MytiLec-1 Shows Glycan-Dependent Toxicity against Brine Shrimp Artemia and Induces Apoptotic Death of Ehrlich Ascites Carcinoma Cells In Vivo
Abstract
1. Introduction
2. Results
2.1. Purification and Confirmation of the Molecular Mass of MytiLec-1
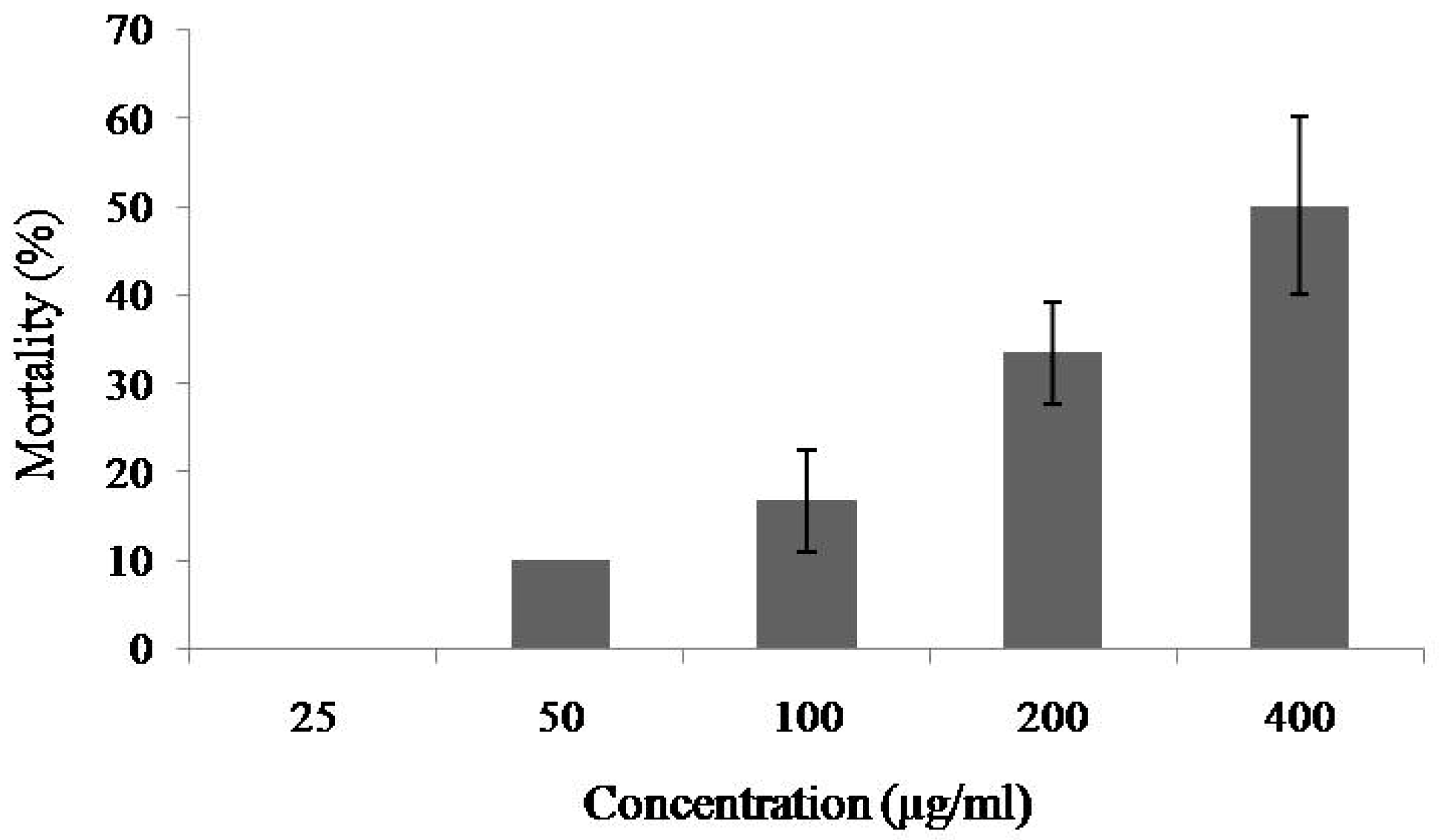
2.2. Toxicity of MytiLec-1 against Brine Shrimp Artemia Nauplii
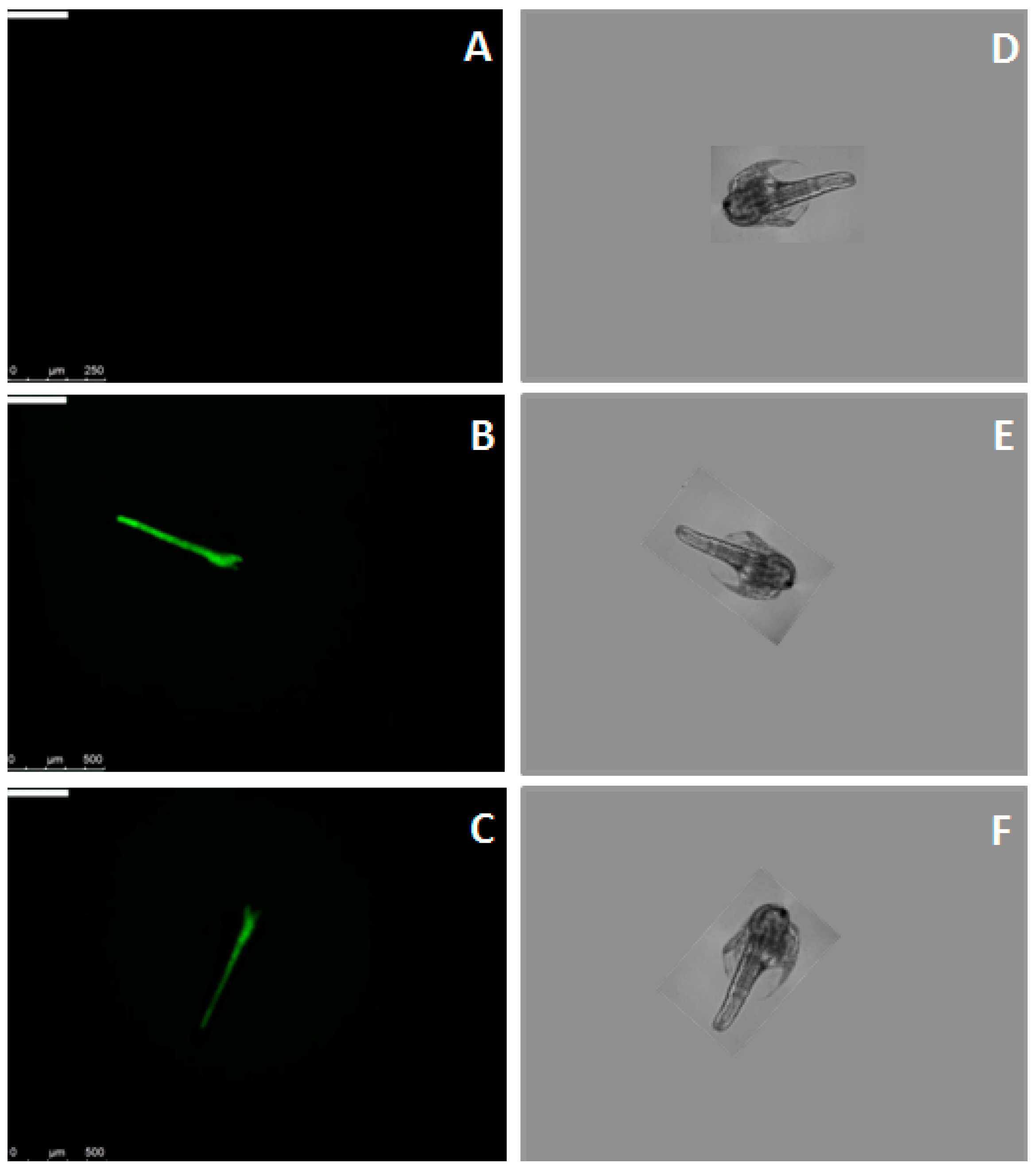
2.3. Binding of FITC-Labeled Lectins to Artemia Nauplii Detected by Fluorescence Microscopy
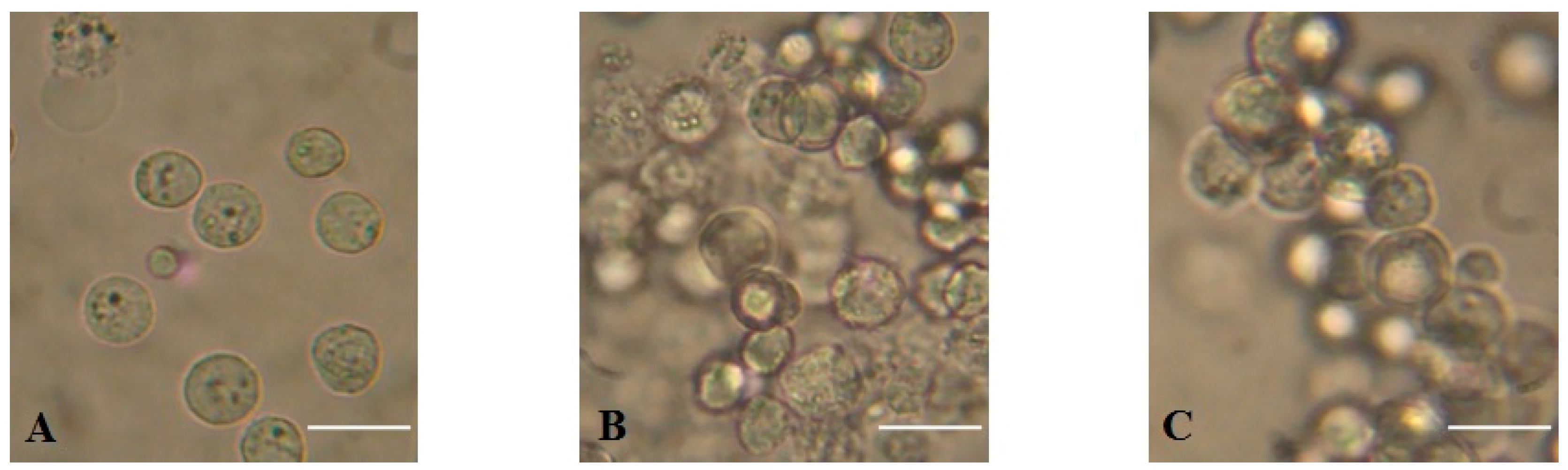
2.4. Agglutination of Ehrlich Ascites Carcinoma Cells
2.5. In Vivo Antitumor Activity of MytiLec-1
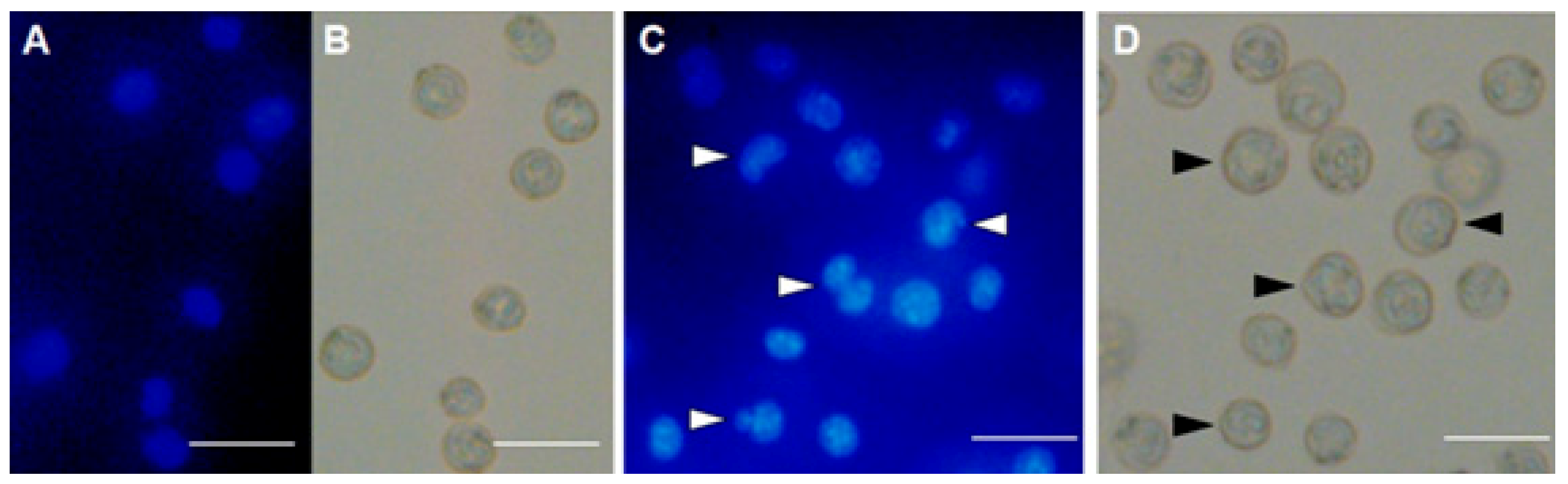
2.6. Morphological Examination of Ehrlich Ascites CarcinomaCells
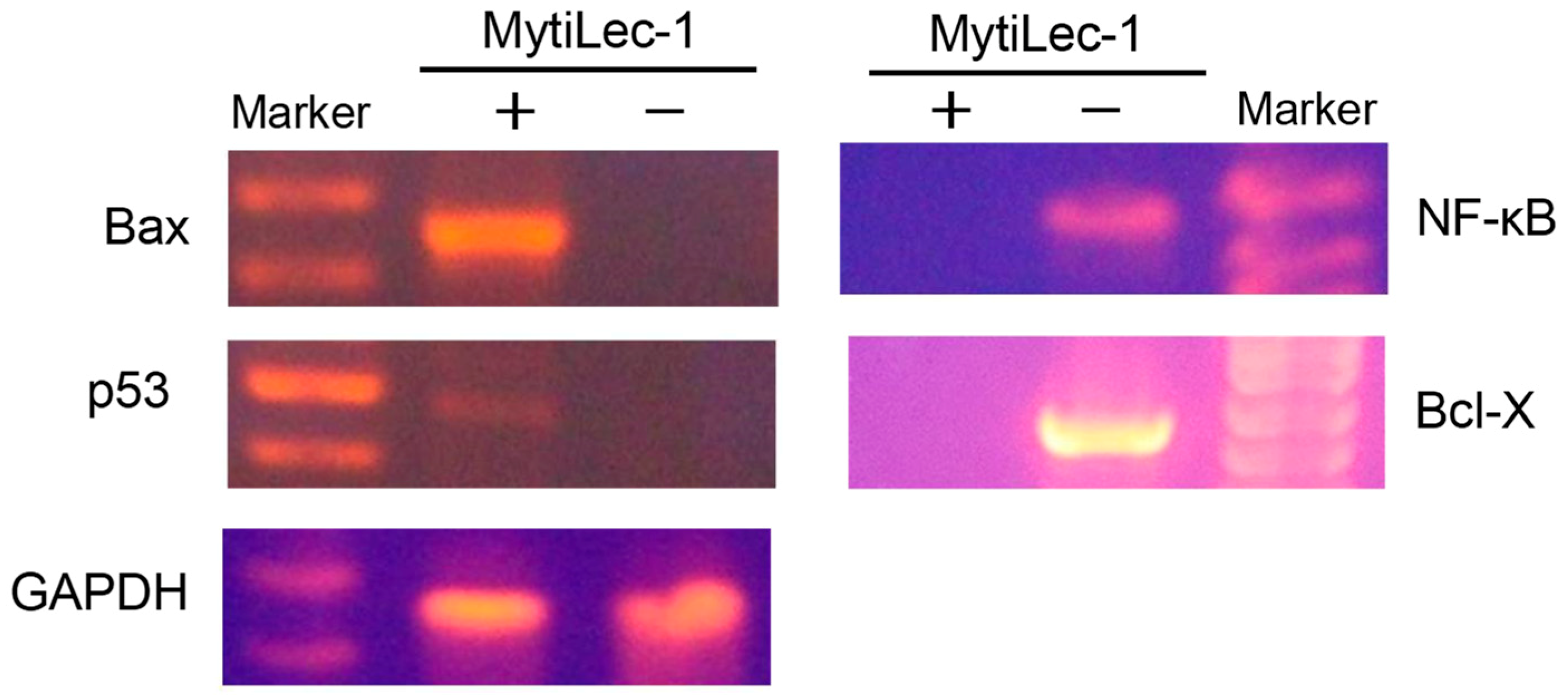
2.7. Expression of Apoptosis-Related Genes
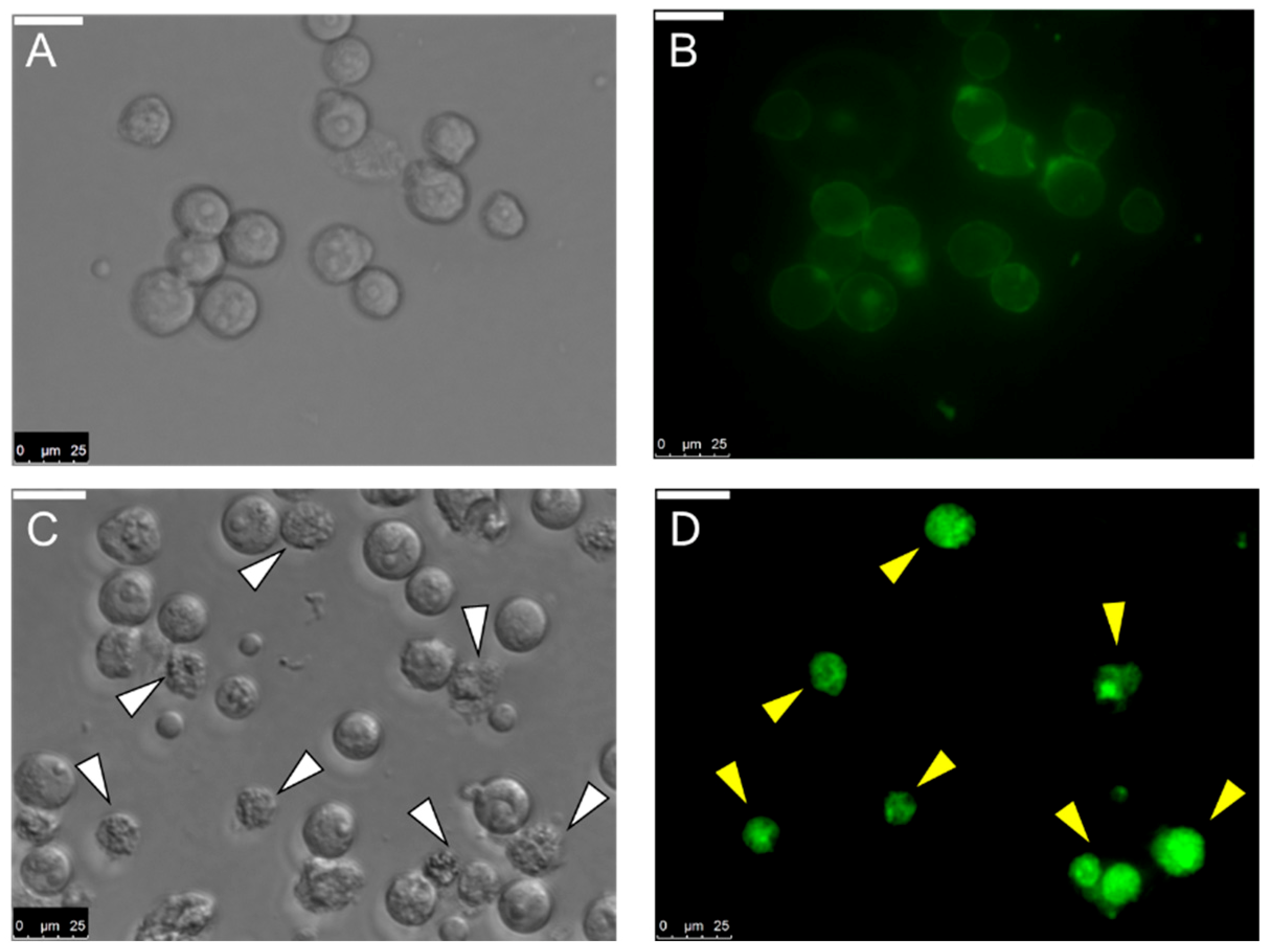
2.8. Internalization of MytiLec-1 into U937 Cells
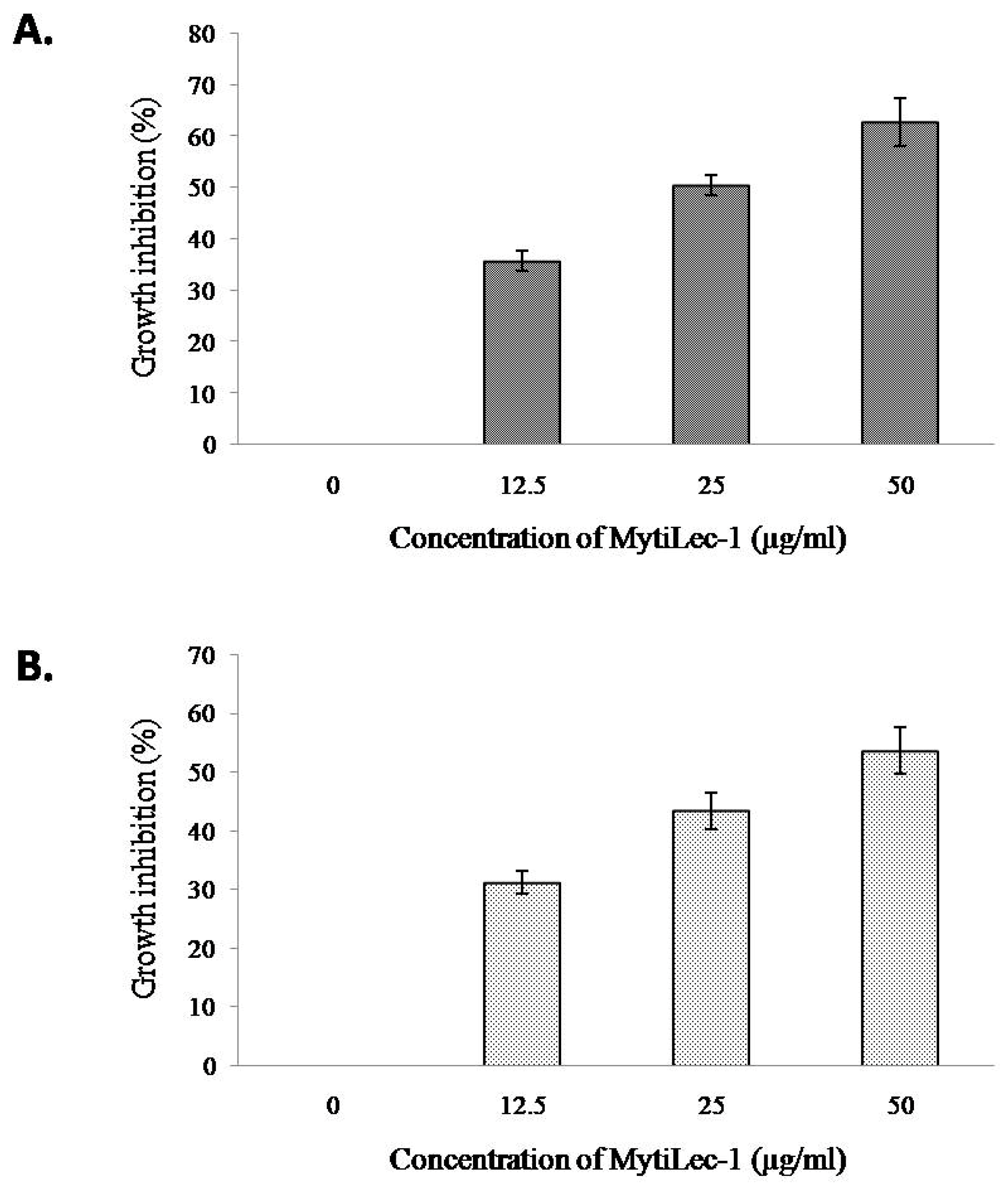
2.9. In Vitro Antiproliferative Activity of MytiLec-1 against U937 and HeLa Cell Lines
3. Discussion
4. Experimental Design
4.1. Materials
4.2. Purification of Protein
4.3. Brine Shrimp Nauplii Lethality Assay
4.4. Preparation of Fluorescein Isothioycanate (FITC)-Labeled Proteins
4.5. Fluorescence Microscopy of Artemia Nauplii
4.6. Experimental Animals and Ethical Clearance
4.7. Ehrlich Ascites Carcinoma Cell Agglutination
4.8. Determination of Growth Inhibition of Ehrlich Ascites Carcinoma Cells
mice) × 100}
4.9. Examination of Morphological Alteration and Nuclear Damages of Ehrlich Ascites Carcinoma Cells by Hoechst Staining
4.10. Isolation of RNA from Ehrlich Ascites Carcinoma Cells and Expression of Apoptosis-Related Genes
4.11. Cell Culture
4.12. Incubation of Fluorescein Isothiocyanate (FITC)-Conjugated MytiLec-1 with U937 Cells
4.13. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Colorimetric Assay of Different Cancer Cell Lines
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia-Maldonado, E.; Cano-Sanchez, P.; Hernandez-Santoyo, A. Molecular and functional characterization of a glycosylated galactose-binding lectin from Mytilus californianus. Fish Shellfish Immunol. 2017, 66, 564–574. [Google Scholar] [CrossRef]
- Rubinstein, N.; Ilarregui, J.M.; Toscano, M.A.; Rabinovich, G.A. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 2004, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bian, H.J.; Bao, J.K. Plant lectins: Potential antineoplastic drugs from bench to clinic. Cancer Lett. 2010, 287, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with potential for anticancer therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef] [PubMed]
- Varrot, A.; Basheer, S.M.; Imberty, A. Fungal lectins: Structure, function and potential applications. Curr. Opin. Struct. Biol. 2013, 23, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Herre, J.; Willment, J.A.; Gordon, S.; Brown, G.D. The role of Dectin-1 in antifungal immunity. Crit. Rev. Immunol. 2004, 24, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, B.C.L.C.; Marcelino, D.S.S.P.; Felix, O.W.; de Moura, M.C.; Viana, P.E.; Soares, G.F.; Guedes, P.P.M.; Napoleao, T.H.; Dos, S.C.M.T. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018, 125, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Gerdol, M.; Fujii, Y.; Rajia, S.; Koide, Y.; Yamamoto, D.; Kawsar, S.M.A.; Ozeki, Y. cDNA and Gene Structure of MytiLec-1, A Bacteriostatic R-Type Lectin from the Mediterranean Mussel (Mytilus galloprovincialis). Mar. Drugs 2016, 14, 92. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Ramessar, K.; O’Keefe, B.R. Antiviral lectins: Selective inhibitors of viral entry. Antiviral. Res. 2017, 142, 37–54. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.; Dan, X.; Ng, T.B. Marine lectins and their medicinal applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755–3773. [Google Scholar] [CrossRef]
- Kilpatrick, D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta. 2002, 1572, 187–197. [Google Scholar] [CrossRef]
- Vasta, G.R.; Ahmed, H.; Tasumi, S.; Odom, E.W.; Saito, K. Biological roles of lectins in innate immunity: Molecular and structural basis for diversity in self/nonself recognition. Adv. Exp. Med. Biol. 2007, 598, 389–406. [Google Scholar] [PubMed]
- Varki, A.; Etzler, M.E.; Cummings, R.D.; Esko, J.D. Discovery and Classification of Glycan-binding Proteins, In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; pp. 375–394. [Google Scholar]
- Fujii, Y.; Dohmae, N.; Takio, K.; Kawsar, S.M.A.; Matsumoto, R.; Hasan, I.; Koide, Y.; Kanaly, R.A.; Yasumitsu, H.; Ogawa, Y.; et al. A lectin from the mussel Mytilus galloprovincialis has a highly novel primary structure and induces glycan-mediated cytotoxicity of globotriaosylceramide-expressing lymphoma cells. J. Biol. Chem. 2012, 287, 44772–44783. [Google Scholar] [CrossRef]
- Belogortseva, N.I.; Molchanova, V.I.; Kurika, A.V.; Skobun, A.S.; Glazkova, V.E. Isolation and characterization of new GalNAc/Gal-specific lectin from the sea mussel Crenomytilus grayanus. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 119, 45–50. [Google Scholar] [CrossRef]
- Kovalchuk, S.N.; Chikalovets, I.V.; Chernikov, O.V.; Molchanova, V.I.; Li, W.; Rasskazov, V.A.; Lukyanov, P.A. cDNA cloning and structural characterization of a lectin from the mussel Crenomytilus grayanus with a unique amino acid sequence and antibacterial activity. Fish Shellfish Immunol. 2013, 35, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Chikalovets, I.V.; Kondrashina, A.S.; Chernikov, O.V.; Molchanova, V.I.; Lukyanov, P.A. Isolation and general characteristics of lectin from the mussel Mytilus trossulus. Chem. Nat. Comp. 2013, 48, 1058–1061. [Google Scholar] [CrossRef]
- Chernikov, O.; Kuzmich, A.; Chikalovets, I.; Molchanova, V.; Hua, K.F. Lectin CGL from the sea mussel Crenomytilus grayanus induces Burkitt’s lymphoma cells death via interaction with surface glycan. Int. J. Biol. Macromol. 2017, 104, 508–514. [Google Scholar] [CrossRef]
- Hasan, I.; Sugawara, S.; Fujii, Y.; Koide, Y.; Terada, D.; Iimura, N.; Fujiwara, T.; Takahashi, K.G.; Kojima, N.; Rajia, S.; et al. MytiLec, a Mussel R-Type Lectin, interacts with surface glycan Gb3 on Burkitt’s lymphoma cells to trigger apoptosis through multiple pathways. Mar. Drugs. 2015, 13, 7377–7389. [Google Scholar] [CrossRef]
- Terada, D.; Kawai, F.; Noguchi, H.; Unzai, S.; Hasan, I.; Fujii, Y.; Park, S.; Ozeki, Y.; Tame, J.R.H. Crystal structure of MytiLec, a galactose-binding lectin from the mussel Mytilus galloprovincialis with cytotoxicity against certain cancer cell types. Sci. Rep. 2016, 6, 28344. [Google Scholar] [CrossRef]
- Moura, R.M.; Melo, A.A.; Carneiro, R.F.; Rodrigues, C.R.; Delatorre, P.; Nascimento, K.S.; Saker-Sampaio, S.; Nagano, C.S.; Cavada, B.S.; Sampaio, A.H. Hemagglutinating/Hemolytic activities in extracts of marine invertebrates from the Brazilian coast and isolation of two lectins from the marine sponge Cliona varians and the sea cucumber Holothuria grisea. An. Acad. Bras. Cienc. 2015, 87, 973–984. [Google Scholar] [CrossRef][Green Version]
- Carneiro, R.F.; de Melo, A.A.; Nascimento, F.E.; Simplicio, C.A.; Nascimento, K.S.; Rocha, B.A.; Saker-Sampaio, S.; Moura, R.M.; Mota, S.S.; Cavada, B.S.; et al. Halilectin 1 (H-1) and Halilectin 2 (H-2): Two new lectins isolated from the marine sponge Haliclona caerulea. J. Mol. Recognit. 2013, 26, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Arruda, F.V.; Melo, A.A.; Vasconcelos, M.A.; Carneiro, R.F.; Barroso-Neto, I.L.; Silva, S.R.; Pereira-Junior, F.N.; Nagano, C.S.; Nascimento, K.S.; Teixeira, E.H.; et al. Toxicity and binding profile of lectins from the Genus canavalia on brine shrimp. Biomed. Res. Int. 2013, 2013, 154542. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, A.; Bardocz, S. Biological effects of plant lectins on the gastrointestinal tract: Metabolic consequences and applications. Trends Glycosci. Glyc. 1996, 8, 149–165. [Google Scholar] [CrossRef]
- Miyake, K.; Tanaka, T.; McNeil, P.L. Lectin-based food poisoning: A new mechanism of protein toxicity. PLoS ONE 2007, 2, e687. [Google Scholar] [CrossRef] [PubMed]
- Terada, D.; Voet, A.R.D.; Noguchi, H.; Kamata, K.; Ohki, M.; Addy, C.; Fujii, Y.; Yamamoto, D.; Ozeki, Y.; Tame, J.R.H.; et al. Computational design of a symmetrical beta-trefoil lectin with cancer cell binding activity. Sci. Rep. 2017, 7, 5943. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.R.S.; Amin, R.; Hasan, I.; Asaduzzaman, A.K.M.; Kabir, S.R. Antitumor properties of a methyl-β-d-galactopyranoside specific lectin from Kaempferia rotunda against Ehrlich ascites carcinoma cells. Int. J. Biol. Macromol. 2017, 102, 952–959. [Google Scholar] [CrossRef]
- Kabir, K.M.A.; Amin, R.; Hasan, I.; Asaduzzaman, A.K.M.; Khatun, H.; Kabir, S.R. Geodorum densiflorum rhizome lectin inhibits Ehrlich ascites carcinoma cell growth by inducing apoptosis through the regulation of BAX, p53 and NF-κB genes expression. Int. J. Biol. Macromol. 2019, 125, 92–98. [Google Scholar] [CrossRef]
- Hasan, I.; Islam, F.; Ozeki, Y.; Kabir, S.R. Antiproliferative activity of cytotoxic tuber lectins from Solanum tuberosum against experimentally induced Ehrlich ascites carcinoma in mice. Afr. J. Biotechnol. 2014, 13, 1679–1685. [Google Scholar]
- Kabir, S.R.; Rahman, M.M.; Amin, R.; Karim, M.R.; Mahmud, Z.H.; Hossain, M.T. Solanum tuberosum lectin inhibits Ehrlich ascites carcinoma cells growth by inducing apoptosis and G2/M cell cycle arrest. Tumour. Biol. 2016, 37, 8437–8444. [Google Scholar] [CrossRef]
- Eckhardt, A.E.; Goldstein, I.J. An α-d-galactopyranosyl-containing glycoprotein from Ehrlich ascites tumor cell plasma membranes. In Glyconjugate Research, 1st ed.; Gregory, J.D., Jeanloz, R.W., Eds.; Academic Press Inc.: New York, NY, USA, 1979; pp. 1043–1045. [Google Scholar]
- Sakakibara, F.; Kawauchi, H.; Takayanagi, G.; Ise, H. Egg lectin of Rana japonica and its receptor glycoprotein of Ehrlich tumor cells. Cancer Res. 1979, 39, 1347–1352. [Google Scholar]
- Ahmed, H.; Chatterjee, B.P.; Debnath, A.K. Interaction and in vivo growth inhibition of Ehrlich ascites tumor cells by jacalin. J. Biosci. 1988, 13, 419–424. [Google Scholar] [CrossRef]
- Kabir, S.R.; Nabi, M.M.; Haque, A.; Zaman, R.U.; Mahmud, Z.H.; Reza, M.A. Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine 2013, 20, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.A.; Akter, Z.; Uddin, M.J.; Ferdaus, K.M.K.B.; Hoque, K.M.F.; Ferdousi, Z.; Reza, M.A. Characterization and evaluation of antibacterial and antiproliferative activities of crude protein extracts isolated from the seeds of Ricinus communis in Bangladesh. BMC Complement. Altern. Med. 2016, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.A.; Husna, J.; Khatun, M.; Hasan, R.; Kamruzzaman, M.; Hoque, K.M.F.; Reza, M.A.; Ferdousi, Z. Assessment of antioxidant, anticancer and antimicrobial activity of two vegetable species of Amaranthus in Bangladesh. BMC Complement. Altern. Med. 2016, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.A.; Kabir, S.R. Antibacterial activity of Kaempferia rotunda rhizome lectin and its induction of apoptosis in Ehrlich ascites carcinoma cells. Appl. Biochem. Biotechnol. 2014, 172, 2866–2876. [Google Scholar]
- Groc, L.; Bezin, L.; Jiang, H.; Jackson, T.S.; Levine, R.A. Bax, Bcl-2, and cyclin expression and apoptosis in rat substantia nigra during development. Neurosci. Lett. 2001, 306, 198–202. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Xu, B.; Zhou, H. Curcumin induces p53-independent necrosis in H1299 cells via a mitochondria-associated pathway. Mol. Med. Rep. 2015, 12, 7806–7814. [Google Scholar] [CrossRef]
- Baichwal, V.R.; Baeuerle, P.A. Activate NF-kappa B or die? Curr. Biol. 1997, 7, R94–R96. [Google Scholar] [CrossRef]
- Tong, Q.S.; Zheng, L.D.; Wang, L.; Liu, J.; Qian, W. BAK overexpression mediates p53-independent apoptosis inducing effects on human gastric cancer cells. BMC Cancer 2004, 4, 33. [Google Scholar] [CrossRef]
- Degenhardt, K.; Chen, G.; Lindsten, T.; White, E. BAX and BAK mediate p53-independent suppression of tumorigenesis. Cancer Cell. 2002, 2, 193–203. [Google Scholar] [CrossRef]
- Strobel, T.; Swanson, L.; Korsmeyer, S.; Cannistra, S.A. BAX enhances paclitaxel-induced apoptosis through a p53-independent pathway. Proc. Natl. Acad. Sci. USA. 1996, 93, 14094–14099. [Google Scholar] [CrossRef] [PubMed]
- Mise, K.; Akifusa, S.; Watarai, S.; Ansai, T.; Nishihara, T.; Takehara, T. Involvement of ganglioside GM3 in G2/M cell cycle arrest of human monocytic cells induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect. Immun. 2005, 73, 4846–4852. [Google Scholar] [CrossRef] [PubMed]
- Matsushima-Hibiya, Y.; Watanabe, M.; Hidari, K.I.J.; Miyamoto, D.; Suzuki, Y.; Kasama, T.; Kanazawa, T.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Identification of glycosphingolipid receptors for Pierisin-1, a guanine-specific ADP-ribosylaing toxin from the cabbage butterfly. J. Biol. Chem. 2003, 278, 9972–9978. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Ozeki, Y. Histochemical localization of N-acetylhexosamine-binding lectin HOL-18 in Halichondria okadai (Japanese black sponge), and its antimicrobial and cytotoxic anticancer effects. Int. J. Biol. Macromol. 2019, 124, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Zurga, S.; Nanut, M.P.; Kos, J.; Sabotic, J. Fungal lectin MpL enables entry of protein drugs into cancer cells and their subcellular targeting. Oncotarget 2017, 8, 26896–26910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oda, T.; Iwaoka, J.; Komatsu, N.; Muramatsu, T. Involvement of N-acetylcysteine-sensitive pathways in ricin-induced apoptotic cell death in U937 cells. Biosci. Biotechnol. Biochem. 1999, 63, 341–348. [Google Scholar] [CrossRef]
- Hasegawa, N.; Kimura, Y.; Oda, T.; Komatsu, N.; Muramatsu, T. Isolated ricin B-chain-mediated apoptosis in U937 cells. Biosci. Biotechnol. Biochem. 2000, 64, 1422–1429. [Google Scholar] [CrossRef]
- Koyama, Y.; Katsuno, Y.; Miyoshi, N.; Hayakawa, S.; Mita, T.; Muto, H.; Isemura, S.; Aoyagi, Y.; Isemura, M. Apoptosis induction by lectin isolated from the mushroom Boletopsis leucomelas in U937 cells. Biosci. Biotechnol. Biochem. 2002, 66, 784–789. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Soares, S.G.; Tamarozzi, M.B.; Rego, E.M.; Roque-Barreira, M.C. The recognition of N-glycans by the lectin ArtinM mediates cell death of a human myeloid leukemia cell line. PLoS ONE 2011, 6, e27892. [Google Scholar] [CrossRef]
- Rabelo, L.; Monteiro, N.; Serquiz, R.; Santos, P.; Oliveira, R.; Oliveira, A.; Rocha, H.; Morais, A.H.; Uchoa, A.; Santos, E. A lactose-binding lectin from the marine sponge Cinachyrella apion (Cal) induces cell death in human cervical adenocarcinoma cells. Mar. Drugs 2012, 10, 727–743. [Google Scholar] [CrossRef]
- Fujii, Y.; Fujiwara, T.; Koide, Y.; Hasan, I.; Sugawara, S.; Rajia, S.; Kawsar, S.M.A.; Yamamoto, D.; Araki, D.; Kanaly, R.A.; et al. Internalization of a novel, huge lectin from Ibacus novemdentatus (slipper lobster) induces apoptosis of mammalian cancer cells. Glycoconj. J. 2017, 34, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.R.; Zubair, M.A.; Nurujjaman, M.; Haque, M.A.; Hasan, I.; Islam, M.F.; Hossain, M.T.; Hossain, M.A.; Rakib, M.A.; Alam, M.T.; et al. Purification and characterization of a Ca2+ dependent novel lectin from Nymphaea nouchali Tuber with antiproliferative activities. Biosci. Rep. 2011, 31, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 333. [Google Scholar]


| Primer | Forward | Reverse |
|---|---|---|
| GAPDH | GTGGAAGGACTCATGACCACAG | CTGGTGCTCAGTGTAGCCCAG |
| Bax | CCTGCTTCTTTCTTCATCGG | AGGTGCCTGGACTCTTGGGT |
| Bcl-X | TTGGACAATGGACTGGTTGA | GTAGAGTGGATGGTCAGTG |
| NF-κB | AACAAAATGCCCCACGGTTA | GGGACGATGCAATGGACTGT |
| p53 | GCGTCTTAGAGACAGTTGCCT | GGATAGGTCGGCGGTTCATGC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, I.; Asaduzzaman, A.K.M.; Swarna, R.R.; Fujii, Y.; Ozeki, Y.; Uddin, M.B.; Kabir, S.R. MytiLec-1 Shows Glycan-Dependent Toxicity against Brine Shrimp Artemia and Induces Apoptotic Death of Ehrlich Ascites Carcinoma Cells In Vivo. Mar. Drugs 2019, 17, 502. https://doi.org/10.3390/md17090502
Hasan I, Asaduzzaman AKM, Swarna RR, Fujii Y, Ozeki Y, Uddin MB, Kabir SR. MytiLec-1 Shows Glycan-Dependent Toxicity against Brine Shrimp Artemia and Induces Apoptotic Death of Ehrlich Ascites Carcinoma Cells In Vivo. Marine Drugs. 2019; 17(9):502. https://doi.org/10.3390/md17090502
Chicago/Turabian StyleHasan, Imtiaj, A.K.M. Asaduzzaman, Rubaiya Rafique Swarna, Yuki Fujii, Yasuhiro Ozeki, Md. Belal Uddin, and Syed Rashel Kabir. 2019. "MytiLec-1 Shows Glycan-Dependent Toxicity against Brine Shrimp Artemia and Induces Apoptotic Death of Ehrlich Ascites Carcinoma Cells In Vivo" Marine Drugs 17, no. 9: 502. https://doi.org/10.3390/md17090502
APA StyleHasan, I., Asaduzzaman, A. K. M., Swarna, R. R., Fujii, Y., Ozeki, Y., Uddin, M. B., & Kabir, S. R. (2019). MytiLec-1 Shows Glycan-Dependent Toxicity against Brine Shrimp Artemia and Induces Apoptotic Death of Ehrlich Ascites Carcinoma Cells In Vivo. Marine Drugs, 17(9), 502. https://doi.org/10.3390/md17090502