Metabolic Profiling of the Soft Coral Erythropodium caribaeorum (Alcyonacea: Anthothelidae) from the Colombian Caribbean Reveals Different Chemotypes
Abstract
1. Introduction
2. Results and Discussion
2.1. MS-Based Metabolic Profiling of E. caribaeorum in the Three Study Locations
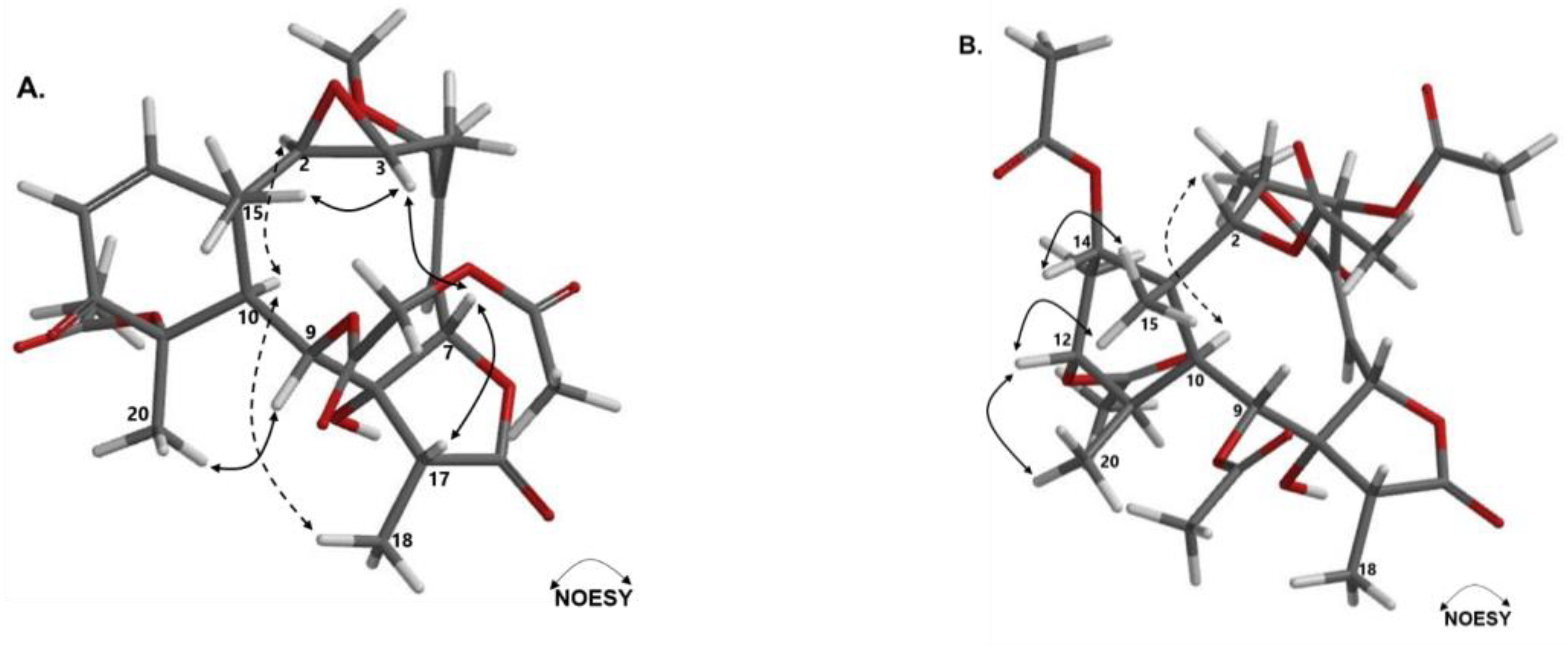
2.2. Chemical Characterization of E. caribaeorum Chemotypes
2.3. Cytotoxic Activity of Erythrolides
3. Materials and Methods
3.1. General
3.2. Animal Material
3.3. Extraction and Fractionation of E. caribaeorum Samples
3.4. LC-MS Analysis
3.5. Preprocessing of LC-MS Data
3.6. Isolation and Identification of Erythrolides and Eleutherobins
3.7. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef]
- Fenical, W.; Pawlik, J. Defensive properties of secondary metabolites from the Caribbean gorgonian coral Erythropodium caribaeorum. Mar. Ecol. Prog. Ser. 1991, 75, 1–8. [Google Scholar] [CrossRef]
- Long, B.H.; Carboni, J.M.; Wasserman, A.J.; Cornell, L.A.; Casazza, A.M.; Jensen, P.R.; Lindel, T.; Fenical, W.; Fairchild, C.R. Eleutherobin, a novel cytotoxic agent that induces tubulin polymerization, is similar to paclitaxel (Taxol). Cancer Res. 1998, 58, 1111–1115. [Google Scholar] [PubMed]
- Look, S.A.; Fenical, W.; Van Engen, D.; Clardy, J. ChemInform Abstract: Erythrolides: Unique Marine Diterpenoids Interrelated by a Naturally Occurring Di-Π-Methane Rearrangement. Chem. Inf. 1984, 15, 5026–5027. [Google Scholar] [CrossRef]
- Dookran, R.; Maharaj, D.; Mootoo, B.S.; Ramsewak, R.; McLean, S.; Reynolds, W.F.; Tinto, W.F. Diterpenes from the Gorgonian Coral Erythropodium caribaeorum from the Southern Caribbean. J. Nat. Prod. 1993, 56, 1051–1056. [Google Scholar] [CrossRef]
- Maharaj, D.; Pascoe, K.O.; Tinto, W.F. Briarane direrpenes from the gorgonian octocoral Erythropodium caribaeorum from the northern Caribbean. J. Nat. Prod. 1999, 62, 313–314. [Google Scholar] [CrossRef]
- Craig, K.S.; Rebérioux, D.; Roberge, M.; Andersen, R.J.; Taglialatela-Scafati, O.; Taglialatela-Scafati, O. Briarane, Erythrane, and Aquariane Diterpenoids from the Caribbean GorgonianErythropodium caribaeorum. Eur. J. Org. Chem. 2003, 2003, 3515–3523. [Google Scholar]
- Taglialatela-Scafati, O.; Deo-Jangra, U.; Campbell, M.; Roberge, M.; Andersen, R.J. Diterpenoids from cultured Erythropodium caribaeorum. Org. Lett. 2002, 4, 4085–4087. [Google Scholar] [CrossRef]
- Puyana, M.; Narvaez, G.; Paz, A.; Osorno, O.; Duque, C. Pseudopterosin content variability of the purple sea whip Pseudopterogorgia elisabethae at the islands of San Andres and Providencia (SW Caribbean). J. Chem. Ecol. 2004, 30, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Sansinenea, E.; Ortiz, A. Antimycobacterial Natural Products from Marine Pseudopterogorgia elisabethae. Curr. Org. Synth. 2015, 13, 556–568. [Google Scholar] [CrossRef]
- Duque, C.; Puyana, M.; Narvaez, G.; Osorno, O.; Hara, N.; Fujimoto, Y. Pseudopterosins P–V, new compounds from the gorgonian octocoral Pseudopterogorgia elisabethae from Providencia island, Colombian Caribbean. Tetrahedron 2004, 60, 10627–10635. [Google Scholar] [CrossRef]
- Correa, H.; Haltli, B.; Duque, C.; Kerr, R.; Haltli, B. Bacterial Communities of the Gorgonian Octocoral Pseudopterogorgia elisabethae. Microb. Ecol. 2013, 66, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Berrue, F.; Withers, S.T.; Haltli, B.; Withers, J.; Kerr, R.G. Chemical Screening Method for the Rapid Identification of Microbial Sources of Marine Invertebrate-Associated Metabolites. Mar. Drugs 2011, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.; Amaya-García, F.; Tello, E.; Ramos, F.A.; Umaña, A.; Puyana, M.; Resende, J.A.L.C.; Castellanos, L. Screening of acetylcholinesterase inhibitors in marine organisms from the Caribbean Sea. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- García, F.A.; Nuñez, M.L.S.; Ramos, F.A.; Puyana, M.; Paixão, I.C.N.D.P.; Teixeira, V.L.; Castellanos, L. Dolabellane diterpenes from the Caribbean soft corals Eunicea laciniata and Eunicea asperula and determination of their anti HSV-1 activity. Rev. Colomb. Química 2017, 46, 5. [Google Scholar] [CrossRef]
- Instituto de Investigaciones Marinos y Costeras “José Benito Vives de Andréis” Siam. Available online: http://siam.invemar.org.co/sibm-busqueda-avanzada (accessed on January 25 2019).
- Mushtaq, M.Y.; Choi, Y.H.; Verpoorte, R.; Wilson, E.G. Extraction for Metabolomics: Access to the Metabolome. Phytochem. Anal. 2014, 25, 291–306. [Google Scholar] [CrossRef]
- MacIntyre, L.; Zhang, T.; Viegelmann, C.; Martinez, I.J.; Cheng, C.; Dowdells, C.; Abdelmohsen, U.R.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R. Metabolomic Tools for Secondary Metabolite Discovery from Marine Microbial Symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar] [CrossRef]
- He, Q.; Sun, R.; Liu, H.; Geng, Z.; Chen, D.; Li, Y.; Han, J.; Lin, W.; Du, S.; Deng, Z. NMR-Based Metabolomic Analysis of Spatial Variation in Soft Corals. Mar. Drugs 2014, 12, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Al-Hammady, M.A.; Hegazy, M.-E.F.; Meyer, A.; Mohamed, T.A.; Westphal, H.; Wessjohann, L.A. Soft Corals Biodiversity in the Egyptian Red Sea: A Comparative MS and NMR Metabolomics Approach of Wild and Aquarium Grown Species. J. Proteome Res. 2016, 15, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Pordesimo, E.O.; Schmitz, F.J.; Ciereszko, L.S.; Hossain, M.B.; Van Der Helm, D. New briarein diterpenes from the Caribbean gorgonians Erythropodium caribaeorum and Briareum sp. J. Org. Chem. 1991, 56, 2344–2357. [Google Scholar] [CrossRef]
- Banjoo, D.; Mootoo, B.S.; Ramsewak, R.S.; Sharma, R.; Lough, A.J.; McLean, S.; Reynolds, W.F. New Erythrolides from the Caribbean Gorgonian Octocoral Erythropodium caribaeorum. J. Nat. Prod. 2002, 65, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Cinel, B.; Roberge, M.; Behrisch, H.; van Ofwegen, L.; Castro, C.B.; Andersen, R.J. Antimitotic Diterpenes from Erythropodium c aribaeorum Test Pharmacophore Models for Microtubule Stabilization. Org. Lett. 2000, 2, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.D. The natural products chemistry of West Indian gorgonian octocorals. Tetrahedron 1995, 51, 4571–4618. [Google Scholar] [CrossRef]
- Su, Y.-D.; Su, J.-H.; Hwang, T.-L.; Wen, Z.-H.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarane Diterpenoids Isolated from Octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef]
- Moon, N.G.; Harned, A.M. Synthetic explorations of the briarane jungle: Progress in developing a synthetic route to a common family of diterpenoid natural products. R. Soc. Open Sci. 2018, 5, 172280. [Google Scholar] [CrossRef]
- Bathe, U.; Tissier, A. Cytochrome P450 enzymes: A driving force of plant diterpene diversity. Phytochemistry 2019, 161, 149–162. [Google Scholar] [CrossRef]
- Hall, A.; Roche, S.; West, L. Synthesis of Briarane Diterpenoids: Biomimetic Transannular Oxa-6π electrocyclization Induced by a UVA/UVC Photoswitch. Org. Lett. 2017, 19, 576–579. [Google Scholar] [CrossRef]
- Lindel, T.; Jensen, P.R.; Fenical, W.; Long, B.H.; Casazza, A.M.; Carboni, J.; Fairchild, C.R. Eleutherobin, a New Cytotoxin that Mimics Paclitaxel (Taxol) by Stabilizing Microtubules. J. Am. Chem. Soc. 1997, 119, 8744–8745. [Google Scholar] [CrossRef]
- Bloor, S.J.; Schmitz, F.J.; Hossain, M.B.; Van der Helm, D. Diterpenoids from the Gorgonian Solenopodium stechei. J. Org. Chem. 1992, 57, 1205–1216. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Sung, P.-J.; Huang, L.-H.; Lee, S.-F.; Wu, T.; Chang, B.-Y.; Duh, C.-Y.; Fang, L.-S.; Soong, K.; Lee, T.-J. New Cytotoxic Briaran Diterpenes from the Formosan Gorgonian Briareum sp. J. Nat. Prod. 1996, 59, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Sheu, J.-H.; Cheng, M.-C.; Duh, C.-Y.; Fang, L.-S.; Sung, P.-J.; Chiang, M.Y. Novel Cytotoxic Diterpenes, Excavatolides A–E, Isolated from the Formosan Gorgonian Briareum excavatum. J. Nat. Prod. 2002, 61, 602–608. [Google Scholar]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Castillo, S.; Gopalacharyulu, P.; Yetukuri, L.; Orešič, M.; Peddinti, G. Algorithms and tools for the preprocessing of LC–MS metabolomics data. Chemom. Intell. Lab. Syst. 2011, 108, 23–32. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Eli Lilly & Company: Indianapolis, IN, USA; National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004.
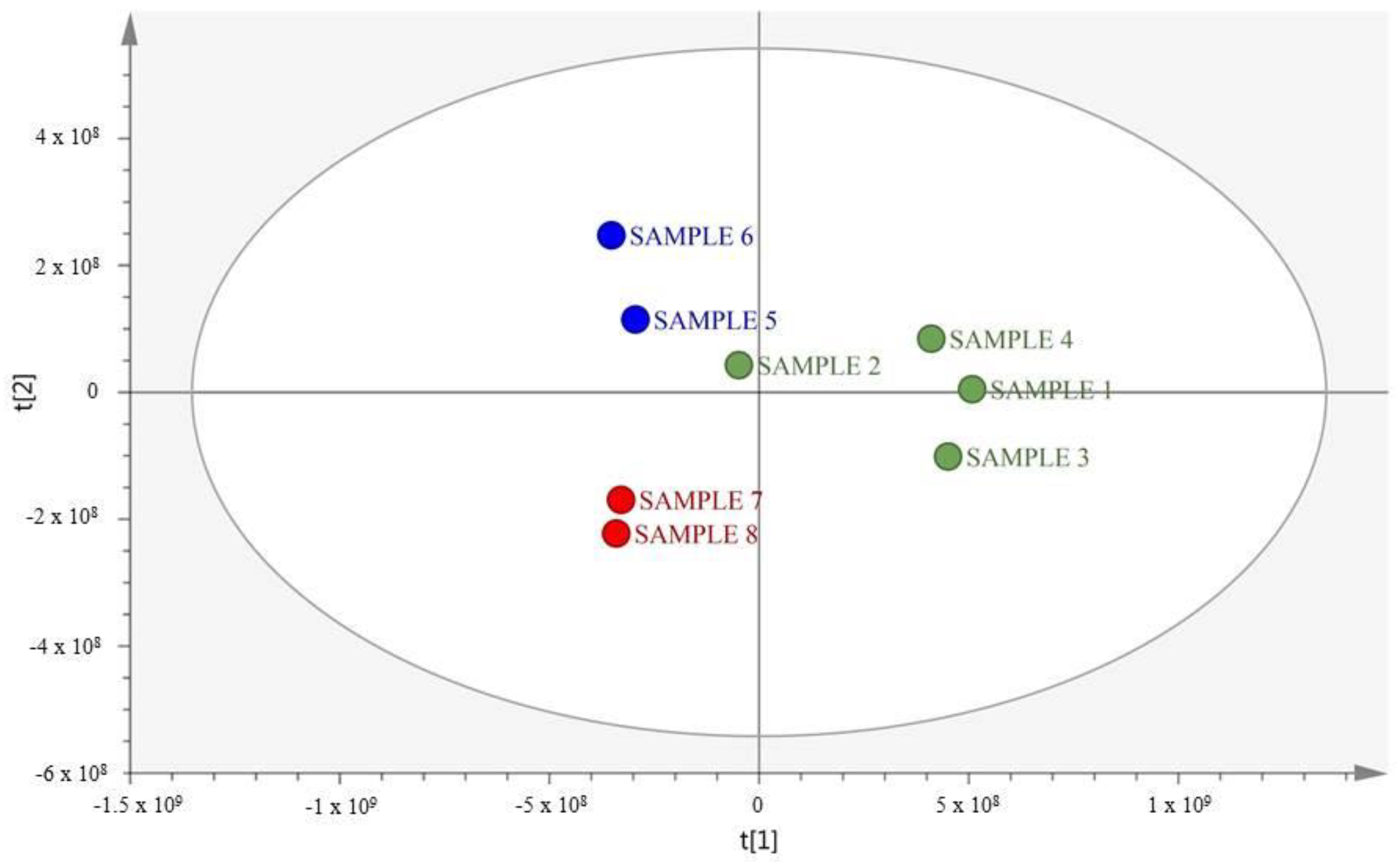
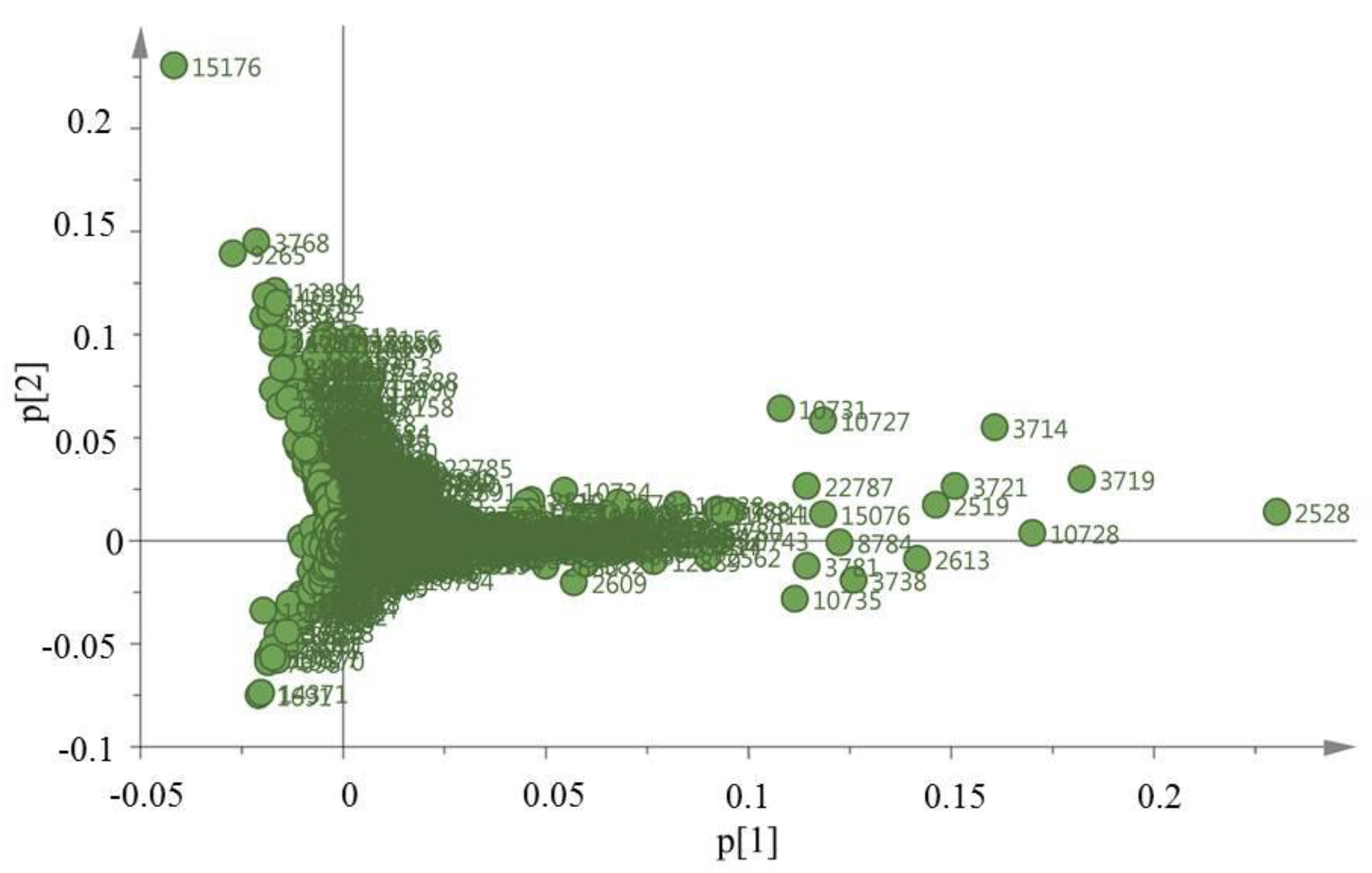
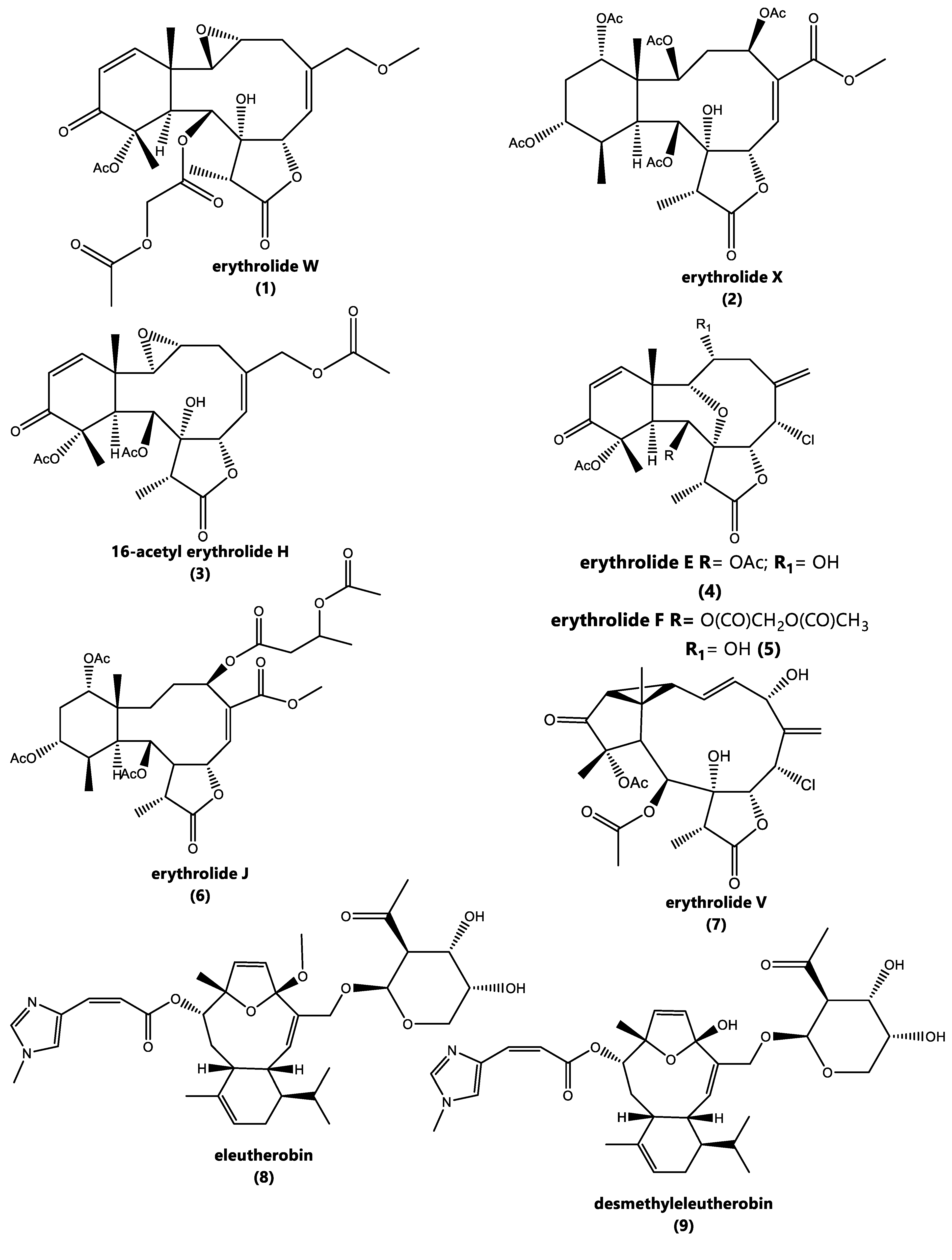

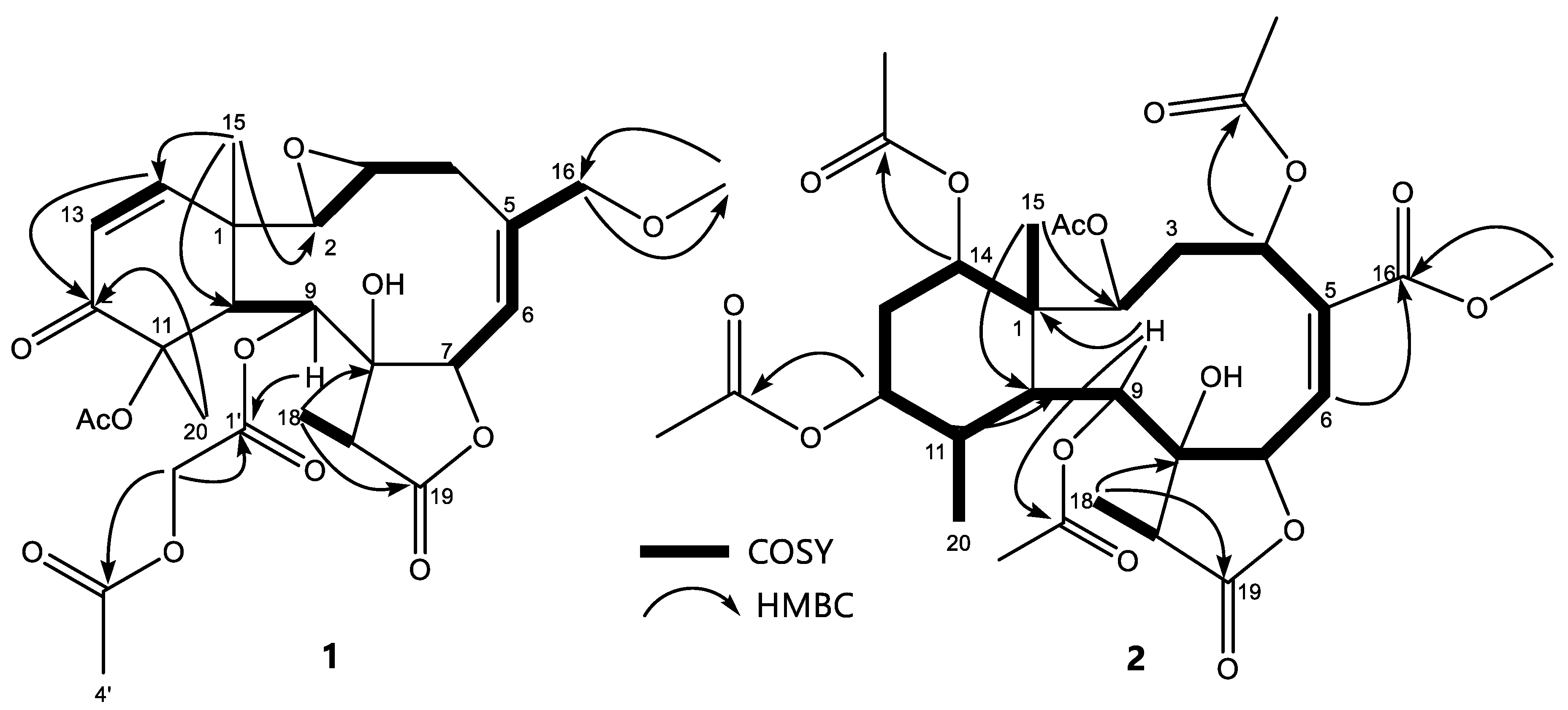
| N° | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| δH integr, mult, (J in Hz) | δC mult. | δH integr, mult, (J in Hz) | δC mult. | |
| 1 | 41.0, C | 45.6, C | ||
| 2 | 2.77, 1H, s | 63.7, CH | 4.90, 1H, bs | 76.0, CH |
| 3a | 3.51, 1H, d, (5.6) | 57.2, CH | 3.00. 1H, m | 37.0, CH2 |
| 3b | 2.12, 1H, m | |||
| 4a | 2.89, 1H, dd, (16.0, 6.5) | 28.6, CH2 | 5.81, 1H, dd, (11.8, 6.1) | 68.3, CH |
| 4b | 2.23, 1H, m | |||
| 5 | 145.4, C | 138.3, C | ||
| 6 | 5.54, 1H, d, (10.1) | 119.4, CH | 6.81, 1H, d, (10.1) | 138.0, CH |
| 7 | 5.14, 1H, d, (10.1) | 77.9, CH | 5.61, 1H, d, (10.1) | 76.0, CH |
| 8 | 81.5, C | 82.8, C | ||
| 9 | 5.79, 1H, bs | 70.4, CH | 5.33, 1H, d, (1.2) | 75.7, CH |
| 10 | 3.59, 1H, bs | 41.2, CH | 2.91, 1H, d, (2.6) | 32.5, CH |
| 11 | 81.6, C | 2.56, 1H, m | 43.5, CH | |
| 12 | 194.8, C | 5.26, 1H, dd, (12.5, 6.6) | 67.0, CH | |
| 13a | 6.00, 1H, d, (10.2) | 124.1, CH | 2.64, 1H, dd, (15.2, 7.5) | 41.4, CH2 |
| 13b | 2.52, 1H, dd, (15.2, 6.3) | |||
| 14 | 6.67, 1H, d, (10.2) | 153.6, CH | 4.85, 1H, d, (8.2) | 73.0, CH |
| 15 | 0.94, 3H, s | 14.4, CH3 | 1.15, 3H, s | 14.9, CH3 |
| 16 | 3.76, 2H, bs | 76.1, CH2 | 168.0, C | |
| 17 | 2.53, 1H, q, (6.5) | 43.6, CH | 2.56, 1H, m | 43.5, CH |
| 18 | 1.24, 3H, d, (6.5) | 6.9, CH3 | 1.28, 3H, d, (6.4) | 6.4, CH3 |
| 19 | 175.8, C | 175.5, C | ||
| 20 | 1.48, 3H, s | 21.3, CH3 | 1.30, 3H, d, (7.1) | 20.1, CH3 |
| 1’ | 166.8, C | |||
| 2’ | 4.64, 2H, bs | 61.2, CH2 | ||
| 3’ | 170.4, C | |||
| 4’ | 2.21, 3H, s | 20.3, CH3 | ||
| AcO-2 | 170.8, C | |||
| 1.99, 3H, s | 20.1, CH3 | |||
| AcO-4 | 169.9, C | |||
| 2.01, 3H, s | 21.4, CH3 | |||
| AcO-9 | 169.2, C | |||
| 2.24, 3H, s | 21.6, CH3 | |||
| AcO-11 | 170.6, C | |||
| 2.05, 3H, s | 21.5, CH3 | |||
| AcO-12 | 170.3, C | |||
| 1.95, 3H, s | 20.7, CH3 | |||
| AcO-14 | 170.4, C | |||
| 1.98, 3H, s | 21.1, CH3 | |||
| OMe-16 | 3.34, 3H, s | 58.7, CH3 | 3.84, 3H, s | 53.0, CH3 |
| Ery A | Ery B | Ery C | Ery D | Ery E | Ery F | Ery G | Ery H | Ery I | Ery J | Ery K | Ery L | Ery M | Ery N | Ery O | Ery P | Ery Q | Ery R | Ery S | Ery T | Ery U | Ery V | Ery W | Ery X | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jamaica [8,24] | ||||||||||||||||||||||||
| Belize [6] | ||||||||||||||||||||||||
| Bahamas [4] | ||||||||||||||||||||||||
| Tobago [25,7] | ||||||||||||||||||||||||
| Dominica [9] | ||||||||||||||||||||||||
| Santa Marta | ||||||||||||||||||||||||
| Islas del Rosario | ||||||||||||||||||||||||
| Providencia |
| ID | IC50 A549 (µM) | IC50 MCF7 (µM) | IC50 PC3 (µM) |
|---|---|---|---|
| erythrolide A | 18.41 ± 3.15 | 6.77 ± 0.98 | 2.45 ± 0.38 |
| erythrolide B | 27.09 ± 4.26 | 15.21 ± 2.31 | 6.46 ± 0.96 |
| erythrolide D | 2.58 ± 0.72 | 42.45 ± 4.34 | 60.00 ± 5.55 |
| erythrolide E | 148.22 ± 12.27 | 73.87 ± 5.03 | 189.11 ± 14.93 |
| erythrolide F | 46.49 ± 7.93 | 116.44 ± 12.97 | >120 |
| erythrolide I | >120 | >120 | >120 |
| erythrolide J | 37.93 ± 3.91 | 56.06 ± 3.88 | 42.49 ± 3.53 |
| erythrolide R | 75.28 ± 18.31 | >120 | >120 |
| erythrolide U | 36.65 ± 5.84 | 124.79 ± 15.01 | >120 |
| erythrolide V | 101.72 ± 20.72 | >120 | >120 |
| erythrolide W | >120 | >120 | >120 |
| erythrolide X | >120 | >120 | >120 |
| 16-acetyl erythrolide H | >120 | 113.11 ± 16.04 | >120 |
| # SAMPLE | LOCATION | DEPTH (m) | DATE | VOUCHER NUMBER |
|---|---|---|---|---|
| 1 | Providencia Island (Santa Catalina Cay) | 6 | 16 July 2012 | CO 0109 |
| 2 | Providencia Island (Lawrence Reef) | 1.5 | 28 February 2013 | CO 0110 |
| 3 | Providencia Island (Lawrence Reef) | 1.5 | 28 February 2013 | CO 0111 |
| 4 | Providencia Island (Convento) | 7.5 | 16 July 2012 | CO 0112 |
| 5 | Santa Marta (Punta Venado) | 4.5 | 10 December 2011 | CO 0113 |
| 6 | Santa Marta (Punta Venado) | 6 | 06 September 2010 | CO 0114 |
| 7 | Islas del Rosario (Botellas, Isla Grande) | 4.5 | 20 September 2012 | CO 0115 |
| 8 | Islas del Rosario (Pavitos) | 17 | 22 September 2012 | CO 0116 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, S.L.; Forero, A.M.; Ayala, F.I.; Puyana, M.; Zea, S.; Castellanos, L.; Muñoz, D.; Arboleda, G.; Sandoval-Hernández, A.G.; Ramos, F.A. Metabolic Profiling of the Soft Coral Erythropodium caribaeorum (Alcyonacea: Anthothelidae) from the Colombian Caribbean Reveals Different Chemotypes. Mar. Drugs 2020, 18, 4. https://doi.org/10.3390/md18010004
Molina SL, Forero AM, Ayala FI, Puyana M, Zea S, Castellanos L, Muñoz D, Arboleda G, Sandoval-Hernández AG, Ramos FA. Metabolic Profiling of the Soft Coral Erythropodium caribaeorum (Alcyonacea: Anthothelidae) from the Colombian Caribbean Reveals Different Chemotypes. Marine Drugs. 2020; 18(1):4. https://doi.org/10.3390/md18010004
Chicago/Turabian StyleMolina, Sandra L., Abel M. Forero, Farja I. Ayala, Mónica Puyana, Sven Zea, Leonardo Castellanos, Diego Muñoz, Gonzalo Arboleda, Adrián G. Sandoval-Hernández, and Freddy A. Ramos. 2020. "Metabolic Profiling of the Soft Coral Erythropodium caribaeorum (Alcyonacea: Anthothelidae) from the Colombian Caribbean Reveals Different Chemotypes" Marine Drugs 18, no. 1: 4. https://doi.org/10.3390/md18010004
APA StyleMolina, S. L., Forero, A. M., Ayala, F. I., Puyana, M., Zea, S., Castellanos, L., Muñoz, D., Arboleda, G., Sandoval-Hernández, A. G., & Ramos, F. A. (2020). Metabolic Profiling of the Soft Coral Erythropodium caribaeorum (Alcyonacea: Anthothelidae) from the Colombian Caribbean Reveals Different Chemotypes. Marine Drugs, 18(1), 4. https://doi.org/10.3390/md18010004







