; Quintans, J.S.; Barreto, R.S.; Quintans-Junior, L.J.; et al. Indole alkaloids from marine sources as potential leads against infectious diseases. Biomed. Res. Int. 2014, 2014, 375423. [Google Scholar] [CrossRef] [PubMed]
- Ashok, P.; Ganguly, S.; Murugesan, S. Manzamine alkaloids: Isolation, cytotoxicity, antimalarial activity and SAR studies. Drug Discovery Today 2014, 19, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, Y.; Torres-Mendoza, D.; Jones, G.E.; Fernandez, P.L. Marine diterpenoids as potential anti-inflammatory agents. Mediators. Inflamm. 2015, 2015, 263543. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; Garcia-Maurino, S.; Avila-Roman, J.; Rodriguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef]
- Planes, N.; Caballero-George, C. Marine and soil derived natural products: A new source of novel cardiovascular protective agents targeting the endothelin system. Planta Med. 2015, 81, 630–636. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant marine algae phlorotannins and radioprotection: A review of experimental evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Shindo, K.; Misawa, N. New and rare carotenoids isolated from marine bacteria and their antioxidant activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef]
- Maeda, H. Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: A review. J. Oleo. Sci. 2015, 64, 125–132. [Google Scholar] [CrossRef]
- Sharifuddin, Y.; Chin, Y.X.; Lim, P.E.; Phang, S.M. Potential bioactive compounds from seaweed for diabetes management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.J.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.; Rodriguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.Y.; Choi, H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharm. Res. 2015, 38, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Leiros, M.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Gracilins: Spongionella-derived promising compounds for Alzheimer disease. Neuropharmacology 2015, 93, 285–293. [Google Scholar] [CrossRef]
- Russo, P.; Kisialiou, A.; Lamonaca, P.; Moroni, R.; Prinzi, G.; Fini, M. New drugs from marine organisms in Alzheimer’s disease. Mar. Drugs 2016, 14, 5. [Google Scholar] [CrossRef]
- Thomas, N.V.; Manivasagan, P.; Kim, S.K. Potential matrix metalloproteinase inhibitors from edible marine algae: A review. Environ. Toxicol. Pharmacol. 2014, 37, 1090–1100. [Google Scholar] [CrossRef]
- Beesoo, R.; Neergheen-Bhujun, V.; Bhagooli, R.; Bahorun, T. Apoptosis inducing lead compounds isolated from marine organisms of potential relevance in cancer treatment. Mutat. Res. 2014, 768, 84–97. [Google Scholar] [CrossRef]
- Mishra, A.; Tandon, R.; Kesarwani, S.; Singh, R.; Tiwari, G.L. Emerging applications of cyanobacterial ultraviolet protecting compound scytonemin. J. Appl. Phycol. 2015, 27, 1045–1051. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications--a review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, S.; Matos, M.J.; Borges, F.; Uriarte, E.; Santana, L. Bioactive coumarins from marine sources: Origin, structural features and pharmacological properties. Curr. Top. Med. Chem. 2015, 15, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
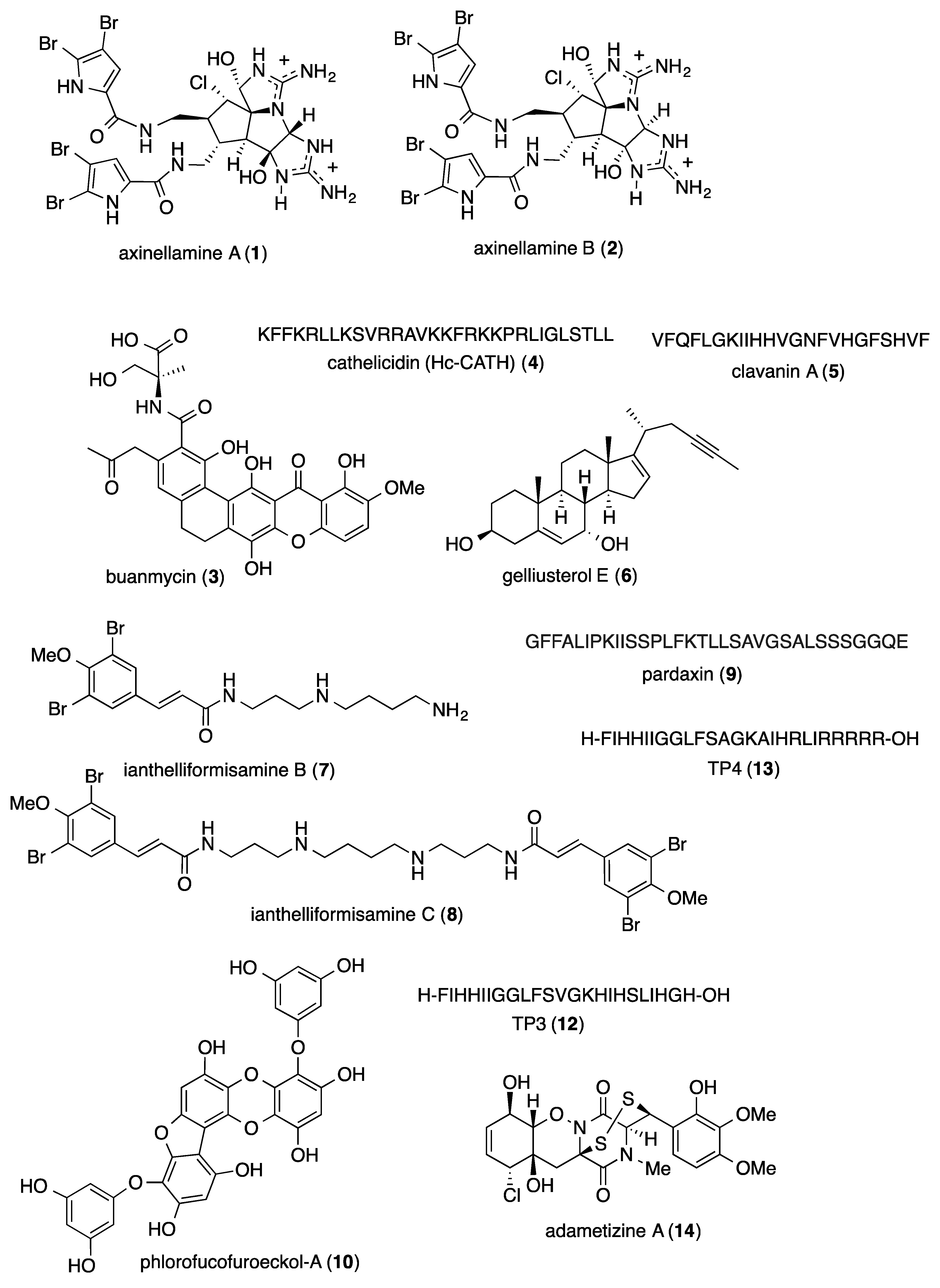
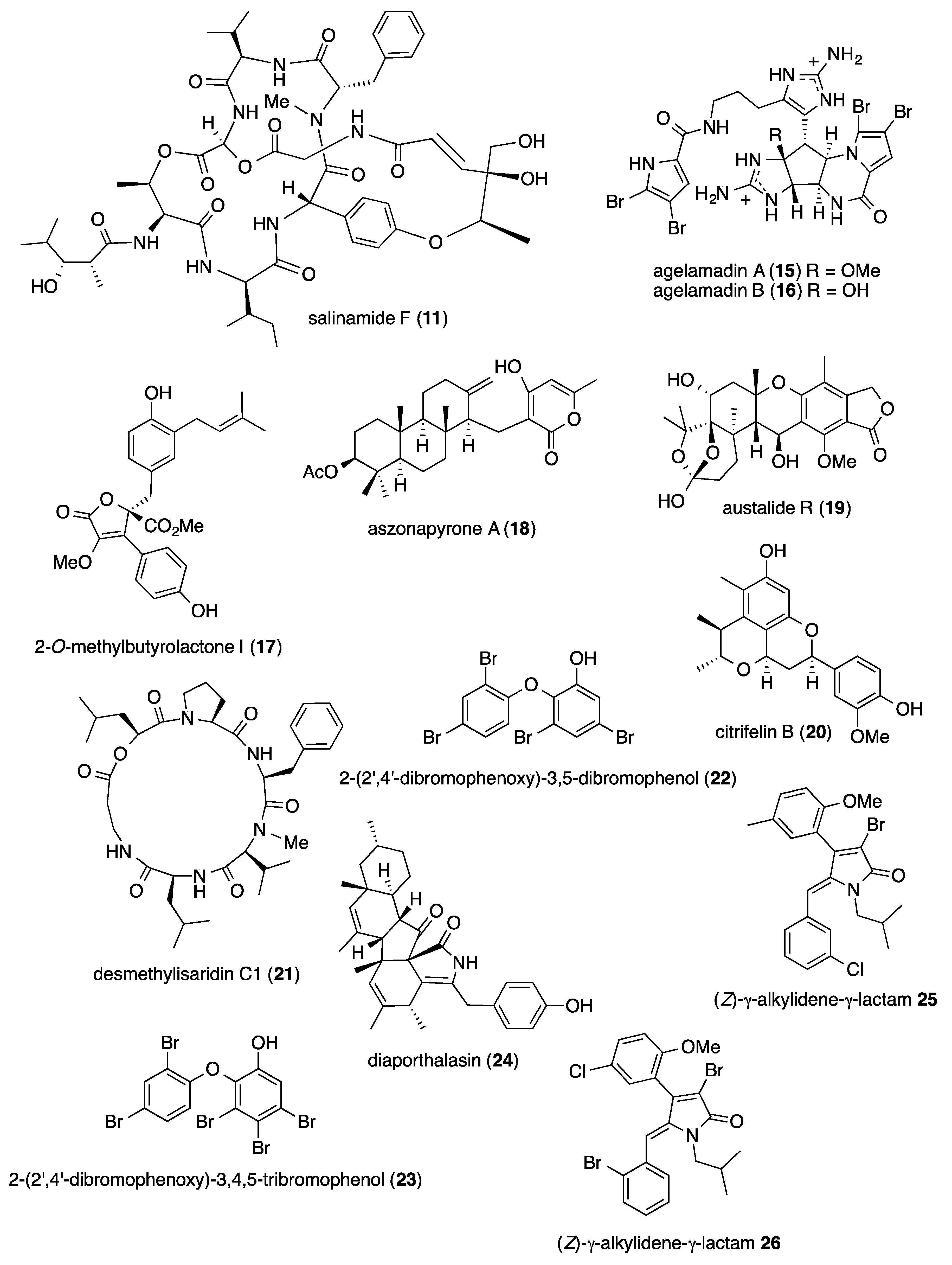

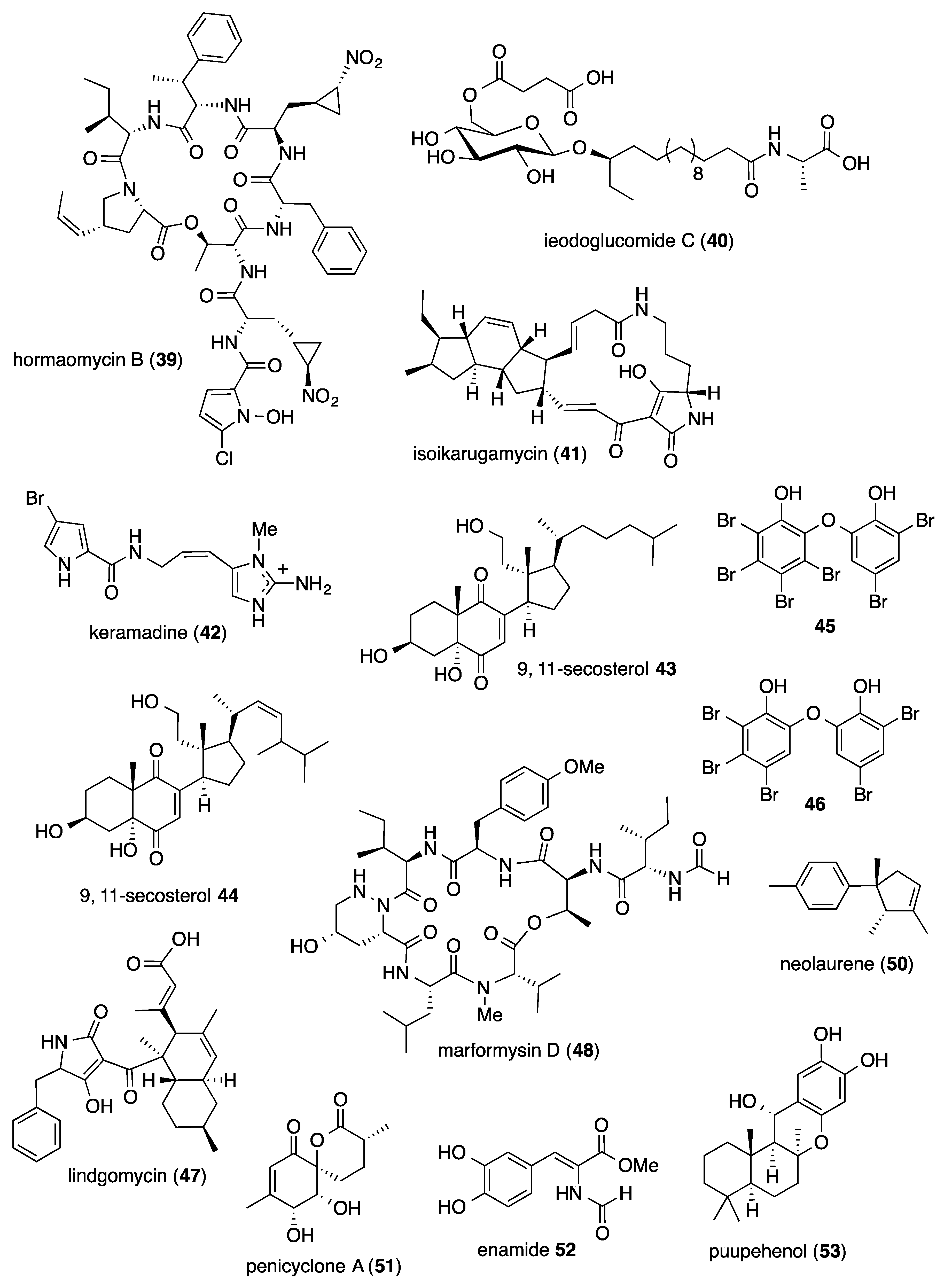
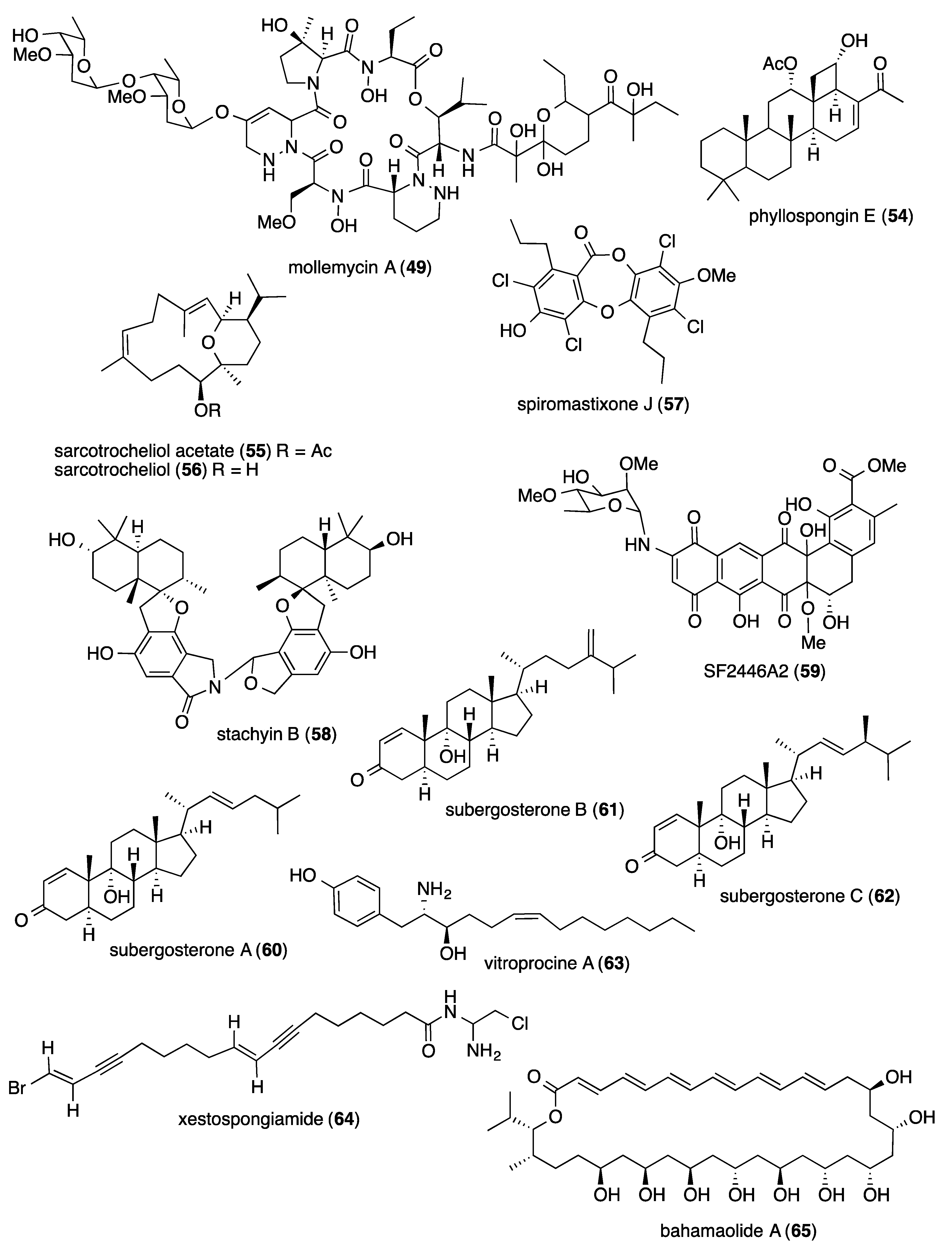

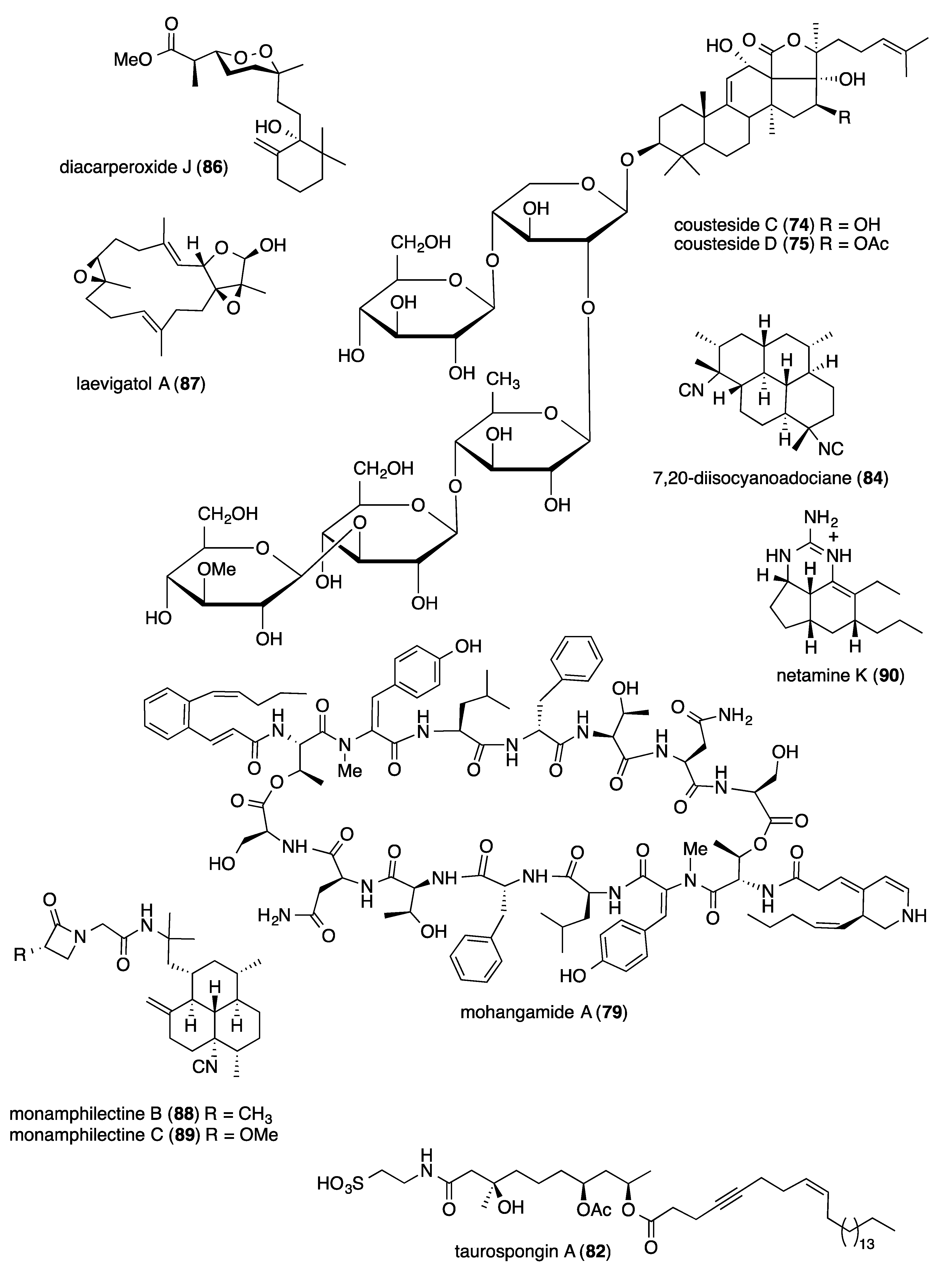
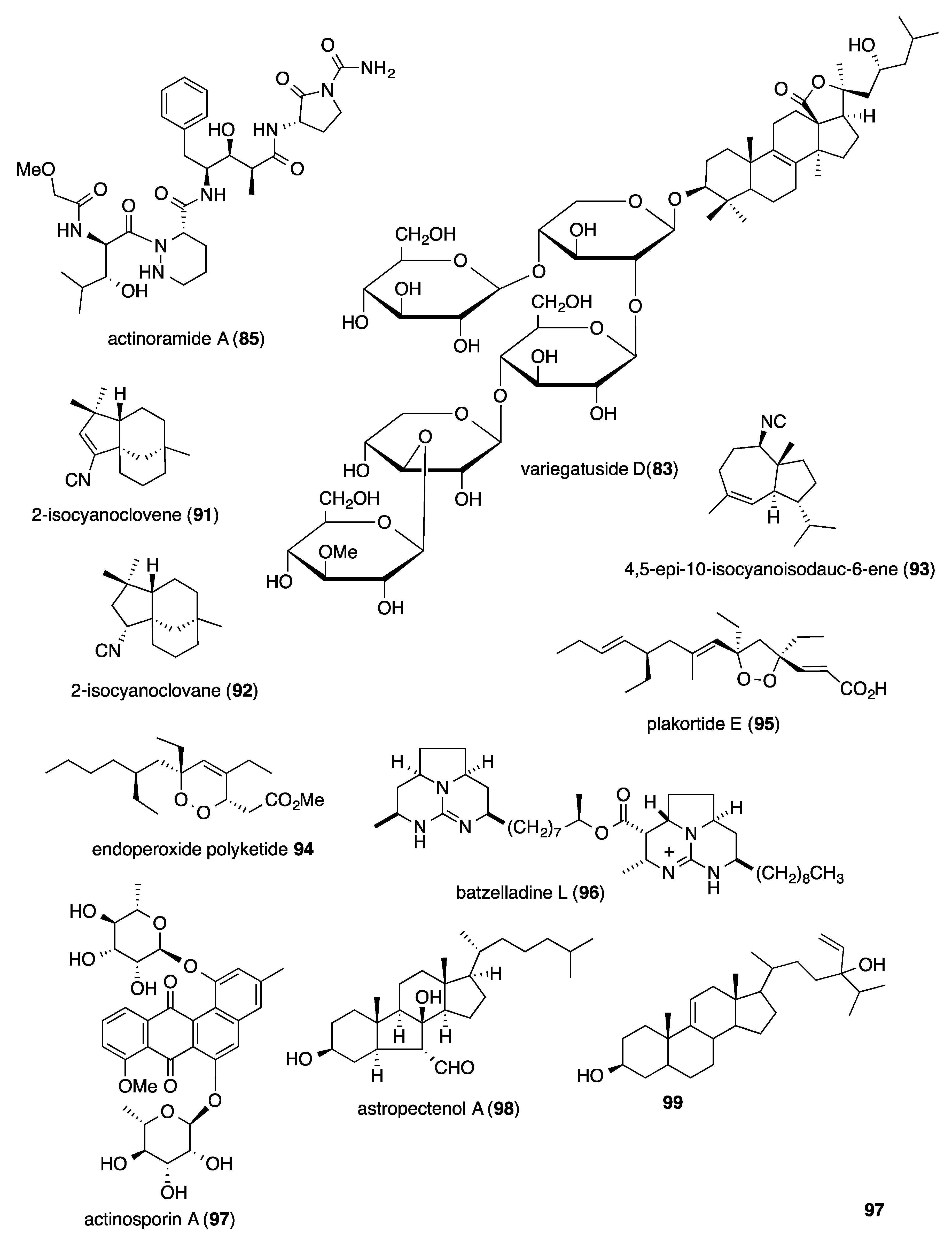
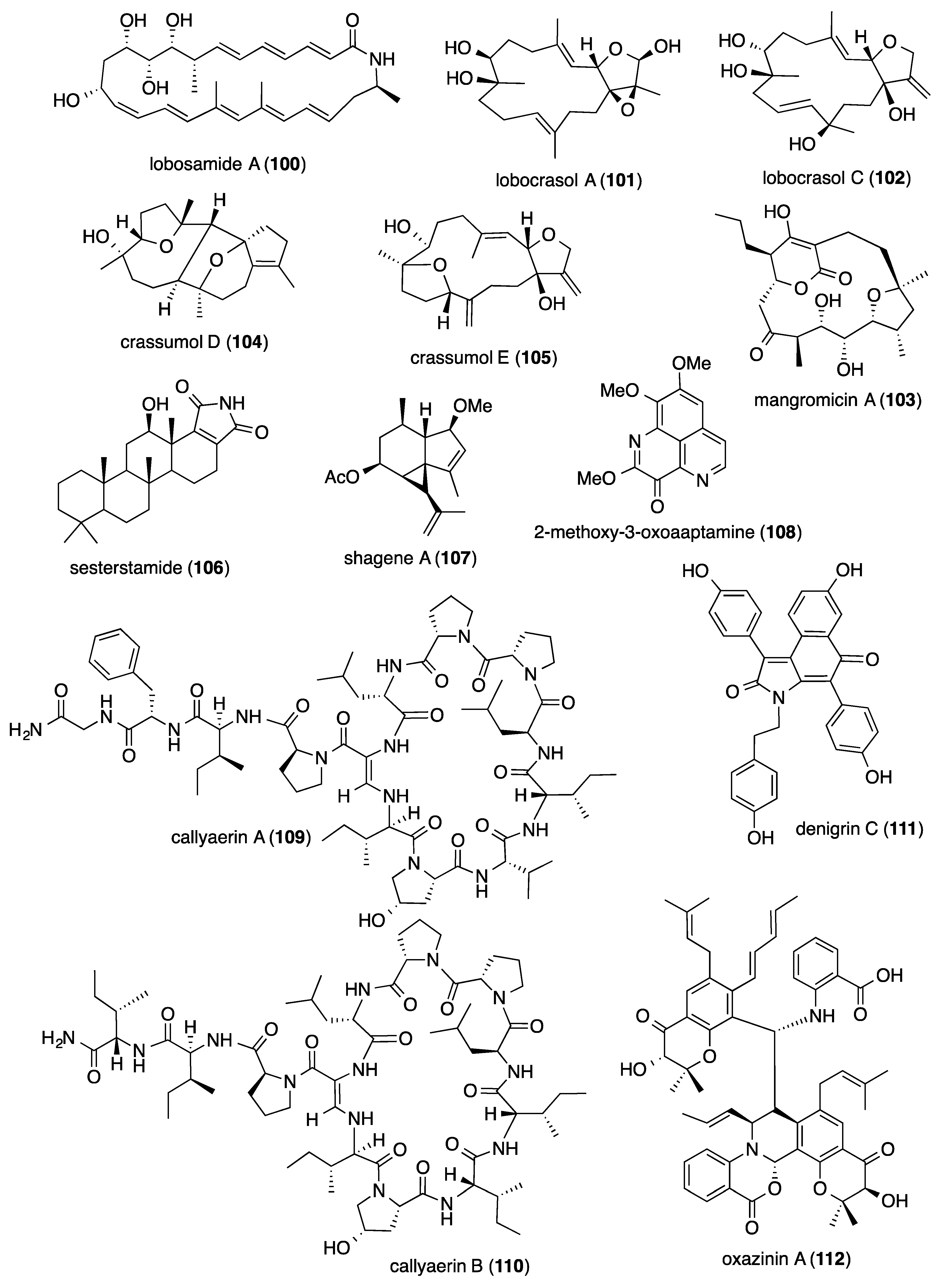
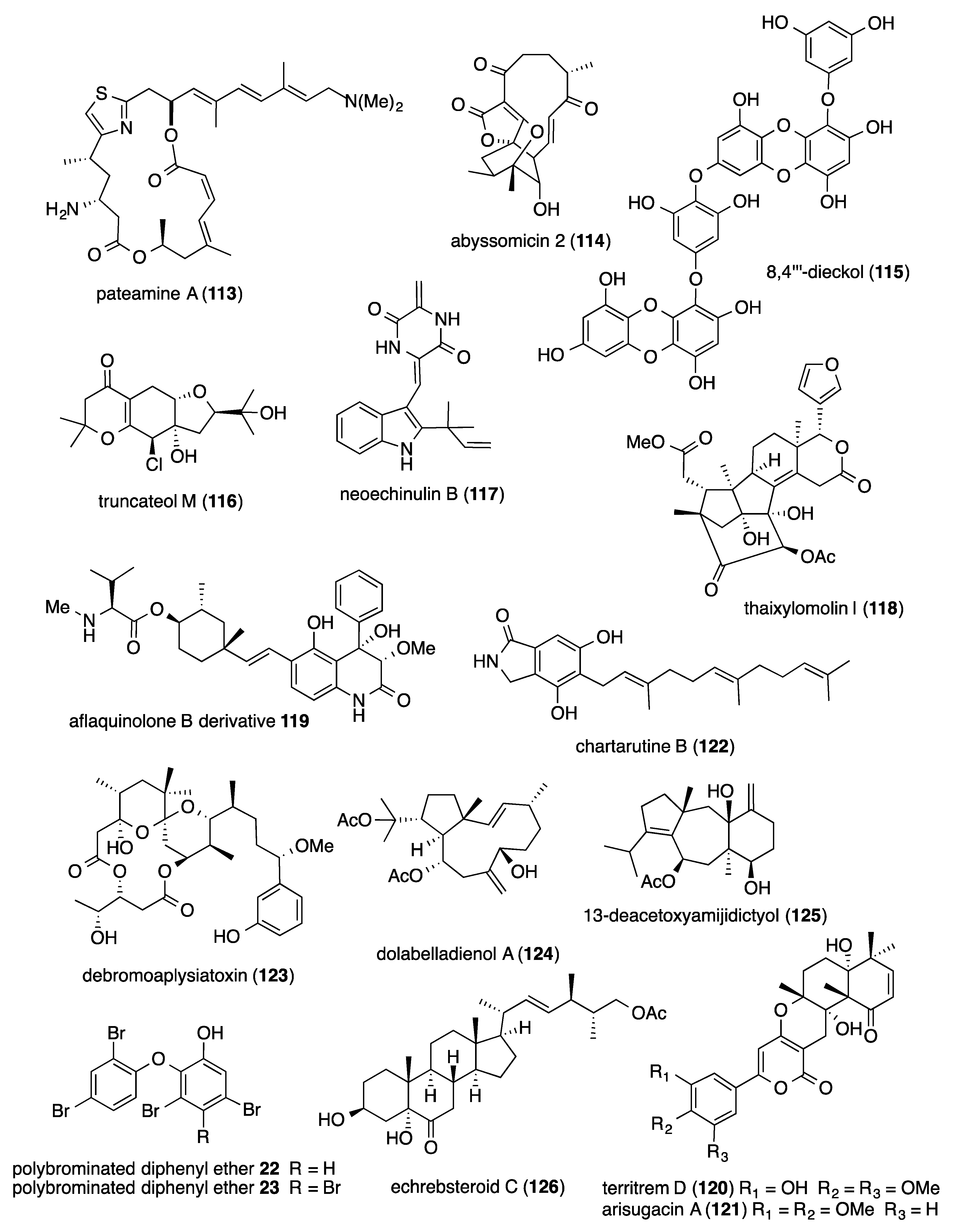

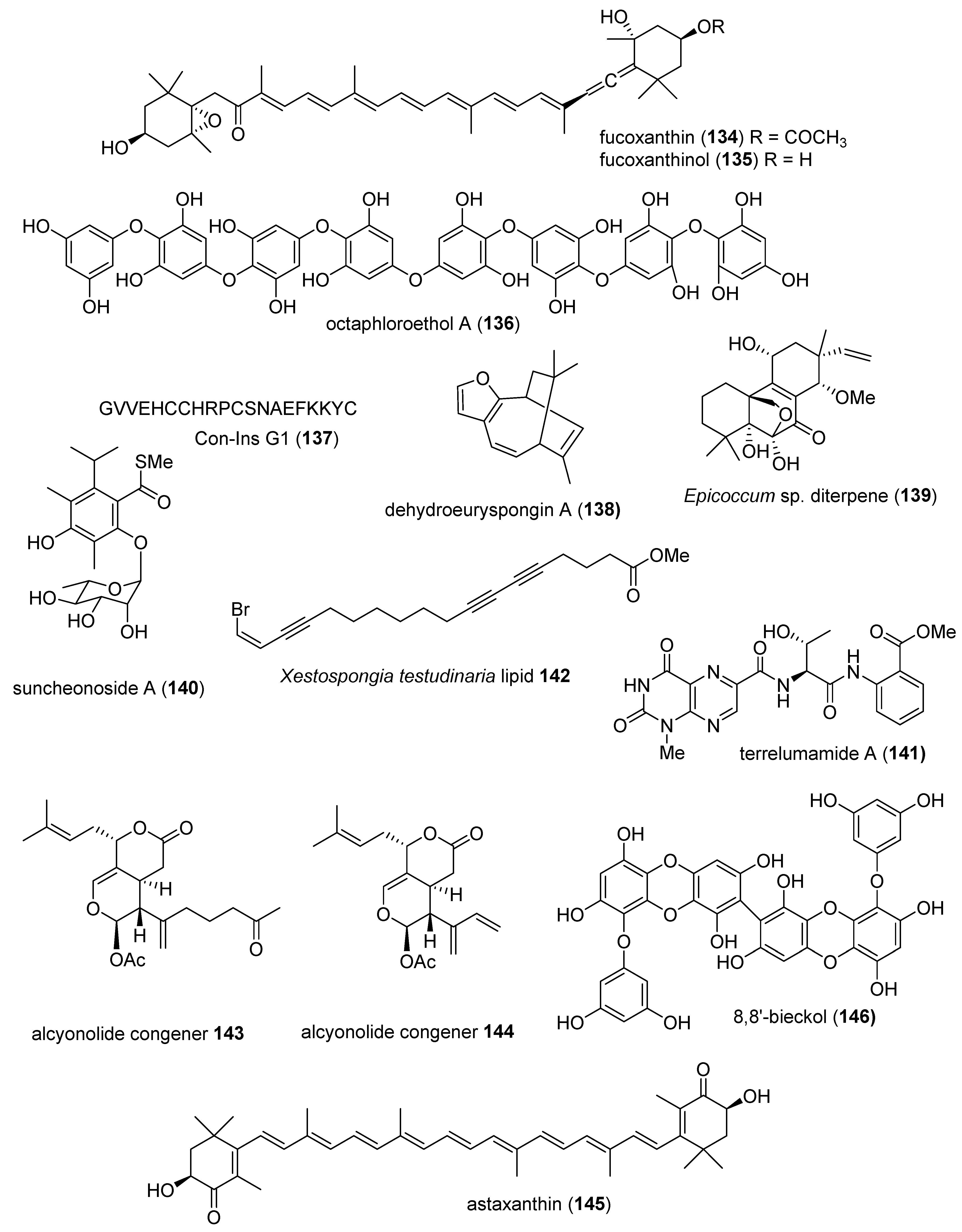
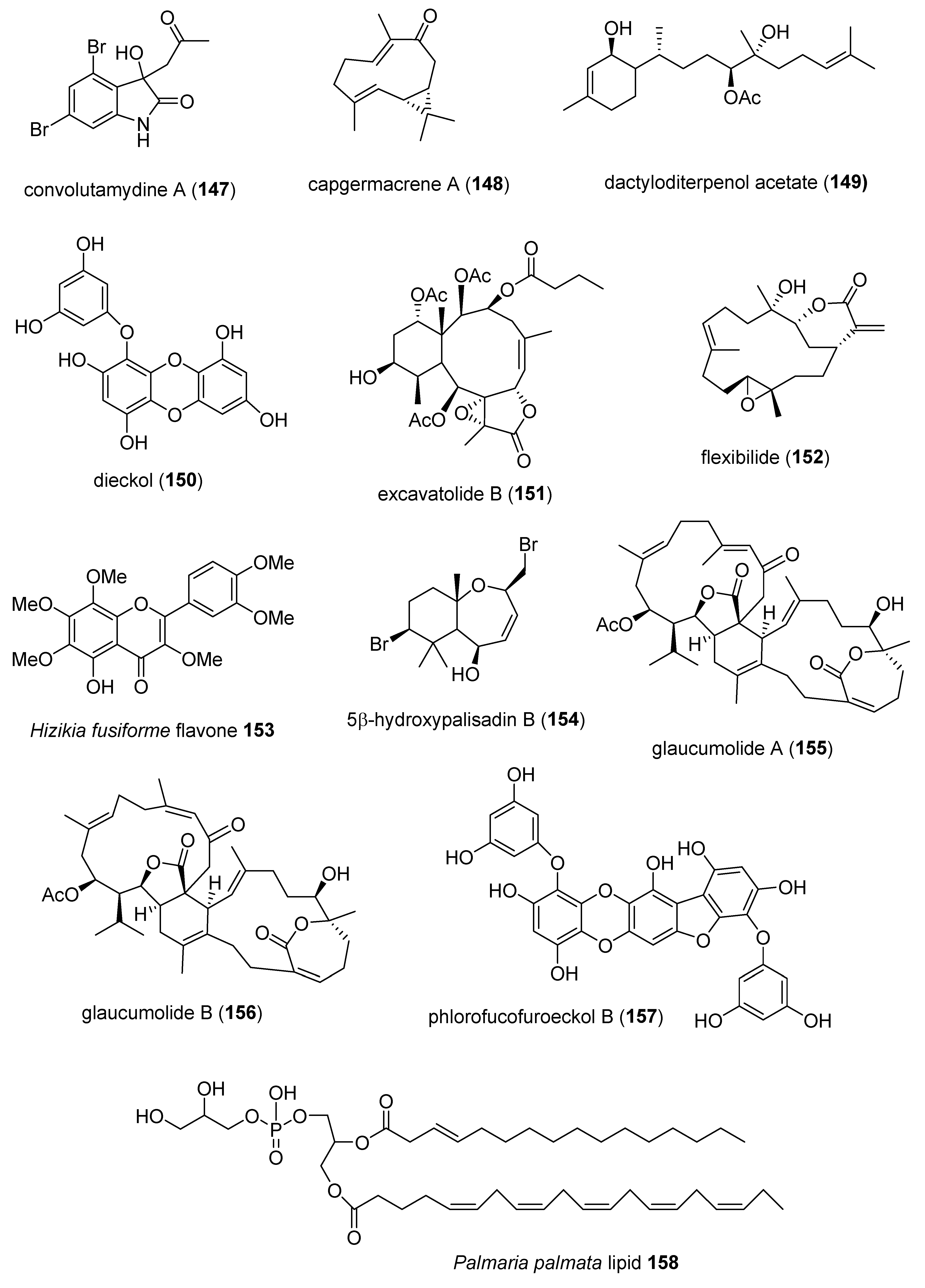
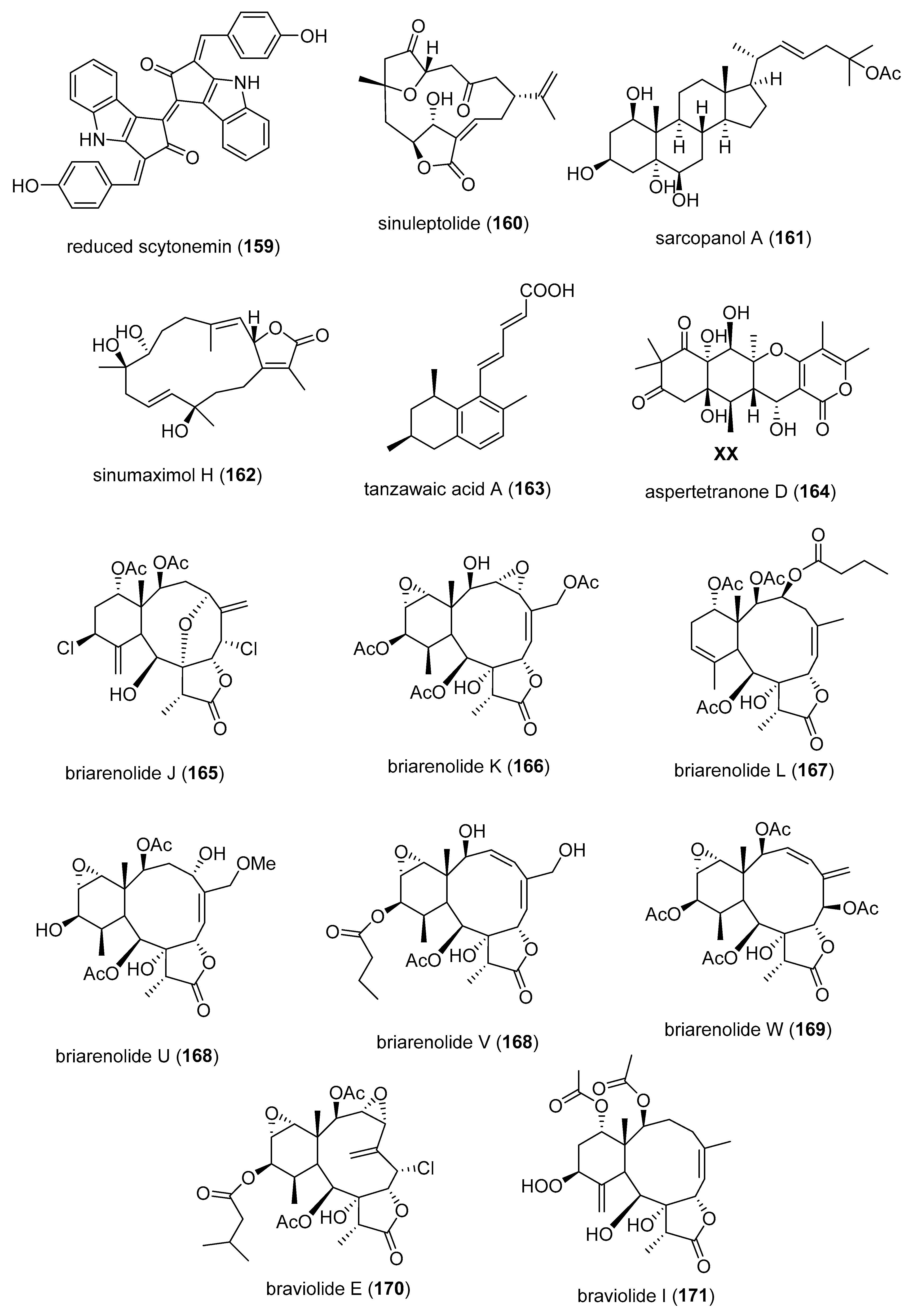
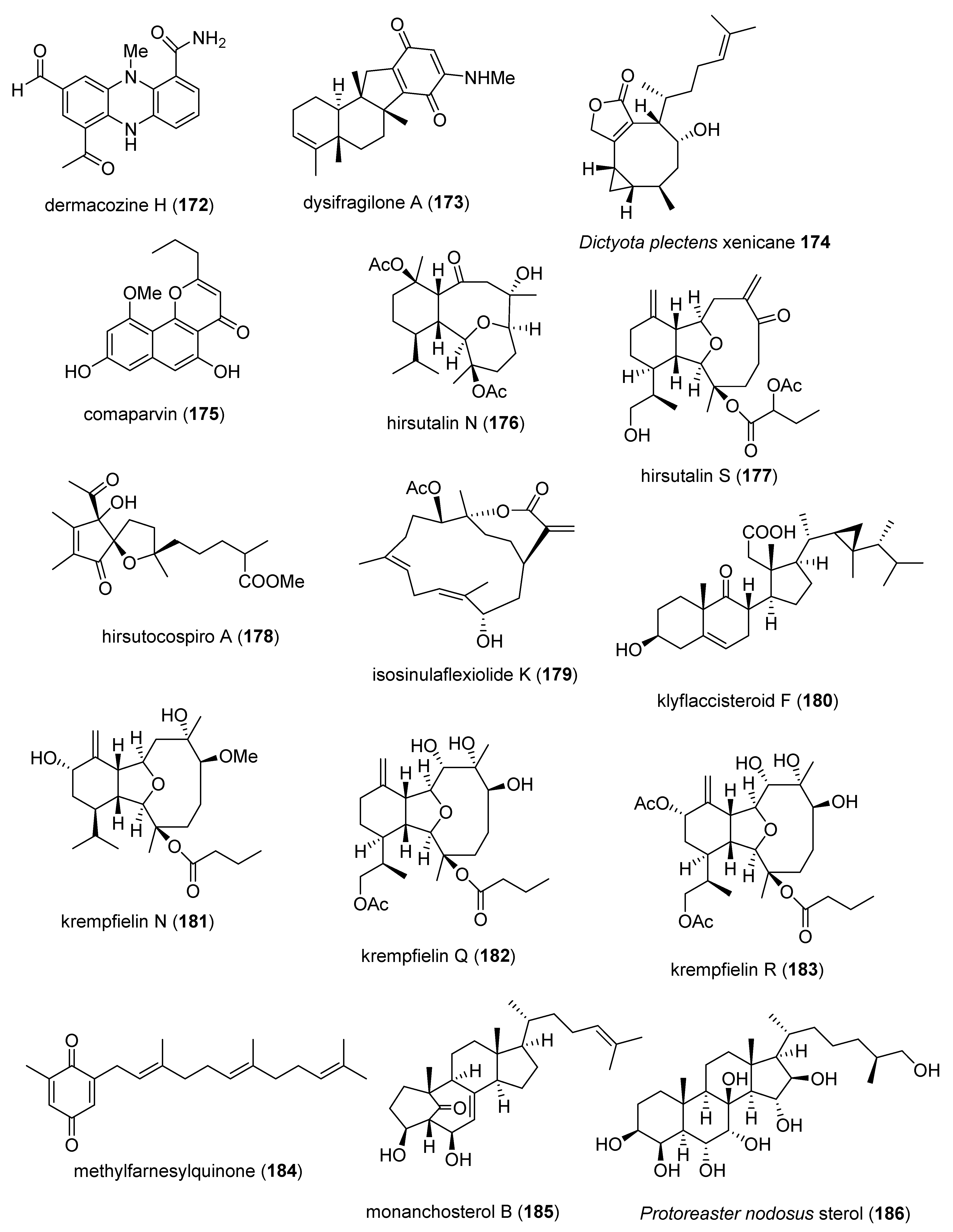



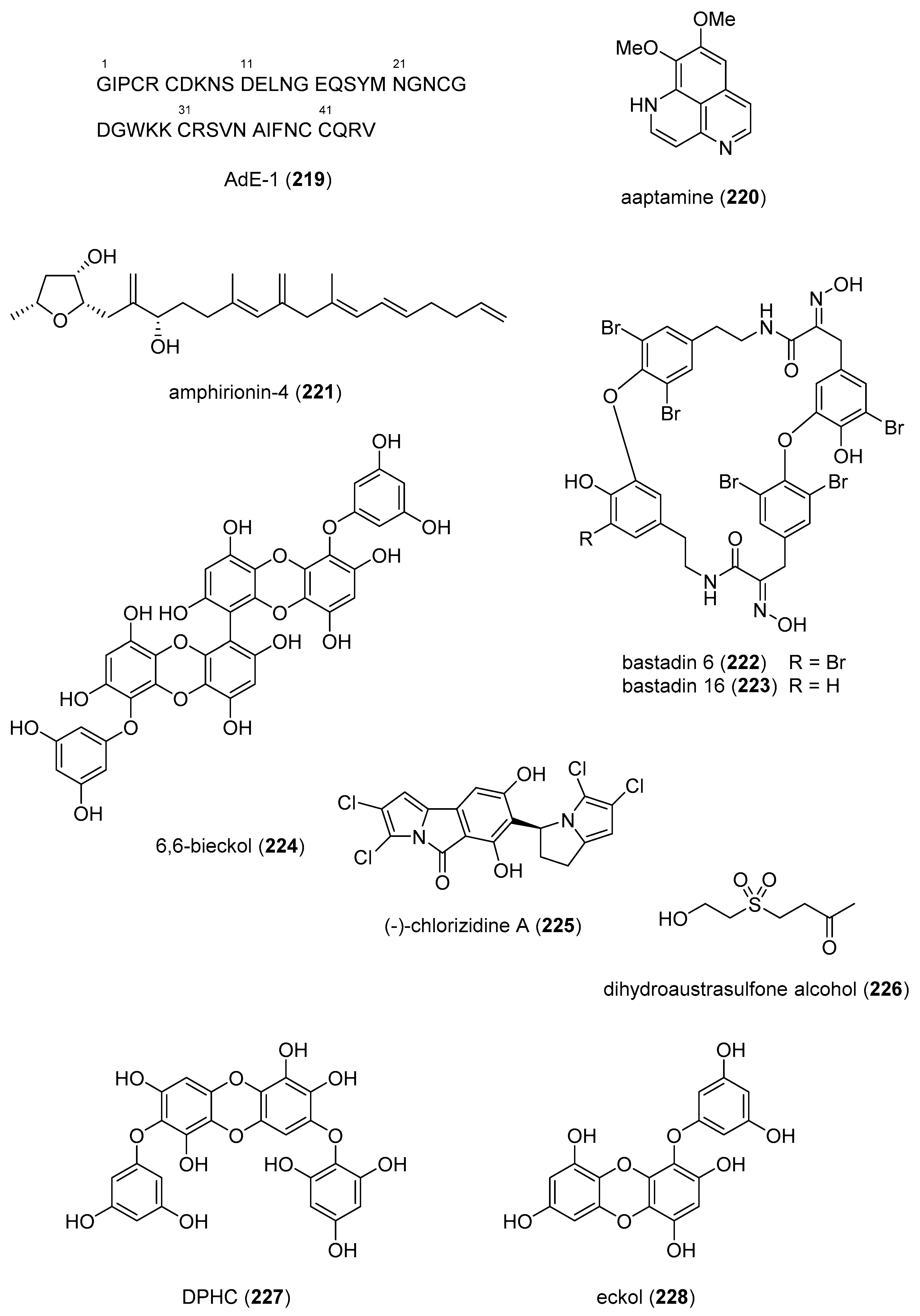

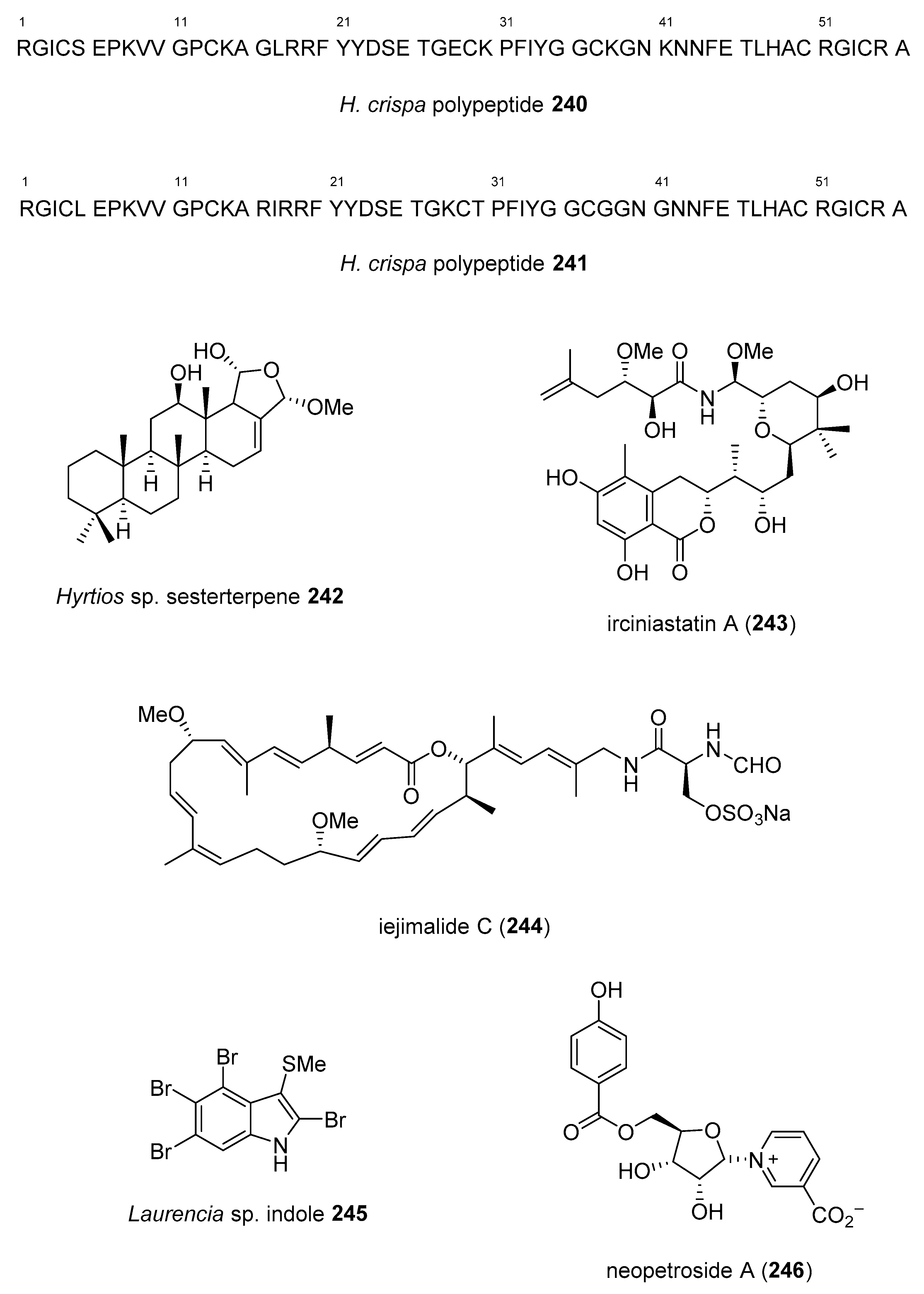


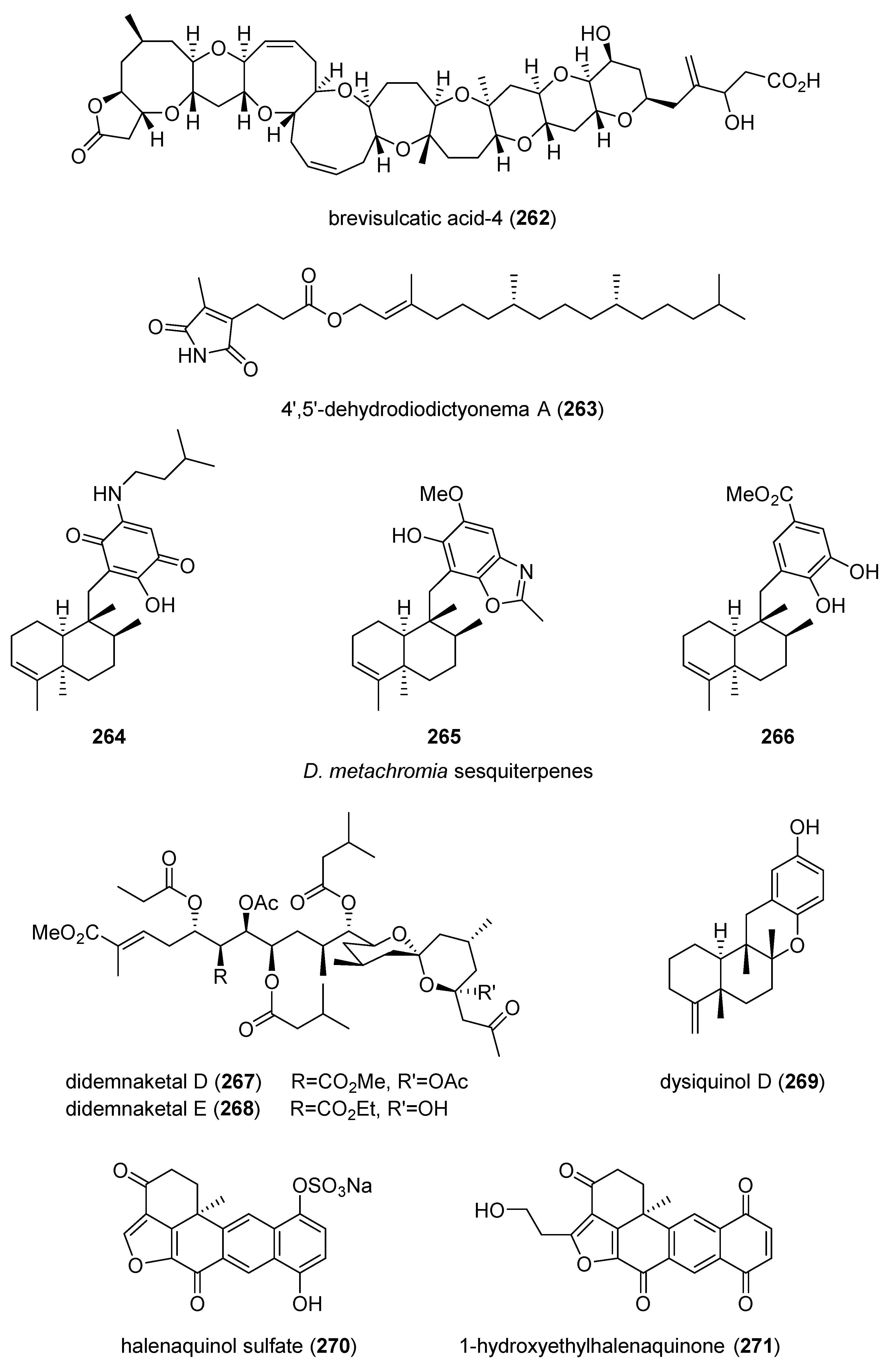


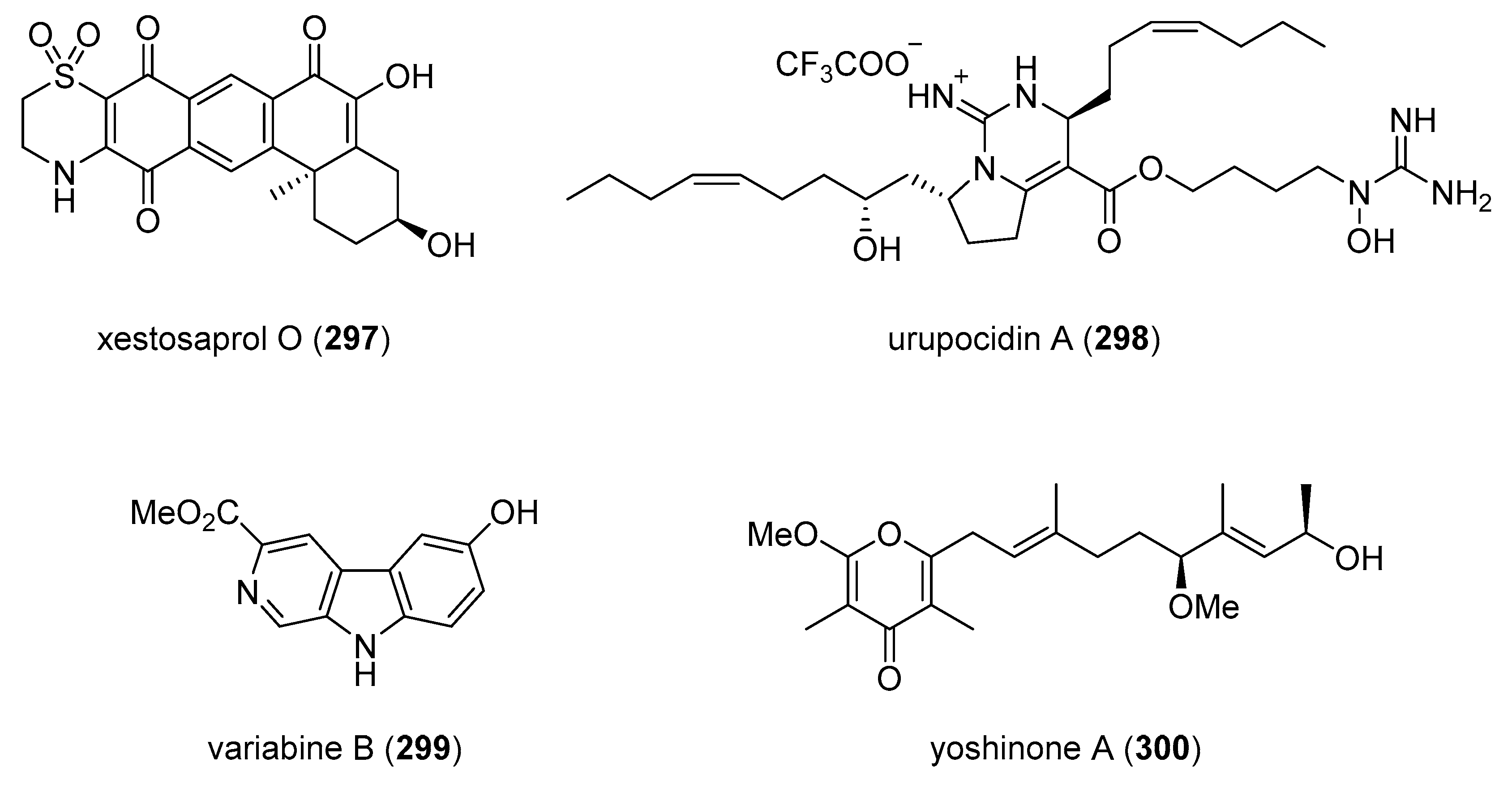
| Drug Class | Compound/Organism a | Chemistry | Pharmacologic Activity | IC50 b | MMOA b | Country c | References |
|---|---|---|---|---|---|---|---|
| Antibacterial | axinellamines A and B (1, 2)/sponge | Alkaloid f | Gram-positive and negative inhibition | 0.5–32 μg/mL + | Normal cellular division inhibition | USA | [24] |
| Antibacterial | buanmycin (3)/bacterium | Polyketide d | S. enterica inhibition | 0.7 μM + | Sortase A inhibition | S. KOR | [25] |
| Antibacterial | cathelicidin (4)/sea snake | Peptide f | Gram-positive and negative inhibition | 0.16–20.7 μg/mL + | Membrane morphology alteration | CHN | [26] |
| Antibacterial | clavanin A (5)/ascidian | Peptide f | S. aureus and E. coli infection inhibition | 10 mg/kg *** | IL-6 and TNF-α inhibition | BRA | [27] |
| Antibacterial | gelliusterol E (6)/sponge | Terpenoid e | C. trachomatis inhibition | 2.34 μM | OmpA protein inhibition | EGY, GBR | [28] |
| Antibacterial | ianthelliformisamimes B and C (7, 8)/sponge | Alkaloid f | Enhanced antibiotics against E. aerogenes, P. aeruginosa, K. Pneumoniae MDR strains in vitro | 3.12–12.5 μM * | Enhancement of drug transporters | FRA | [29] |
| Antibacterial | pardaxin (9)/flatfish | Peptide f | MR S. aureus inhibition in vivo | 8 mg/mL * | MCP-1, IL-6, and TNF-α inhibition | TWN | [30] |
| Antibacterial | phlorofucofuroeckol-A (10)/alga | Polyketide d | MR S. aureus inhibition | 32 μg/mL + | PBP2a suppresion | S. KOR | [31] |
| Antibacterial | salinamide F (11)/bacterium | Peptide f | E. coli inhibition | 0.2 μg/mL + | RNAP inhibition | USA | [32] |
| Antibacterial | piscidins (12, 13)/fish | Peptide f | K. pneumonia and A. baumannii inhibition in vitro | 1.5–3.1 μM + | Undetermined | TWN | [33] |
| Antibacterial | adametizine A (14)/fungus | Terpenoid e | S. aureus inhibition | 8 μg/mL + | Undetermined | CHN | [34] |
| Antibacterial | agelamadins A and B (15, 16)/sponge | Alkaloid f | M. luteus and C. neoformans inhibition | 5–8 μg/mL + | Undetermined | AUS, JPN | [35] |
| Antibacterial | Aspergillus sp. butyrolactone (17)/fungus | Terpenoid e | S. aureus and B. cereus inhibition | 1.56 μM + | Undetermined | CHN | [36] |
| Antibacterial | aszonapyrone A (18)/fungus | Terpenoid e | S. aureus and B. subtilis inhibition | 8 μg/mL + | Undetermined | PRT, THAI | [37] |
| Antibacterial | austalide R (19)/fungus | Terpenoid e | Marine bacteria inhibition | 0.1 μg/mL + | Undetermined | CHN, DEU, GBR | [38] |
| Antibacterial | citrifelin B (20)/fungus | Polyketide d | S. aureus inhibition | 4 μg/mL + | Undetermined | CHN | [39] |
| Antibacterial | desmethylisaridin C1 (21)/fungus | Peptide f | E. coli inhibition | 8 μg/mL | Undetermined | CHN | [40] |
| Antibacterial | D. granulosa diphenyl ethers (22, 23)/sponge | Polyketide d | Gram-positive and negative inhibition | 1–16 μg/mL + | Undetermined | USA | [41] |
| Antibacterial | diaporthalasin (24) /fungus | Terpenoid e | MR S. aureus inhibition | 2 μg/mL + | Undetermined | THAI | [42] |
| Antibacterial | D. pulchra furanones (25, 26)/alga | Alkaloid f | P. aeruginosa biofilm inhibition | 1.3 μM + | Undetermined | BRA, FRA, USA | [43] |
| Antibacterial | aureol B (27)/ sponge | Terpenoid e | Gram-positive and negative inhibition | 1 μg/mL + | Undetermined | S. KOR | [44] |
| Antibacterial | dysidinoid A (28)/sponge | Terpenoid e | MR S. aureus inhibition | 8 μg/mL ** | Undetermined | CHN | [45] |
| Antibacterial | Eunicea sp. compounds (29, 30)/sponge | Terpenoid e | P. aeruginosa and S. aureus biofilm inhibition | 0.5 mg/mL + | Undetermined | COL | [46] |
| Antibacterial | flavipesin A (31)/fungus | Polyketide d | S. aureus and B. subtillis inhibition | 0.25–8 μg/mL + | Undetermined | CHN | [47] |
| Antibacterial | gageopeptides A–D (32–35)/bacterium | Peptide f | S. aureus and B. subtillis inhibition | 0.04–0.08 μM + | Undetermined | BGD, S. KOR | [48] |
| Antibacterial | gageotetrins A–C (36–38)/bacterium | Peptide f | S. aureus and B. subtillis inhibition | 0.02–0.04 μM + | Undetermined | BGD, S. KOR | [49] |
| Antibacterial | hormaomycin B (39)/bacterium | Peptide f | S. aureus and K. rhizophila inhibition | 0.4–7 μM + | Undetermined | S. KOR | [50] |
| Antibacterial | ieodoglucomide C (40)/bacterium | Glycolipid | Gram-positive and negative inhibition | 0.01–0.05 μM + | Undetermined | S. KOR | [51] |
| Antibacterial | isoikarugamycin (41)/bacterium | Alkaloid f/ Terpenoid e | MR S.aureus | 2–4 μg/mL + | Undetermined | ESP | [52] |
| Antibacterial | keramadine (42)/sponge | Alkaloid f | M. luteus inhibition | 4 μg/mL + | Undetermined | AUS, JPN | [53] |
| Antibacterial | Ircinia sp. secosterols (43, 44)/sponge | Terpenoid e | M. luteus and S. epidermidis inhibition | 3.1, 6.3 μg/mL | Undetermined | S. KOR | [54,55] |
| Antibacterial | L. dendyi terpenoids (45, 46)/sponge | Polyketide d | MR S. aureus inhibition | 0.05–0.29 μM | Undetermined | USA | [56] |
| Antibacterial | lindgomycin (47)/fungus | Polyketide d | MR S. aureus inhibition | 5.1 μM | Undetermined | CHN, DEU | [57] |
| Antibacterial | marformysin D (48)/bacterium | Peptide f | M. luteus inhibition | 0.063 μg/mL + | Undetermined | CHN | [58] |
| Antibacterial | mollemycin A (49)/bacterium | Polyketide d | S. aureus and S. epidermidis inhibition | 0.05 μM | Undetermined | AUS | [59] |
| Antibacterial | neolaurene (50)/alga | Terpenoid e | S. typhi and S. aureus inhibition | 7.5 μg/mL | Undetermined | MYS | [60] |
| Antibacterial | penicyclone A (51)/fungus | Polyketide d | S. aureus inhibition | 0.3 μg/mL + | Undetermined | CHN | [61] |
| Antibacterial | P. oxalicum enamide (52)/fungus | Polyketide d | S. aureus inhibition | 2 μg/mL + | Undetermined | CHN | [62] |
| Antibacterial | puupehenol (53)/ sponge | Terpenoid e | B. cereus and S. aureus inhibition | 10 μg/disk + | Undetermined | AUS, USA | [63] |
| Antibacterial | phyllospongin E (54)/sponge | Terpenoid e | B. cereus and S. aureus inhibition | 2.5–3.3 μg/mL + | Undetermined | EGY, GBR | [64] |
| Antibacterial | sarcotrocheliols (55, 56)/soft coral | Terpenoid e | MR S. aureus inhibition | 1.5–4.3 μM + | Undetermined | SAU | [65] |
| Antibacterial | spiromastixone J (57)/fungus | Polyketide d | MR S. aureus inhibition | 2 μM | Undetermined | CHN, DEU | [66] |
| Antibacterial | stachyin B (58)/fungus | Alkaloid f /Terpenoid e | MR S. aureus and B. subtillis inhibition | 1.4–1.7 μM | Undetermined | CHN, DEU | [67] |
| Antibacterial | Streptomyces sp. glycoside (59)/bacterium | Polyketide d | C. trachomatis inhibition | 4.03 μM | Undetermined | EGY, DEU | [68] |
| Antibacterial | subergosterones A–C (60–62)/gorgonian coral | Terpenoid e | B. cereus inhibition | 1.6–3.1 μM + | Undetermined | CHN | [69] |
| Antibacterial | vitroprocine A (63)/bacterium | Polyketide d | A. baumannii inhibition | 8 μg/mL + | Undetermined | TWN, USA | [70] |
| Antibacterial | xestospongiamide (64)/sponge | Polyketide d | Gram-positive and negative inhibition | 2.5 μM + | Undetermined | EGY, SAU | [71] |
| Antifungal | bahamaolide A (65)/bacterium | Polyketide d | C. albicans inhibition | 1.5–3.1 μg/mL + | ICL inhibition | S. KOR | [72] |
| Antifungal | heronamide C (66)/bacterium | Polyketal/ alkaloid f | S. pombe cell inhibition | 5.8 μM + | Alteration of membrane microdomains | JPN | [73] |
| Antifungal | forazoline A (67)/bacterium | Polyketide d | C. albicans inhibition | 16 μg/mL + | Affected membrane integrity | USA | [74] |
| Antifungal | aaptamine derivative (68)/sponge | Alkaloid f | T. rubrum inhibition | 4 μg/mL + | Undetermined | CHN | [75] |
| Antifungal | amphidinin G (69)/dinoflagellate | Polyketide d | T. mentagrophytes inhibition | 8 μg/mL | Undetermined | JPN | [76] |
| Antifungal | amphidinol 18 (70)/dinoflagellate | Polyketide d | C. albicans inhibition | 9 μg/mL + | Undetermined | ITA | [77] |
| Antifungal | crambescin homologues (71–73)/sponge | Alkaloid f | C. neoformans var. gattii inhibition | 0.85–2.6 μM + | Undetermined | USA | [78] |
| Antifungal | coustesides C and D (74, 75)/sea cucumber | Terpenoid glycoside e | C. albicans inhibition | 1 mg/mL ++ | Undetermined | EGY, S.KOR | [79] |
| Antifungal | L. okamurai laurenes (76–78)/alga | Terpenoid e | C. glabrata inhibition | 2–4 μg/mL ** | Undetermined | CHN | [80,81] |
| Antifungal | mohangamide A (79)/bacterium | Peptide f | C. albicans isocitrate lyase inhibition | 4.4 μM | Undetermined | S. KOR | [82] |
| Antifungal | pleosporallin E (80)/fungus | Polyketide d | C. albicans inhibition | 7.44 μg/mL + | Undetermined | CHN | [83] |
| Antifungal | S. purpurea lysophospholipid (81)/sponge | Phospholipid | C. glabrata and C. neoformans inhibition | 4 μg/mL + | Undetermined | CHN | [84] |
| Antifungal | taurospongin A (82)/sponge | Polyketide d | C. neoformans inhibition | 1 μg/mL + | Undetermined | AUS, JPN | [85] |
| Antifungal | variegatuside D (83)/sea cucumber | Terpenoid glycoside e | Several Candida species inhibition | 3.4–13.6 μg/mL + | Undetermined | CHN | [86] |
| Antifungal | xestospongiamide (64)/sponge | Polyketide d | A. niger and C. albicans inhibition | >5 μM + | Undetermined | EGY, SAU | [71] |
| Antimalarial | C. hooperi isonitrile (84)/sponge | Terpenoid e | P. falciparum D6 and W2 strain inhibition | 4.3–4.7 nM | β-hematin inhibition | USA, ZAF | [87] |
| Antimalarial | actinoramide A (85)/bacterium | Peptide f | P. falciparum strains inhibition | 0.2 μM | Undetermined | CRI, USA | [88] |
| Antimalarial | diacarperoxide J (86)/sponge | Terpenoid e | P. falciparum D6 and W2 strain inhibition | 1.6–1.8 μM | Undetermined | CHN, USA | [89] |
| Antimalarial | laevigatol A (87)/soft coral | Terpenoid e | P. falciparum NF54 strain inhibition | 3.0 μM | Undetermined | CHE, DEU, S. KOR, VNM | [90] |
| Antimalarial | mollemycin A (49)/bacterium | Polyketide d | P. falciparum 3D7and Dd2 strain inhibition | 7–9 nM | Undetermined | AUS | [59] |
| Antimalarial | mon amphilectines B and C (88, 89)/sponge | Terpenoid e | P. falciparum 3D7strain inhibition | 44 nM | Undetermined | USA | [91] |
| Antimalarial | netamine K (90)/sponge | Alkaloid f | P. falciparum inhibition | 2.4 μM | Undetermined | BEL, FRA, ISR | [92] |
| Antimalarial | P. ocellata sesquiterpenes (91–93)/nudibranch | Terpenoid e | P. falciparum inhibition | 0.26–0.3 μM | Undetermined | AUS, ITA | [93] |
| Antimalarial | P. simplex polyketide (94)/sponge | Polyketide d | P. falciparum D10 and W2 strain inhibition | 2.7–4.0 μM | Undetermined | CHN, ITA | [94] |
| Antiprotozoal | plakortide E (95)/sponge | Polyketide d | T. brucei inhibition | 5 μM | Rhodesain inhibition | EGY, DEU | [95] |
| Antiprotozoal | batzelladine L (96)/sponge | Alkaloid f | T. cruzi and L. infantum inhibition | 2 μM | Enhanced ROS generation | BRA, CAN | [96] |
| Antiprotozoal | actinoporin A (97)/ bacterium | Polyketide d | T. b. brucei inhibition | 15 μM | Undetermined | AUS, DEU, EGY, GBR | [97] |
| Antiprotozoal | astropectenol A (98)/soft coral | Terpenoid e | T. brucei inhibition | 1.6 μM | Undetermined | DEU, VNM, S. KOR | [98] |
| Antiprotozoal | H. simulans sterol (99) sponge | Terpenoid e | T. b. brucei inhibition | 4.6 μM + | Undetermined | IRL, GBR | [99] |
| Antiprotozoal | lobosamide A (100)/bacterium | Alkaloid f | T. b. brucei inhibition | 0.8 μM | Undetermined | USA | [100] |
| Antiprotozoal | lobocrasols A and C (101, 102)/soft corals | Terpenoid e | L. donovani inhibition | 0.18 μM | Undetermined | CHE, DEU, S. KOR, VNM | [90] |
| Antiprotozoal | mangromicin A (103)/fungus | Polyketide d | T. b. brucei inhibition | 2.44 µg/mL | Undetermined | JPN | [101] |
| Antiprotozoal | crassumols D and E (104, 105)/soft corals | Terpenoid e | T. b. rhodesiense inhibition | 0.61 and 0.72 μM | Undetermined | CHE, DEU, S. KOR, VNM | [90] |
| Antiprotozoal | sesterstamide (106)/sponge | Terpenoid e | L. donovani inhibition | 32.9 µg/mL | Undetermined | CHN | [102] |
| Antiprotozoal | shagene A (107)/soft coral | Terpenoid e | L. donovani inhibition | 5 μM | Undetermined | AUS, USA | [103] |
| Antituberculosis | aaptamine analog (108)/sponge | Alkaloid f | M. smegmatis inhibition | 6.25 μg/mL + | Undetermined | JPN | [104] |
| Antituberculosis | callyaerins A and B (109, 110)/sponge | Peptide f | M. tuberculosis inhibition | 2, 5 μM ** | Undetermined | CHN, DEU, NLD | [105] |
| Antituberculosis | denigrin C (111)/sponge | Alkaloid f | M. tuberculosis H37Rv inhibition | 4 μg/mL + | Undetermined | IND | [106] |
| Antituberculosis | oxazinin A (112)/fungus | Polyketide d | M. tuberculosis inhibition | 2.9 μM | Undetermined | USA | [107] |
| Antiviral | pateamine A (113)/sponge | Mixed Biogenesis | Sindbis virus mRNA translation inhibition | >100 nM | nsP1 or nsP2 viral protein synthesis inhibition | CAN, ESP, NZL | [108] |
| Antiviral | abyssomicin 2 (114)/bacterium | Polyketide d | HIV-1 reactivation | 13.9 μM | Increased viral RNA in CD4+ T cells | USA | [109] |
| Antiviral | 8,4′’’-dieckol (115)/alga | Polyketide d | HIV-1 inhibition | 10 μM * | Reverse transcriptase inhibition | S. KOR | [110] |
| Antiviral | truncateol M (116)/fungus | Terpenoid e | H1N1 influenza A virus inhibition | 8.8 μM | Virion assembly/release inhibition | CHN, DEU | [111] |
| Antiviral | neoechinulin B (117)/fungus | Alkaloid f | H3N2, H1N1 A influenza virus inhibition | 17-22 μM | Hemagglutinin inhibition | CHN, DEU | [112] |
| Antiviral | thaixylomolin I (118)/mangrove | Terpenoid e | H1N1 influenza A virus inhibition | 77 μM | Undetermined | CHN, DEU, THAI | [113] |
| Antiviral | aaptamine derivative (68)/sponge | Alkaloid f | HIV-1 inhibition | 10 μM * | Undetermined | CHN | [75] |
| Antiviral | aflaquinolone B derivative (119)/fungus | Mixed biogenesis | RSV inhibition | 0.042 μM | Undetermined | CHN | [114] |
| Antiviral | A. terreus lactones (120, 121)/fungus | Polyketide d | HSV-1 inhibition | 6.34 μg/mL | Undetermined | CHN | [115] |
| Antiviral | chartarutine B (122)/fungus | Alkaloid f/terpenoid e | HIV-1 inhibition | 4.9 μM | Undetermined | CHN, DEU | [116] |
| Antiviral | debromoaplysiatoxin (123)/cyanobacterium | Polyketide d | CHIKV inhibition | 1.4 μM | Undetermined | NZL, SGP | [117] |
| Antiviral | dolabelladienol A (124)/alga | Terpenoid e | HIV-1 inhibition | 2.9 μM | Undetermined | BRA, COL, ESP | [118] |
| Antiviral | D. plectens diterpene (125)/alga | Terpenoid e | HIV-1 inhibition | 16.1 μM | Undetermined | CHN | [119] |
| Antiviral | Dysidea sp. PBDEs (22, 23)/sponge | Polyketide d | Hepatitis B inhibition | 0.23–0.80 μM | Core promoter inhibition | IDN, JPN, NLD | [120] |
| Antiviral | echrebsteroid C (126)/gorgonian | Terpenoid e | RSV inhibition | 0.19 μM | Undetermined | CHN | [121] |
| Antiviral | (+)-pestaloxazine A (127)/fungus | Alkaloid f | Enterovirus 71 inhibition | 14.2 μM | Undetermined | CHN | [122] |
| Antiviral | phlorofucofuroeckol-A(10)/alga | Polyketide d | MNV inhibition | 0.9 μM | Undetermined | S. KOR | [123] |
| Antiviral | secocrassumol (128)/soft coral | Terpenoid e | HCMV inhibition | 5 μg/mL | Undetermined | TWN | [124] |
| Antiviral | sporolide B (129)/bacterium | Polyketide d | HIV-reverse transcriptase inhibition | 14 μM | Undetermined | IND | [125] |
| Antiviral | stellettapeptins A and B (130, 131)/sponge | Peptide f | HIV-1 infection inhibition | 23–27 nM | Undetermined | USA | [126] |
| Antiviral | trichobotrysin A (132)/fungus | Polyketide d /Alkaloid f | HSV-1 inhibition | 3.08 μM | Undetermined | CHN | [127] |
| Anthelmintic | phorioadenine A (133)/sponge | Alkaloid f | H. contortus inhibition | 31 μg/mL +++ | Undetermined | AUS | [128] |
| Drug Class | Compound/Organism a+ | Chemistry | Pharmacological Activity | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|---|
| Antidiabetic | fucoxanthin and fucoxanthinol (134, 135)/alga | Terpenoid f | Improved glucose tolerance in vitro and in vivo | 50 µM * | Cytokine inhibition | JPN, S. KOR | [154,155] |
| Antidiabetic | octaphlorethol A (136)/alga | Shikimate h | α-glucosidase inhibition | 110 µM | Molecular docking on active site | CAN, S. KOR | [156] |
| Antidiabetic | phlorofucofuroeckol-A (10)/alga | Polyketide d | Decreased glucose levels in vivo | 10 mg/kg ** | α-glucosidase inhibition | S. KOR | [157] |
| Antidiabetic | Con-Ins G1 (137)/cone snail | Peptide g | Hypoglycemia induction | 65 ng/g * | Undetermined | AUS, DNK, USA | [158] |
| Antidiabetic | dehydroeuryspongin A (138)/sponge | Terpenoid f | PTP1B inhibition | 3.58 μM | Undetermined | IDN, JPN | [159] |
| Antidiabetic | Epicoccum sp. diterpene (139)/fungus | Terpenoid f | α-glucosidase inhibition | 4.6 μM | Undetermined | CHN | [160] |
| Antidiabetic | suncheonoside A (140)/bacterium | Terpenoid f | Adiponectin production | 10 μM * | Undetermined | S. KOR | [161] |
| Antidiabetic | terrelumamide A (141)/fungus | Peptide g | Adiponectin production | 37 μM * | Undetermined | S. KOR | [162] |
| Antidiabetic | X. testudinaria lipid (142)/sponge | Polyketide d | PTP1B inhibition | 5.3 μM | Undetermined | CHN | [163] |
| Anti-inflammatory | alcyonolide congeners (143, 144)/soft coral | Terpenoid f | Macrophage NO inhibition | 2 μM * | iNOS expression inhibition | JPN | [164] |
| Anti-inflammatory | astaxanthin (145)/alga | Terpenoid f | Oxidative stress inhibition in vivo | 10 mg/kg ** | CAT and SOD enhancement | CHN | [165] |
| Anti-inflammatory | 8,8′-bieckol (146)/alga | Polyketide e | Macrophage NO and PGE2 release inhibition | 50 μM * | Inhibition of NFκB | S. KOR | [166] |
| Anti-inflammatory | convolutamydine A (147)/ bryozoa | Alkaloid g | Formalin-induced licking behavior inhibition | 0.01 mg/kg * | TNF-α, IL-6 release inhibition | BRA | [167] |
| Anti-inflammatory | capgermacrene A (148)/ soft coral | Terpenoid f | Macrophage NO and IL-1β inhibition | <10 μg/mL * | iNOS expression inhibition | MYS, S. KOR | [168] |
| Anti-inflammatory | cathelicidin (4)/sea snake | Peptide g | Binding of LPS to TLR4 inhibition | 4 μg/mL * | Inflammatory cytokine inhibition | CHN | [26] |
| Anti-inflammatory | dactyloditerpenol acetate (149)/ sea hare | Terpenoid f | LPS- activated microglia in vitro inhibition | 0.4–1 μM | O2- and TXB2 inhibition | USA | [169] |
| Anti-inflammatory | dieckol (150)/alga | Shikimate h | Macrophage iNOS transcription inhibition | 30 μM * | Inhibition of NFκB and p38MAPK | S. KOR | [170] |
| Anti-inflammatory | dieckol (150)/alga | Shikimate h | Human keratinocyte MDC/CCL22 inhibition | 12.5 μM * | STAT1 phosphorylation inhibition | S. KOR | [171] |
| Anti-inflammatory | excavatolide B (151)/gorgonian | Terpenoid f | Macrophage iNOS and COX-2 transcription inhibition | 25 μM * | In vivo iNOS protein expression reduction | TWN | [172] |
| Anti-inflammatory | flexibilide (152)/soft coral | Terpenoid f | Neuropathic pain inhibition | 10 µg * | Upregulation of TGF-β1 | TWN | [173] |
| Anti-inflammatory | fucoxanthinol (135)/alga | Terpenoid f | Macrophage TNF-α and MCP-1 release inhibition | 10 μM | COX-2 expression inhibition | JPN | [155] |
| Anti-inflammatory | H. fusiforme flavone (153)/ alga | Shikimate h/Polyketide d | Macrophage NO and PGE2 release inhibition | 10 μg/mL * | iNOS, COX-2 expression inhibition | S. KOR | [174] |
| Anti-inflammatory | 5β-hydroxypalisadin B (154)/alga | Terpenoid f | Macrophage NO release inhibition | 17 μM | Partial iNOS expression inhibition | LKA, MYS, S. KOR | [175] |
| Anti-inflammatory | glaucumolides A and B (155, 156)/soft coral | Terpenoid f | Neutrophil SOX and elastase inhibition | 2.8–4 µM * | iNOS, COX-2 inhibition | TWN | [176] |
| Anti-inflammatory | phlorofucofuroeckol-B (157)/alga | Polyketide d | Microglia activation inhibition | 0.1 μg/mL * | iNOS, COX-2 inhibition | S. KOR | [177] |
| Anti-inflammatory | P. palmata lipid (158)/alga | Polyketide d | Macrophage NO release inhibition | 16.7 μM | iNOS expression inhibition | CAN | [178] |
| Anti-inflammatory | reduced scytonemin (159)/alga | Alkaloid g | Macrophage NO release inhibition | 1 µM * | HO-1 expression induction | JPN | [179] |
| Anti-inflammatory | sinuleptolide (160)/soft coral | Terpenoid f | LPS-activated rat microglia in vitro inhibition | 0.5–2.9 μM | Cytokine release inhibition | ESP, FIN, IND, ITA, | [180] |
| Anti-inflammatory | sarcopanol A (161)/soft coral | Terpenoid f | iNOS, COX-2, and ICAM-1 transcription inhibition | 8.3 µM | NFκB inhibition | S. KOR, VNM | [181] |
| Anti-inflammatory | sinumaximol H (162)/sponge | Terpenoid f | iNOS and ICAM-1 transcription inhibition | 1 µM * | NFκB inhibition | S. KOR, VNM | [182] |
| Anti-inflammatory | tanzawaic acid A (163)/fungus | Polyketide d | NO inhibition | 7.1 µM | iNOS and PTP1B inhibition | VNM, S. KOR | [183] |
| Anti-inflammatory | aspertetranone D (164)/fungus | Terpenoid f | IL-6 inhibition | 40 µM * | Undetermined | CHN, USA | [184] |
| Anti-inflammatory | briarenolide J (165)/soft coral | Terpenoid f | Neutrophil SOX and elastase inhibition | 10–15 µM | Undetermined | TWN | [185] |
| Anti-inflammatory | briarenolides K and L (166, 167)/soft coral | Terpenoid f | Macrophage iNOS inhibition | >10 μg/mL * | Undetermined | TWN | [186] |
| Anti-inflammatory | briarenolides U, V, W (168, 169)/soft coral | Terpenoid f | Macrophage COX-2 and iNOS expression inhibition | >10 μg/mL * | Undetermined | TWN | [187] |
| Anti-inflammatory | briaviolides E and I (170, 171)/soft coral | Terpenoid f | Neutrophil SOX and elastase inhibition | >10 μg/mL * | Undetermined | TWN | [188] |
| Anti-inflammatory | dermacozine H (172)/bacterium | Alkaloid g | Radical scavenging activity | 18.8 µM | Undetermined | DEU, EGY, UK | [189] |
| Anti-inflammatory | dysifragilone A (173)/sponge | Terpenoid f | Macrophage NO release inhibition | 6.6 µM | Undetermined | CHN | [190] |
| Anti-inflammatory | D. plectens xenicane (174)/alga | Terpenoid f | Macrophage NO release inhibition | 10 µM | Undetermined | CHN | [191] |
| Anti-inflammatory | comaparvin (175)/crinoid | Polyketide d | Carrageenan-induced hyperalgesia inhibition | 30 mg/kg * | iNOS expression inhibition | TWN | [192] |
| Anti-inflammatory | hirsutalins N and S (176, 177)/ soft coral | Terpenoid f | Neutrophil elastase inhibition | 10 μM * | Undetermined | TWN | [193,194] |
| Anti-inflammatory | hirsutocospiro A (178)/soft coral | Terpenoid f | Neutrophil SOX and elastase inhibition | 3.7–4.1 μM | Undetermined | TWN | [195] |
| Anti-inflammatory | isosinulaflexiolide K (179)/soft coral | Terpenoid f | Macrophage COX-2 and iNOS expression inhibition | >10 μM * | Undetermined | TWN | [196] |
| Anti-inflammatory | klyflaccisteroid F (180)/soft coral | Terpenoid f | Neutrophil SOX and elastase inhibition | 0.34 μM | Undetermined | TWN | [197] |
| Anti-inflammatory | krempfielin N (181)/soft coral | Terpenoid f | Neutrophil SOX and elastase inhibition | >10 μM * | Undetermined | TWN | [198] |
| Anti-inflammatory | krempfielins Q and R (182, 183)/soft coral | Terpenoid f | Neutrophil SOX and elastase inhibition | >10 μM * | Undetermined | TWN | [199] |
| Anti-inflammatory | methylfarnesylquinone (184)/alga | Shikimate h/Terpenoid f | Neutrophil SOX and elastase inhibition | 0.2–0.48 µg/mL | Undetermined | TWN | [200] |
| Anti-inflammatory | monanchosterol B (185)/sponge | Terpenoid f | Macrophage IL-6 expression inhibition | 5 µM | Undetermined | S. KOR | [201] |
| Anti-inflammatory | P. nodosus sterol (186)/starfish | Terpenoid f | IL-12 and IL-6 inhibition | 1.3–3.1 µM | Undetermined | VNM, S. KOR | [202] |
| Anti-inflammatory | rhytidenone C (187)/fungus | Polyketide d | Macrophage NO inhibition | 0.31 µM | Undetermined | THA | [203] |
| Anti-inflammatory | sarcocrassocolide E (188)/soft coral | Terpenoid f | Macrophage COX-2 and iNOS expression inhibition | <10 μM * | Undetermined | TWN | [204] |
| Anti-inflammatory | sinulacembranolide A (189)/octocoral | Terpenoid f | Macrophage iNOS expression inhibition | <10 μM | Undetermined | TWN | [205] |
| Anti-inflammatory | thomimarine B (190)/fungus | Terpenoid f | Macrophage NO inhibition | >10 μM | Undetermined | RUS, VNM | [206] |
| Anti-inflammatory | tortuosene A (191)/soft coral | Terpenoid f | Neutrophil SOX inhibition | 7.3 μM | Undetermined | TWN | [207] |
| Immune system | grassypeptolide A (192)/cyanobacterium | Peptide g | IL-2 and T-cell proliferation inhibition | 1 μM * | Dipeptidyl peptidase 8 inhibition | CHN, JPN, USA | [208] |
| Immune system | F. reticulata alkaloids (193)/sponge | Alkaloid g | IL-2 inhibition | 5–50 µM * | Undetermined | CHN, NLD | [209] |
| Immune system | luzonicoside A (194)/starfish | Terpenoid f | Macrophage NO and ROS stimulation | 0.01–0.1 µM * | Undetermined | RUS, VNM | [210] |
| Immune system | typicoside C1(195)/sea cucumber | Terpenoid f | Macrophage ROS stimulation | <1 ng/mL * | Undetermined | IND, RUS | [211] |
| Nervous system | aurone glycoside (196)/fungus | Shikimate h/Polyketide d | Oxidative stress neuroprotection | 1 µM * | Apoptosis inhibition | CHN | [212] |
| Nervous system | azaspiracid-1 (197)/alga | Polyketide d/ Alkaloid g | Peripherin-labelled neurite process | 15 nM * | Peripherin isoform downregulation | NOR | [213] |
| Nervous system | caulerpine (198)/alga | Alkaloid g | Antinociceptive activity | 40 mg/kg * | Involves α2 and 5-HT3 receptors | BRA | [214] |
| Nervous systema | 6-bromohypaphorine (199)/sea slug | Alkaloid g | Human α7 nAChR agonist | 23 µM | Rise [Ca2+ ]i | RUS | [215] |
| Nervous system | piscidin (200)/fish | Peptide g | Antinociceptive activity | 20 µg/rat * | Phosphor-mTOR inhibition | TWN | [216] |
| Nervous system | C. marmoreus conotoxin Mr1.7 (201)/cone snail | Peptide g | Ach-evoked membrane current inhibition | 53.1 nM | A3β2 nAChR inhibition | CHN | [217] |
| Nervous system | C. litteratus conotoxin lt16a (202)/cone snail | Peptide g | Neuronal Na+ current inhibition | 1 µM * | Undetermined | CHN | [218] |
| Nervous system | C. vitulinus peptide (203)/ cone snail | Peptide g | Neuronal BK channel inhibition | 8.5 µM | Electrostatic interaction with β4 subunits | CHN, USA | [219] |
| Nervous system | echinochrome A (204)/sea urchin | Polyketide d | Acetylcholinesterase inhibition and NO scavenging | 16.4 µM | Irreversible and uncompetitive inhibition | S. KOR, RUS | [220] |
| Nervous system | ganglioside LLG-3 (205)/starfish | Glycolipid | Neuritogenesis stimulation in vitro | 1 nM * | MAPK signaling stimulation | JPN | [221] |
| Nervous system | heteronemin (206)/sponge | Terpenoid f | TDP-43 binding to DNA inhibition | 10.1 nM | Promoted aggregation of insoluble TDP-43 | ITA | [222] |
| Nervous system | pinnatoxin A (207)/mollusc | Polyketide d/ Alkaloid g | Muscle and neuronal nAChRs receptor inhibition | 0.086–47.5 nM | EF-ketal ring confers nAChR subtype specificity | FRA, USA | [223] |
| Nervous tissue | PhcrTx1 (208)/sea anemone | Peptide g | ASIC inhibition | 100 nM | Lower potency on Kv channels | BEL, BRA, CUB, DEU, ESP, MEX | [224] |
| Nervous tissue | phlorofucofuroeckol-A (10)/alga | Polyketide d | Butyrylcholinesterase inhibition | 0.95 µM | β-secretase inhibition | S. KOR | [225] |
| Nervous tissue | S. auritum ceramide (209)/soft coral | Polyketide d | Anxiolytic and CNS depressing activity in vivo | 1 mg/kg ** | GABA(A) receptor modulation | EGY, USA | [226] |
| Nervous system | spirolide C (210)/dinoflagellate | Polyketide d/ Alkaloid g | nAChR inhibition | 1.5–3 nM * | Muscle and neuronal -type nAChR inhibition | FRA | [227] |
| Nervous system | zonarol (211)/alga | Meroterpenoid f | Glutamate toxicity inhibition in vitro | 0.22 µM | Nrf2/ARE pathway activation | JPN, USA | [228] |
| Nervous system | aplysinellamide-1 (212)/sponge | Alkaloid g | ApoE secretion modulation | 30 µM * | Undetermined | AUS, CAN | [229] |
| Nervous system | A. terreus lactones (120, 121)/fungus | Polyketide d | acetylcholinesterase inhibition | 4.2 µM | Undetermined | CHN | [115] |
| Nervous system | C. araneosus ar3j conotoxin (213)/cone snail | Peptide g | Sleep induction | 2 nM * | Undetermined | IND | [230] |
| Nervous system | D. cejpii steroid (214)/fungus | Terpenoid f | Amyloid β-42 production inhibition | 10 µM * | Undetermined | DEU, FRA | [231] |
| Nervous system | genuanine (215)/cone snail | Alkaloid g | Paralysis in vivo | 40 nM * | Undetermined | PRT, USA | [232] |
| Nervous system | homoaerothionin (216)/sponge | Alkaloid g | acetylcholinesterase inhibition | 2.9–6.2 µM | Undetermined | THA | [233] |
| Nervous system | mooreamide A (217)/bacterium | Polyketide d | CB1 binding | 0.47 µM ** | Undetermined | ITA, PNG, USA | [234] |
| Nervous system | S. spinosulus hydroquinone (218)/sponge | Shikimate h/Polyketide d | Enhance glutamate and ACh release | 10 µM * | Undetermined | ITA | [235] |
| Compound/Organism a | Chemistry | Pharmacological Activity i | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|
| AdE-1 (219)/sea anemone | Peptide g | Cardiomyocyte action potential modulation | 2 nM * | Na+ and K+ current increase | ISR | [236] |
| aaptamine (220)/sponge | Alkaloid g | ROS inhibition | 10 μM * | Cytokine inhibition | S. KOR | [237] |
| amphirionin-4 (221)/dinoflagellate | Polyketide e | Bone marrow stromal cells proliferation stimulation | <0.1 ng/mL | Cytoskeleton protein synthesis | JPN | [238] |
| astaxanthin (145)/alga | Terpenoid f | Leydig cell steroidogenesis protection | 10 µg/mL * | ROS scavenging | TWN | [239] |
| bastadins 6 and 16 (222, 223)/sponge | Alkaloid g | Foam cell formation inhibition | 5 μM * | ACAT inhibition | JPN | [240] |
| 6,6-bieckol (224)/alga | Polyketide e | Adipocyte differentiation inhibition | 50 µg/mL * | Adipogenesis inhibition | S. KOR | [241] |
| (-)-chlorizidine A (225)/bacterium | Alkaloid g | Increase G1 cell cycle phase | 2 μM * | GAPDH and hENO1 binding | BRA, USA | [242] |
| dihydroaustrasulfone alcohol (226)/soft coral | Polyketide e | PDGF-induced HASMC proliferation and angiogenesis inhibition | 10 μM * | DNA synthesis and VEGF signaling inhibition | TWN | [243,244] |
| DPHC (227)/alga | Terpenoid f | UVB radiation-induced DNA damage protection | 20 μM * | Nucleotide excision repair system induction | S. KOR | [245] |
| echinochrome A (204)/sea urchin | Alkaloid g | Cardiac contractility inhibition | 3 μM * | SERCA2A inhibition | BEL, S. KOR, RUS | [246] |
| echinochrome A (204)/sea urchin | Alkaloid g | Increased mitochondria biogenesis and function | 5 μM * | Mitochondrial biogenesis genes upregulation | S. KOR, RUS | [247] |
| eckol (228)/alga | Polyketide e | ROS suppression in cells | 10 μM * | Increased HO-1 expression | S. KOR | [248] |
| emindole SB (229)/fungus | Terpenoid f | Nonselective CB1/CB2 antagonist | 2.2–7.0 μM ** | Undetermined | CHE, DEU | [249] |
| farnesylquinone (230)/bacterium | Polyketide e | Decreased lipid accumulation | 1 μM * | Increased PPARα activity | CHN, DEU | [250] |
| FGFC1 (231)/fungus | Alkaloid g | Thrombolysis induction in vivo | 5 mg/kg * | Fibrin hydrolysis induction in vitro | CHN | [251] |
| formosusin A (232)/fungus | Alkaloid g | Mammalian DNA polymerase β inhibition | 35.6 μM | Competitive and non-competitive inhibition | JPN | [252] |
| fucodiphlorethol (233)/alga | Polyketide e | ROS inhibition | 10 μM * | Decreased mitochondrial loss, and caspase-9 expression | S. KOR | [253] |
| gallic acid (234)/alga | Shikimate h | NO-dependent vasorelaxant effect | 12.5 µg/mL | Phospho-eNOS increase | S. KOR | [254] |
| gallinamide A (235)/bacterium | Peptide f | Human cathepsin L inhibition | 5 nM | Covalent inhibition | USA | [255] |
| girolline (236)/sponge | Alkaloid g | TLR 5 inhibition | 2 µg/mL | IL-8 and IL-6 inhibition | CAN, NLD, USA | [256] |
| gracilins A, H, L (237–239)/sponge | Terpenoid f | mPTP opening inhibition | 1 μM * | Binding to CypD | EGY, ESP, GBR | [257] |
| H. crispa polypeptides (240, 241)/sea anemone | Peptide f | Macrophage TNF-α, IL-6, and proIL-1β inhibition | Trypsin and α-chemotrypsin inhibition | RUS, TWN | [258] | |
| Hyrtios sp. sesterterpene (242)/sponge | Terpenoid f | TDP-43 inhibition | 0.4 nM | TDP-43 to DNA binding inhibition | ITA, PYF | [259] |
| irciniastatin A (243)/sponge | Polyketide e | TNF-α receptor 1 ectodomain shedding | 10 nM * | ERK activation induced | JPN | [260] |
| iejimalide C (244)/ascidian | Polyketide e | V-ATPase inhibitor | 0.12 μM | Bafilomycin site binding | JPN | [261] |
| Laurencia sp. indole (245)/alga | Alkaloid g | Aryl hydrocarbon receptor agonist | 10 μM * | DNA binding stimulation and CYP1A1 induction | JPN, USA | [262] |
| neopetroside A (246)/sponge | Alkaloid g | Cardiomyocyte mitochondrial upregulation | 10 µM * | Increased ATP levels and O2 consumption | RUS, S. KOR | [263] |
| Phlorofucofuroeckol-A (10)/alga | Polyketide e | Lipid accumulation inhibition | 18 µM | Decreased PPARγ expression | S. KOR | [264] |
| polytheonamide B (247)/sponge | Peptide g | One-ion pore channel permeation determined | NA | Two ion binding sites defined, but second ion excluded | JPN | [265] |
| siphonaxanthin (248)/alga | Terpenoid f | Adipogenesis inhibition | 5 µM | Transcription factor inhibition | JPN | [266] |
| spiromastixones J and L (57, 249)/fungus | Polyketide e | Cholesterol uptake inhibition | 10 μM * | PPARγ upregulation | CHN | [267] |
| thalassospiramide C (250)/bacterium | Peptide g | HCAN1 inhibition | 3.4 nM | Binding to Cys115 residue | CHN, USA | [268] |
| xyloketal B (251)/fungus | Polyketide e | Atherosclerotic plaque attenuation | 14 mg/kg *** | Increased eNOS activity | CAN, CHN | [269] |
| xyloketal B (251)/fungus | Polyketide e | P450 3a activity and expression regulation | 14 mg/kg *** | Active site binding determined by docking studies | CHN, USA | [270] |
| X. testudinaria lipid (252)/sponge | Polyketide e | Pancreatic lipase inhibition | 3.11 μM | Triglyceride level decrease in vivo | CHN, ITA | [271] |
| acuminolide A (253)/dinoflagellate | Polyketide e | Stimulation of actomyosin ATPase | 1 μM * | Undetermined | S. KOR | [272] |
| ahpatinin Ac (254)/bacterium | Peptide g | Pepsin inhibition | 11 nM | Undetermined | JPN | [273] |
| alternariol derivatives (255–257)/sponge | Shikimate h | HCV protease inhibition | 12–52 µg/mL | Undetermined | EGY, SAU | [136] |
| apliamide D (258)/ascidian | Peptide g | Na+/K+-ATPase inhibition | 3.2 μM | Undetermined | S. KOR | [274] |
| austalides 4 and 9 (259, 260)/fungus | Terpenoid f | Endo-1,3-β-D-glucanase inhibition | 0.01 μM | Undetermined | RUS | [275] |
| axinelline A (261)/bacterium | Alkaloid g | COX-2 inhibition | 2.8 μM | Undetermined | CHN | [276] |
| brevisulcatic acid-4 (262)/dinoflagellate | Polyketide e | Activation of sodium channels | 20 ng/mL | Undetermined | JPN, NZL | [277] |
| 4′,5′-dehydrodiodictyonema (263)/alga | Terpenoid f | PTP1B inhibition | 2.3 μM | Undetermined | CHN | [278] |
| D. metachromia sesquiterpenes (264–266)/sponge | Terpenoid f | Multiple kinases inhibition | 0.97–4.8 μM | Undetermined | CHN, NLD, DEU | [279] |
| didemnaketals D and E (267, 268)/ascidian | Terpenoid f | Multiple kinases inhibition | 10 μg/mL * | Undetermined | EGY | [280] |
| dysiquinol D (269)/sponge | Terpenoid f | NF-κB inhibition | 0.81 μM | Undetermined | AUS, CHN | [281] |
| fucoxanthin (134)/alga | Terpenoid f | Hydroxyl radical-scavenging | 10 μg/mL * | Undetermined | CHN | [282] |
| halenaquinol sulfate (270)/sponge | Polyketide e | CDK9 and DYRK1A inhibition | 0.5–0.61 μM | Undetermined | FRA, NZL | [283] |
| 1-hydroxyethylhalenaquinone (271)/sponge | Polyketide e | Proteasome-chymotrypsin-like activity inhibition | 0.19 μM | Undetermined | IDN, JPN, NLD | [284] |
| hymenialdisine derivatives (272–274)/sponge | Alkaloid g | PfGSK-3 inhibition | 0.07–0.2 μM | Undetermined | DEU, EGY, FRA, NLD | [285] |
| hymenialdisine (275)/sponge | Alkaloid g | CK1, CDK5, GSK3β inhibition | 0.03–0.16 μM | Undetermined | AUS | [286] |
| hyattellactone A (276)/sponge | Terpenoid f | PTP1B inhibition | 7.45 μM | Undetermined | IDN, JPN | [287] |
| H. lachne sesterterpenoid (277)/sponge | Terpenoid f | PTP1B inhibition | 5.2 μM | Undetermined | CHN | [288] |
| ianthelliformisamines A–C (278, 7, 8)/ sponge | Alkaloid g | Carbonic anhydrase inhibition | 0.2–0.85 μM ** | Undetermined | AUS, ITA | [289] |
| incisterols A5 and A6 (279, 280)/sponge | Terpenoid f | PXR agonists | 10 μM * | Undetermined | ITA | [290] |
| L. crassum cembranoid (281)/soft coral | Terpenoid f | PPAR transcription activation | 2.07 μM | Undetermined | VNM | [291] |
| L. okamurai terpenoid (282)/alga | Terpenoid f | PTP1B inhibition | 4.9 μg/mL | Undetermined | CHN | [292] |
| malonganenone L (283)/sea whip | Alkaloid g | PDE4D inhibition | 8.5 μM | Undetermined | CHN | [293] |
| punctaporonin K (284)/fungus | Terpenoid f | Lipid-lowering effect | 10 μM * | Undetermined | CHN, DEU | [294] |
| psychrophilin G (285)/fungus | Peptide g | Lipid-lowering effect | 10 μM * | Undetermined | CHN | [295] |
| racemosin (286)/alga | Alkaloid g | PTP1B inhibition | 5.9 µM | Undetermined | CHN | [296] |
| sarcoehrendin B (287)/soft coral | Polyketide e | PDE4 inhibition | 3.7 µM | Undetermined | CHN | [297] |
| sarsolilide A (288)/soft coral | Terpenoid f | PTP1B inhibition | 6.8 µM | Undetermined | CHN, HUN | [298] |
| Stachybotry sp. xanthone (289)/fungus | Polyketide e | COX-2 inhibition | 8.9 µM | Undetermined | CHN | [299] |
| S. thunbergii alkapolyene (290)/alga | Polyketide e | Soybean LOX inhibition | 5 µM | Undetermined | JPN, S. KOR | [300] |
| shinorine (291)/alga | Alkaloid g | C. histolyticum collagenase inhibition | 104 µM | Undetermined | AUT | [301] |
| sinularone D (292)/soft coral | Polyketide e | NF-κB inhibition | 10 μg/mL * | Undetermined | CHN | [302] |
| N-(2-benzenepropanoic acid) stachybotrylactam (293)/fungus | Alkaloid g | Triglyceride and cholesterol inhibition | 10 μM * | Undetermined | CHN, DEU | [303] |
| strongylophorine-13/-14 (294)/sponge | Terpenoid f | Hu proteasome 20S inhibition | 2.1 μM | Undetermined | JPN | [304] |
| swinhoeisterol A (295)/sponge | Terpenoid f | (h)P300 acetyltransferase inhibition | 2.7 μM | Undetermined | ITA, CHN, USA | [305] |
| thunberol (296)/alga | Terpenoid f | PTP1B inhibition | 2.24 μg/mL | Undetermined | CHN | [306] |
| xestosaprol O (297)/sponge | Terpenoid f | IDO1 inhibition | 4 μM | Undetermined | CAN, NLD | [307] |
| urupocidin A (298)/sponge | Alkaloid g | iNOS expression induction | 10 μM * | Undetermined | RUS, TWN | [308] |
| variabine B (299)/sponge | Alkaloid g | Proteasome-chymotrypsin-like activity inhibition | 4 µg/mL | Undetermined | IDN, JPN, NLD | [309] |
| yoshinone A (300)/cyanobacterium | Polyketide e | Triglyceride inhibition | 0.4 μM | Undetermined | JPN | [310] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2020, 18, 5. https://doi.org/10.3390/md18010005
Mayer AMS, Guerrero AJ, Rodríguez AD, Taglialatela-Scafati O, Nakamura F, Fusetani N. Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Marine Drugs. 2020; 18(1):5. https://doi.org/10.3390/md18010005
Chicago/Turabian StyleMayer, Alejandro M. S., Aimee J. Guerrero, Abimael D. Rodríguez, Orazio Taglialatela-Scafati, Fumiaki Nakamura, and Nobuhiro Fusetani. 2020. "Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action" Marine Drugs 18, no. 1: 5. https://doi.org/10.3390/md18010005
APA StyleMayer, A. M. S., Guerrero, A. J., Rodríguez, A. D., Taglialatela-Scafati, O., Nakamura, F., & Fusetani, N. (2020). Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Marine Drugs, 18(1), 5. https://doi.org/10.3390/md18010005






