Abstract
Bioceramic scaffolds are crucial in tissue engineering for bone regeneration. They usually provide hierarchical porosity, bioactivity, and mechanical support supplying osteoconductive properties and allowing for 3D cell culture. In the case of age-related diseases such as osteoarthritis and osteoporosis, or other bone alterations as alveolar bone resorption or spinal fractures, functional tissue recovery usually requires the use of grafts. These bone grafts or bone void fillers are usually based on porous calcium phosphate grains which, once disposed into the bone defect, act as scaffolds by incorporating, to their own porosity, the intergranular one. Despite their routine use in traumatology and dental applications, specific graft requirements such as osteoinductivity or balanced dissolution rate are still not completely fulfilled. Marine origin bioceramics research opens the possibility to find new sources of bone grafts given the wide diversity of marine materials still largely unexplored. The interest in this field has also been urged by the limitations of synthetic or mammalian-derived grafts already in use and broadly investigated. The present review covers the current stage of major marine origin bioceramic grafts for bone tissue regeneration and their promising properties. Both products already available on the market and those in preclinical phases are included. To understand their clear contribution to the field, the main clinical requirements and the current available biological-derived ceramic grafts with their advantages and limitations have been collected.
1. Introduction
Cell culture in two dimensions has traditionally been used to test the biological response to different biomaterials or to perform drug screening by growing cells on flat surfaces. These conventional cell monolayer cultures do not fully reflect the essential physiology of real tissues as they modify the tissue-specific architecture (forced polarity, flattened cell shape), mechanical/biochemical signals, and cell-to-cell communications [1,2]. Therefore, the simplicity of this method supposes an advantage but, at the same time, a disadvantage since the “in vivo” 3D complex environment is not represented, resulting in non-predictive data for clinical applications [3].
Current alternatives include three-dimensional cell culture techniques based on organoids, cell encapsulation on hydrogels, or growing the cells on scaffolds instead of flat surfaces. Organoids are 3D multicellular tissue constructs that can growth either in gels or in suspension generating organ-like structures [4]. Cell encapsulation allows cell survival and extracellular matrix deposition while permitting the analysis of complex cell interactions [5]. On the other hand, scaffolds provide convenient cell support due to their porosity, facilitating oxygen, nutrients, and waste transportation [6]. These systems resemble more closely the tissue physiological characteristics by providing structural tensile strength, cell adhesion, polarity, migration, and proliferation [7].
Moreover, for tissue regeneration purposes, scaffolds can be implanted to help tissue reconstruction, and then be either removed or biodegraded after fulfilling its purpose [8]. Bone graft materials or void fillers are used as direct cell support in certain patients to promote regeneration [9]. They are usually based on ceramics as calcium phosphate (CaP) grains of different sizes (in dentistry around 0.5–1 mm) depending on the bone defect volume to be filled. When bone grafts are placed into the defect a 3D scaffold with intergranular porosity is generated in addition to the intrinsic porosity of the grains. These bioceramics are commonly tested in the laboratory in 3D cell culture by their seeding using variable defect volumes to obtain cellular responses closer to the clinic [8,10].
An intense research interest exists on bioceramic structure, including combinations of different CaP phases (hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP)) to provide the optimal balance in stability/resorbability able to ensure bone regeneration. This need promotes searching for new bioceramic sources including marine derived structures with their chemical and morphological particularities. These bioceramics should follow the ethics requirements, the animal welfare awareness (avoiding animal sacrifices), as well as sustainability regulations. Therefore, the use of animal discards and fishing by-products or wastes as calcium phosphate sources has recently gained increased attention. Finally, 3D composite materials by including ceramic granules into polymeric hydrogels, typically collagen, with or without growth factors are also used. Traditional chemical methodologies or advanced ones, such as 3D printing, are being investigated [10].
The present review covers the current stage of the main marine origin bioceramic grafts tested in 3D in vitro culture and designed for bone tissue regeneration including their promising properties. Both products already available on the market and those in preclinical phases are included. To understand their clear contribution to the field, main clinical requirements and current available biological-derived ceramic grafts, with their advantages and limitations, have been collected.
2. Bone Grafts: Limitations of Auto and Allografts
Bone-related diseases suppose nowadays one of the main causes of disability and involve a high number of surgical interventions. Many of the required interventions are caused by pathologies related to aging, associated with the large number of cellular changes [11]. Osteoarthritis and osteoporosis are the age-related diseases with the highest incidence in the field of traumatology. Moreover, bone defects can also be derived from car accidents, falls or sport-related injuries, trauma or tumors, and congenital diseases, such as spinal fractures, deterioration of intervertebral discs, and narrowing of spinal canal (stenosis). The dentistry field must also be considered where, again aging, genetic factors, incorrect oral hygiene, or oral trauma, influence the appearance of periodontitis and caries which, at the same time, will contribute to the increase of partial or total edentulism incidence, including alveolar bone resorption. All these bone alterations require the use of grafts to promote functional tissue recovery [12]. This high demand of bone tissue implies over two million of bone grafting procedures performed annually worldwide [13] with an estimated global market of $2.6B [14].
The ideal bone substitute, according to the clinicians, should be biocompatible, structurally similar to bone, easily moldable within the osseous defect, not produce inflammatory response, osteoconductive, osteoinductive, and resorbable. It must also be non-thermally conductive, sterilizable, and easily accessible at a reasonable cost [15,16,17,18]. In terms of porosity, the recommended pore size for the growth of capillaries is 50 μm, and a size of 200 µm is needed for the growth of new osteons in the pores [19,20]. It is important to consider the colonization of these macropores by mesenchymal cells, allowing bone apposition, begins at two or three weeks post-implantation, so the resorption of the graft should not be too fast [20]. Moreover, micropores of less than 10 μm allow circulation of organic liquids and diffusion of substances through the matrix while increasing the specific surface area, improving the metabolic environment for bone-producing cells, and accelerating remodeling. Finally, the presence of interconnected porosity favors the appearance of a capillary force that actively absorbs the patient’s blood and bone marrow into the matrix of the material. Furthermore, to be able to talk about osteoconduction, porosity must allow vascularization and cell growth, also having a crucial role in graft biodegradation [21,22].
Autografts remain as the gold standard in bone regeneration for both trauma and dentistry given their osteoinductive properties. In the case of traumatology, grafts are usually obtained from non-essential bone such as the iliac crest or the fibula [15]. In dentistry, they are obtained from intraoral sites for small defects (chin, maxillary tuberosity, ascending branch) or from extraoral sites when a higher volume is required (iliac crest, tibia, or calotte). The choice will depend on the type, size, and shape of the bone cavity, clinical experience, and professional preference. Autogenous cancellous bone has greater osteogenic capacity while cortical bone provides greater stability [23]. Autografts have disadvantages such as postoperative morbidity of the donor area with bruising, residual pain, fractures, infection, hemorrhage, muscle weakness, neurological injury, and scarring [24,25]. Besides, their availability is limited as, in some cases, the amount of graft extracted is insufficient. On the other hand, the considerable increase in surgical time and the requirement of an additional anesthetic procedure also imply clear limitations [25].
On the other hand, allografts result from bone tissue obtained from another human, which can be alive or a cadaveric donor (bone donation), and they must be processed in a tissue bank [15]. Allografts can be used mineralized (tissue bank) or demineralized (after being subjected to different chemical processes). These latter are the ones usually commercialized under various trademarks (see Table 1). Mineralized allografts, preserved under freezing, show the advantages of autografts but with a slightly lower osteoinductive capacity due to the loss of growth factors and inferior mechanical properties. Allografts present high availability, important quantities, and different shapes and sizes, avoiding sacrifices of host structures and donor site morbidity together with a reduced surgical time [13]. They are widely used in osteoarticular reconstructive surgery, in hospitals with a bone bank, given their ease of use and good results in bone fractures and defects filling [26,27]. They are also the most common type currently used in foot and ankle surgery [28]. Their limitations are related to the quality of the regenerated bone tissue that is not always predictable due to its limited osteoinduction, in addition to requiring costly and laborious processing to eliminate its antigenic capacity [23]. They also present limitations in their mechanical resistance and the potential risk of disease transmission from the donor such as HIV, hepatitis B, hepatitis C, and human T-lymphotropic virus [29].

Table 1.
Commercially available demineralized allografts (DBMs) suitable for bone defects not intrinsic to the bony structure stability 1.
Demineralized allografts or demineralized bone matrices (DBMs) are composed of the organic matrix of human bone: mainly collagen and inherent growth factors including bone morphogenic proteins (BMPs) stored in the tissue after the removal of at least 40% of the mineral content [13]. DBMs maintain the collagen matrix, which reproduces the three-dimensional architecture of bone facilitating and guiding the invasion, growth, and differentiation of the host cells [13]. They are thought to present superior osteoinductive capacity to that of mineralized allografts due to the greater availability of bone morphogenetic protein and growth factors. These non-bioceramic grafts maintain part of the structure and components of the mineralized tissue of origin and, in certain products, a residual mineral content. They are, however, limited to be used in bone defects without load bearing given their low biomechanical resistance [30]. Moreover, their osteoinductive capacity varies depending on donor age, mineral content, nature, sterilization and processing, receptor species (more osteoinduction in animal models than in humans), recipient region, or implant site. Removal or inactivation of viruses must be performed to avoid disease transmission [31].
Demineralized allografts are used combined with other compounds such as autologous bone, allografts, blood and autologous marrow or with synthetic materials. Numerous studies suggest that DBM enriched with bone marrow could be comparable to autografts to treat long bone fractures [32,33,34]. Other possibilities include, outside the scope of this review, the use of bone morphogenetic proteins in collagen sponges, these molecules are considered the most potent inducers of bone consolidation. An example of a commercial product is InfuseTM Bone Graft (Medtronic), which consists on the recombinant human BMP-2 applied to an absorbable collagen sponge carrier (bovine origin). It is indicated for non-load applications on spinal fusion procedures in skeletally mature patients, open tibia shaft fractures, and oral-maxillofacial procedures as an alternative to autologous bone grafts. Contraindications include patients with hypersensitivity to any of the components, skeletally immature, with active infections at the surgical site, with inadequate neurovascular status or in the vicinity of resected or existing tumors, among others [35].
Biological grafts of human origin, reviewed in this section, meet the osteoconduction requirement, especially autografts and mineralized allografts. In the case of the autografts, limited to small defects, they also provide certain osteoinduction and osteogenesis. In order to overcome the limited availability of autografts, and the rest of the already mentioned disadvantages, when larger volumes are required for applications requiring loads, CaP grafts of mammalian origins are also available on the market.
3. Bioceramic Xenografts: Mammalian Origin
The bone tissue physiological similarities between humans and mammals make easy to consider the mammalian mineral tissue as an adequate source of bone xenografts for human use. To develop these xenografts, donors’ bone is treated using different protocols to avoid disease transmission and immunologic reactions while ensuring biocompatibility and restoring bone structure and function [36]. A good number of xenografts are obtained after bone tissue treatment with the BioCleanse® process. This process consists of the tissue sterilization and cleansing procedure using low temperature by combining mechanical and chemical processes removing cells, lipids, and other sources of antigenic material [37,38]. However, other companies decide to use their proprietary manufacturing process. Xenografts can not only be used alone but also combined with autografts, as mentioned above, to decrease the amount of autogenous bone needed reducing patient morbidity and showing improved regeneration when compared to xenografts alone [39,40].
Bovine based grafts are the most commonly used bone xenografts in orthopedic surgery [41]. Several bovine based bone grafts are already on the market with variable preparations, from blocks to granules, shown in Table 2. Moreover, the close genotype between humans and pigs have made this mammal donor another commonly used source for bone grafts with similar results to bovine xenografts [42,43,44]. The nowadays commercialized porcine bone grafts are also described in Table 2. Equine bone tissue is also used as source of bone grafts. These mammal derived grafts are thought to have osteogenic and bone inductive properties to assist bone healing and can be incorporated into the bone host tissue acting as support for bone colonization. However, they do not allow remodeling and they stay mainly unaltered on the host bone [45]. To allow osteoclast function, the addition of collagen could promote graft resorption [45]. In agreement with this, the use of collagenated porcine bone grafts indicated the possibility of graft resorption [45]. Moreover, the coating of bovine graft with poly(l-lactide-co-ɛ-caprolactone) (PLCL) and polysaccharides promoted an increased proliferation of mesenchymal stem cells and bone formation when compared to un-treated bovine bone grafts [46]. Furthermore, the use of collagenated porcine bone xenografts showed better clinical results in ridge preservation procedures when compared to cortical porcine bone [47]. Already commercialized combinations of ceramic bone xenografts with polymers or extracellular matrix proteins are shown Table 2 in the last four rows. In general, bone xenografts present a high success rate without major complications after use [48].

Table 2.
Currently commercialized bone xenografts from mammal origin. The last four rows include the commercialized combinations of polymers or extracellular matrix proteins with mammalian origin xenografts 2.
Mammalian Xenografts in Research
In addition to the already commercialized bone grafts from mammal origin, new approaches have been proposed to enhance their osteoconductivity. To this end, several strategies have been explored. Park and coworkers modified the surface of deproteinized porcine cancellous bone to introduce magnesium ions on their surface. The resulting bone xenograft showed an apparent increase in bone ingrowth when compared to non-treated deproteinized bovine and porcine grafts [49]. In a similar way, fluoride ions were added to porcine bone xenografts leading to an increase in mesenchymal stem cell proliferation and osteogenic differentiation together with an accelerated “in vivo” bone ingrowth [50]. Additionally, different authors added polymers and proteins as collagen to bioceramic xenografts to increase their hydrophilicity and improve mechanical properties [51]. High molecular weight hyaluronic acid combined with bovine xenografts has shown an increase in bone healing when compared to bovine xenografts alone [52]. Moreover, the addition of biomembrane fraction 1 protein, obtained from latex, to bone grafts was able to modulate the expression of extracellular matrix degradative enzymes “in vivo” [53]. Another attempt to enhance bone regeneration has been the implantation of bovine xenografts after soaking with “Hypericum perforatum” extract leading to an improvement in bone healing [54].
Other research aims in the mammalian xenograft field are to obtain and characterize other tissue donors suitable as bone xenografts for humans. One example is the use of calcinated antler cancellous bone obtained from deer (Cervidae spp.) due to their similar structure and composition when compared to human bone [55]. Moreover, antlers’ growth cycle guarantees a high annual availability of the graft source since the animal discards them each year and, therefore, their use does not involve animal sacrifice promoting the re-use of waste and discards [56,57,58]. Zhang and coworkers [59] demonstrated the utility of these grafts for bone regeneration, inducing neovascularization and osteogenesis in mandible defects at similar levels to grafts already on the market. As for all the animal donors, there are several differences between donor characteristics such as age that modulate xenografts porosity and crystallinity [60].
4. Bioceramic Xenografts: Marine Origin
The ocean provides bioceramics with interconnected porosity in a hierarchical structure similar to those of trabecular human bone, making them suitable materials as bone grafts with osteoconductive properties [61]. One example is the exoskeleton of several coral species, mainly composed of the crystalline ceramic structure aragonite (calcium carbonate). Two reef-building coral skeletons are commercially used as bone grafts, Porites and Goniopora, given their availability in large quantities and highly consistent structure. These bioceramics are subjected to thermal treatments to avoid immunogenic responses with only tiny quantities of intra-crystalline proteins remaining. However, they possess inherent weakness in compression and the absorption of calcium carbonate is too quick, limiting the use of these grafts [61,62].
To increase the strength of coral skeletons, so they can support the high compressive forces exerted in load bearing long bones, a chemical transformation can be performed from the native calcium carbonate composition to hydroxyapatite by a hydrothermal conversion [63]. This procedure increases graft durability as the resulting hydroxyapatite degrades slowly, with complete resorption achieved after a year or even longer. In fact, a clinical evaluation by Korovessis and coworkers in dorsal and lateral fusion for degenerative lumbar spine disease, proved the complete resorption of this coralline hydroxyapatite (mixed with local bone and bone marrow) one year after surgery [64]. This biomaterial was also tested by Coughlin and coworkers in hindfoot arthrodesis concluding their effectiveness as a bone graft in clinical foot procedures [65]. However, they reported difficulty of containing it, with extrusion present in all patients, and a too slow resorption rate (graft presence up to 6 years after surgery). More recently, Messina provided a recipient site surgical preparation protocol in dental implantation using coralline HA granules and homologous fibrin glue to minimize risks of failure related to mechanical instability and low retention at the surgical site [66].
Another variant already on the market consists of grafts produced from converted coral skeletons not entirely transformed to hydroxyapatite, so that some parts remain as calcium carbonate. In this way, the biodegradation properties are improved to suit bone remodeling, turnover, and natural bone healing [63]. Finally, to overcome the contaminant issues (intracrystalline proteins that accompany biogenic crystals) and limit ecological impact, farming techniques are being implemented, growing these marine structures in artificial aquaria [62]. The coral growth in aquariums will allow the strict control of conditions and the addition of determined ions of interest during the growth period (as silicate or phosphate ions). This technique allows to enrich the calcium carbonate structure obtaining higher bioactivity at the final mineral product [67]. The commercial coralline-derived grafts currently available for clinicians are shown at Table 3.

Table 3.
Commercially available bioceramic xenografts of marine origin.
There are products on the market that combine DBM allografts with coralline-graft granules, as is the case of StaGraftTM DBM PLUS (Zimmer Biomet) with coralline hydroxyapatite/calcium carbonate granules of Pro-Osteon® 500R. These products are indicated for filling bony voids or gaps in extremities and pelvis that are not intrinsic to the bony stability of the structure, autograft extender in the spine, bone void filler in the spine (posterolateral spine), and craniofacial defects fillers in craniotomies no larger than 25 cm2.
Marine Xenografts in Research
The commercially available unconverted coral (calcium carbonate) is still not ideal for most long-term implant purposes due to its very fast dissolution rate and poor longevity and stability. Moreover, coralline grafts with partial conversion of coral to hydroxyapatite provide poor mechanical properties for load-bearing applications when high structural strength is required. Therefore, new developments have been investigated, such as a complete conversion of coral to pure hydroxyapatite and its subsequent coating with a sol-gel-derived HA to cover micro- and nano-pores within the intra-pore material, whilst maintaining the large pores [71]. Following this strategy, the biaxial strength was improved two-fold providing enhanced durability, longevity, and strength in the physiological environment for load-bearing bone graft applications where high strength requirements are required. In this case, the coralline origin is practically anecdotal as, apart from compositional modifications, morphological changes on the porosity of the original structure are also performed. Other previous attempts focused on the mechanical strength improvement were based on the preparation of fluorine- and zirconia-doped coralline HA [72], and the recent incorporation of Sr ions on conventional coralline HA to stimulate bone formation and inhibit bone resorption [73]. Nano-hydroxyapatite/coralline grafts coated with vascular endothelial growth factor (VEGF) have also been investigated and recently tested in an alveolar defect animal model leading to significantly improved neovascularization and mineralization [74].
Apart from corals, other traditionally investigated marine bioceramic sources are nacre, seashells (foraminifera, bivalve mollusks as oyster and mussels), sponge skeletons, diatom frustules, sea urchin spines, cuttlefish bone [61], other fish bones such as tuna [75], and shark teeth [76,77]. When obtained as powders, these ceramics showed rod-like shape particles with submicron average size as shown in Figure 1A and excellent “in vitro” biocompatibility independently of the fish source (Figure 1B) [75]. Several of the marine bioceramic sources such as certain seashells, fish bones, and shark teeth, are waste or by-products from the fishing and food industries guaranteeing source abundance. Their re-use as bone grafts contributes to sustainability, by taking advantage of this waste and re-valorizing it into products with a higher added value. Again, calcium carbonate is the composition that mainly predominates in their structure, as is the case for nacre, oyster and mussel shells, calcareous sponges’ skeleton, sea urchin spines, and cuttlefish bone [61,78]. Others are based on silicon compounds as certain sponge skeletons and diatom frustules [79]. In addition, finally, fish bones and shark teeth are sources of great interest given their composition based on calcium phosphates, as human bones [80].
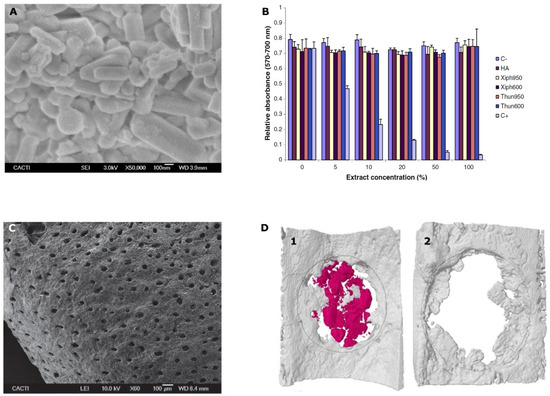
Figure 1.
(A) FSEM micrograph showing the appearance of the obtained powders from fish bones at 600 °C; (B) cell viability of osteoblasts (MC3T3-E1) after incubation with sword fish (Xiph) or tuna (Thun) samples treated at 600 or 950 °C together with commercial hydroxyapatite (HA) and control extracts for 24 h. C+: positive control. C−: negative control; (C) SEM micrographs of the shark teeth bioapatites morphology at 60×; (D) micro-CT reconstructions of an extracted bilateral parietal rat bone defect either treated with shark teeth bioapatites (1) or correspondent critical defect control (2) after 3 weeks of implantation. Marine shark teeth bioapatites are colored in red and new bone tissue in gray. Areas of interest are delimited by gray lines. Figure 1A,B reprinted from [76] with permission from Elsevier. Figure 1C,D reprinted from [78] with permission from John Wiley and Sons.
From all the calcium carbonate sources, nacre is, by far, the most studied as bone graft. It consists of a highly crystallized acellular calcium carbonate powder as pseudohexagonal aragonite nanograins encapsulated into the intracrystalline organic matrix [81]. The organic material supposes around 1.7% or less [82], and could be removed by thermal treatment at around 550–600 °C, temperature at which aragonite is already transformed to calcite (it occurs at 300–400 °C) [83]. A recent review [84] collected the “in vivo” and “in vitro” studies which revealed its osteoinductive potential, together with osteoconductivity, biocompatibility, and biodegradability. Stimulation of new bone formation was observed when it was implanted at various sites for different uses such as human maxillary alveolar bone, load-bearing sites fillers, cranial defects fillers as well as intervertebral fusion [84,85] using different animal models [86]. This material would, therefore, solve the still existing demands for bone fillers suitable in applications that require load support, being also osteoinductive. According to Zhang et al. [84], nacre presents a great potential in the field of bone substitutes given its remarkable mechanical properties, with a Young’s modulus of 70 GPa (dry) or 60 GPa (wet) equivalent to steel [61]. The absence of nacre commercial products available for clinical use could be explained by the existence of regulatory aspects or purely commercial barriers (such as profitability) that hinder its entrance in the bone grafts market. Interestingly, there is currently a large amount of literature based on the synthesis of nacre-mimetic composites. One example is the infiltration of a thermally switchable Diels-Alder polymeric network into a lamellar scaffold of alumina, recently published by Du and coworkers [87].
Another promising source currently in development is the already mentioned fishing by-product shark tooth, which directly provides calcium phosphate. In fact, according to López-Álvarez et al. [76], the two sections of shark tooth, enameloid and dentine, constitute two direct sources of bioapatites, once subjected to a thermal processing to remove the organic material. Dentine offers a porous biphasic bioceramic with an apatitic phase of HA apatite-CaF in ≈60% and a non-apatitic one with whitlockite/β-TCP in ≈40%, together with a globular morphology and bimodal porosity (~50 μm and 0.5–1.0 μm). The shark enameloid provides mainly an apatitic phase of fluorapatite in 91%, as aligned elongated crystals of 0.5–1.0 μm in the shortest dimension, and a small contribution of around 9% of whitlockite/β-TCP. This enameloid bioceramic will contribute to improve bone graft mechanical properties since, according Enax et al. [88], it is about six times harder than dentine, providing high bulk moduli and stiffness constants. Moreover, the incorporation of Fluor ions into the apatite lattice will provide protection against acids [89], and is thought to have a potential role as a cell growth factor enhancer, acting primarily on the osteoprogenitor cells and/or undifferentiated osteoblasts. Thus, its presence could contribute to bone healing and regeneration by inducing the differentiation of osteoprogenitor and undifferentiated precursor cells to osteoblasts [90].
Moreover, the presence of fluorapatite provides low resorption levels. The resorption rate of the graft would be increased and modulated (as desired) by the contribution of the non-apatitic phase from the shark dentine. Moreover, the presence of trace elements with relevant roles in bone metabolism as Mg, together with Na, Sr, K, Al, and Fe dopes these grafts with osteoinductive properties [91]. “In vitro” [76] and “in vivo” evaluation [77] of these shark teeth grafts have confirmed their good results for bone regeneration, with higher early osteogenic activity (p < 0.01) on MC3T3-E1 pre-osteoblasts after 21 days of incubation than commercial synthetic and bovine bone grafts [77]. Moreover, the porosity provided by dentine together with the inter-granular cavities allowed the ingrowth of new bone cells (osteoconductive properties) after three weeks of implantation in a rodent model, showing higher osteointegration than commercial synthetic granules (biphasic HA/β-TCP (60%/40%)) [77]. Bone formation was observed from the critical defect surroundings but also at its central area indicating also potential osteoinductive properties (Figure 1D) that could be associated to the porous structure of the ceramic as shown in Figure 1C. Furthermore, significantly higher bone mineral density (p < 0.05) was quantified on these grafts when compared to the commercial synthetic graft [77]. Shark tooth is, therefore, an interesting source of bone grafts with great potential presenting osteoinductive properties and moldable resorption level, features that are not yet commercially available in any bone graft of biological origin.
5. Concluding Remarks
In this review we summarized the current available biological-derived ceramic bone grafts, including their advantages, limitations, and applications that are collected and summarized in Table 4. Despite the wide variety of bone graft alternatives currently clinically available, there is no perfect material able to fulfill bone tissue requirements. While autografts are the gold standard, the limited volume available is their main drawback. On the other hand, allografts do not present this problem but present diminished osteoinductive and mechanical properties. The use of mammalian and marine xenografts allow for availability in large quantities but did not present the exact human bone tissue morphology and their osteoinductive capacities and biological responses are decreased when compared to autographs and allographs.

Table 4.
Advantages, limitations, and applications of biological-derived ceramic bone grafts.
The bone grafts market is expected to keep steadily increasing in the near future and the search for materials able to fulfill all the requirements for adequate bone function, promoting osteoinduction and osteoconduction, continues to be a point of interest for the companies. A gradual transition is emerging from current procedures based on autografts and allografts to commercial grafts of synthetic or biological origin (xenografts). However, the higher biological response obtained with natural calcium phosphate based grafts, when compared to those of synthetic origin, make them “a priori” a better choice for bone regeneration. The wide variety of marine derived ceramic grafts allowing for the second use of animal discards and fishing by-products provides a viable source of bone grafts while contributing to sustainability. The research recently performed on the marine field for new bone grafts sources has led to the discovery of new naturally-derived bone grafts with promising mechanical and biological features. However, further studies should be performed to ensure their adequate performance in terms of 3D bone regeneration and toxicity to allow their commercial exploitation and future clinical use.
Author Contributions
P.D.-R. and M.L.-A. analyzed the bibliography and prepared the tables and figure. P.D.-R., M.L.-A., J.S., P.G., M.L. designed the project and wrote the manuscript. All authors reviewed the manuscript.
Funding
This research was partially supported by the European Union projects 0245_IBEROS_1_E and 0302_CVMAR_I_1_P, both from Interreg V-A Spain-Portugal (POCTEP 2015) and BLUEHUMAN EAPA_151/2016 from INTERREG Atlantic Area, European Regional Development Fund. Moreover, regional funds from Competitive Reference Groups (GRC) ED431C 2016/008 and ED431C 2017_51 and Research networks ED431D 2017/13 both from Xunta de Galicia (Spain) are also acknowledged. P. Diaz-Rodriguez and M. López-Alvarez are thankful for the funding support provided by 0245_IBEROS_1_E from EU Interreg V-A Spain-Portugal (POCTEP) project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoarau-Vechot, J.; Rafii, A.; Touboul, C. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology (Bethesda) 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, J.; Bertero, A.; Coccini, T.; Baderna, D.; Buzanska, L.; Caloni, F. Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol. JAT 2019. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; Erndt-Marino, J.; Chen, H.; Diaz-Quiroz, J.F.; Samavedi, S.; Hahn, M.S. A Bioengineered in vitro osteoarthritis model with tunable inflammatory environments indicates context-dependent therapeutic potential of human mesenchymal stem cells. Regen. Eng. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, R.I. Multi-Parametric Live Cell Microscopy of 3D Tissue Models; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Sánchez, M.; Landin, M. Drug-Loaded Biomimetic Ceramics for Tissue Engineering. Pharmaceutics 2018, 10, 272. [Google Scholar] [CrossRef]
- Crane, M.M.; Kaeberlein, M. The paths of mortality: How understanding the biology of aging can help explain systems behavior of single cells. Curr. Opin. Syst. Biol. 2018, 8, 25–31. [Google Scholar] [CrossRef]
- Lalzawmliana, V.A.A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine organisms as a source of natural matrix for bone tissue engineering. Ceram. Int. 2019, 45A, 1469–1481. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- MediPoint. Available online: https://www.reportlinker.com/p02013605/MediPoint-Bone-Grafts-and-Substitutes-Global-Analysis-and-Market-Forecasts.html (accessed on 09 July 2019).
- Fernández-Hernández, O. Terapias para el hueso: Sustitutivos óseos. Mon. Act. Soc. Esp. Med. Cir. Pie Tobillo. 2017, 8, 45–53. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25, S101–S103. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.A.; Cook, J.J. Bone graft substitutes and allografts for reconstruction of the foot and ankle. Clin. Podiatr. Med. Surg. 2009, 26, 589–605. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002. [Google Scholar] [CrossRef]
- Tatay Díaz, A.; Pérez Sánchez, J.M.; RiberaZabalbeascoa, J.; Cordero Fernández, J.A.; Mella Sousa, M. Sustitutos óseos. Revista de la Sociedad Andaluza de Traumatología y Ortopedia 2008, 26, 2–13. [Google Scholar]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Tortolini, P.; Rubio, S. Diferentes alternativas de rellenos óseos. Avances en Periodoncia e Implantología Oral 2012, 24, 133–138. [Google Scholar] [CrossRef]
- Müller, S.A.; Barg, A.; Vavken, P.; Valderrabano, V.; Müller, A.M. Autograft versus sterilized allograft for lateral calcaneal lengthening osteotomies: Comparison of 50 patients. Medicine (Baltimore) 2016, 95, e4343. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Ferreira, J.M.F. Synthetic and Marine-Derived Porous Scaffolds for Bone Tissue Engineering. Materials (Basel) 2018, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Tomford, W.W.; Bloem, R.M.; Mankin, H.J. Osteoarticular allografts. Acta Orthop. Belg. 1991, 57, 98–102. [Google Scholar] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Yeoh, J.C.; Taylor, B.A. Osseous Healing in Foot and Ankle Surgery with Autograft, Allograft, and Other Orthobiologics. Orthop. Clin. North Am. 2017, 48, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, D.; Singh, A. Radiation sterilization of tissue allografts: A review. World J. Radiol. 2016, 8, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Shehadi, J.A.; Elzein, S.M. Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm. Surg. Neurol. Int. 2017, 8, 203. [Google Scholar] [CrossRef]
- Lindsey, R.W.; Wood, G.W.; Sadasivian, K.K.; Stubbs, H.A.; Block, J.E. Grafting long bone fractures with demineralized bone matrix putty enriched with bone marrow: Pilot findings. Orthopedics 2006, 29, 939–941. [Google Scholar]
- Ajiboye, R.M.; Eckardt, M.A.; Hamamoto, J.T.; Plotkin, B.; Daubs, M.D.; Wang, J.C. Outcomes of Demineralized Bone Matrix Enriched with Concentrated Bone Marrow Aspirate in Lumbar Fusion. Int. J. Spine Surg. 2016, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Al Kayal, T.; Panetta, D.; Canciani, B.; Losi, P.; Tripodi, M.; Burchielli, S.; Ottoni, P.; Salvadori, P.A.; Soldani, G. Evaluation of the effect of a gamma irradiated DBM-pluronic F127 composite on bone regeneration in Wistar rat. PLoS ONE 2015, 10, e0125110. [Google Scholar] [CrossRef] [PubMed]
- MedTronic. Available online: https://www.medtronic.com/us-en/healthcare-professionals/products/spinal-orthopaedic/bone-grafting/infuse-bone-graft.html (accessed on 09 July 2019).
- Kim, S.H.; Shin, J.W.; Park, S.A.; Kim, Y.K.; Park, M.S.; Mok, J.M.; Yang, W.I.; Lee, J.W. Chemical, structural properties, and osteoconductive effectiveness of bone block derived from porcine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 68, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N.; Davison, N.; Fitzpatrick, D.; Marshall, R.; Lin, A.; Mundy, K.; Cobb, R.R. Characterization of the mechanical properties of bovine cortical bone treated with a novel tissue sterilization process. Cell Tissue Bank. 2011, 12, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Supronowicz, P.; Zhukauskas, R.; York-Ely, A.; Wicomb, W.; Thula, T.; Fleming, L.; Cobb, R.R. Immunologic analyses of bovine bone treated with a novel tissue sterilization process. Xenotransplantation 2008, 15, 398–406. [Google Scholar] [CrossRef]
- Alayan, J.; Ivanovski, S. A prospective controlled trial comparing xenograft/autogenous bone and collagen-stabilized xenograft for maxillary sinus augmentation-Complications, patient-reported outcomes and volumetric analysis. Clin. Oral Implant. Res. 2018, 29, 248–262. [Google Scholar] [CrossRef]
- Serrano, C.A.; Castellanos, P.; Botticelli, D. Use of Combination of Allografts and Xenografts for Alveolar Ridge Preservation Procedures: A Clinical and Histological Case Series. Implant. Dent. 2018, 27, 467–473. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Calvo Guirado, J.L.; Ramirez Fernandez, M.P.; Negri, B.; Delgado Ruiz, R.A.; Mate Sanchez de-Val, J.E.; Gomez-Moreno, G. Experimental model of bone response to collagenized xenografts of porcine origin (OsteoBiol(R) mp3): A radiological and histomorphometric study. Clin. Implant Dent. Relat. Res. 2013, 15, 143–151. [Google Scholar] [CrossRef]
- Orsini, G.; Scarano, A.; Piattelli, M.; Piccirilli, M.; Caputi, S.; Piattelli, A. Histologic and ultrastructural analysis of regenerated bone in maxillary sinus augmentation using a porcine bone-derived biomaterial. J. Periodontol. 2006, 77, 1984–1990. [Google Scholar] [CrossRef]
- Lee, J.H.; Yi, G.S. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal. Implant Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Pagliani, L.; Andersson, P.; Lanza, M.; Nappo, A.; Verrocchi, D.; Volpe, S.; Sennerby, L. A collagenated porcine bone substitute for augmentation at Neoss implant sites: A prospective 1-year multicenter case series study with histology. Clin. Implant Dent. Relat. Res. 2012, 14, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Mayer, Y.; Ginesin, O.; Khutaba, A.; Machtei, E.E.; Zigdon Giladi, H. Biocompatibility and osteoconductivity of PLCL coated and noncoated xenografts: An in vitro and preclinical trial. Clin. Implant Dent. Relat. Res. 2018, 20, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Calvo-Guirado, J.L.; Negri, B.; Di Felice, R.; Covani, U. Volumetric analysis of remodelling pattern after ridge preservation comparing use of two types of xenografts. A multicentre randomized clinical trial. Clin. Oral Implants Res. 2016, 27, e105–e115. [Google Scholar] [CrossRef]
- de Azambuja Carvalho, P.H.; Dos Santos Trento, G.; Moura, L.B.; Cunha, G.; Gabrielli, M.A.C.; Pereira-Filho, V.A. Horizontal ridge augmentation using xenogenous bone graft-systematic review. Oral Maxillofac. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Ko, H.J.; Jang, J.H.; Kang, H.; Suh, J.Y. Increased new bone formation with a surface magnesium-incorporated deproteinized porcine bone substitute in rabbit calvarial defects. J. Biomed. Mater. Res. Part A 2012, 100, 834–840. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, R.; Li, Z.; Luo, X.; Huang, B.; Liu, Q.; Chen, Z. Contribution of the in situ release of endogenous cations from xenograft bone driven by fluoride incorporation toward enhanced bone regeneration. Biomater. Sci. 2018, 6, 2951–2964. [Google Scholar] [CrossRef]
- Cingolani, A.; Grottoli, C.F.; Esposito, R.; Villa, T.; Rossi, F.; Perale, G. Improving Bovine Bone Mechanical Characteristics for the Development of Xenohybrid Bone Grafts. Curr. Pharm. Biotechnol. 2018, 19, 1005–1013. [Google Scholar] [CrossRef]
- Arpag, O.F.; Damlar, I.; Altan, A.; Tatli, U.; Gunay, A. To what extent does hyaluronic acid affect healing of xenografts? A histomorphometric study in a rabbit model. J. Appl. Oral Sci. Rev. FOB 2018, 26, e20170004. [Google Scholar] [CrossRef]
- Santos Kotake, B.G.; Gonzaga, M.G.; Coutinho-Netto, J.; Ervolino, E.; de Figueiredo, F.A.T.; Issa, J.P.M. Bone repair of critical-sized defects in Wistar rats treated with autogenic, allogenic or xenogenic bone grafts alone or in combination with natural latex fraction F1. Biomed. Mater. (Bristol Engl.) 2018, 13, 025022. [Google Scholar] [CrossRef]
- Damlar, I.; Arpag, O.F.; Tatli, U.; Altan, A. Effects of Hypericum perforatum on the healing of xenografts: A histomorphometric study in rabbits. Br. J. Oral Maxillofac. Surg. 2017, 55, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, Q.; Liu, H.; Heng, B.C.; Peng, H.; Song, Y.; Yang, Z.; Deng, X. Osteoconductive effectiveness of bone graft derived from antler cancellous bone: An experimental study in the rabbit mandible defect model. Int. J. Oral Maxillofac. Surg. 2012, 41, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, U.; Stoffels, D.; Kierdorf, H. Element concentrations and element ratios in antler and pedicle bone of yearling red deer (Cervus elaphus) stags-a quantitative X-ray fluorescence study. Biol. Trace Elem. Res. 2014, 162, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.J. 2-the Diversity of Deer. In Deer Antlers; Goss, R.J., Ed.; Academic Press: San Diego, CA, USA, 1983; pp. 6–51. [Google Scholar]
- Gaspar-López, E.; Landete-Castillejos, T.; Estevez, J.; Ceacero, F.; Gallego, L.; García, A. Biometrics, Testosterone, Cortisol and Antler Growth Cycle in Iberian Red Deer Stags (Cervus elaphus hispanicus). Reprod. Domest. Anim. 2010, 45, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, M.; Song, L.; Wei, Y.; Lin, Y.; Liu, W.; Heng, B.C.; Peng, H.; Wang, Y.; Deng, X. Effects of compatibility of deproteinized antler cancellous bone with various bioactive factors on their osteogenic potential. Biomaterials 2013, 34, 9103–9114. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhang, X.; Xu, M.; Heng, B.C.; Dai, X.; Mo, X.; Wei, J.; Wei, Y.; Deng, X. Effects of deer age on the physicochemical properties of deproteinized antler cancellous bone: An approach to optimize osteoconductivity of bone graft. Biomed. Mater. (Bristol Engl.) 2015, 10, 035006. [Google Scholar] [CrossRef] [PubMed]
- Macha, I.J.; Ben-Nissan, B. Marine Skeletons: Towards Hard Tissue Repair and Regeneration. Mar. Drugs 2018, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Lai, W.F.; Jung, H.S. Evolving marine biomimetics for regenerative dentistry. Mar. Drugs 2014, 12, 2877–2912. [Google Scholar] [CrossRef]
- Fu, K.; Xu, Q.; Czernuszka, J.; Triffitt, J.T.; Xia, Z. Characterization of a biodegradable coralline hydroxyapatite/calcium carbonate composite and its clinical implementation. Biomed. Mater. (Bristol Engl.) 2013, 8, 065007. [Google Scholar] [CrossRef]
- Korovessis, P.; Koureas, G.; Zacharatos, S.; Papazisis, Z.; Lambiris, E. Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus coralline hydroxyapatite. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2005, 14, 630–638. [Google Scholar] [CrossRef]
- Coughlin, M.J.; Grimes, J.S.; Kennedy, M.P. Coralline hydroxyapatite bone graft substitute in hindfoot surgery. Foot Ankle Int. 2006, 27, 19–22. [Google Scholar] [CrossRef]
- Messina, A.M.; Marini, L.; Oh, D.S.; Marini, E. A Step-by-Step Procedure for Bone Regeneration Using Calcium Phosphate Scaffolds: From Site Preparation to Graft Placement. J. Craniofacial Surg. 2019, 30, 149–153. [Google Scholar] [CrossRef]
- Schwartz, O.; Binderman, I. Coral Bone Graft Substitute. U.S. Patent 8936638B2, 2011. [Google Scholar]
- Damien, E.; Revell, P.A. Coralline hydroxyapatite bone graft substitute: A review of experimental studies and biomedical applications. J. Appl. Biomater. Biomech. JABB 2004, 2, 65–73. [Google Scholar]
- Schopper, C.; Moser, D.; Sabbas, A.; Lagogiannis, G.; Spassova, E.; Konig, F.; Donath, K.; Ewers, R. The fluorohydroxyapatite (FHA) FRIOS Algipore is a suitable biomaterial for the reconstruction of severely atrophic human maxillae. Clin. Oral Implants Res. 2003, 14, 743–749. [Google Scholar]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Milev, A.; Vago, R. Morphology of sol-gel derived nano-coated coralline hydroxyapatite. Biomaterials 2004, 25, 4971–4975. [Google Scholar] [CrossRef]
- Sivakumar, M.; Manjubala, I. Preparation of hydroxyapatite/fluoroapatite-zirconia composites using Indian corals for biomedical applications. Mater. Lett. 2001, 50, 199–205. [Google Scholar] [CrossRef]
- Sethmann, I.; Luft, C.; Kleebe, H.J. Development of Phosphatized Calcium Carbonate Biominerals as Bioactive Bone Graft Substitute Materials, Part I: Incorporation of Magnesium and Strontium Ions. J. Funct. Biomater. 2018, 9, 69. [Google Scholar] [CrossRef]
- Du, B.; Gao, Y.; Deng, Y.; Zhao, Y.; Lai, C.; Guo, Z.; Rong, M.; Zhou, L. Local delivery of rhVEGF165 through biocoated nHA/coral block grafts in critical-sized dog mandible defects: A histological study at the early stages of bone healing. Int. J. Clin. Exp. Med. 2015, 8, 4940–4953. [Google Scholar]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng.C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Lopez-Alvarez, M.; Perez-Davila, S.; Rodriguez-Valencia, C.; Gonzalez, P.; Serra, J. The improved biological response of shark tooth bioapatites in a comparative in vitro study with synthetic and bovine bone grafts. Biomed. Mater. (Bristol Engl.) 2016, 11, 035011. [Google Scholar] [CrossRef]
- Lopez-Alvarez, M.; Vigo, E.; Rodriguez-Valencia, C.; Outeirino-Iglesias, V.; Gonzalez, P.; Serra, J. In vivo evaluation of shark teeth-derived bioapatites. Clin. Oral Implants Res. 2017, 28, e91–e100. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Garcia-Triñanes, P.; Echezarreta López, M.M.; Santoveña, A.; Landin, M. Mineralized alginate hydrogels using marine carbonates for bone tissue engineering applications. Carbohydr. Polym. 2018, 195, 235–242. [Google Scholar] [CrossRef]
- Maldonado, M.; Navarro, L.; Grasa, A.; Gonzalez, A.; Vaquerizo, I. Silicon uptake by sponges: A twist to understanding nutrient cycling on continental margins. Sci. Rep. 2011, 1, 30. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Rousseau, M.; Lopez, E.; Stempfle, P.; Brendle, M.; Franke, L.; Guette, A.; Naslain, R.; Bourrat, X. Multiscale structure of sheet nacre. Biomaterials 2005, 26, 6254–6262. [Google Scholar] [CrossRef]
- Lamghari, M.; Huet, H.; Laurent, A.; Berland, S.; Lopez, E. A model for evaluating injectable bone replacements in the vertebrae of sheep: Radiological and histological study. Biomaterials 1999, 20, 2107–2114. [Google Scholar] [CrossRef]
- Balmain, J.; Hannoyer, B.; Lopez, E. Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction analyses of mineral and organic matrix during heating of mother of pearl (nacre) from the shell of the mollusc Pinctada maxima. J. Biomed. Mater. Res. 1999, 48, 749–754. [Google Scholar] [CrossRef]
- Zhang, G.; Brion, A.; Willemin, A.S.; Piet, M.H.; Moby, V.; Bianchi, A.; Mainard, D.; Galois, L.; Gillet, P.; Rousseau, M. Nacre, a natural, multi-use and timely biomaterial for bone graft substitution. J. Biomed. Mater. Res. Part A 2017, 105, 662–671. [Google Scholar] [CrossRef]
- Atlan, G.; Balmain, N.; Berland, S.; Vidal, B.; Lopez, E. Reconstruction of human maxillary defects with nacre powder: Histological evidence for bone regeneration. Comptes Rendus De l’Academie Des Sci. Ser. III Sci. De La Vie 1997, 320, 253–258. [Google Scholar] [CrossRef]
- Pascaretti-Grizon, F.; Libouban, H.; Camprasse, G.; Camprasse, S.; Mallet, R.; Chappard, D. The interface between nacre and bone after implantation in the sheep: A nanotomographic and Raman study. J. Raman Spectrosc. 2014, 45, 558–564. [Google Scholar] [CrossRef]
- Du, G.; Mao, A.; Yu, J.; Hou, J.; Zhao, N.; Han, J.; Zhao, Q.; Gao, W.; Xie, T.; Bai, H. Nacre-mimetic composite with intrinsic self-healing and shape-programming capability. Nat. Commun. 2019, 10, 800. [Google Scholar] [CrossRef]
- Enax, J.; Prymak, O.; Raabe, D.; Epple, M. Structure, composition, and mechanical properties of shark teeth. J. Struct. Biol. 2012, 178, 290–299. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Chemical and crystallographic events in the caries process. J. Dent. Res. 1990, 69, 567–574. [Google Scholar] [CrossRef]
- Ellingsen, J.E.; Thomsen, P.; Lyngstadaas, S.P. Advances in dental implant materials and tissue regeneration. Periodontol. 2000 2006, 41, 13–156. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, M.E.; Canalejo, A.; Herencia, C.; Martinez-Moreno, J.M.; Peralta-Ramirez, A.; Perez-Martinez, P.; Navarro-Gonzalez, J.F.; Rodriguez, M.; Peter, M.; Gundlach, K.; et al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol. Dial. Transplant. Off. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2014, 29, 282–289. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).