Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba
Abstract
1. Introduction
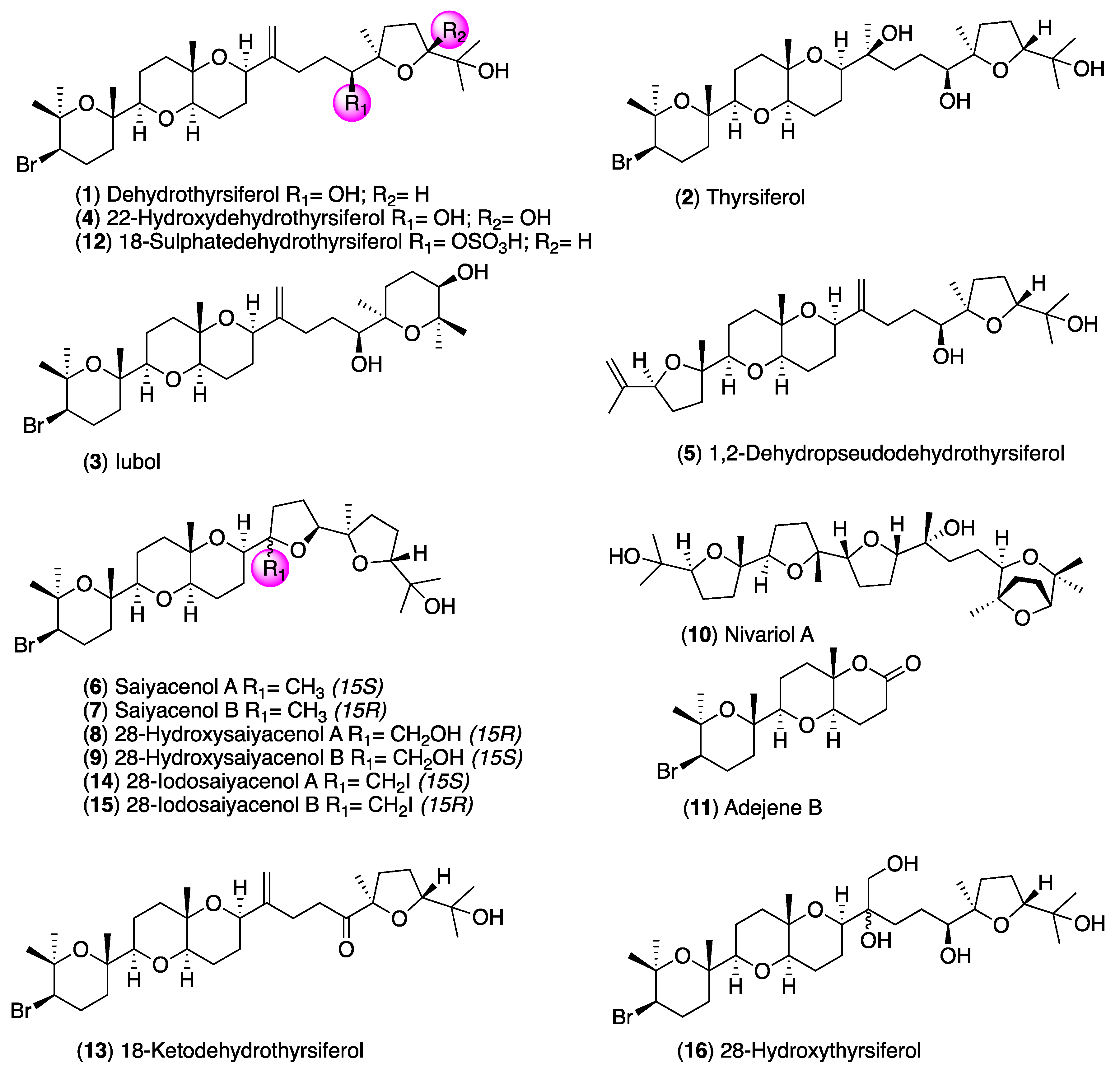
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Isolation of Laurencia Metabolites
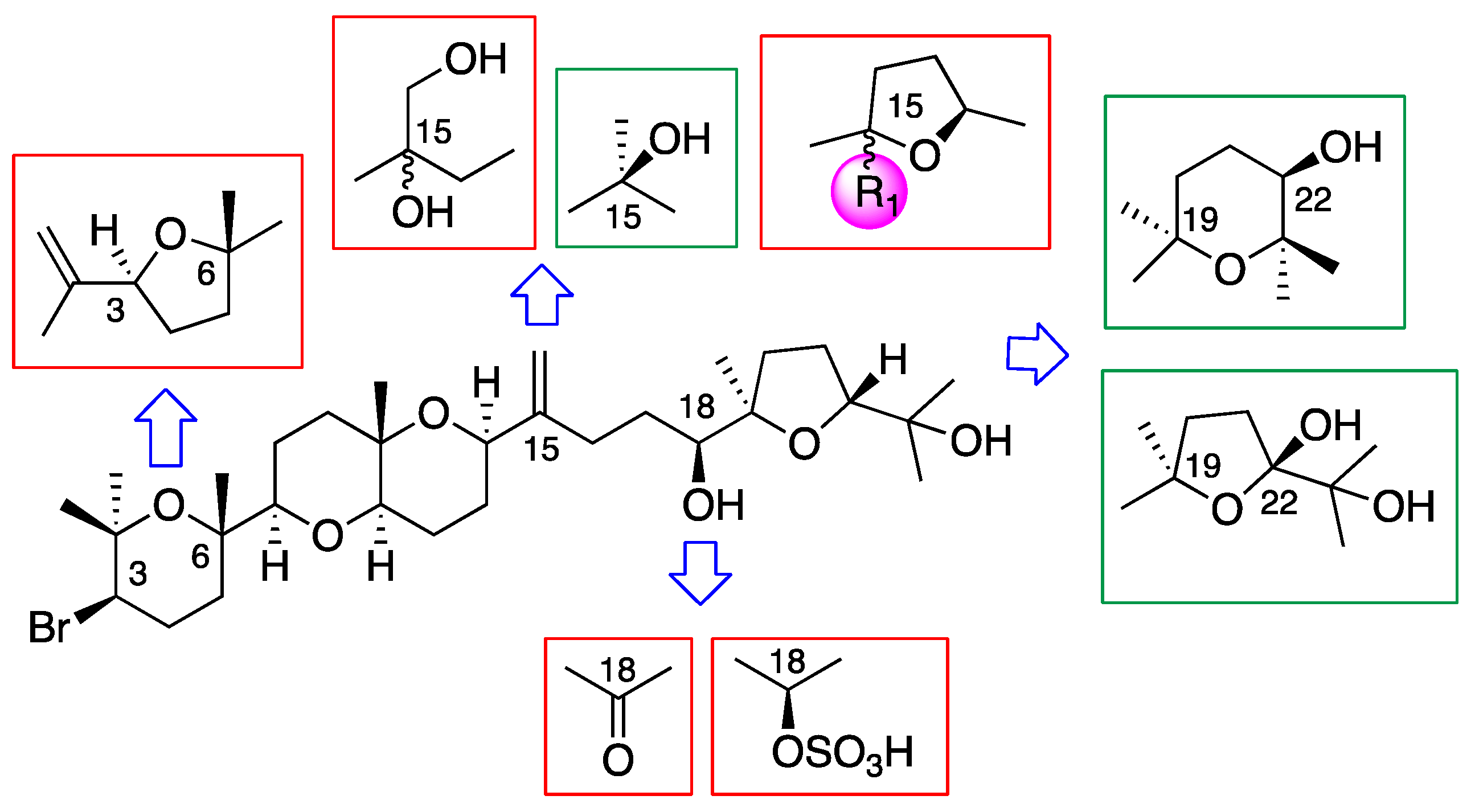
3.3. Chemical Transformations of Dehydrothyrsiferol (1)
3.3.1. Preparation of 18-sulphatedehydrothyrsiferol (12)
3.3.2. Chemical Transformation of Dehydrothyrsiferol (1) into 18-Ketodehydrothyrsiferol (13)
3.3.3. Chemical Transformations of Dehydrothyrsiferol (1) into 28-Iodosaiyacenol A and B (14 and 15)
3.3.4. Synthesis of 15,28 Diol-Compounds (16)
3.3.5. Preparation of Synthetic 28-Hydroxysaiyacenol A and B (8 and 9)
3.4. Cell Strains and Chemical Inhibitors
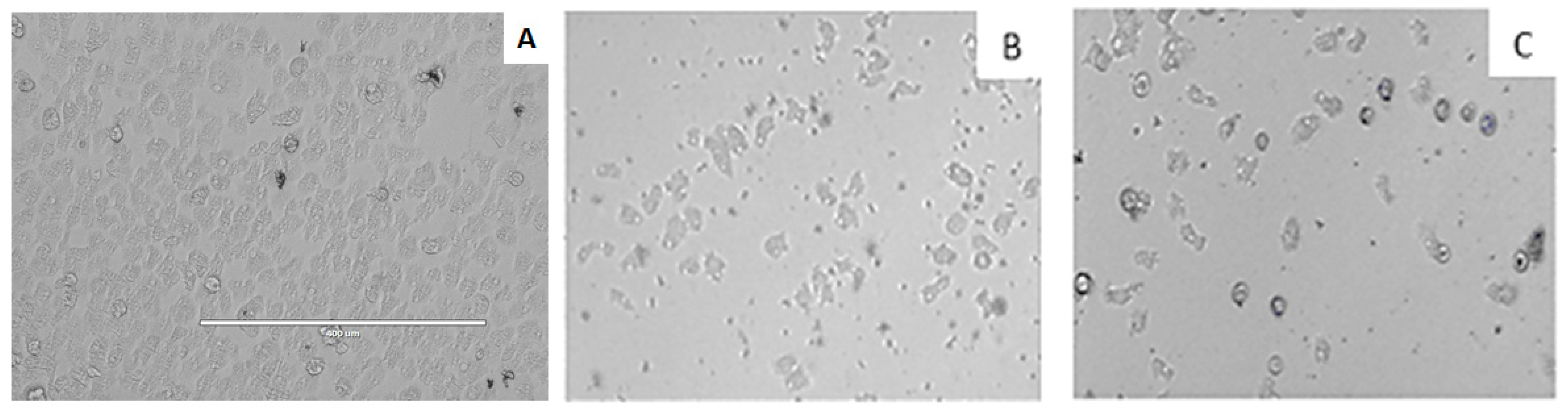
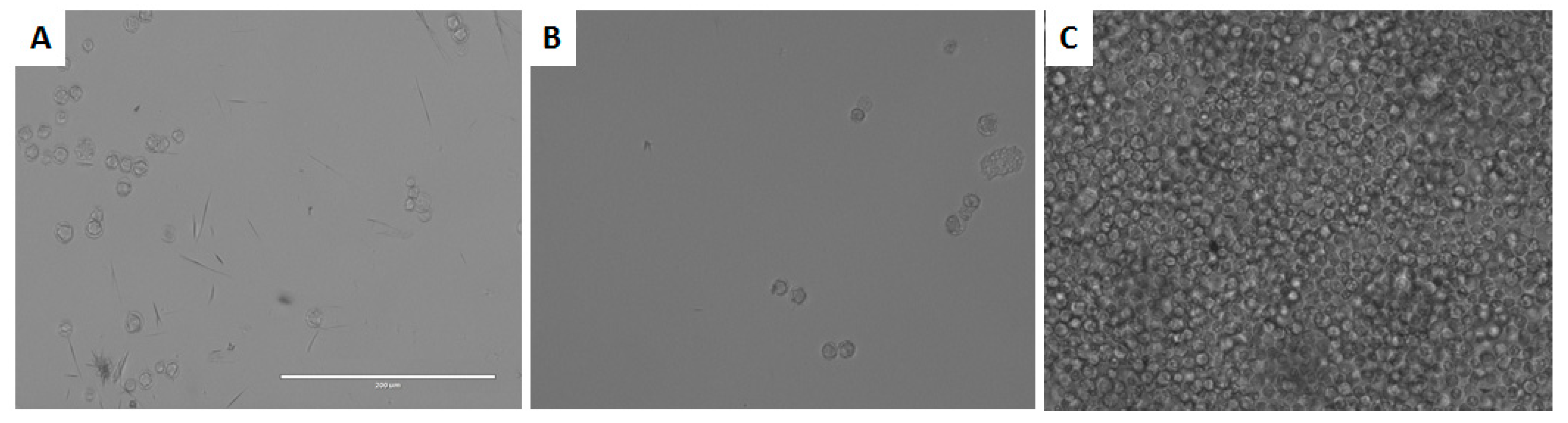
3.5. In Vitro Activity against Acanthamoeba spp. Trophozoites
3.6. In vitro Activity against Acanthamoeba castellanii Neff cysts
3.7. Cytotoxicity Assay
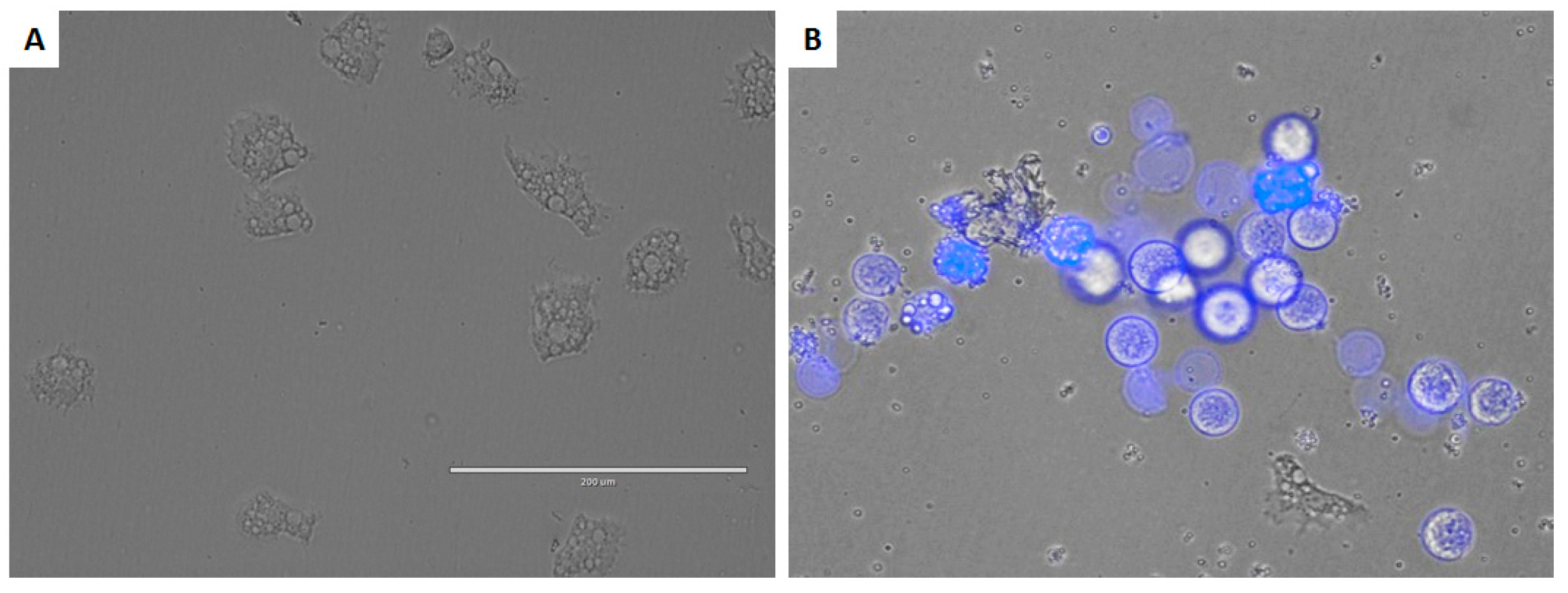
3.8. Double-Stain Assay for Programmed Cell Death Determination
3.9. Intracellular Reactive Oxygen Species (ROS) Production Using CellROX® Deep Red Staining
3.10. Analysis of Mitochondrial Membrane Potential
3.11. Measurement of ATP Levels
3.12. Plasma Membrane Permeability
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabral, G.; Marciano-Cabral, F. Acanthamoeba spp. as Agents of Disease in Humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Abd El Wahab, W.M.; El-Badry, A.A.; Hamdy, D.A. Molecular characterization and phylogenetic analysis of Acanthamoeba isolates in tap water of Beni-Suef, Egypt. Acta Parasitol. 2018, 63, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Batlle, M.; Hernández-Piñero, I.; Rizo-Liendo, A.; López-Arencibia, A.; Sifaoui, I.; Bethencourt-Estrella, C.J.; Chiboub, O.; Valladares, B.; Piñero, J.E.; Lorenzo-Morales, J. Isolation and molecular identification of Free-Living Amoebae from dishcloths in Tenerife, Canary Islands, Spain. Parasitol. Res. 2019, 118, 927–933. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, A.; Salazar-Villatoro, L.; Omaña-Molina, M.; Reyes-Batlle, M.; Martín-Navarro, C.M.; Lorenzo-Morales, J. Morphological features and in vitro cytopathic effect of Acanthamoeba griffini trophozoites isolated from a clinical case. J. Parasitol. Res. 2014, 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Visvesvara, G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004, 34, 1001–1027. [Google Scholar] [CrossRef] [PubMed]
- Alkharashi, M.; Lindsley, K.; Law, H.A.; Sikder, S. Medical interventions for Acanthamoeba keratitis. Cochrane Database Syst. Rev. 2015, 2, CD010792. [Google Scholar] [CrossRef]
- Maycock, N.J.R.; Jayaswal, R. Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 2016, 35, 713–720. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Neelam, S.; Niederkorn, J.Y. Pathobiology and immunobiology of Acanthamoeba keratitis: Insights from animal models. Yale J. Biol. Med. 2017, 90, 261–268. [Google Scholar] [PubMed]
- Carrijo-Carvalho, L.C.; Santana, V.P.; Foronda, A.S.; De Freitas, D.; De Souza Carvalho, F.R. Therapeutic agents and biocides for ocular infections by free-living amoebae of Acanthamoeba genus. Surv. Ophthalmol. 2017, 62, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Harizani, M.; Ioannou, E.; Roussis, V. The Laurencia Paradox: An Endless Source of chemodiversity. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Galk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Berlin, Germany, 2016; Volume 102, pp. 91–252. [Google Scholar]
- García-Davis, S.; Sifaoui, I.; Reyes-Batlle, M.; Viveros-Valdez, E.; Piñero, J.E.; Lorenzo-Morales, J.; Fernández, J.J.; Díaz-Marrero, A.R. Anti-Acanthamoeba Activity of Brominated Sesquiterpenes from Laurencia johnstonii. Mar. Drugs 2018, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Arteaga, J.M.; Fernandez, J.J.; Martin, J.D.; Norte, M.; Ruano, J.Z. Terpenoids of rhe Red Alga Laurencia pinnatifida. Tetrahedron 1984, 40, 2751–2755. [Google Scholar] [CrossRef]
- Blunt, J.W.; Hartshorn, M.P.; McLennan, T.J.; Munro, M.H.G.; Robinson, W.T.; Yorke, S.C. Thyrsiferol: A squalene-derived metabolite of Laurencia thyrsifera. Tetrahedron Lett. 1978, 19, 69–72. [Google Scholar] [CrossRef]
- Cen-Pacheco, F.; Villa-Pulgarin, J.A.; Mollinedo, F.; Martín, M.N.; Fernández, J.J.; Daranas, A.H. New polyether triterpenoids from Laurencia viridis and their biological evalution. Mar. Drugs 2011, 9, 2220–2235. [Google Scholar] [CrossRef]
- Cen-Pacheco, F.; Mollinedo, F.; Villa-Pulgarin, J.; Martín, M.N.; Fernández, J.J.; Daranas, A.H. Saiyacenols A and B: The key to solve the controversy about the configuration of aplysiols. Tetrahedron 2012, 68, 7275–7279. [Google Scholar] [CrossRef]
- Cen-Pacheco, F.; Santiago-Benítez, A.J.; García, C.; Álvarez-Méndez, S.J.; Martín-Rodríguez, A.J.; Martín, M.N.; Martín, V.S.; Gavín, J.A.; Fernández, J.J.; Daranas, A.H. Oxasqualenoids from Laurencia viridis: Combined spectroscopic–computational analysis and antifouling potential. J. Nat. Prod. 2015, 78, 712–721. [Google Scholar] [CrossRef]
- Cen-Pacheco, F.; Rodríguez, J.; Martín, M.N.; Fernández, J.J.; Daranas, A.H. Connecting discrete stereoclusters by using DFT and NMR spectroscopy: The case of nivariol. Chem. Eur. J. 2013, 19, 8525–8532. [Google Scholar] [CrossRef]
- Cen-Pacheco, F.; Nordström, L.; Souto, M.L.; Martín, M.N.; Fernández, J.J.; Daranas, A.H. Studies on Polyethers Produced by Red Algae. Mar. Drugs 2010, 8, 1178–1188. [Google Scholar] [CrossRef]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasites Vectors 2011, 4, 44. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Kliescikova, J.; Martinez-Carretero, E.; De Pablos, L.M.; Profotova, B.; Nohynkova, E.; Osuna, A.; Valladares, B. Glycogen phosphorylase in Acanthamoeba spp.: Determining the role of the enzyme during the encystment process using RNA interference. Eukaryot. Cell. 2008, 7, 509–517. [Google Scholar] [CrossRef]
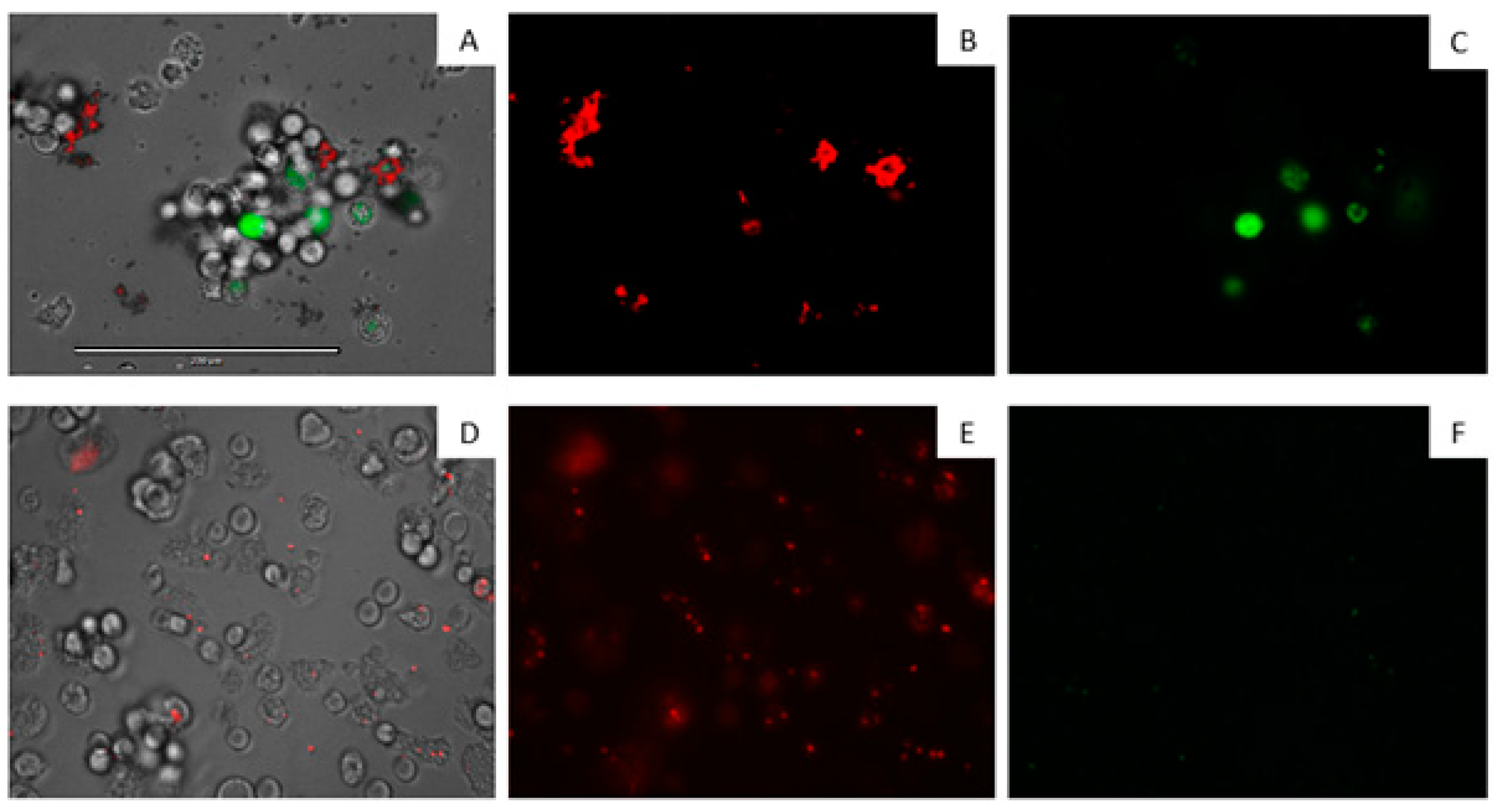
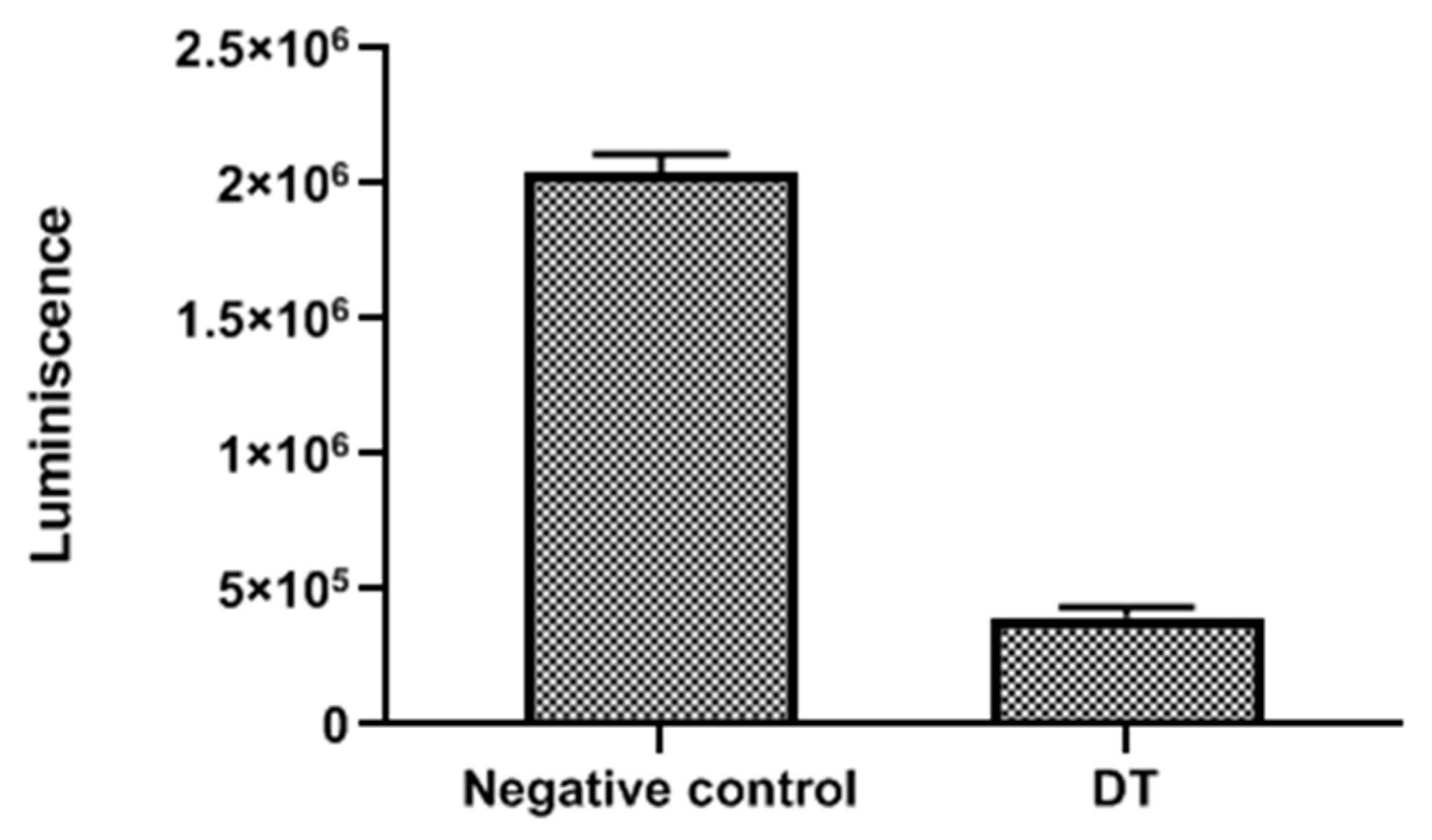
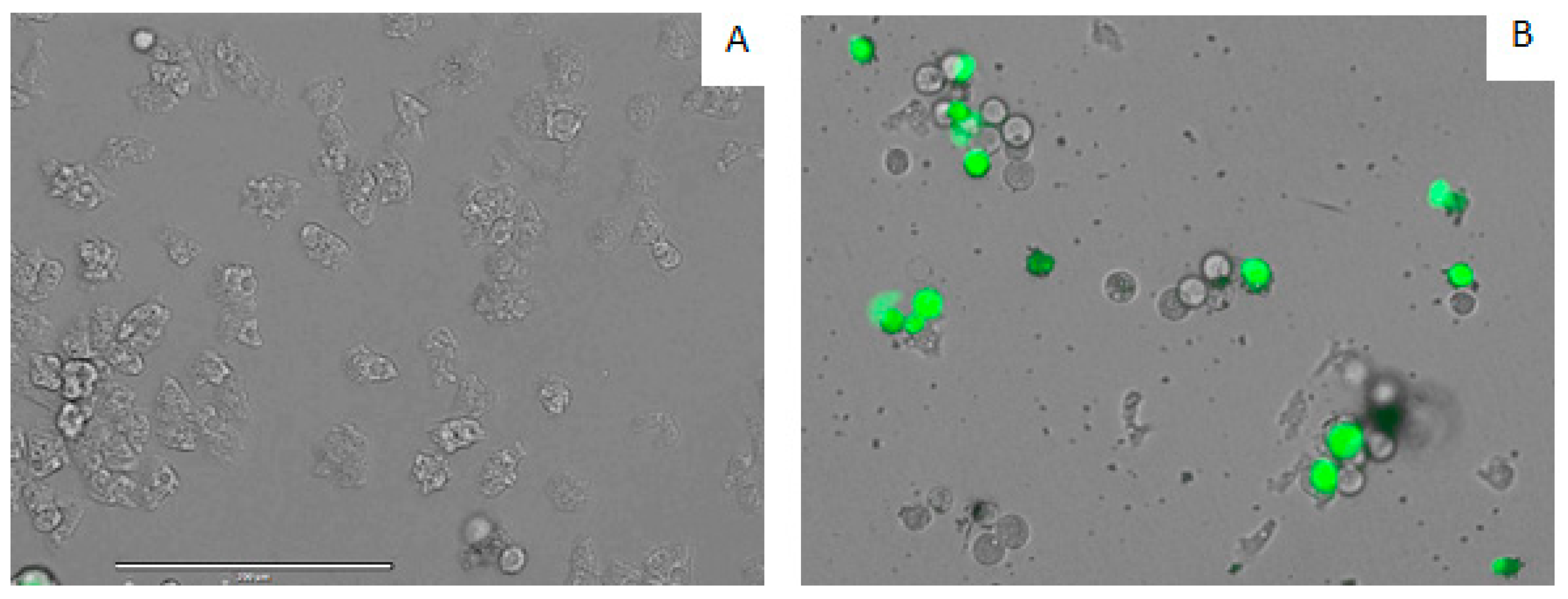
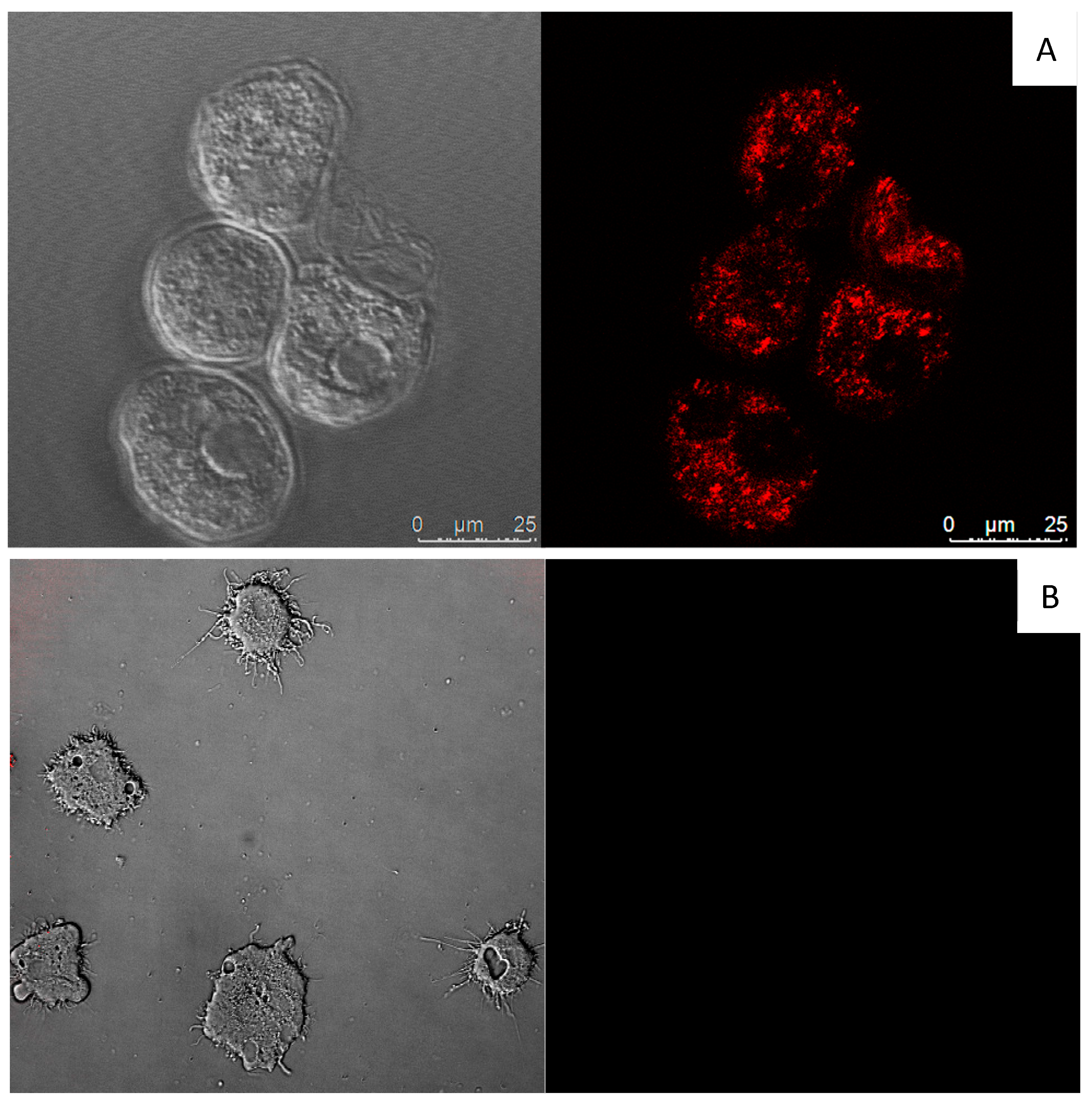
| Compound | IC50 (µM) | CC50 (µM) |
|---|---|---|
| Dehydrothyrsiferol (DT) (1) | 12.83 ± 1.38 ab | 28.77 ±3.10 B |
| Thyrsiferol (2) | 13.97 ± 1.57 ab | >100 D |
| Iubol (3) | 5.30 ± 0.87 ab | 7.72 ±0.22 A |
| 22-Hydroxydehydrothyrsiferol (4) | 17.00 ± 4.57 ab | >100 D |
| 1,2-Dehydropseudodehydrothyrsiferol (5) | 104.76 ± 1.72 f | >100 D |
| Saiyacenol A (6) | 55.43 ± 6.56 de | 59.91 ±8.50 C |
| Saiyacenol B (7) | 77.89 ± 3.30 ef | >100 D |
| 28-Hydroxysaiyacenol A (8) | 66.22 ± 3.81 de | >100 D |
| 28-Hydroxysaiyacenol B (9) | 59.92 ± 10.07 de | >100 D |
| Nivariol A (10) | 101.70 ± 9.57 f | >100 D |
| Adejene B (11) | 48.34 ± 0.55 cd | >100 D |
| 18-Sulphatedehydrothyrsiferol (12) | 43.18 ± 0.14 cd | >100 D |
| 18-Ketodehydrothyrsiferol (13) | 29.09 ± 2.84 bc | 23.37 ±1.76 B |
| 28-Iodosaiyacenol A (14) | 50.46 ± 3.05 cd | 29.45 ±0.20 B |
| 28-Iodosaiyacenol B (15) | 54.34 ± 6.56 de | >100 D |
| 28-Hydroxythyrsiferol (16) 1 | 103.66 ± 8.42 f | >100 D |
| Chlorhexidine * | 3.02 ± 0.89 a | 6.64 ±0.35 A |
| Voriconazole * | 0.94 ± 0.29 a | 2.64 ±0.27 A |
| Amphotericin B * | 39.65 ± 0.56 bcd | >100 D |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Morales, J.; Díaz-Marrero, A.R.; Cen-Pacheco, F.; Sifaoui, I.; Reyes-Batlle, M.; Souto, M.L.; Hernández Daranas, A.; Piñero, J.E.; Fernández, J.J. Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba. Mar. Drugs 2019, 17, 420. https://doi.org/10.3390/md17070420
Lorenzo-Morales J, Díaz-Marrero AR, Cen-Pacheco F, Sifaoui I, Reyes-Batlle M, Souto ML, Hernández Daranas A, Piñero JE, Fernández JJ. Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba. Marine Drugs. 2019; 17(7):420. https://doi.org/10.3390/md17070420
Chicago/Turabian StyleLorenzo-Morales, Jacob, Ana R. Díaz-Marrero, Francisco Cen-Pacheco, Ines Sifaoui, María Reyes-Batlle, María L. Souto, Antonio Hernández Daranas, José E. Piñero, and José J. Fernández. 2019. "Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba" Marine Drugs 17, no. 7: 420. https://doi.org/10.3390/md17070420
APA StyleLorenzo-Morales, J., Díaz-Marrero, A. R., Cen-Pacheco, F., Sifaoui, I., Reyes-Batlle, M., Souto, M. L., Hernández Daranas, A., Piñero, J. E., & Fernández, J. J. (2019). Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba. Marine Drugs, 17(7), 420. https://doi.org/10.3390/md17070420









