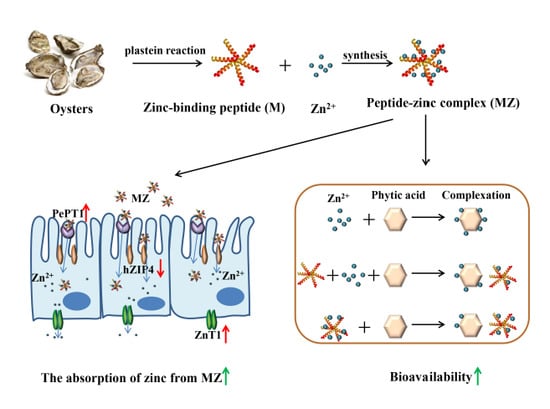
Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc
Abstract
1. Introduction
2. Results and Discussion
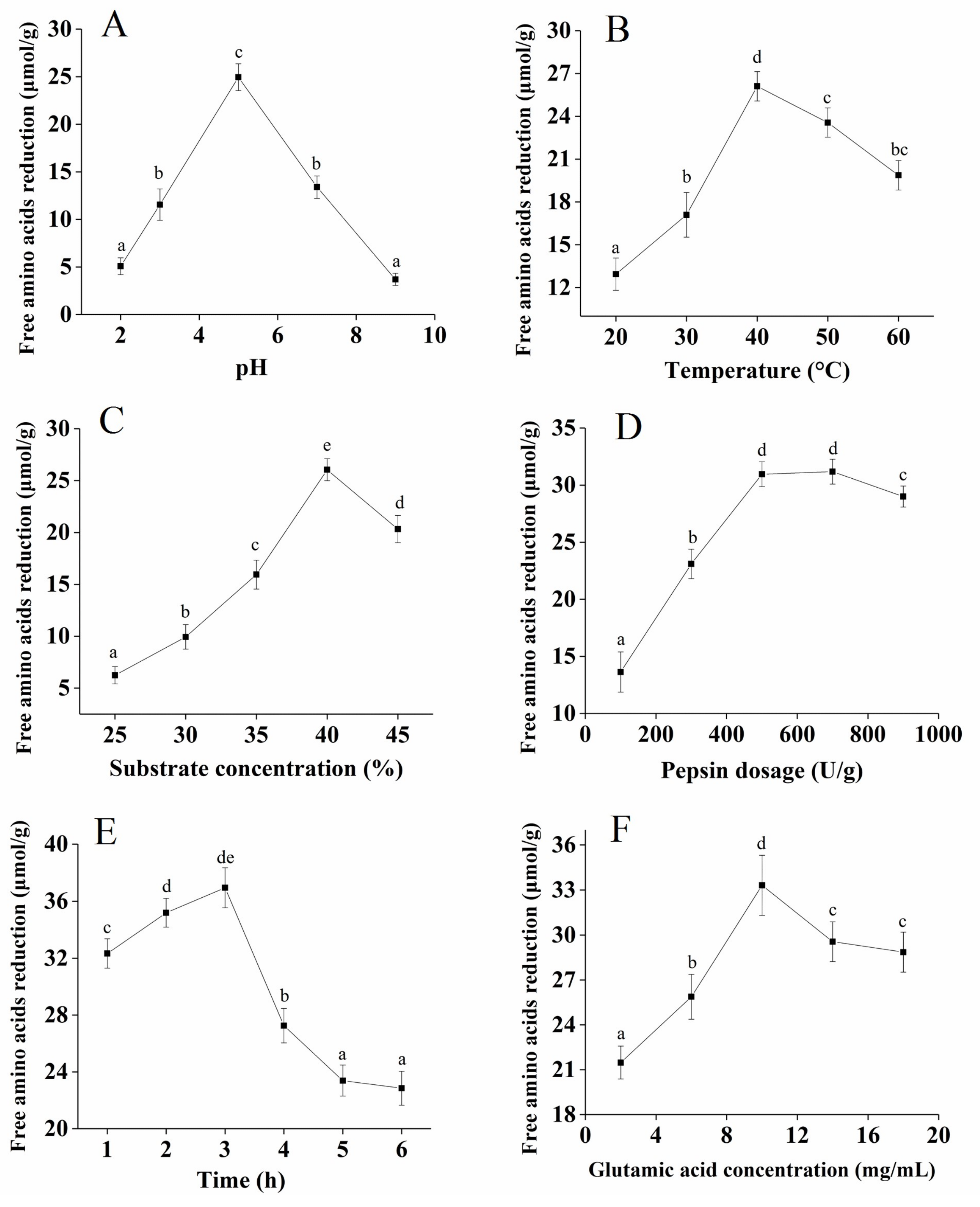
2.1. Optimization of the Plastein Reaction Conditions
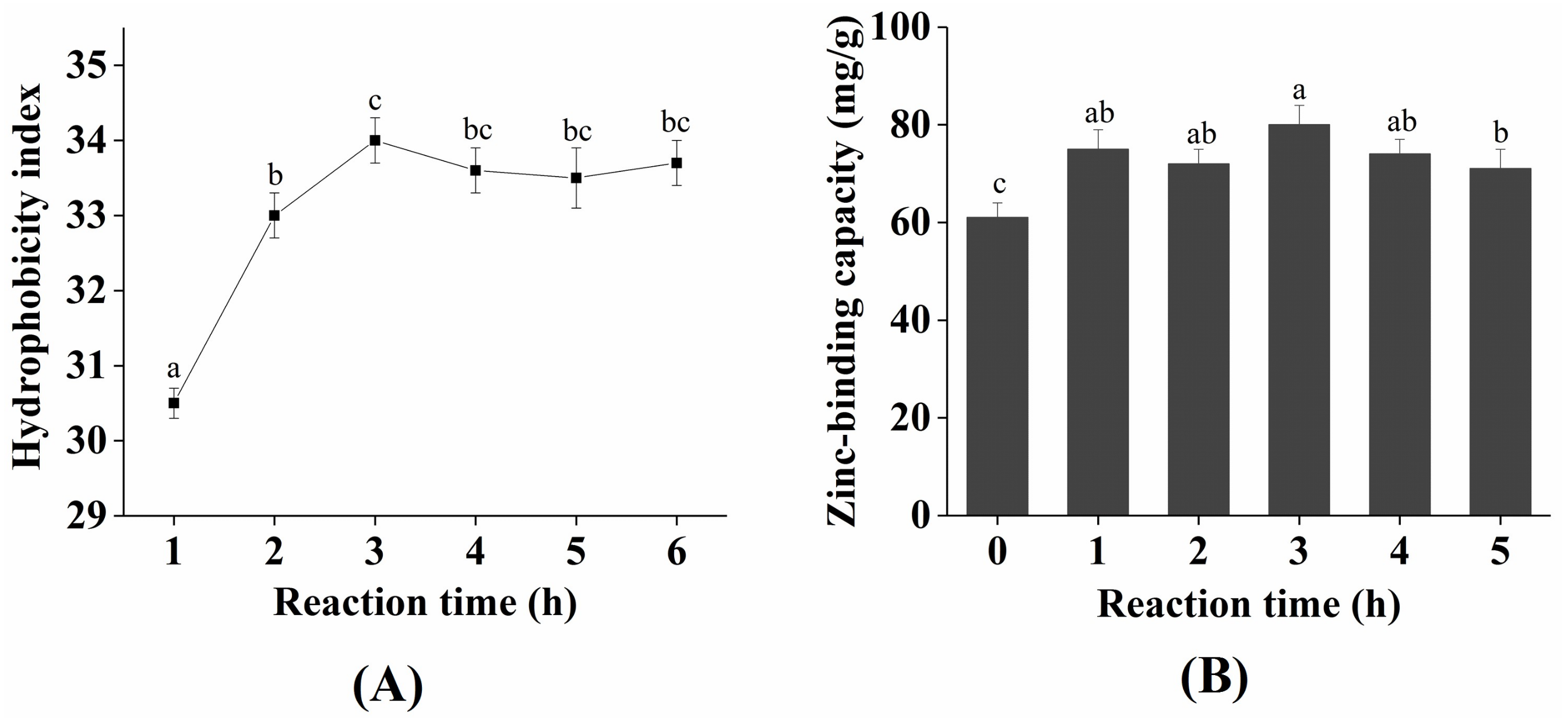
2.2. Change in Zinc-Binding Capacity and Hydrophobicity during the Plastein Reaction
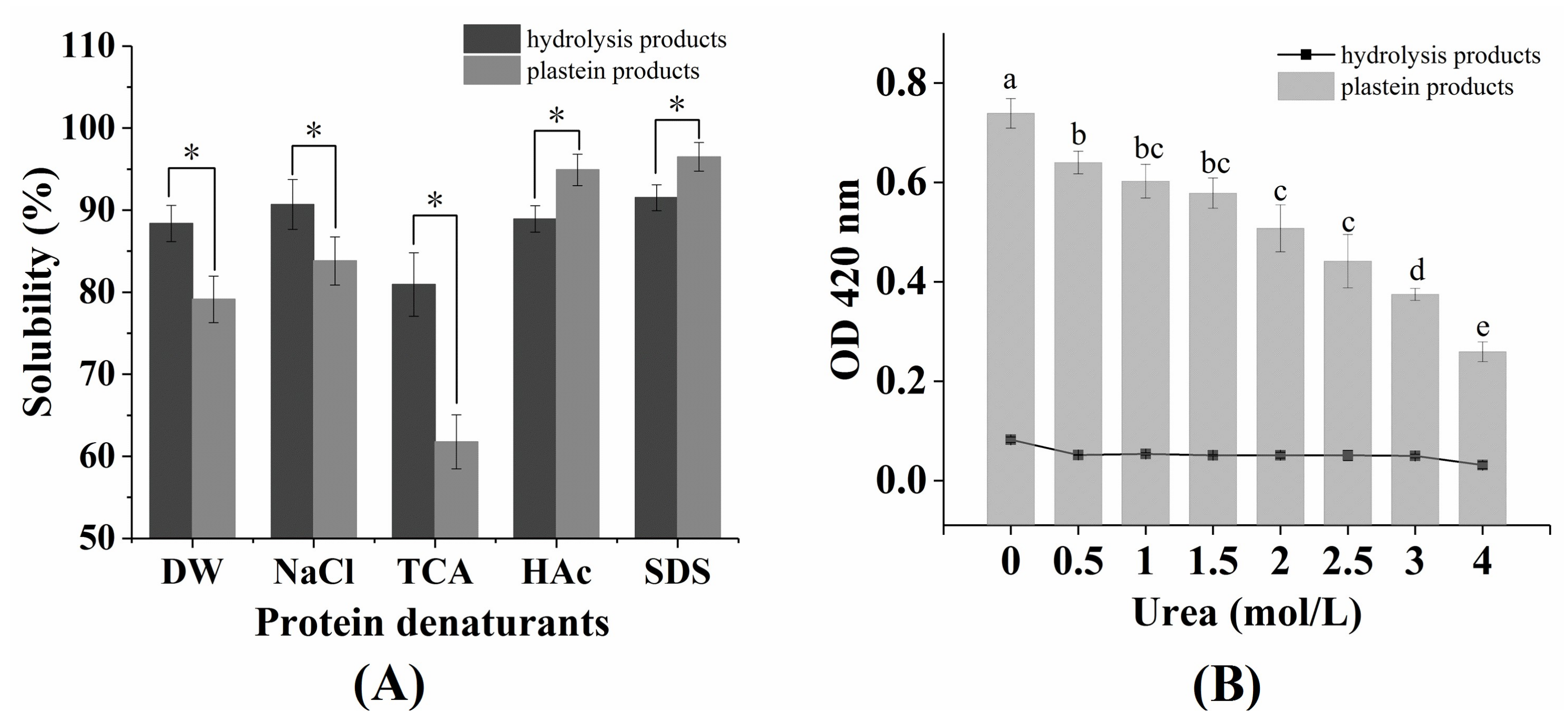
2.3. The Effects of Protein Denaturants on the Stability of Plastein Products
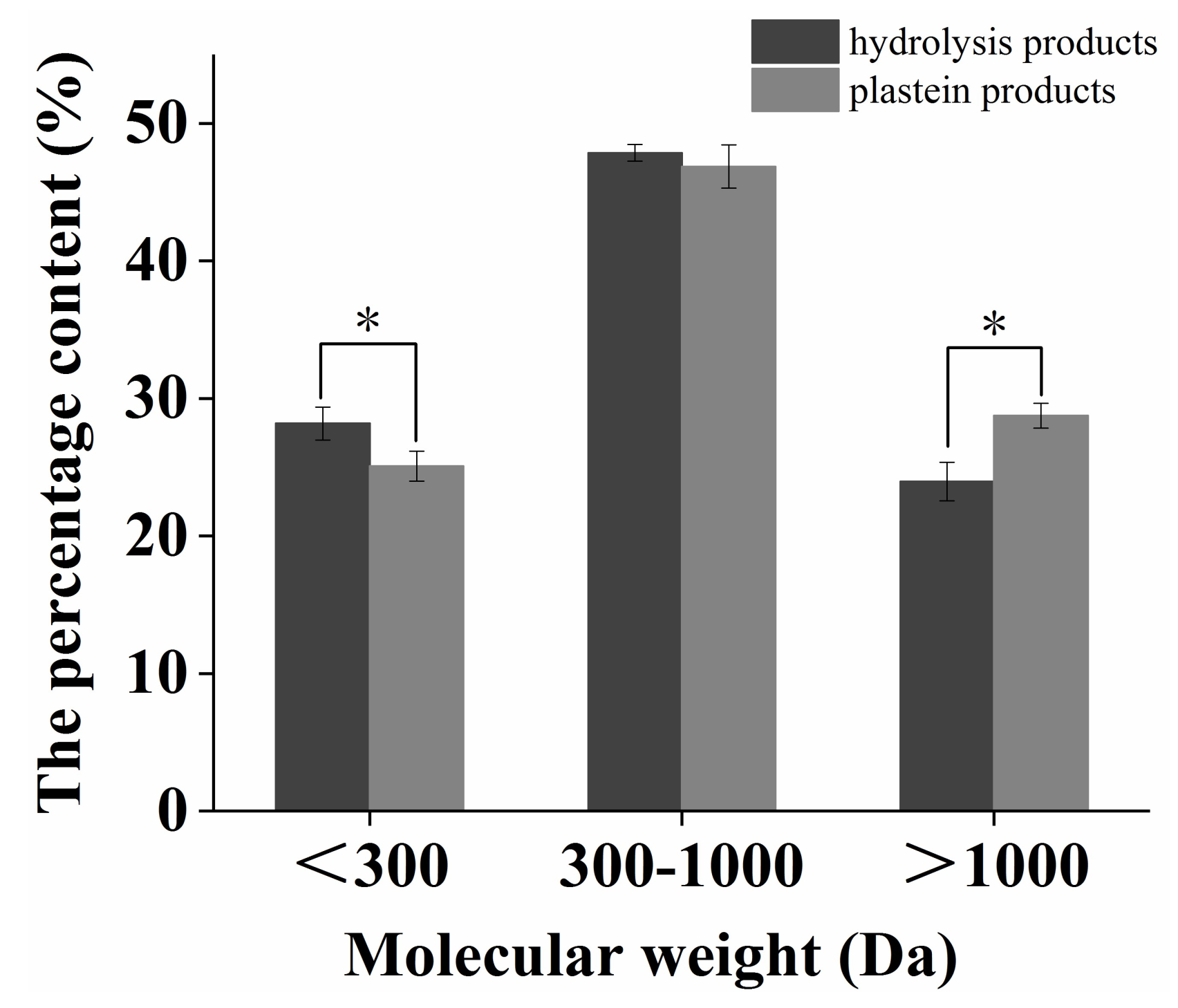
2.4. Change in Molecular Weight Distribution during the Plastein Reaction
2.5. Zinc-Binding Capacity and l-[1-13C]Glutamate Abundance of Different Components of Plastein Products
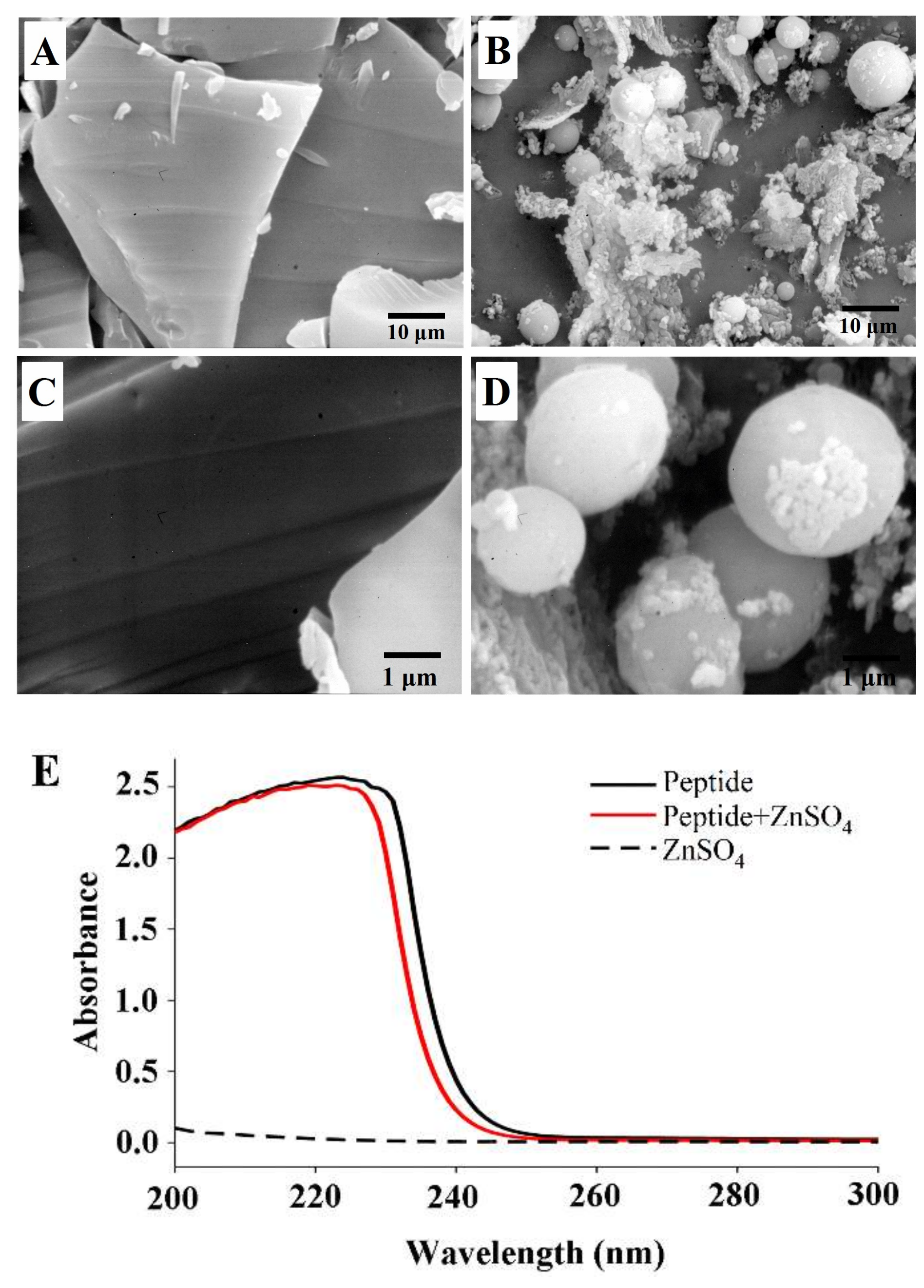
2.6. Scanning electron microscopy (SEM) Photograph and UV-Vis Absorption of the peptide-zinc complex (MZ)
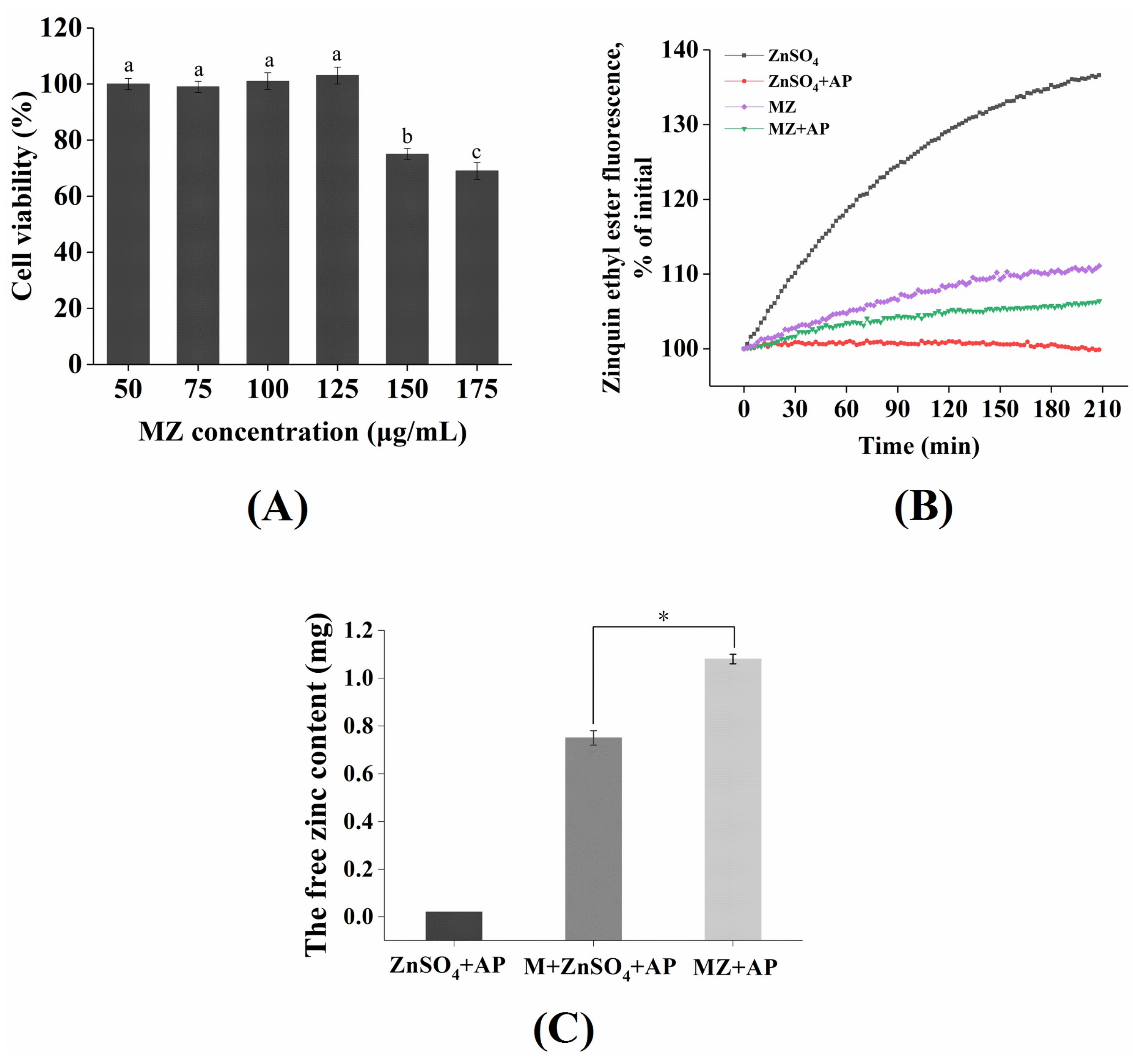
2.7. Cytotoxicity of MZ Against Caco-2 Cells
2.8. Absorption of Zinc from MZ in Caco-2 Cells
2.9. The Effect of Phytic Acid on Zinc Bioavailability
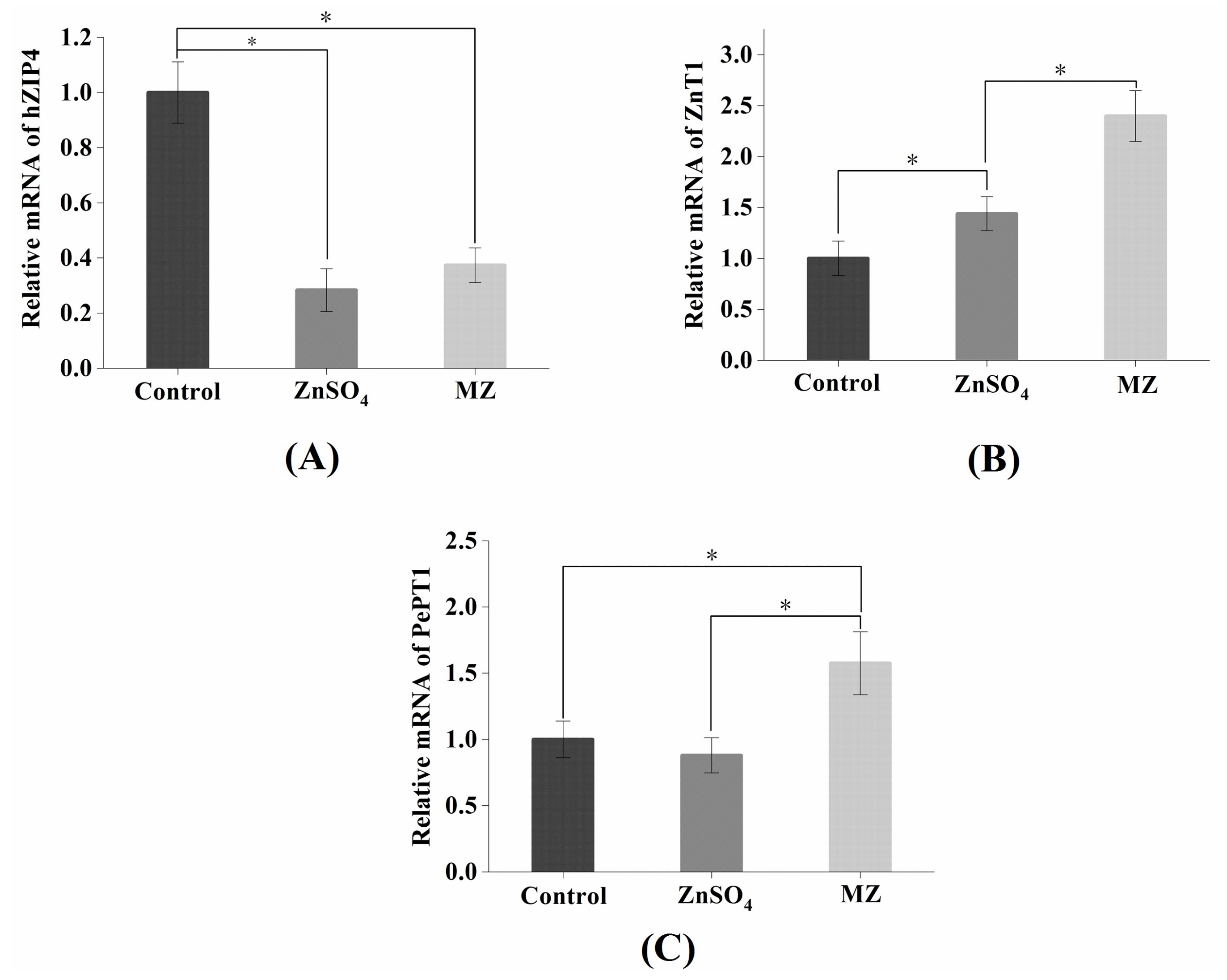
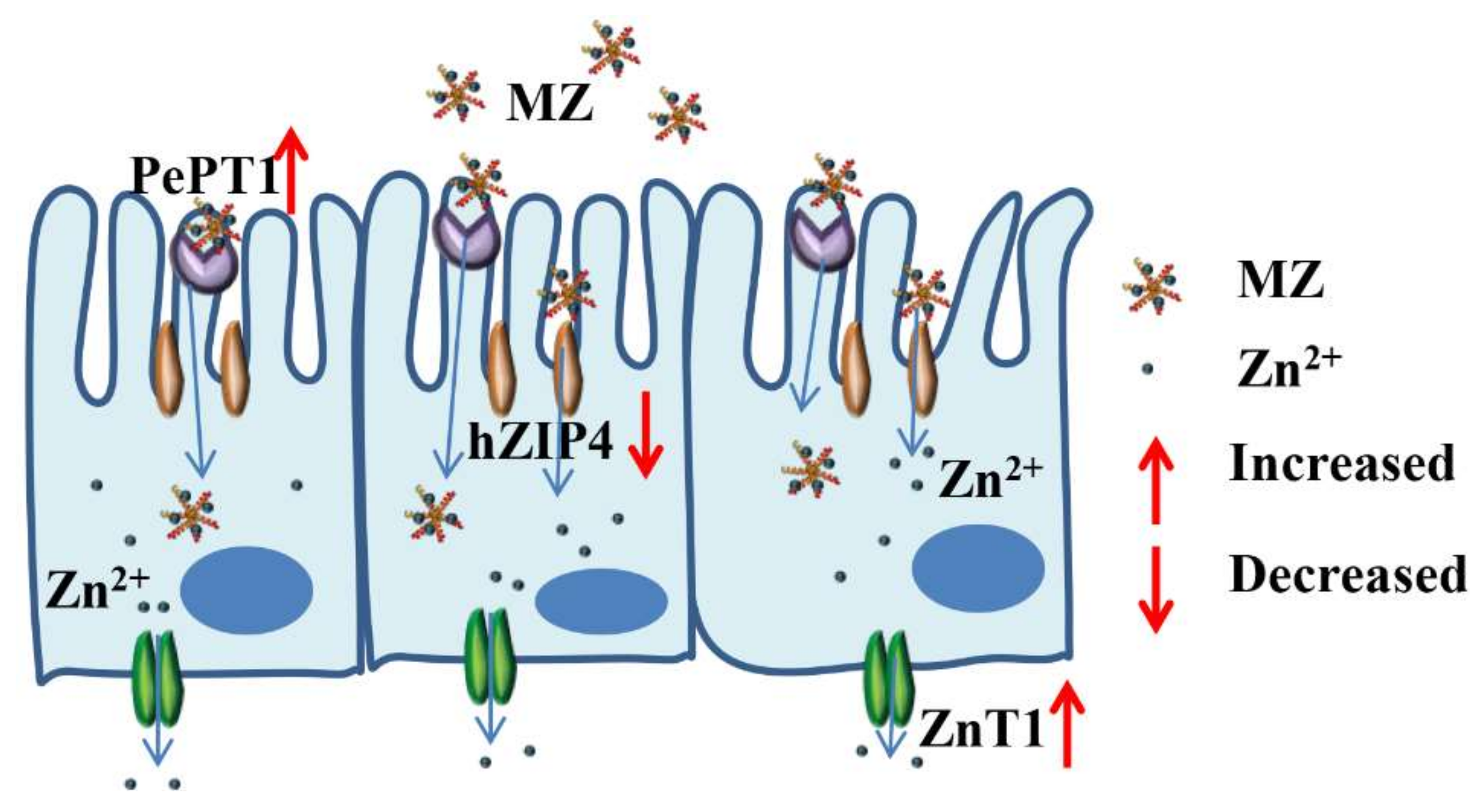
2.10. Effect of MZ on hZIP4 and ZnT1 mRNA Levels in Caco-2 Cells
2.11. Effect of MZ on PePT1 mRNA Levels in Caco-2 Cells
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Oyster Hydrolysate Preparation
3.3. Modification of Oyster Hydrolysates by Plastein Reaction
3.4. Free Amino Acids Determination
3.5. Zinc-Binding Capacity Determination
3.6. Hydrophobic Changes during the Plastein Reaction
3.7. Effects of Protein Denaturants on Plastein Products
3.8. Change in the Molecular Weight Distribution Profile
3.9. Determination of the Abundance of l-[1-13C]Glutamate
3.10. Characterization of the MZ
3.10.1. Preparation of the MZ
3.10.2. UV-Visible
3.10.3. Morphology Analysis
3.11. Cytotoxicity of the MZ against Caco-2 Cells
3.12. Zinc Absorption Assay in Caco-2 Cells
3.13. Effect of MZ on hZIP4, PepT-1 and ZnT1 mRNA Levels in Caco-2 Cells
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prasad, A.S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. 2014, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Trame, S.; Wessels, I.; Haase, H.; Rink, L. A short 18 items food frequency questionnaire biochemically validated to estimate zinc status in humans. J. Trace Elem. Med. Biol. 2018, 49, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Spenser, R.; Hadar, N.; Sharon, M.; Glahn, R.P.; Omry, K.; Elad, T. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutr. 2011, 142, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Puckett, B.J.; Eggleston, D.B. Oyster demographics in a network of no-take reserves: Recruitment, growth, survival, and density dependence. Mar. Coast. Fish. 2012, 4, 605–627. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, S.; Xu, J.; Zeng, M.; Song, H.; Zhao, Y. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control 2008, 19, 231–235. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Zeng, M.; Cui, W.; Zhao, Y.; Liu, Z.; Dong, S.; Guo, Y. Antiviral active peptide from oyster. Chin. J. Oceanol. Limn. 2008, 26, 307–312. [Google Scholar] [CrossRef]
- Shiozaki, K.; Shiozaki, M.; Masuda, J.; Yamauchi, A.; Ohwada, S.; Nakano, T.; Yamaguchi, T.; Saito, T.; Muramoto, K.; Sato, M. Identification of oyster-derived hypotensive peptide acting as angiotensin-I-converting enzyme inhibitor. Fish. Sci. 2010, 76, 865–872. [Google Scholar] [CrossRef]
- Coombs, T. The distribution of zinc in the oyster Ostrea edulis and its relation to enzymic activity and to other metals. Mar. Biol. 1972, 12, 170–178. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Huang, W.; Zhao, Y.; Dong, S.; Zeng, M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J. Funct. Foods 2013, 5, 689–697. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Liu, X.; Zhao, M. Particulate nanocomposite from oyster (Crassostrea rivularis) hydrolysates via zinc chelation improves zinc solubility and peptide activity. Food Chem. 2018, 258, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, J.; Wang, Z.; Zhao, Y. Separation and identification of oyster peptide modified by plastein reaction and characterization of peptide-zinc complexes. Chem. J. Chin. Univ. 2018, 39, 470–475. [Google Scholar]
- Li, J.; Liu, Z.; Zhao, Y.; Zhu, X.; Yu, R.; Dong, S.; Wu, H. Novel natural angiotensin converting enzyme (ACE)-inhibitory peptides derived from sea cucumber-modified hydrolysates by adding exogenous proline and a study of their structure–activity relationship. Mar. Drugs 2018, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Brownsell, V.; Williams, R.; Andrews, A. Application of the plastein reaction to mycoprotein: II. Plastein properties. Food Chem. 2001, 72, 337–346. [Google Scholar] [CrossRef]
- Suisui, J.; Yuanhui, Z.; Qingqing, S.; Xiaojie, Z.; Shiyuan, D.; Zunying, L.; Haohao, W.; Mingyong, Z. Modification of ACE-inhibitory peptides from Acaudina molpadioidea using the plastein reaction and examination of its mechanism. Food Biosci. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and identification of ACE inhibitory peptides from the marine macroalga ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Piper, D.W.; Fenton, B.H. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965, 6, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Condés, M.C.; Añón, M.C.; Mauri, A.N.; Dufresne, A. Amaranth protein films reinforced with maize starch nanocrystals. Food Hydrocolloid. 2015, 47, 146–157. [Google Scholar] [CrossRef]
- Grallert, A.; Hagan, I.M. Preparation of protein extracts from Schizosaccharomyces pombe using trichloroacetic acid precipitation. Cold Spring Harb. Protoc. 2017, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Makhatadze, G.I.; Privalov, P.L. Protein interactions with urea and guanidinium chloride: A calorimetric study. J. Mol. Biol. 1992, 226, 491–505. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pavlova, S.T.; Kim, J.; Kim, J.; Mirica, L.M. The effect of Cu2+ and Zn2+ on the Aβ42 peptide aggregation and cellular toxicity. Metallomics 2013, 5, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Feng, Y.; Guo, T.; Yang, Y.; Guo, W.; Huang, M.; Wu, H.; Zeng, M. Biogenic polyphosphate nanoparticles from Synechococcus sp. PCC 7002 exhibit intestinal protective potential in human intestinal epithelial cells in vitro and murine small intestine ex vivo. J. Agric. Food Chem. 2018, 66, 8026–8035. [Google Scholar] [CrossRef] [PubMed]
- Ranaldi, G.; Ferruzza, S.; Canali, R.; Leoni, G.; Zalewski, P.D.; Sambuy, Y.; Perozzi, G.; Murgia, C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J. Nutr. Biochem. 2013, 24, 967–976. [Google Scholar] [CrossRef]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, C.; Liu, L.; Gong, Y.; Peng, S.; Xie, Y.; Cao, Y. 3-Hydroxyflavone enhances the toxicity of ZnO nanoparticles in vitro. J. Appl. Toxicol. 2018, 38, 1206–1214. [Google Scholar] [CrossRef]
- Tacnet, F.; Lauthier, F.; Ripoche, P. Mechanisms of zinc transport into pig small intestine brush-border membrane vesicles. J. Physiol. 1993, 465, 57–72. [Google Scholar] [CrossRef]
- Cousins, R.J. Gastrointestinal factors influencing zinc absorption and homeostasis. Int. J. Vitam. Nutr. Res. 2010, 80, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ojima, K.; Naito, H.; Ichinose, N.; Tateishi, T. Preparation, solubility, and cytocompatibility of zinc-releasing calcium phosphate ceramics. J. Biomed. Mater. Res. A 2015, 50, 178–183. [Google Scholar] [CrossRef]
- Hansen, M.; Sandström, B.; Lönnerdal, B. The effect of casein phosphopetides on zinc and calcium absorption from high phytate infant diets assessed in rat pups and Caco-2 cells. Pediatr. Res. 1996, 40, 547. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.X.; Wang, X.P.; Guo, X.N. Isolation and characterization of zinc-chelating peptides from wheat germ protein hydrolysates. J. Funct. Foods 2015, 12, 23–32. [Google Scholar] [CrossRef]
- Deng, B.; Zhou, X.; Wu, J.; Long, C.; Yao, Y.; Peng, H.; Wan, D.; Wu, X. Effects of dietary supplementation with tribasic zinc sulfate or zinc sulfate on growth performance, zinc content and expression of zinc transporters in young pigs. Anim. Sci. J. 2017, 88, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.W.; Prasad, A.S.; Butler, C.E.; Sakr, W.A.; Kucuk, O.; Sarkar, F.H. Differential expression of hZnT-4 in Human prostate tissues. Prostate 2004, 58, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Jappar, D.; Wu, S.P.; Hu, Y.; Smith, D.E. Significance and regional dependency of peptide transporter (PEPT) 1 in the intestinal permeability of glycylsarcosine: In situ single-pass perfusion studies in wild-type and Pept1 knockout mice. Drug Metab. Dispos. 2010, 38, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Maubon, N.; Le Vee, M.; Fossati, L.; Audry, M.; Le Ferrec, E.; Bolze, S.; Fardel, O. Analysis of drug transporter expression in human intestinal Caco-2 cells by real-time PCR. Fund. Clin. Pharmacol. 2007, 21, 659–663. [Google Scholar] [CrossRef]
- Ledoux, M.; Lamy, F. Determination of proteins and sulfobetaine with the Folin-phenol reagent. Anal. Biochem. 1986, 157, 28–31. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, Z.Y.; Dong, S.Y.; Mao, X.Z.; Zhao, Y.H. Stability of modified peptide using zinc binding and plastein reaction. Mod. Food Sci. Technol. 2015, 31, 150–154. [Google Scholar]
- Moro, A.; Gatti, C.; Delorenzi, N. Hydrophobicity of whey protein concentrates measured by fluorescence quenching and its relation with surface functional properties. J. Agric. Food Chem. 2001, 49, 4784–4789. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, E.K.; Satterlee, L.D. The formation of heat and enzyme induced (plastein) gels from pepsin-hydrolyzed soy protein isolate. J. Food Biochem. 2010, 14, 1–13. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, L.; Feng, G.; Miao, Y.; Wu, H.; Zeng, M. Characterization of key factors of anchovy (Engraulis japonicus) meat in the nanoparticle-mediated enhancement of non-heme iron absorption. J. Agric. Food Chem. 2017, 65, 11212–11219. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Xie, Y.; Luo, H.; Li, G.; Wu, T.; Zhang, T. Transport characteristics of isorhamnetin across intestinal Caco-2 cell monolayers and the effects of transporters on it. Food Chem. Toxicol. 2014, 66, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhu, S.; Zeng, M.; Liu, Z.; Dong, S.; Zhao, Y.; Huang, H.; Lo, Y.M. Enhancement of non-heme iron absorption by anchovy (Engraulis japonicus) muscle protein hydrolysate involves a nanoparticle-mediated mechanism. J. Agric. Food Chem. 2014, 62, 8632–8639. [Google Scholar] [CrossRef]
- Makhov, P.; Golovine, K.; Uzzo, R.G.; Rothman, J.; Crispen, P.L.; Shaw, T.; Scoll, B.J.; Kolenko, V.M. Zinc chelation induces rapid depletion of the X-linked inhibitor of apoptosis and sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Cell Death Differ. 2008, 15, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, K.; Raghu, P.; Ravinder, P.; Nair, K.M. Effect of dietary ligands and food matrices on zinc uptake in Caco-2 cells: Implications in assessing zinc bioavailability. J. Agric. Food Chem. 2008, 56, 10967–10972. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Shi, G.; Chang, J.; Liu, Z.; Zeng, M. Cooperation of lactic acid bacteria regulated by the AI-2/LuxS system involve in the biopreservation of refrigerated shrimp. Food Res. Int. 2019, 120, 679–687. [Google Scholar] [CrossRef]
- Moslehi-Jenabian, S.; Gori, K.; Jespersen, L. AI-2 signalling is induced by acidic shock in probiotic strains of Lactobacillus spp. Int. J. Food Microbiol. 2009, 135, 295–302. [Google Scholar] [CrossRef]
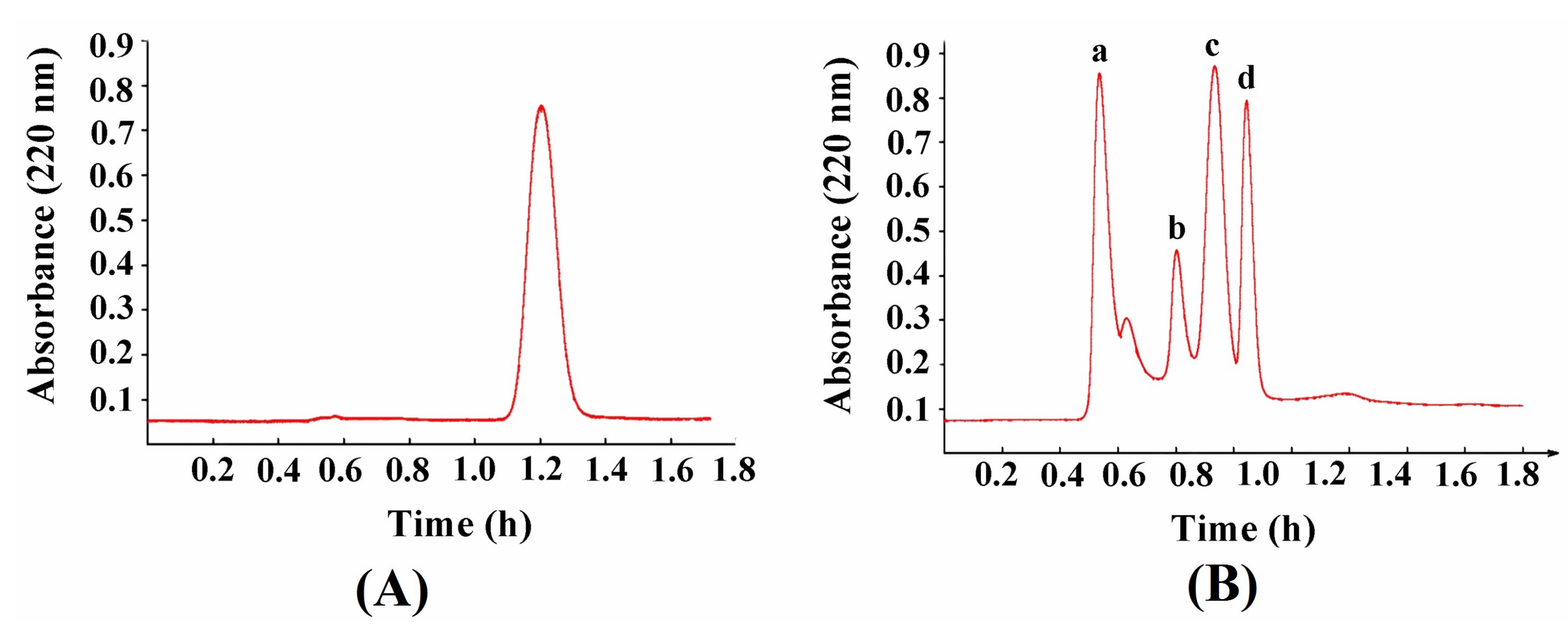
| Sample Name | Zinc-Binding Capacity (mg/g) | l-[1-13C]glutamate Abundance (‰) |
|---|---|---|
| Control | 69.12 ± 1.54 c | −21.87 |
| a | 94.43 ± 2.07 b | 255.73 |
| b | 101.08 ± 3.10 a | 1108.22 |
| c | 72.21 ± 2.68 c | 7.16 |
| d | 26.54 ± 1.43 d | −4.54 |
| Genes | Oligonucleotide Sequence (5′–3′) |
|---|---|
| β-actin | Forward GGAGATTACTGCCCTGGCTCCTA |
| Reverse GACTCATCGTACTCCTGCTTGCTG | |
| ZnT1 | Forward ATGGGGGCTCTGGTGAACGC |
| Reverse CCTGGTCGGGACCCTGCTCG | |
| PepT1 | Forward GCTCTTATCGCCGACTCGTG |
| Reverse GGGTTTGATTCCTCCAGTCC | |
| hZIP4 | Forward TGGTCTCTACGTGGCACTC |
| Reverse GGGTCCCGTACTTTCAACATC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Gong, C.; Wang, Z.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Xiao, F.; Cao, Y.; et al. Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Mar. Drugs 2019, 17, 341. https://doi.org/10.3390/md17060341
Li J, Gong C, Wang Z, Gao R, Ren J, Zhou X, Wang H, Xu H, Xiao F, Cao Y, et al. Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Marine Drugs. 2019; 17(6):341. https://doi.org/10.3390/md17060341
Chicago/Turabian StyleLi, Jianpeng, Chen Gong, Zaiyang Wang, Ruichang Gao, Jiaoyan Ren, Xiaodong Zhou, Haiyan Wang, He Xu, Feng Xiao, Yuhui Cao, and et al. 2019. "Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc" Marine Drugs 17, no. 6: 341. https://doi.org/10.3390/md17060341
APA StyleLi, J., Gong, C., Wang, Z., Gao, R., Ren, J., Zhou, X., Wang, H., Xu, H., Xiao, F., Cao, Y., & Zhao, Y. (2019). Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Marine Drugs, 17(6), 341. https://doi.org/10.3390/md17060341