Ulva lactuca, A Source of Troubles and Potential Riches
Abstract
1. Introduction
2. Potential Riches of Ulva
2.1. Ulva Bioactive Compounds
2.2. Ulva for Food
2.3. Production of Biofuels
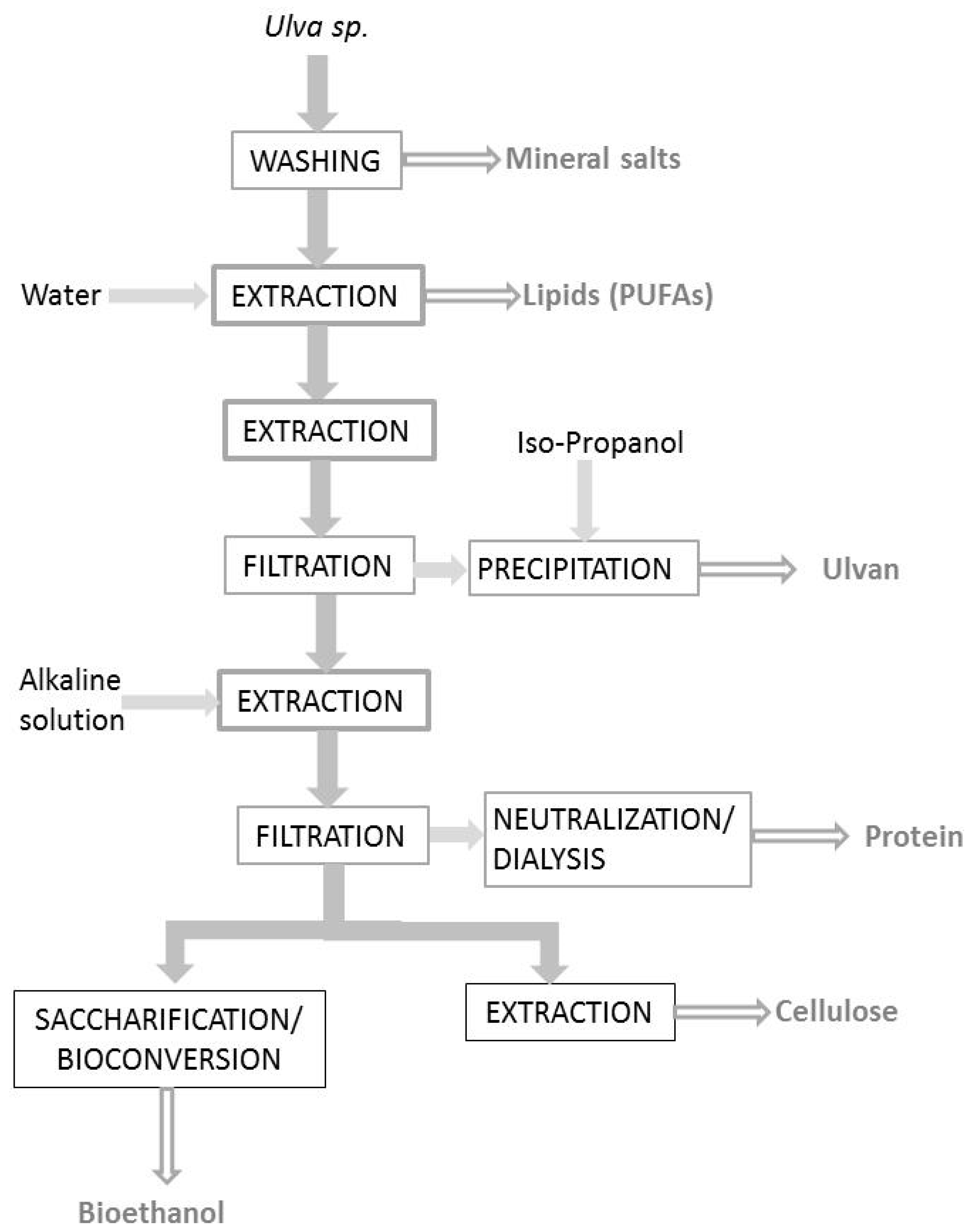
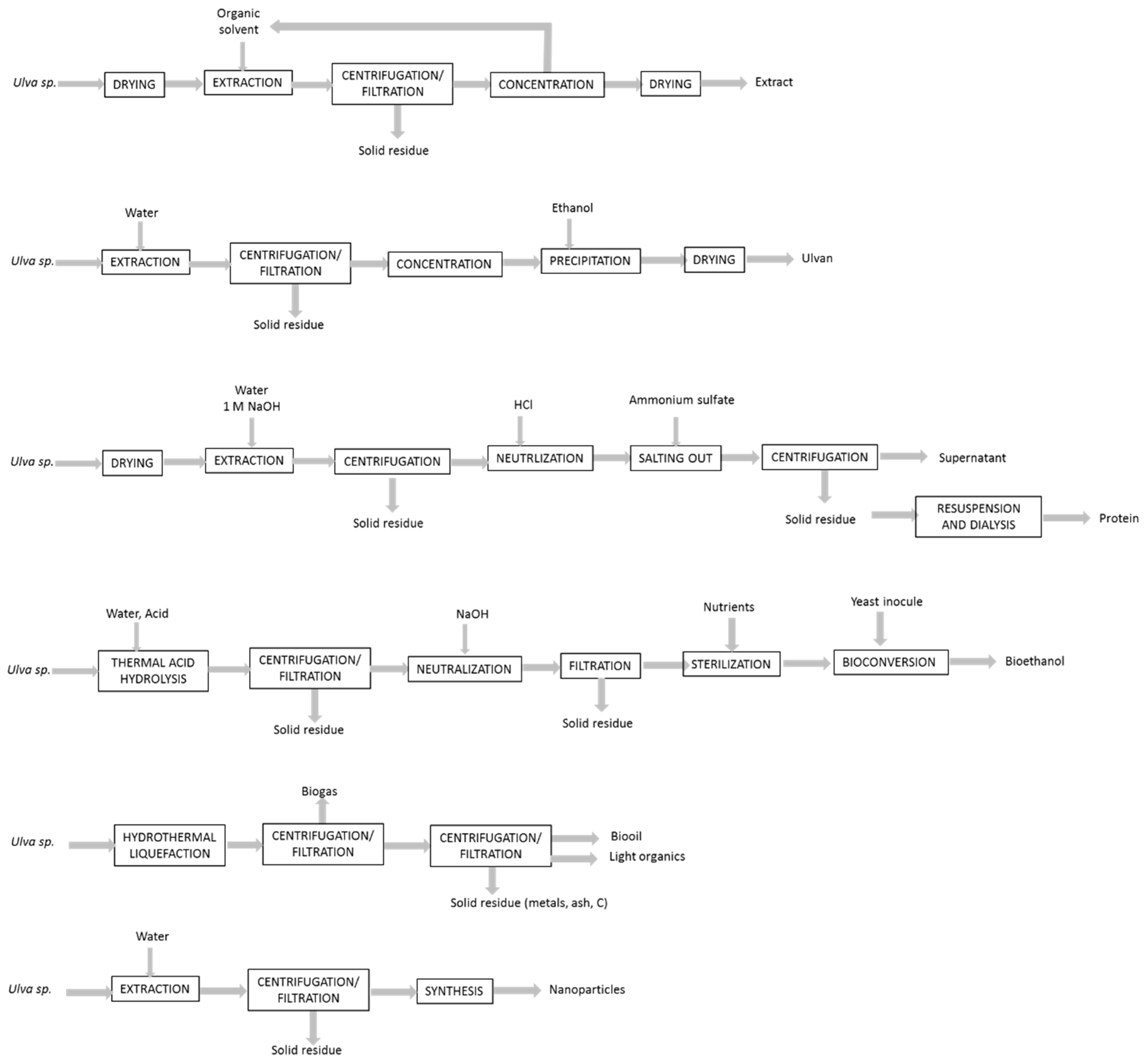
3. Processes and Strategies to Extract Component from Ulva
3.1. Conventional Processes
3.2. Biorefineries
4. Emerging Technologies
4.1. Pressurized Solvent Extraction
4.2. Microwave Assisted Hydrothermal Extraction
4.3. Ultrasound
4.4. Supercritical Fluid Extraction
4.5. Enzyme Extraction
5. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wichard, T.; Charrier, B.; Mineur, F.; Bothwell, J.H.; Clerck, O.D.; Coates, J.C. The green seaweed Ulva: A model system to study morphogenesis. Front. Plant Sci. 2015, 6, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Chevassus-au-Louis, B.; Andral, B.; Femenias, A.; Buvier, M. Bilan des Connaissances scientifiques sur Les Causes de Prolifération de Macroalgues Vertes; Rapport Pour Le Gouvernement Français 2012. Report No.: CGEDD 007942-01 et CGAAER 11128; Conseil Général de L’environnement et du Développement Durable: Paris, France, 2012. (In French) [Google Scholar]
- Pillard, S. Mise au Point sur Les Algues Vertes: Risques Environnementaux et Valorisations en 2016; Sciences Pharmaceutiques 2016, Dumas-01393938; Universite de Picardie Jules Verne: Amiens, France, 2016. (In French) [Google Scholar]
- Rybak, A. Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol. Indic. 2018, 85, 253–261. [Google Scholar] [CrossRef]
- Kraan, S. Pigments and minor compounds in algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 205–251. [Google Scholar]
- Robin, A.; Sack, M.; Israel, A.; Frey, W.; Müller, G.; Golberg, A. Deashing macroalgae biomass by pulsed electric field treatment. Bioresour. Technol. 2018, 255, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp.; Fucus vesiculosus and Saccharina latissima as functional ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [PubMed]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Reis, R.L.; Gomes, M.E. Seaweed polysaccharide-based hydrogels used for the regeneration of articular cartilage. Crit. Rev. Biotechnol 2014, 3, 410–424. [Google Scholar] [CrossRef]
- Morelli, A.; Betti, M.; Puppi, D.; Chiellini, F. Design, preparation and characterization of Ulvan based thermosensitive hydrogels. Carbohydr. Polym. 2016, 136, 1108–1117. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Hayden, H.S.; Blomster, J.; Maggs, C.A.; Silva, P.C.; Stanhope, M.J.; Waaland, J.R. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur. J. Phycol. 2003, 38, 277–294. [Google Scholar] [CrossRef]
- Kirkendale, L.; Saunders, G.W.; Winberg, P. A Molecular survey of Ulva (Chlorophyta) in temperate Australia reveals enhanced levels of cosmopolitanism. J. Phycol. 2012, 49, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Letts, E.A.; Richards, E.H. Report on green seaweeds and especially Ulva latissima in relation to the pollution of the waters in which they occur. In Royal Commission on Sewage Disposal; 7th Report, Appendix III, Section II; HMSO: London, UK, 1911. [Google Scholar]
- Schiffner, V.; Vatova, A. The Algae of the Lagoon: Chlorophyceae, Phaeophyceae, Rhodophyceae, Myxophyceae; Minio, M., Ed.; The Lagoon of Venice: Venezia, Italy, 1938; Volume 3, p. 250. (In Italian) [Google Scholar]
- Curiel, D.; Rismondo, A.; Bellemo, G.; Marzocchi, M. Macroalgal biomass and species variations in the lagoon of Venice (Northern Adriatic Sea, Italy): 1981–1998. Sci. Mar. 2004, 68, 57–67. [Google Scholar] [CrossRef]
- Villares, R.; Puente, X.; Carballeira, A. Nitrogen and phosphorus in Ulva sp. in the Galician Rias Bajas (northwest Spain): Seasonal fluctuations and influence on growth. Bol. Inst. Esp. Oceanogr. 1999, 15, 337–341. [Google Scholar]
- Yabe, T.; Ishii, Y.; Amano, Y.; Koga, T.; Hayashi, S.; Nohara, S.; Tatsumoto, H. Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology 2009, 10, 239–245. [Google Scholar] [CrossRef]
- Charlier, R.H.; Morand, P.; Finkl, C.W. How Brittany and Florida coasts cope with green tides. Int. J. Environ. Stud. 2008, 65, 191–208. [Google Scholar] [CrossRef]
- Li, J.-Y.; Yang, F.; Jin, L.; Wang, Q.; Yin, J.; He, P.; Chen, Y. Safety and quality of the green tide algal species Ulva prolifera for option of human consumption: A nutrition and contamination study. Chemosphere 2018, 210, 1021–1028. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Gann, E.R.; Wilhelm, S.W. Infection by a giant virus (AaV) induces widespread physiological reprogramming in Aureococcus anophagefferens CCMP1984, a Harmful Bloom Algae. Front. Microbiol. 2018, 9, 752–758. [Google Scholar] [CrossRef]
- Schvarcz, C.R.; Steward, G.F. A giant virus infecting green algae encodes key fermentation genes. Virology 2018, 518, 423–433. [Google Scholar] [CrossRef]
- La Scola, B.; Audic, S.; Robert, C.; Jungang, L.; de Lamballerie, X.; Drancourt, M.; Birtles, R.; Claverie, J.M.; Raoult, D. A giant virus in amoebae. Science 2003, 299, 2033–2038. [Google Scholar] [CrossRef]
- Abergel, C.; Legendre, M.; Claverie, J.M. The rapidly expanding universe of giantviruses: Mimivirus, Pandoravirus, Pithovirus and Mollivirus. FEMS Microbiol. Rev. 2015, 39, 779–796. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Carvalho, L.G.; Silva, P.J.; Rodrigues, M.S.; Pereira, O.R.; Pereira, L. Bioproducts from seaweeds: A review with special focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp.; for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Isolation and characterization of angiotensin I-converting enzyme (ACE) inhibitory peptides from Ulva rigida C. Agardh protein hydrolysate. J. Funct. Foods 2016, 26, 65–76. [Google Scholar] [CrossRef]
- Cian, R.E.; Hernández-Chirlaque, C.; Gámez-Belmonte, R.; Drago, S.R.; Sánchez de Medina, F.; Martínez-Augustin, O. Green alga Ulva sp. hydrolysates and their peptide fractions regulate cytokine production in splenic macrophages and lymphocytes involving the TLR4-NFκB/MAPK pathways. Mar. Drugs 2018, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Sari-Chmayssem, N.; Taha, S.; Mawlawi, H.; Guégan, J.-P.; Jeftić, J.; Benvegnu, T. Extracted Ulvans from green algae Ulva linza of Lebanese origin and amphiphilic derivatives: Evaluation of their physico-chemical and rheological properties. J. Appl. Phycol. 2018, 3, 1–16. [Google Scholar] [CrossRef]
- Violle, N.; Rozan, P.; Demais, H.; Nyvall Collen, P.; Bisson, J.-F. Evaluation of the antidepressant- and anxiolytic-like effects of a hydrophilic extract from the green seaweed Ulva sp. in rats. Nutr. Neurosci. 2018, 21, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Cesário, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M.D. Marine algal carbohydrates as carbon sources for the production of biochemicals and biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef]
- Dumbrava, A.; Berger, D.; Matei, C.; Radu, M.D.; Gheorghe, E. Characterization and applications of a new composite material obtained by green synthesis, through deposition of zinc oxide onto calcium carbonate precipitated in green seaweeds extract. Ceram. Int. 2018, 44, 4931–4936. [Google Scholar] [CrossRef]
- McCauley, J.I.; Winberg, P.C.; Meyer, B.J.; Skropeta, D. Effects of nutrients and processing on the nutritionally important metabolites of Ulva sp. (Chlorophyta). Algal Res. 2018, 35, 586–594. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Wu, J.; Luo, Y. Phycoremediation of coastal waters contaminated with bisphenol A by green tidal algae Ulva prolifera. Sci. Total Environ. 2019, 661, 55–62. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Laramore, S.; Baptiste, R.; Wills, P.S.; Hanisak, M.D. Utilization of IMTA-produced Ulva lactuca to supplement or partially replace pelleted diets in shrimp (Litopenaeus vannamei) reared in a clear water production system. J. Appl. Phycol. 2018, 2, 3603–3610. [Google Scholar] [CrossRef]
- Cruz-Suárez, L.E.; León, A.; Peña-Rodríguez, A.; Rodríguez-Peña, G.; Moll, B.; Ricque-Marie, D. Shrimp/Ulva co-culture: A sustainable alternative to diminish the need for artificial feed and improve shrimp quality. Aquaculture 2010, 301, 64–68. [Google Scholar] [CrossRef]
- Mansori, M.; Chernane, H.; Latique, S.; Benaliat, A.; Hsissou, D.; El Kaoua, M. Seaweed extract effect on water deficit and antioxidative mechanisms in bean plants (Phaseolus vulgaris L.). J. Appl. Phycol. 2015, 27, 1689–1698. [Google Scholar] [CrossRef]
- Mansori, M.; Chernane, H.; Latique, S.; Benaliat, A.; Hsissou, D.; El Kaoua, M. Effect of seaweed extract (Ulva rigida) on the water deficit tolerance of Salvia officinalis L. J. Appl. Phycol. 2016, 28, 1363–1370. [Google Scholar] [CrossRef]
- Balboa, E.M.; Soto, M.L.; Nogueira, D.R.; González-López, N.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crop Prod. 2014, 58, 104–110. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Rodríguez-Argüelles, M.C.; Prado-López, S.; Lastra, M.; Grimaldi, M.; Cavazza, A.; Nasi, L.; Salviati, G.; Bigi, F. Macroalgae to nanoparticles: Study of Ulva lactuca L. role in biosynthesis of gold and silver nanoparticles and of their cytotoxicity on colon cancer cell lines. Mater. Sci. Eng. C 2019, 97, 498–509. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Mata, L.; Magnusson, M.; Paul, N.A.; de Nys, R. The intensive land-based production of the green seaweeds Derbesia tenuissima and Ulva ohnoi: Biomass and bioproducts. J. Appl. Phycol. 2016, 28, 365–375. [Google Scholar] [CrossRef]
- Chemodanov, A.; Jinjikhashvily, G.; Habiby, O.; Liberzon, A.; Israel, A.; Yakhini, Z.; Golberg, A. Net primary productivity, biofuel production and CO2 emissions reduction potential of Ulva sp. (Chlorophyta) biomass in a coastal area of the Eastern Mediterranean. Energy Convers. Manag. 2018, 166, 772–779. [Google Scholar] [CrossRef]
- Raikova, S.; Le, C.D.; Beacham, T.A.; Jenkins, R.W.; Allen, M.J.; Chuck, C.J. Towards a marine biorefinery through the hydrothermal liquefaction of macroalgae native to the United Kingdom. Biomass Bioenergy 2017, 107, 244–253. [Google Scholar] [CrossRef]
- Soliman, R.M.; Younis, S.A.; El-Gendy, N.S.; Mostafa, S.S.M.; El-Temtamy, S.A.; Hashim, A.I. Batch bioethanol production via the biological and chemical saccharification of some Egyptian marine macroalgae. J. Appl. Microbiol. 2018, 125, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Saqib, A.; Tabbssum, M.R.; Rashid, U.; Ibrahim, M.; Gill, S.S.; Mehmood, M.A. Marine macroalgae Ulva: A potential feed-stock for bioethanol and biogas production. Asian J. Agric. Biol. 2013, 3, 155–163. [Google Scholar]
- Suganya, T.; Renganathan, S. Optimization and kinetic studies on algal oil extraction from marine macroalgae Ulva lactuca. Bioresour. Technol. 2012, 107, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renew. Sustain. Energy Rev. 2017, 68, 136–146. [Google Scholar] [CrossRef]
- Jiang, R.; Ingle, K.N.; Golberg, A. Macroalgae (seaweed) for liquid transportation biofuel production: What is next? Algal Res. 2016, 14, 48–57. [Google Scholar] [CrossRef]
- El Harchi, M.; Fakihi Kachkach, F.Z.; El Mtili, N. Optimization of thermal acid hydrolysis for bioethanol production from Ulva rigida with yeast Pachysolen tannophilus. S. Afr. J. Bot. 2018, 115, 161–169. [Google Scholar] [CrossRef]
- Van der Wal, H.; Sperber, B.L.; Houweling-Tan, B.; Bakker, R.R.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef]
- Karray, R.; Hamza, M.; Sayadi, S. Evaluation of ultrasonic, acid, thermo-alkaline and enzymatic pre-treatment on anaerobic digestion of Ulva rigida for biogas production. Bioresour. Technol. 2015, 187, 205–213. [Google Scholar] [CrossRef]
- Koçer, A.T.; Özçimen, D. Investigation of the biogas production potential from algal wastes. Waste Manag. Res. 2018, 36, 1100–1105. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-I.; Lee, J.; Sim, S.J.; Lee, J.-H. Production of hydrogen from marine macro-algae biomass using anaerobic sewage sludge microflora. Biotechnol. Bioprocess Eng. 2009, 14, 307–315. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefin. 2010, 4, 287–295. [Google Scholar] [CrossRef]
- Mhatre, A.; Gore, S.; Mhatre, A.; Trivedi, N.; Sharma, M.; Pandit, R.; Anil, A.; Lali, A. Effect of multiple product extractions on bio-methane potential of marine macrophytic green alga Ulva lactuca. Renew. Energy 2018, 132, 742–751. [Google Scholar] [CrossRef]
- Wijesekara, I.; Lang, M.; Marty, C.; Gemin, M.-P.; Boulho, R.; Douzenel, P.; Wickramasinghe, I.; Bedoux, G.; Bourgougnon, N. Different extraction procedures and analysis of protein from Ulva sp. in Brittany. J. Appl. Phycol. 2017, 29, 2503–2511. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of Ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of Ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef]
- Hernández-Garibay, E.; Zertuche-González, J.A.; Pacheco-Ruíz, I. Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J. Appl. Phycol. 2011, 23, 537–542. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Kanno, K.; Fujita, Y.; Honda, S.; Takahashi, S.; Kato, S. Urethane foam of sulfated polysaccharide Ulvan derived from green-tide-forming chlorophyta: Synthesis and application in the removal of heavy metal ions from aqueous solutions. Polym. J. 2014, 46, 813–818. [Google Scholar] [CrossRef]
- Kajiwara, T.; Matsui, K.; Akakabe, Y.; Murakawa, T.; Arai, C. Antimicrobial browning-inhibitory effect of flavor compounds in seaweeds. J. Appl. Phycol. 2006, 18, 413–422. [Google Scholar] [CrossRef]
- De Lima, R.L.; Pires-Cavalcante, K.M.S.; de Alencar, D.B.; Viana, F.A.; Sampaio, A.H.; Saker-Sampaio, S. In vitro evaluation of antioxidant activity of methanolic extracts obtained from seaweeds endemic to the coast of Ceará, Brazil. Acta Sci. Technol. 2016, 38, 247–255. [Google Scholar] [CrossRef]
- Kellogg, J.; Lila, M.A. Chemical and in vitro assessment of Alaskan coastal vegetation antioxidant capacity. J. Agric. Food Chem. 2013, 61, 11025–11032. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Hemaiswarya, S.; Arunkumar, K.; Carvalho, I.S. Antioxidant activity and lipid profile of three seaweeds of Faro, Portugal. Rev. Bras. Bot. 2016, 39, 9–17. [Google Scholar] [CrossRef]
- Cho, M.; Kang, I.-J.; Won, M.-H.; Lee, H.-S.; You, S. The antioxidant properties of ethanol extracts and their solvent-partitioned fractions from various green seaweeds. J. Med. Food 2010, 13, 1232–1239. [Google Scholar] [CrossRef]
- Goh, C.S.; Lee, K.T. A visionary and conceptual macroalgae-based third-generation bioethanol (TGB) biorefinery in Sabah, Malaysia as an underlay for renewable and sustainable development. Renew. Sustain. Energy Rev. 2010, 14, 842–848. [Google Scholar] [CrossRef]
- Golberg, A.; Vitkin, E.; Linshiz, G.; Khan, S.A.; Hillson, N.J.; Yakhini, Z.; Yarmush, M.L. Proposed design of distributed macroalgal biorefineries: Thermodynamics, bioconversion technology, and sustainability implications for developing economies. Biofuels Bioprod. Biorefin. 2014, 8, 67–82. [Google Scholar] [CrossRef]
- Robin, A.; Chavel, P.; Chemodanov, A.; Israel, A.; Golberg, A. Diversity of monosaccharides in marine macroalgae from the Eastern Mediterranean Sea. Algal Res. 2017, 28, 118–127. [Google Scholar] [CrossRef]
- Pezoa-Conte, R.; Leyton, A.; Baccini, A.; Ravanal, M.C.; Mäki-Arvela, P.; Grénman, H.; Xu, C.; Willför, S.; Lienqueo, M.E.; Mikkola, J.-P. Aqueous extraction of the sulfated polysaccharide Ulvan from the green alga Ulva rigida-Kinetics and modeling. Bioenergy Res. 2017, 10, 915–928. [Google Scholar] [CrossRef]
- Chiesa, S.; Gnansounou, E. Protein extraction from biomass in a bioethanol refinery—Possible dietary applications: Use as animal feed and potential extension to human consumption. Bioresour. Technol. 2011, 102, 427–436. [Google Scholar] [CrossRef]
- Ge, H.; Ni, Q.; Chen, Z.; Li, J.; Zhao, F. Effects of short period feeding polysaccharides from marine macroalga, Ulva prolifera on growth and resistance of Litopenaeus vannamei against Vibrio parahaemolyticus infection. J. Appl. Phycol. 2018, 10, 1663–1669. [Google Scholar] [CrossRef]
- Bikker, P.; van Krimpen, M.M.; van Wikselaar, P.; Houweling-Tan, B.; Scaccia, N.; van Hal, J.W.; Huijgen, W.J.J.; Cone, J.W.; López-Contreras, A.M. Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Carl, C.; Mata, L.; de Nys, R.; Paul, N.A. Seaweed salt from Ulva: A novel first step in a cascading biorefinery model. Algal Res. 2016, 16, 308–316. [Google Scholar] [CrossRef]
- Schiener, P.; Atack, T.; Wareing, R.A.; Kelly, M.S.; Hughes, A.D. The by-products from marine biofuels as a feed source for the aquaculture industry: A novel example of the biorefinery approach. Biomass Convers. Biorefin. 2016, 6, 281–287. [Google Scholar] [CrossRef]
- Raikova, S.; Olsson, J.; Mayers, J.J.; Nylund, G.M.; Albers, E.; Chuck, C.J. Effect of geographical location on the variation in products formed from the hydrothermal liquefaction of Ulva intestinalis. Energy Fuels 2018, 10, 1021–1028. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; González-Muñoz, M.J.; Domínguez, H. Introduction. In Water Extraction of Bioactive Compounds; Domínguez, H., González-Muñoz, M.J., Eds.; Elsevier: Kidlington, UK, 2017. [Google Scholar]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.-G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 10, 1–16. [Google Scholar] [CrossRef]
- Boisvert, C.; Beaulieu, L.; Bonnet, C.; Pelletier, E. Assessment of the antioxidant and antibacterial activities of three species of edible seaweeds. J. Food Biochem. 2015, 39, 377–387. [Google Scholar] [CrossRef]
- Singh, R.; Bhaskar, T.; Balagurumurthy, B. Effect of solvent on the hydrothermal liquefaction of macroalgae Ulva fasciata. Process Saf. Environ. Prot. 2015, 93, 154–160. [Google Scholar] [CrossRef]
- Su, D.; Li, S.; Zhao, F.; Cao, Y. Drying dynamics of Ulva prolifera with the heat pump microwave method. Int. J. Simul. Syst. Sci. Technol. 2016, 17, 1–7. [Google Scholar]
- Piccini, M.; Raikova, S.; Allen, M.J.; Chuck, C.J. A synergistic use of microalgae and macroalgae for heavy metal bioremediation and bioenergy production through hydrothermal liquefaction. Sustain. Energy Fuels 2019, 3, 292–301. [Google Scholar] [CrossRef]
- Choi, W.Y.; Han, J.G.; Lee, C.G.; Song, C.H.; Kim, J.S.; Seo, Y.C.; Lee, S.E.; Jung, K.H.; Kang, D.H.; Heo, S.J.; et al. Bioethanol production from Ulva pertusa Kjellman by high-temperature liquefaction. Chem. Biochem. Eng. Q. 2012, 26, 15–21. [Google Scholar]
- Jmel, M.A.; Anders, N.; Yahmed, N.B.; Schmitz, C.; Marzouki, M.N.; Spiess, A.; Smaali, I. Variations in physicochemical properties and bioconversion efficiency of Ulva lactuca polysaccharides after different biomass pretreatment techniques. Appl. Biochem. Biotechnol. 2018, 184, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food Chem. 2016, 210, 311–316. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Kim, S.H.; Baek, K.-H. Antioxidant and free radical-scavenging potential of essential oil from Enteromorpha linza L. prepared by microwave-assisted hydrodistillation. J. Food Biochem. 2015, 39, 80–90. [Google Scholar] [CrossRef]
- Khan, A.M.; Fatima, N. Synthesis of biodiesel from the oily content of marine green alga Ulva fasciata. J. Chem. Soc. Pak. 2015, 37, 1040–1046. [Google Scholar]
- Zhuang, Y.; Guo, J.; Chen, L.; Li, D.; Liu, J.; Ye, N. Microwave-assisted direct liquefaction of Ulva prolifera for bio-oil production by acid catalysis. Bioresour. Technol. 2012, 116, 133–139. [Google Scholar] [CrossRef]
- Kong, X.; Wu, S.; Li, X.; Liu, J. Microwave-assisted liquefaction of Ulva prolifera over Fe2O3-modified HY catalyst. J. Energy Eng. 2018, 144, 401–407. [Google Scholar] [CrossRef]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Ueda, T.; Onda, A.; Yanagisawa, K.; Azuma, J.-I. Hydrolysis of green-tide forming Ulva spp. by microwave irradiation with polyoxometalate clusters. Green Chem. 2014, 16, 2227–2233. [Google Scholar] [CrossRef]
- Kim, J.; Ha, S.H. Hydrothermal pretreatment of Ulva pertusa Kjellman using microwave irradiation for enhanced enzymatic hydrolysis. Korean Chem. Eng. Res. 2015, 53, 570–575. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, 253–260. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of ultrasound-assisted extraction. In Water Extraction of Bioactive Compounds; Domínguez, H., González-Muñoz, M.J., Eds.; Elsevier: Kidlington, UK, 2017; pp. 291–316. [Google Scholar]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Barthélemy, J.-P.; Paquot, M.; Blecker, C.; Attia, H. Impact of extraction procedures on the chemical, rheological and textural properties of Ulvan from Ulva lactuca of Tunisia coast. Food Hydrocoll. 2014, 40, 53–63. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Fleurence, J. The enzymatic degradation of algal cell walls: A useful approach for improving protein accessibility? J. Appl. Phycol. 1999, 11, 313–314. [Google Scholar] [CrossRef]
- Bobin-Dubigeon, C.; Lahaye, M.; Guillon, F.; Barry, J.-L.; Gallant, D.J. Factors limiting the biodegradation of Ulva sp cell-wall polysaccharides. J. Sci. Food Agric. 1997, 75, 341–351. [Google Scholar] [CrossRef]
- Konasani, V.R.; Jin, C.; Karlsson, N.G.; Albers, E. Ulvan lyase from Formosa agariphila and its applicability in depolymerisation of Ulvan extracted from three different Ulva species. Algal Res. 2018, 36, 106–114. [Google Scholar] [CrossRef]
- Reisky, L.; Stanetty, C.; Mihovilovic, M.D.; Schweder, T.; Hehemann, J.-H.; Bornscheuer, U.T. Biochemical characterization of an Ulvan lyase from the marine flavobacterium Formosa agariphila KMM 3901T. Appl. Microbiol. Biotechnol. 2018, 102, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Tsai, W.S.; Chiu, T.H. Antioxidant properties of seven cultivated and natural edible seaweed extracts from Taiwan. J. Aquat. Food Prod. Technol. 2012, 21, 248–264. [Google Scholar] [CrossRef]
- Robin, A.; Kazir, M.; Sack, M.; Israel, A.; Frey, W.; Mueller, G.; Livney, Y.D.; Golberg, A. Functional protein concentrates extracted from the green marine macroalga Ulva sp.; by High Voltage Pulsed Electric Fields and Mechanical Press. ACS Sustain. Chem. Eng. 2018, 6, 13696–13705. [Google Scholar] [CrossRef]
- Polikovsky, M.; Fernand, F.; Sack, M.; Frey, W.; Müller, G.; Golberg, A. In silico food allergenic risk evaluation of proteins extracted from macroalgae Ulva sp. with pulsed electric fields. Food Chem. 2019, 276, 735–744. [Google Scholar] [CrossRef] [PubMed]
- El-Belghiti, K.; Vorobiev, E. Modelling of solute aqueous extraction from carrots subjected to a pulsed electric field pre-treatment. Biosyst. Eng. 2005, 90, 289–294. [Google Scholar] [CrossRef]
- Postma, P.R.; Cerezo-Chinarro, O.; Akkerman, R.J.; Olivieri, G.; Wijffels, R.H.; Brandenburg, W.A.; Eppink, M.H.M. Biorefinery of the macroalgae Ulva lactuca: Extraction of proteins and carbohydrates by mild disintegration. J. Appl. Phycol. 2018, 30, 1281–1293. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Sherif, S.A.; Ghareeb, M.M. Bioethanol production by various hydrolysis and fermentation processes with micro and macro green lgae. Waste Biomass Valoriz. 2018, 9, 1495–1501. [Google Scholar] [CrossRef]
- Trivedi, N.; Gupta, V.; Reddy, C.R.K.; Jha, B. Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour. Technol. 2013, 150, 106–112. [Google Scholar] [CrossRef]
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from green algae Ulva intestinalis: Optimization of ultrasound-assisted extraction and antioxidant activity. J. Appl. Phycol. 2016, 28, 2979–2990. [Google Scholar] [CrossRef]
- Praptijanto, A.; Muharam, A.; Nur, A.; Putrasari, Y. Effect of Ethanol Percentage for Diesel Engine Performance Using Virtual Engine Simulation Tool. Energy Procedia 2015, 68, 345–354. [Google Scholar] [CrossRef]
- Loret, E.P.; Luis, J.; Nuccio, C.; Villard, C.; Mansuelle, P.; Lebrun, R.; Villard, P.H. A low molecular weight protein from the Sea anemone Anemonia viridis with an anti-angiogenic activity. Mar. Drugs 2018, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Härtl, K.; Denton, A.; Franz-Oberdorf, K.; Hoffmann, T.; Spornraft, M.; Usadel, B.; Schwab, W. Early metabolic and transcriptional variations in fruit of natural white-fruited Fragaria vesca genotypes. Sci. Rep. 2017, 7, 45113–45117. [Google Scholar] [CrossRef] [PubMed]
- Loret, E.P.; Darque, A.; Jouve, E.; Loret, E.A.; Nicolino-Brunet, C.; Morange, S.; Castanier, E.; Casanova, J.; Caloustian, C.; Bornet, C.; et al. Intradermal injection of a Tat Oyi-based therapeutic HIV vaccine reduces of 1.5 log copies/ml the HIV RNA rebound median and no HIV DNA rebound following cART interruption in a phase I/II randomized controlled clinical trial. Retrovirology 2016, 13, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Weinbauer, M. Randomly Amplified Polymorphic DNA Reveals Tight Links between Viruses and Microbes in the Bathypelagic Zone of the Northwestern Mediterranean Sea. Appl. Environ. Microbiol. 2010, 20, 6724–6732. [Google Scholar] [CrossRef] [PubMed]
- Angione, C.; Conway, M.; Lió, P. Multiplex methods provide effective integration of multi-omic data in genome-scale models. BMC Bioinform. 2016, 17, 83–89. [Google Scholar] [CrossRef] [PubMed]
| Extraction and Pretreatment Stages of Ulva lactuca (U. l.) | Products | Ref. |
|---|---|---|
| U. l. clathrata. Extraction (0.2 N HCl, 60 °C, 2 h + 0.1 M NaOH, 60 °C, 2 h) | U, Pr | [64] |
| Supercritical (sc)-CO2 (50 MPa, 40 °C, 300–810 min) | A, Cy, M, Ph | [106] |
| U. l. fasciata. Ultrasound pretreatment (24 kHz, 50 °C, 6 min) + chloroform:methanol:hexane (90 °C, 3 h) or sc-CO2 (200 °C, 150 bar, 2.14 L/h) | O | [56] |
| Acid (1–3% H2SO4, 100–121 °C, 0.3–1 h) or alkaline (NaOH, 121 °C, 20.3 h) or buffer (pH 4.8, 120 °C, 1 h) or dry heat (120 °C, 1 h) or liquid ammonia (2%, 0.25 h) + enzyme saccharification + bioconversion | E | [114,115] |
| Dichloromethane, 7 days, 3 stages; adsorption, transesterification | BD | [97] |
| U. l. flexuosa. Sc-CO2 extraction (40 °C, 30 MPa, 11.4%) | Car, Chl | [104] |
| U. l. intestinalis. Liquefaction (deionized water, LSR 5; 345 °C; 30 K/min) | BO | [44] |
| US assisted extraction (66 °C, 40 min, LSR 50, pH 7.0) | U | [116] |
| U. l. Aqueous pretreatment (150 °C) + enzyme extraction + bioconversion | Pr, Ac, B, E | [81] |
| Water, thermal treatment (60 °C, 45 min) or water (pH 2, 95 °C, 3 h) or alkaline extraction (0.25M NaOH; 60 °C, 1 h) + anaerobic digestion | S, U, Pr, BG | [59] |
| Acid pretreatment (H2SO4, pH 2, 150 °C, 10 min); enzyme saccharification | Ac, B, E | [52] |
| MA water extraction (500 W, 70 °C, 20 min) or acid extraction (pH 1.5, 90 °C) | U | [62,95] |
| Bioconversion | BG | [54] |
| US water extraction or alkaline extraction or accelerated extraction | Pr | [87] |
| Incubation with water/heat treatment + lipid extraction + water extraction (90 °C, 2 h) + protein extraction (1 N NaOH, 80 °C) | S, O, U, Pr, C | [83] |
| US pretreatment (LSR 3, 24 kHz, 50 °C, 5 min); solvent extraction | O | [48] |
| OS (30 °C, 24 h) or enzyme (pH 4, 25 °C, 0.5% pectinase, 4 h) or PEF (7.5 kV/cm, 0.05 ms pulses, 6.6 kWh/kg prot) | Pr, U | [113] |
| Liquefaction (deionized water, LSR 5; 345 °C; 30 K/min) | BO | [44] |
| Water or buffer or PEF (75 pulses, 2.9 kV/cm, 5.7 μs, 0.5 Hz), pressing (5 min, 45 daN/cm2) | U, Pr | [111] |
| PEF (247 kJ/kg fw, 50 pulses, 50 kV, 70.3 mm electrode gap) | Pr | [115] |
| EA aqueous extraction (0.5%, 50 °C, 5 h) | Ph, Pr, U | [100] |
| U. l. linza. Extraction (pH 1.5–2, 60 °C, 2 h); re-extraction (pH = 2, 60 °C, 1 h) | U | [30] |
| U. l. meridionalis and U. l. ohnoi. MA hydrothermal extraction (4 + 10 min, 160 °C) | U | [94] |
| U. pertusa. Liquefaction (150 °C, 15 MPa, 15 min); enzyme saccharification | E | [91] |
| U. l. prolifera. MA liquefaction (6% H2SO4, 600 W, 30 min, 180 °C) | BO | [98] |
| MA liquefaction (ethylene glycol, 4.93% H2SO4), 600 W, 30 min, 165 °C | BO | [21] |
| U. l. rigida. Aqueous extraction (100 °C, 1 h) | Ph | [38] |
| Aqueous extraction (125 °C, 1.5 bar, 1 h) + SSF | U, Pr, E | [75] |
| EA extraction (pepsin + bromelain, 37 °C, pH 2.0, 20 + 20 h) | Pr | [28] |
| Acid hydrolysis (2–4% acid, 121 °C, 30–60 min) | E | [50,51] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, H.; Loret, E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. https://doi.org/10.3390/md17060357
Dominguez H, Loret EP. Ulva lactuca, A Source of Troubles and Potential Riches. Marine Drugs. 2019; 17(6):357. https://doi.org/10.3390/md17060357
Chicago/Turabian StyleDominguez, Herminia, and Erwann P. Loret. 2019. "Ulva lactuca, A Source of Troubles and Potential Riches" Marine Drugs 17, no. 6: 357. https://doi.org/10.3390/md17060357
APA StyleDominguez, H., & Loret, E. P. (2019). Ulva lactuca, A Source of Troubles and Potential Riches. Marine Drugs, 17(6), 357. https://doi.org/10.3390/md17060357





