Epigenetic Modification and Differentiation Induction of Malignant Glioma Cells by Oligo-Fucoidan
Abstract
1. Introduction
2. Results
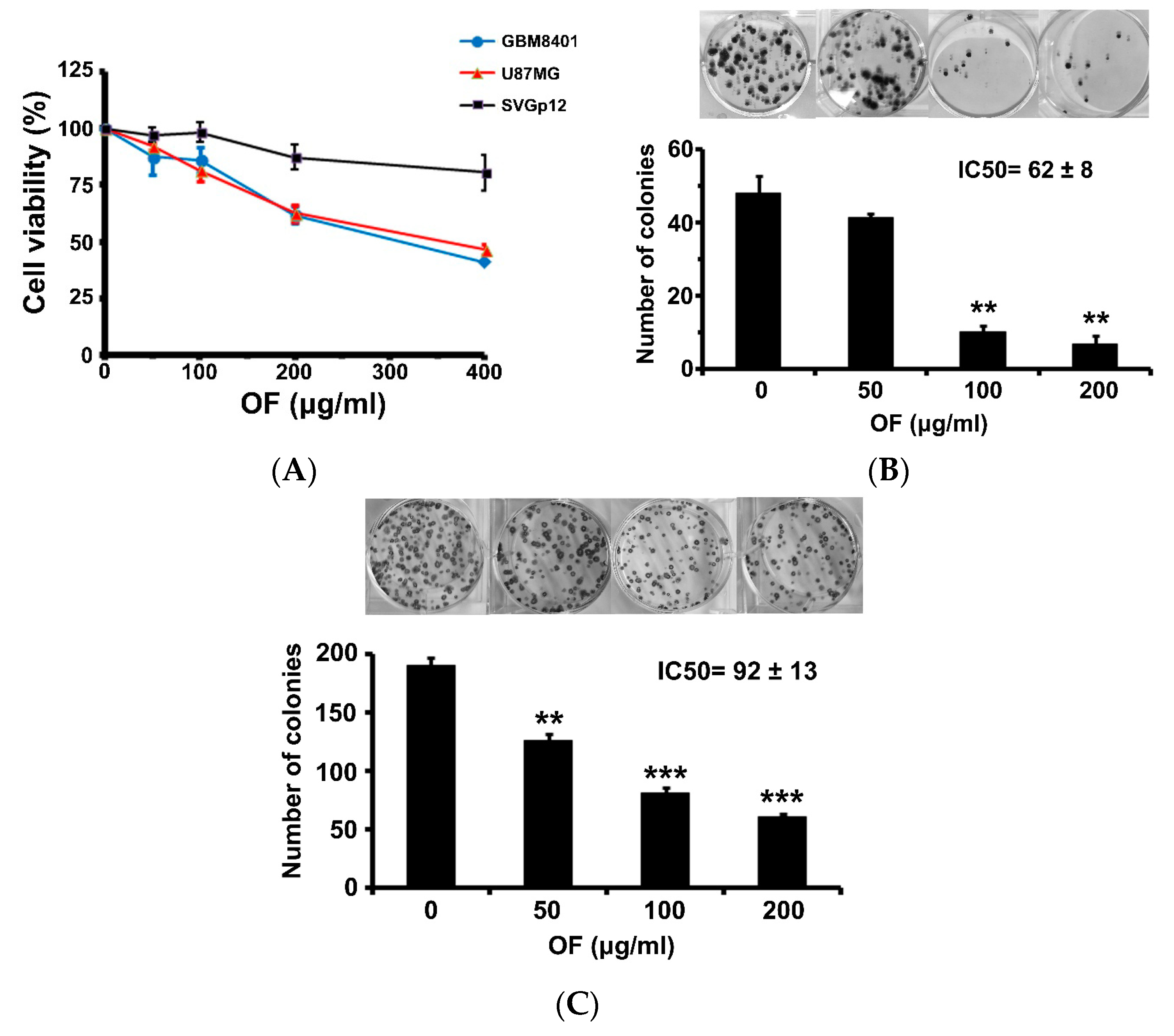
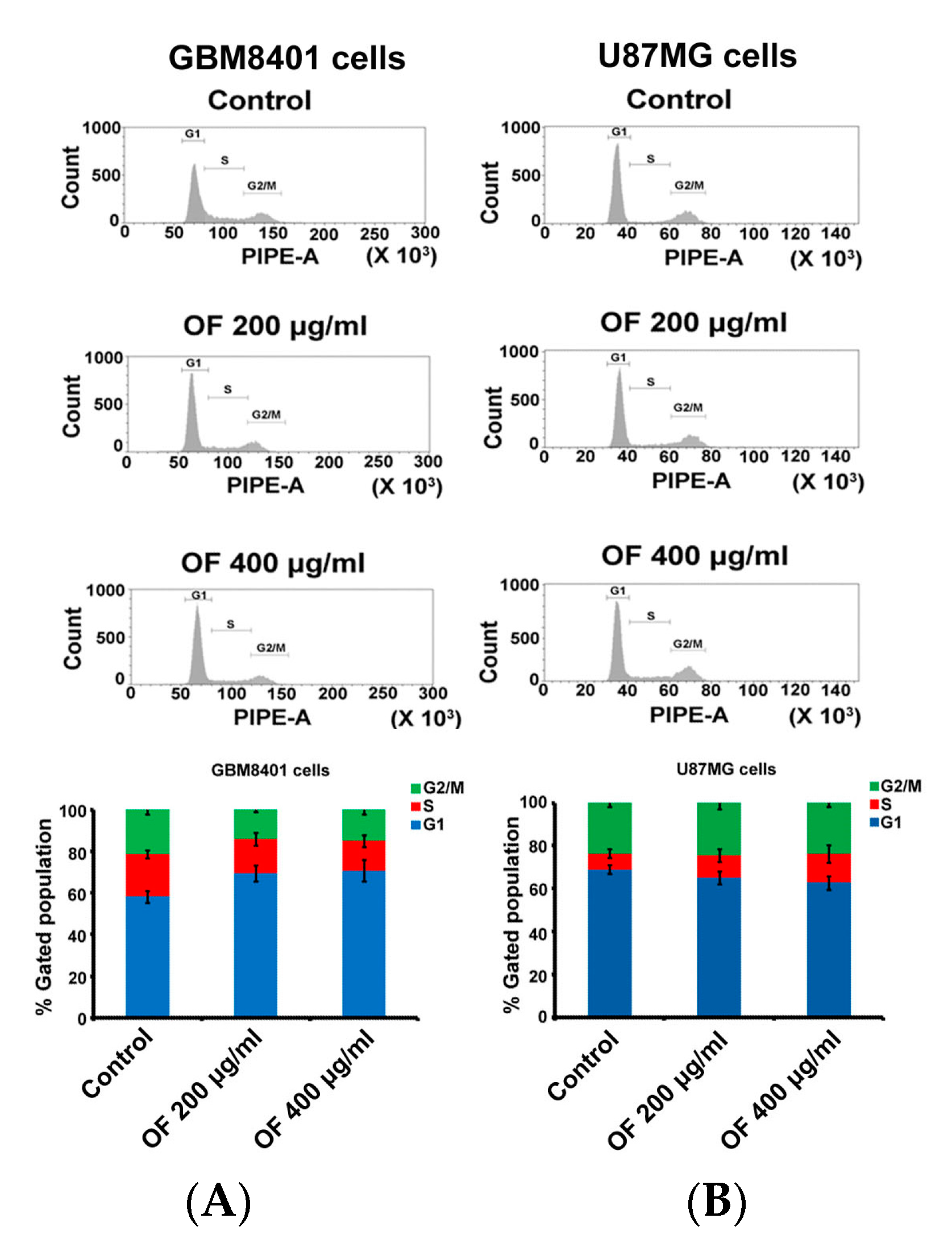
2.1. Oligo-Fucoidan Inhibits Proliferation and Clonogenicity, and Arrests Cell Cycle in Human Malignant Glioma Cells
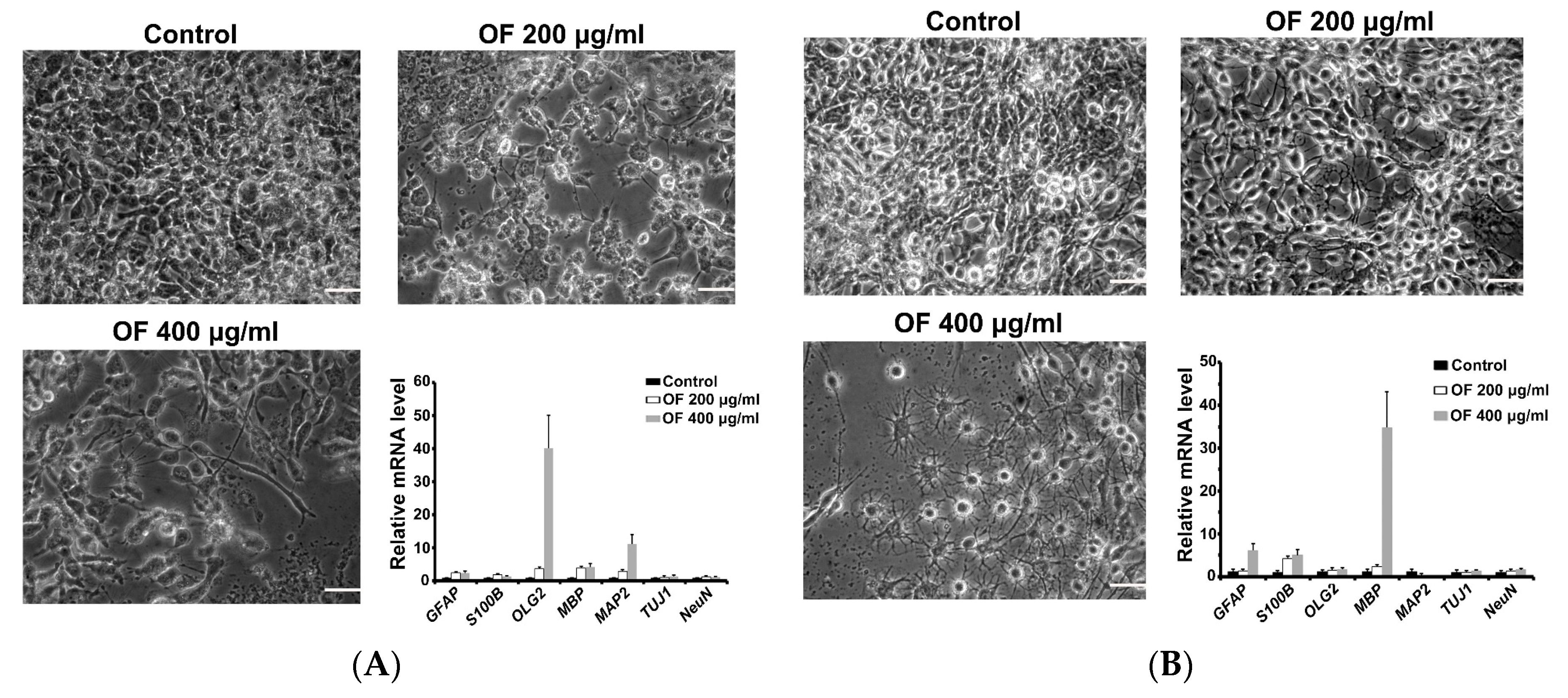
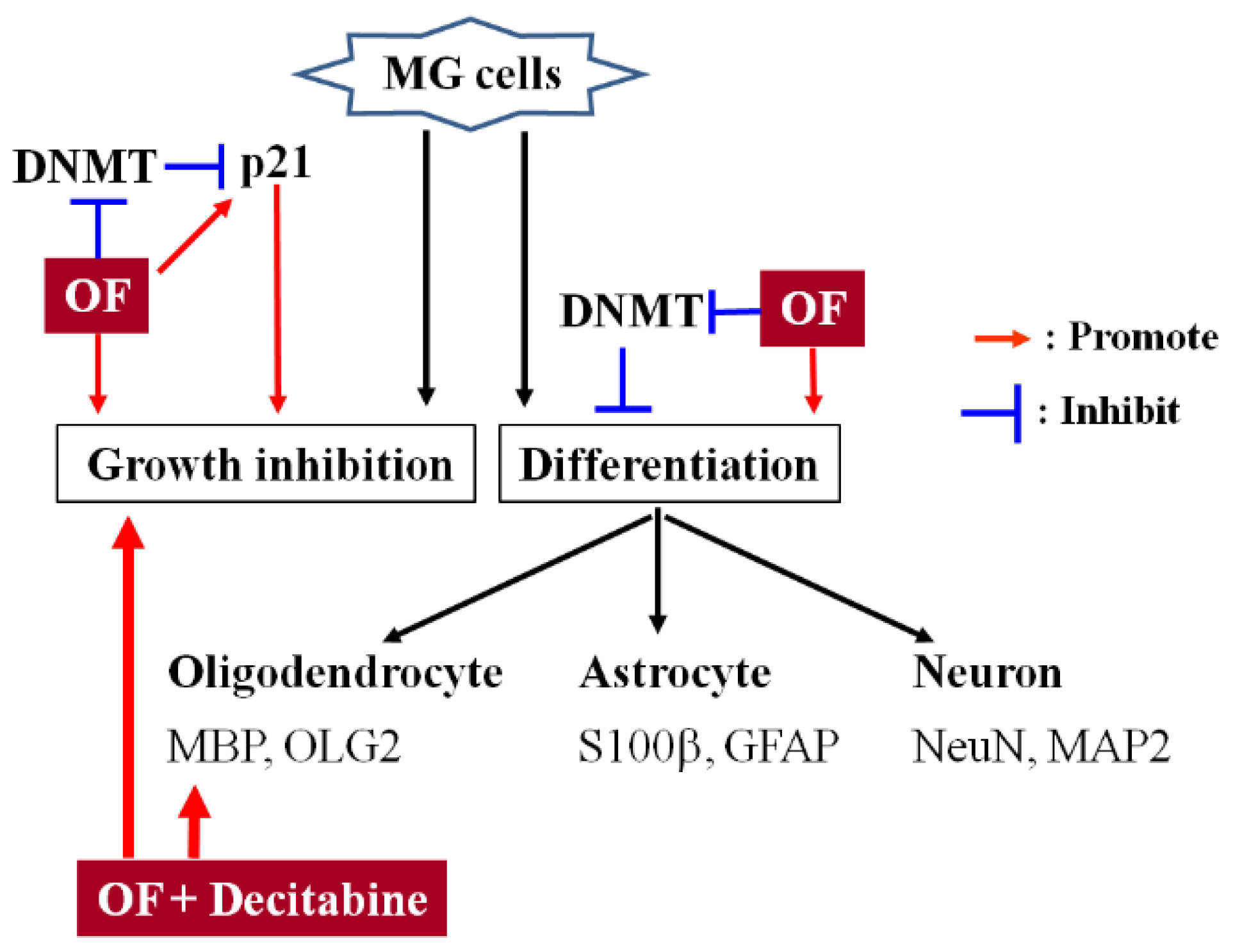
2.2. Oligo-Fucoidan Induces Differentiation of Malignant Glioma Cells
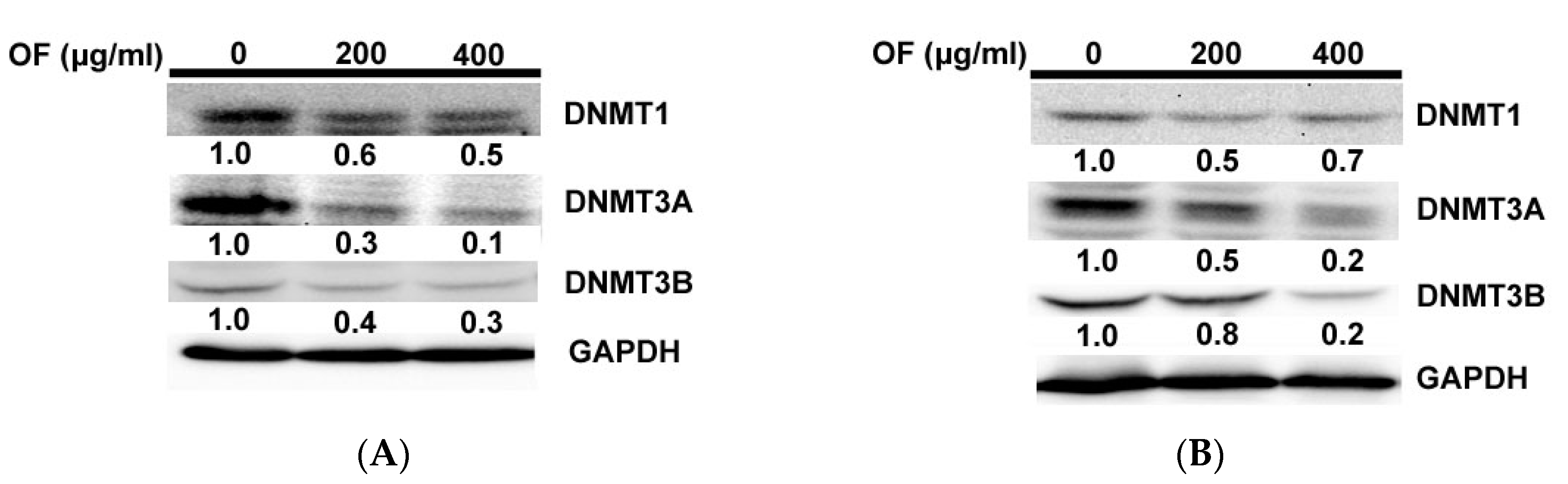
2.3. Oligo-Fucoidan Inhibits DNA Methyltransferases (DNMTs) in Human Malignant Glioma Cells
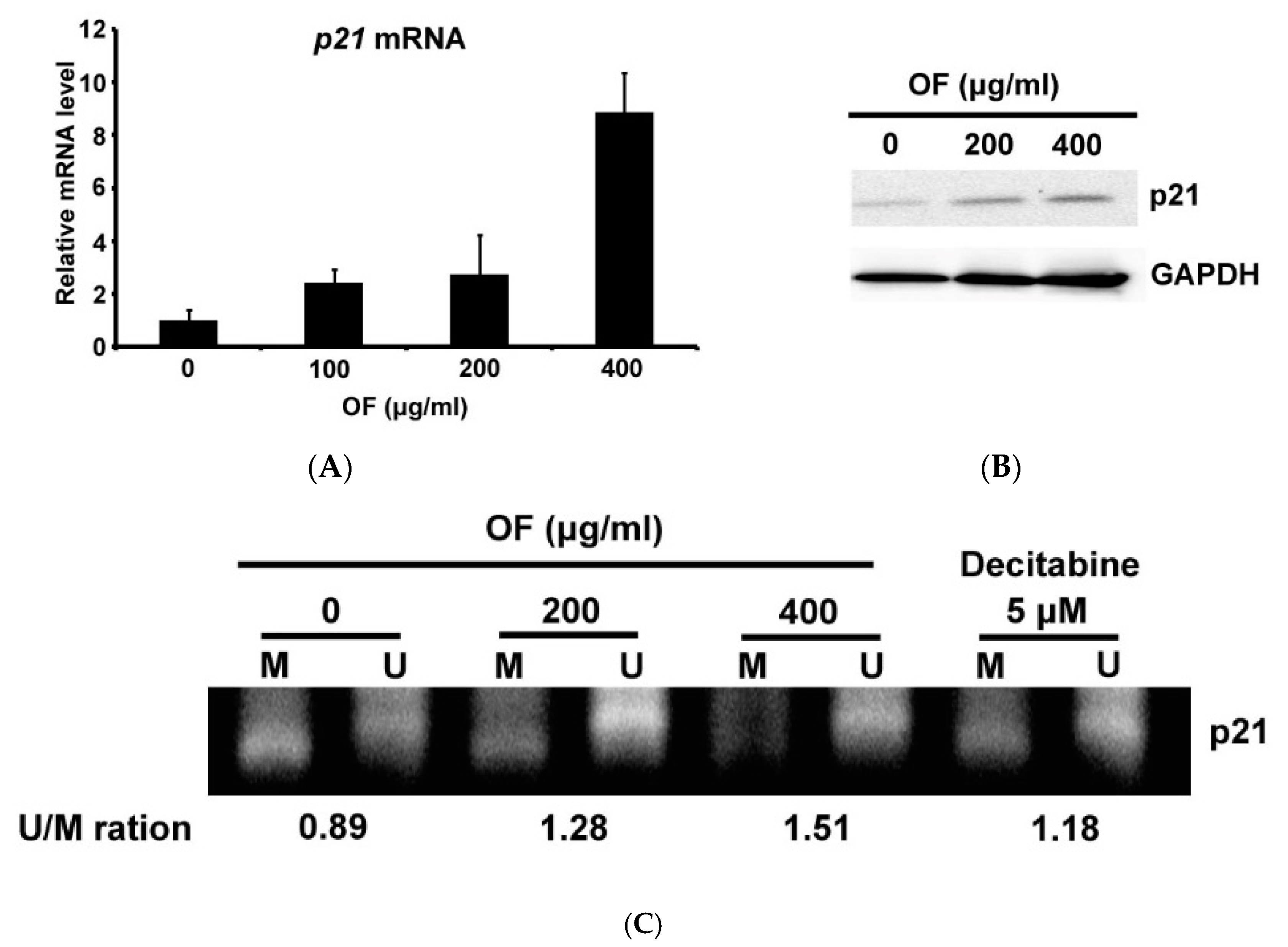
2.4. Oligo-Fucoidan Decreases the Methylation of p21 Gene Accompanied with Induction of Its Expression in Human Malignant Glioma Cells
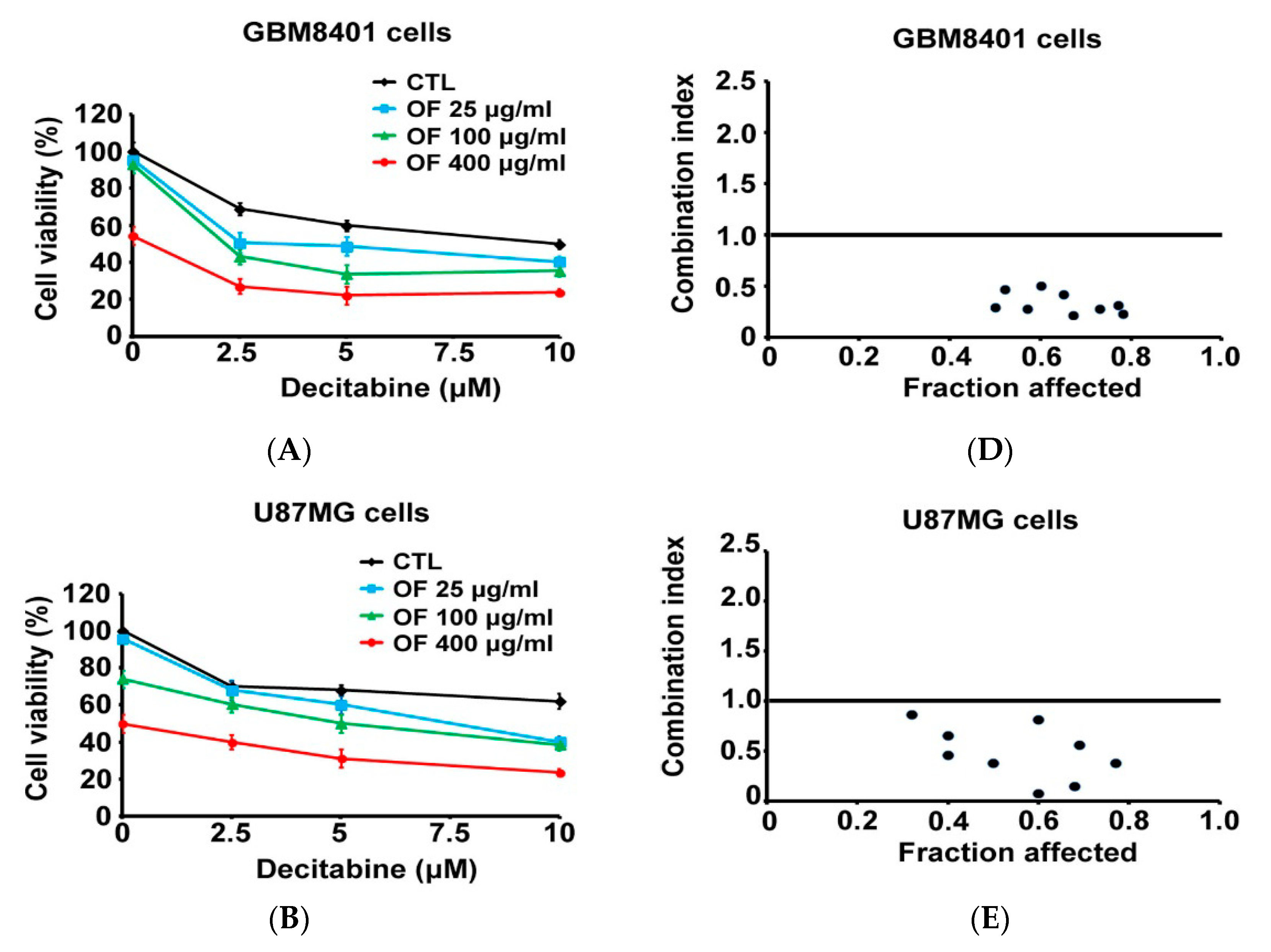
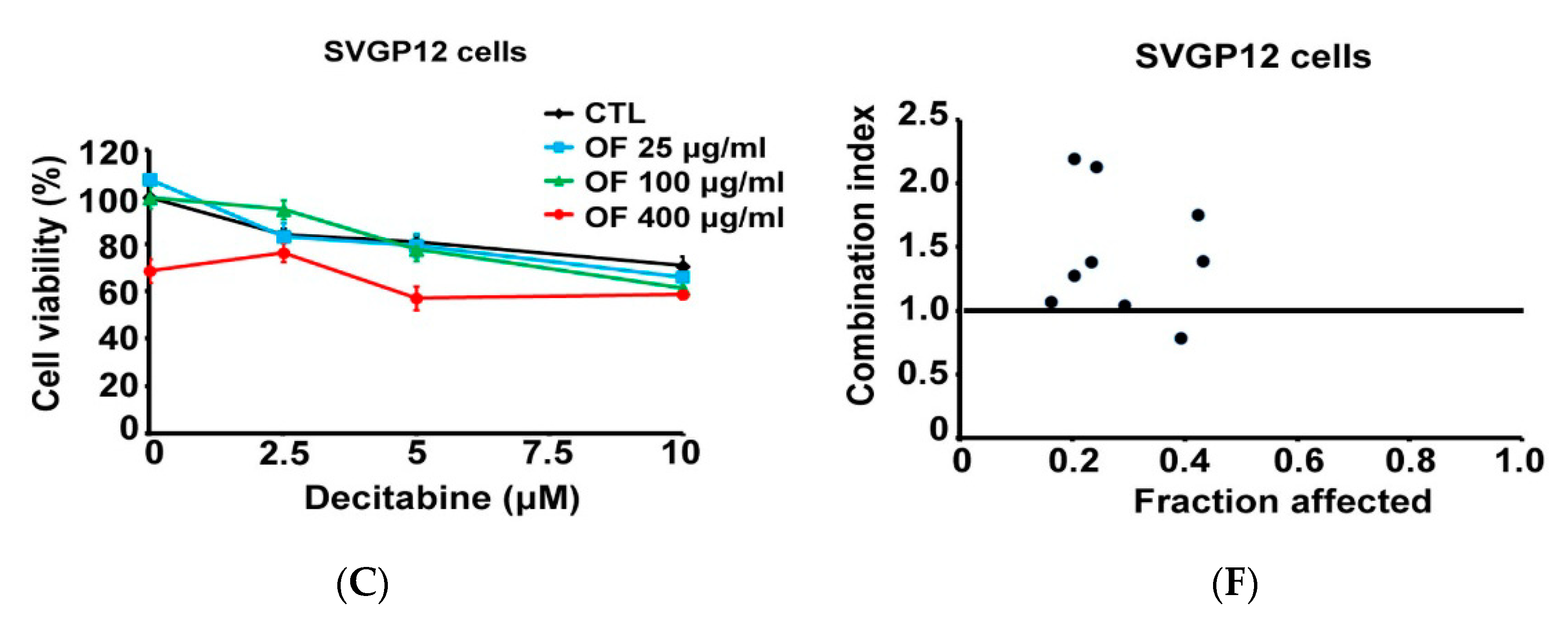
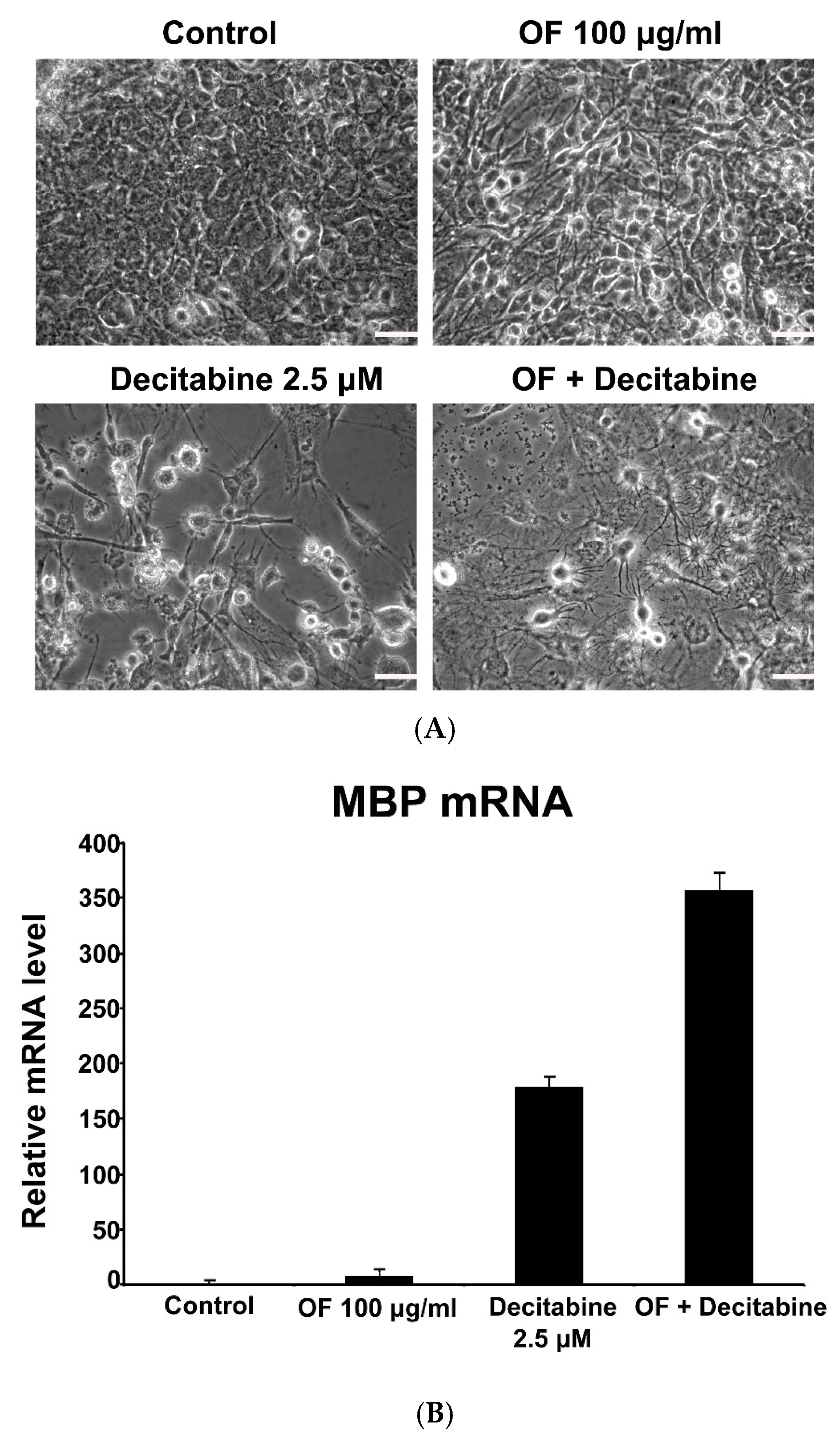
2.5. Combination with Oligo-Fucoidan (OF) Enhances Decitabine-Mediated Growth Inhibition and Differentiation Induction in MG Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cellular Viability
4.4. Clonogenicity
4.5. Photograph of the Cells
4.6. Cell-Cycle Distribution Analysis
4.7. Quantitative RT-PCR
4.8. Western Blotting
4.9. DNA Isolation and Sodium Bisulfite Conversion
4.10. Methylation Specific PCR (MSP)
4.11. Synergistic Combination Effect
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Wangenheim, K.H.; Peterson, H.P. The role of cell differentiation in controlling cell multiplication and cancer. J. Cancer Res. Clin. Oncol. 2008, 134, 725–741. [Google Scholar] [CrossRef]
- Capp, J.P. Stochastic gene expression, disruption of tissue averaging effects and cancer as a disease of development. Bioessays 2005, 27, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Harris, P.J.; Warren, R.Q.; Ivy, S.P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 2011, 8, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sell, S. Stem cell origin of cancer and differentiation therapy. Crit. Rev. Oncol. Hematol. 2004, 51, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Degos, L. Differentiating agents in the treatment of leukemia. Leuk. Res. 1990, 14, 717–719. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Schwartzbaum, J.A.; Fisher, J.L.; Aldape, K.D.; Wrensch, M. Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2006, 2, 494–503. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Alderton, G.K. Tumorigenesis: The origins of glioma. Nat. Rev. Cancer 2011, 11, 627. [Google Scholar]
- Friedmann-Morvinski, D.; et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 2012, 338, 1080–1084. [Google Scholar] [CrossRef]
- Lee, D.Y.; Gianino, S.M.; Gutmann, D.H. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell 2012, 22, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, S.G.; Reynolds, B.A.; Zanetti, N.; Lamorte, G.; Binda, E.; Broggi, G.; Brem, H.; Olivi, A.; Dimeco, F.; Vescovi, A.L. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 2006, 444, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Chen, C.L.; Lee, Y.C.; Kao, T.J.; Chen, K.Y.; Fang, C.Y.; Chang, W.C.; Chiang, Y.H.; Huang, C.C. Znf179 induces differentiation and growth arrest of human primary glioblastoma multiforme in a p53-dependent cell cycle pathway. Sci. Rep. 2017, 7, 4787. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Choi, S.W.; Yang, S.R.; Shin, T.H.; Kim, H.S.; Yu, K.R.; Hong, I.S.; Ro, S.; Cho, J.M.; Kang, K.S. Growth arrest and forced differentiation of human primary glioblastoma multiforme by a novel small molecule. Sci. Rep. 2014, 4, 5546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alcazar, O.; Achberger, S.; Aldrich, W.; Hu, Z.; Negrotto, S.; Saunthararajah, Y.; Triozzi, P. Epigenetic regulation by decitabine of melanoma differentiation in vitro and in vivo. Int. J. Cancer 2012. Int. J. Cancer 2012, 131, 18–29. [Google Scholar] [CrossRef]
- Jones, P.A.; Taylor, M.S. Cellular differentiation, cytidine analogs and DNA methylation. Cell 1980, 20, 85–93. [Google Scholar] [CrossRef]
- Steensma, D.P. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J. 2018, 8, 47. [Google Scholar] [CrossRef]
- Bohl, S.R.; Bullinger, L.; Rucker, F.G. Epigenetic therapy: Azacytidine and decitabine in acute myeloid leukemia. Expert. Rev. Hematol. 2018, 11, 361–371. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef]
- Kwak, J.Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, S.Y.; Cho, H.; Yoo, E.S.; Kang, H.K. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother. Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, Y.; Teruya, K.; Katakura, Y.; Ichikawa, A.; Eto, H.; Hosoi, M.; Hosoi, M.; Nishimoto, S.; Shirahata, S. Enzyme-digested Fucoidan Extracts Derived from Seaweed Mozuku of Cladosiphon novae-caledoniae kylin Inhibit Invasion and Angiogenesis of Tumor Cells. Cytotechnology 2005, 47, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.O.; Park, Y.S.; Lee, J.H.; Seo, J.B.; Koo, K.I.; Jeong, S.C.; Jin, S.D.; Lee, Y.H.; Eom, H.S.; Yun, I. Effect of extracts from safflower seeds on osteoblast differentiation and intracellular calcium ion concentration in MC3T3-E1 cells. Nat. Prod. Res. 2007, 21, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, K.W.; Lim, D.S.; Lee, S. The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem. Cells Dev. 2012, 21, 2204–2211. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee., J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef]
- Hwang, P.A.; Yan, M.D.; Lin, H.T.; Li, K.L.; Lin, Y.C. Toxicological Evaluation of Low Molecular Weight Fucoidan in Vitro and in Vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, T.Y.; Hwang, P.A.; Tseng, L.M.; Chen, R.H.; Tsao, S.M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFbeta receptor degradation in breast cancer. Carcinogenesis 2013, 34, 874–884. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, T.Y.; Wu, Y.C.; Tsao, S.M.; Hwang, P.A.; Shih, Y.W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurf2-dependent ubiquitin proteasome pathway in TGFbeta receptor degradation. Oncotarget 2014, 5, 7870–7885. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.D.; Yao, C.; Chow, J.M.; Chang, C.L.; Hwang, P.A.; Chuang, S.E.; Whang-Peng, J.; Lai, G.M. Fucoidan Elevates MicroRNA-29b to Regulate DNMT3B-MTSS1 Axis and Inhibit EMT in Human Hepatocellular Carcinoma Cells. Mar. Drugs 2015, 13, 6099–6116. [Google Scholar] [CrossRef] [PubMed]
- Turcan, S.; Fabius, A.W.; Borodovsky, A.; Pedraza, A.; Brennan, C.; Huse, J.; Viale, A.; Riggins, G.J.; Chan, T.A. Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT Inhibitor Decitabine. Oncotarget 2013, 4, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Liu, S.; Fabbri, M.; Liu, Z.; Heaphy, C.E.; Callegari, E.; Schwind, S.; Pang, J.; Yu, J.; Muthusamy, N.; et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood 2009, 113, 6411–6418. [Google Scholar] [CrossRef] [PubMed]
- Blanc, R.S.; Vogel, G.; Chen, T.; Crist, C.; Richard, S. PRMT7 Preserves Satellite Cell Regenerative Capacity. Cell Rep. 2016, 14, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.P.; Kantarjian, H.M. Targeting DNA methylation. Clin. Cancer Res. 2009, 15, 3938–3946. [Google Scholar] [CrossRef]
- Chou, T.-C. The combination index (CI1) as the definition of synergism and of synergy claims. Synergy 2018, 7, 49. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.Y.; You, G.S. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2010, 16, 291–297. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Das, S.; Srikanth, M.; Kessler, J.A. Cancer stem cells and glioma. Nat. Clin. Pract. Neurol. 2008, 4, 427–435. [Google Scholar] [CrossRef]
- Yan, M.; Liu, Q. Differentiation therapy: A promising strategy for cancer treatment. Chin. J. Cancer 2016, 35, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, W.; Wang, J.; Zheng, M.; Liao, X.H.; Zhang, N.; Lu, W.; Wang, L.; Chen, A.Z.; Wu, W.G.; et al. A novel controlled release formulation of the Pin1 inhibitor ATRA to improve liver cancer therapy by simultaneously blocking multiple cancer pathways. J. Control. Release 2018, 269, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Nervi, C.; De Marinis, E.; Codacci-Pisanelli, G. Epigenetic treatment of solid tumours: A review of clinical trials. Clin. Epigenetics 2015, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, G.; Shanmuganandam, K.; Bendre, A.; Muzumdar, D.; Goel, A.; Shiras, A. Epigenetic regulation of DNA methyltransferases: DNMT1 and DNMT3B in gliomas. J. Neurooncol. 2011, 104, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Takouda, J.; Yoshimoto, K.; Nakashima, K. New aspects of glioblastoma multiforme revealed by similarities between neural and glioblastoma stem cells. Cell Biol. Toxicol. 2018, 34, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Fouse, S.D.; Costello, J.F. Epigenetics of neurological cancers. Future Oncol. 2009, 5, 1615–1629. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, S.; Delgado, M.; Kremer, L.; Garcia-Sanz, J.A. Will a mAb-Based Immunotherapy Directed against Cancer Stem Cells Be Feasible? Front. Immunol. 2017, 8, 1509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, T.Y.; Hu, C.H.; Shu, D.T.F.; Lu, M.K. Fucoidan upregulates TLR4/CHOP-mediated caspase-3 and PARP activation to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Cancer Lett. 2018, 432, 112–120. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and Tissue Distribution of Fucoidan from Fucus vesiculosus after Oral Administration to Rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Tan, Y.; Dai, S.; Zhu, Y.; Meng, T.; Yang, X.; Liu, Y.; Liu, X.; Yuan, H.; Hu, F. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 2017, 24, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kubota, T.; Sato, K.; Arishima, H. Ultrastructure of capillary endothelium in pilocytic astrocytomas. Brain Tumor Pathol. 2004, 21, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Lin, H.V.; Lin, H.Y.; Lo, S.K. Dietary Supplementation with Low-Molecular-Weight Fucoidan Enhances Innate and Adaptive Immune Responses and Protects against Mycoplasma pneumoniae Antigen Stimulation. Mar. Drugs 2019, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-H.; Lai, I.-C.; Kuo, H.-C.; Chuang, S.-E.; Lee, H.-L.; Whang-Peng, J.; Yao, C.-J.; Lai, G.-M. Epigenetic Modification and Differentiation Induction of Malignant Glioma Cells by Oligo-Fucoidan. Mar. Drugs 2019, 17, 525. https://doi.org/10.3390/md17090525
Liao C-H, Lai I-C, Kuo H-C, Chuang S-E, Lee H-L, Whang-Peng J, Yao C-J, Lai G-M. Epigenetic Modification and Differentiation Induction of Malignant Glioma Cells by Oligo-Fucoidan. Marine Drugs. 2019; 17(9):525. https://doi.org/10.3390/md17090525
Chicago/Turabian StyleLiao, Chien-Huang, I-Chun Lai, Hui-Ching Kuo, Shuang-En Chuang, Hsin-Lun Lee, Jacqueline Whang-Peng, Chih-Jung Yao, and Gi-Ming Lai. 2019. "Epigenetic Modification and Differentiation Induction of Malignant Glioma Cells by Oligo-Fucoidan" Marine Drugs 17, no. 9: 525. https://doi.org/10.3390/md17090525
APA StyleLiao, C.-H., Lai, I.-C., Kuo, H.-C., Chuang, S.-E., Lee, H.-L., Whang-Peng, J., Yao, C.-J., & Lai, G.-M. (2019). Epigenetic Modification and Differentiation Induction of Malignant Glioma Cells by Oligo-Fucoidan. Marine Drugs, 17(9), 525. https://doi.org/10.3390/md17090525



