Biochemical Characterization and Elucidation of Action Pattern of a Novel Polysaccharide Lyase 6 Family Alginate Lyase from Marine Bacterium Flammeovirga sp. NJ-04
Abstract
1. Introduction
2. Results and Discussion
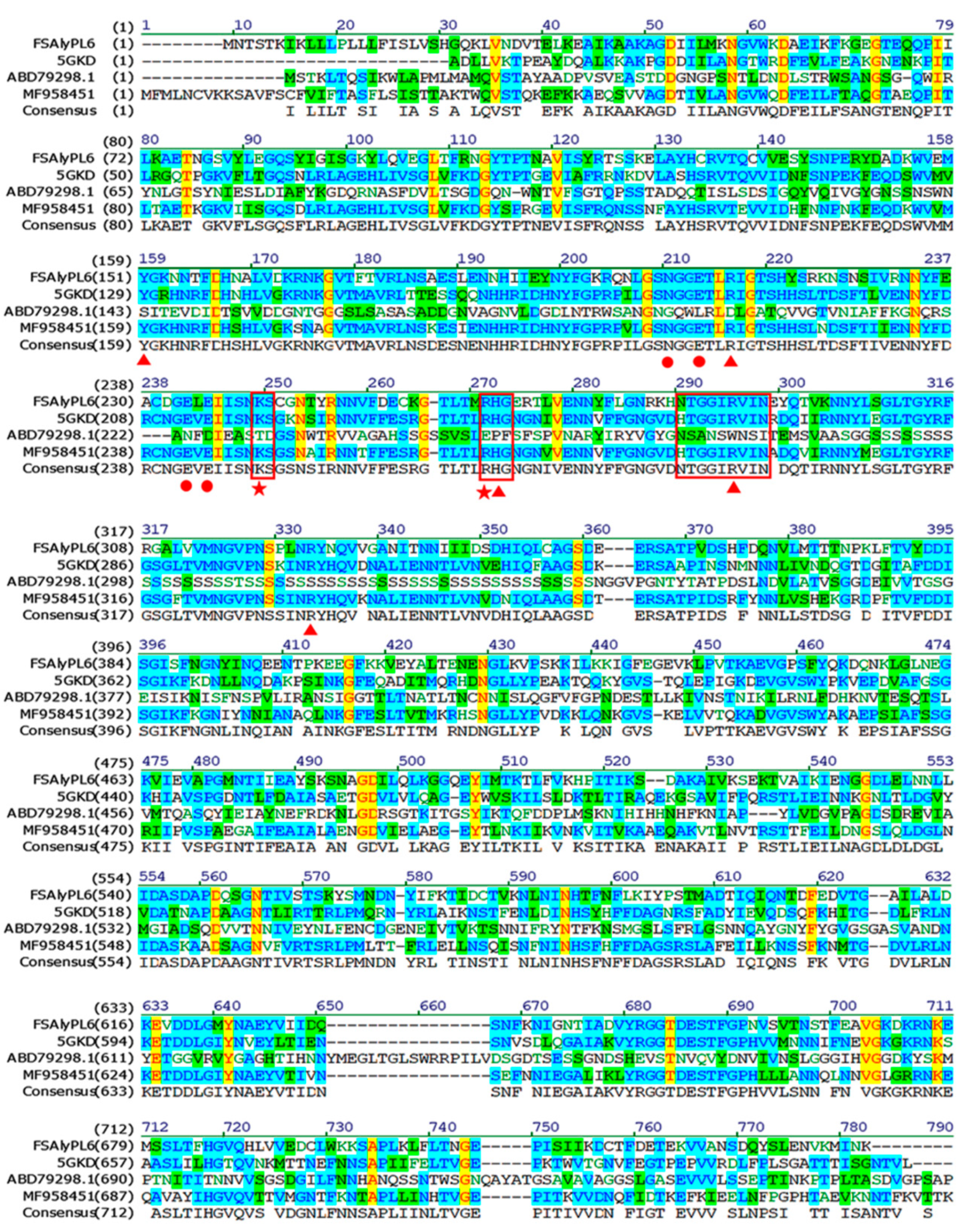
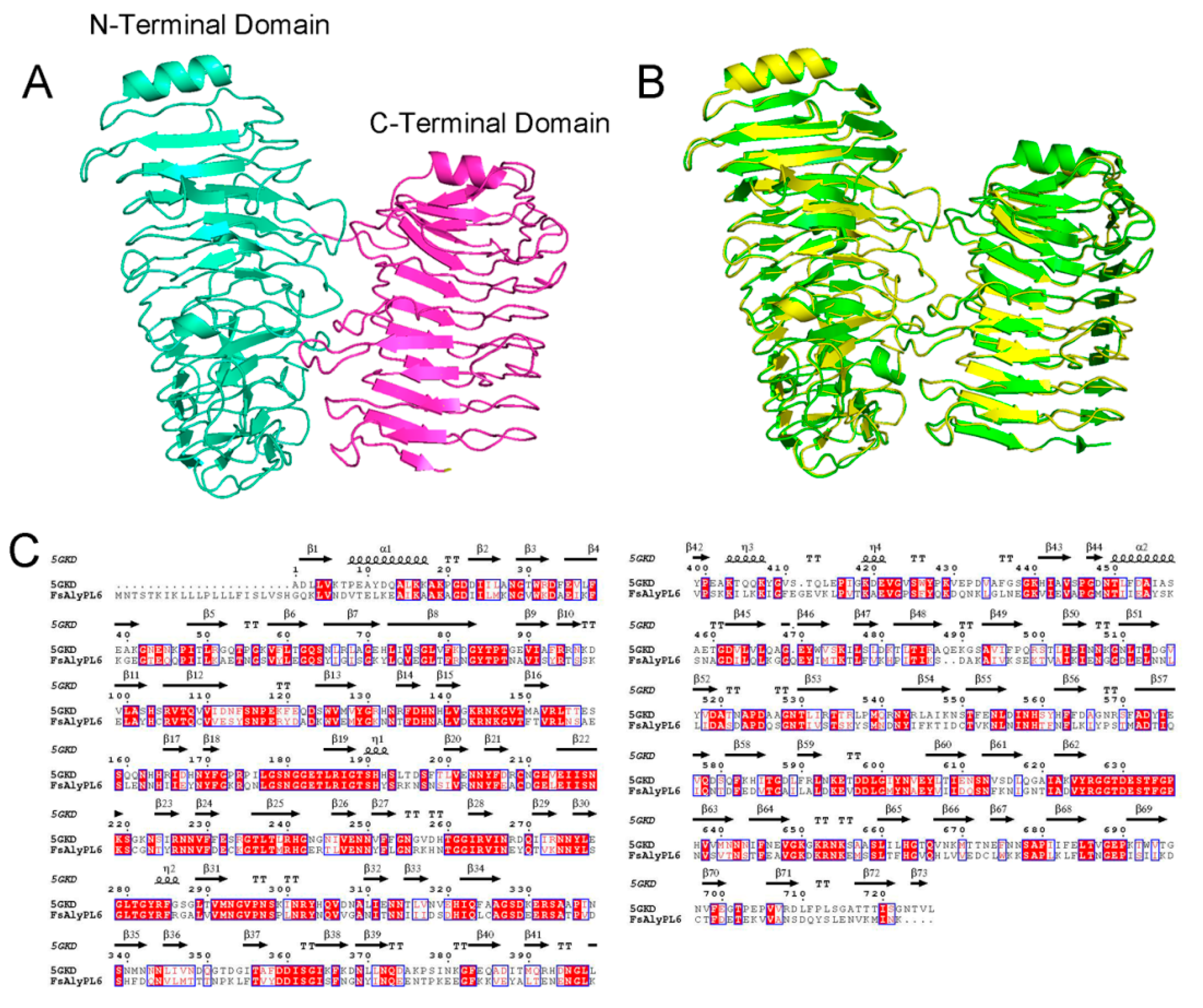
2.1. Sequence Analysis of FsAlyPL6
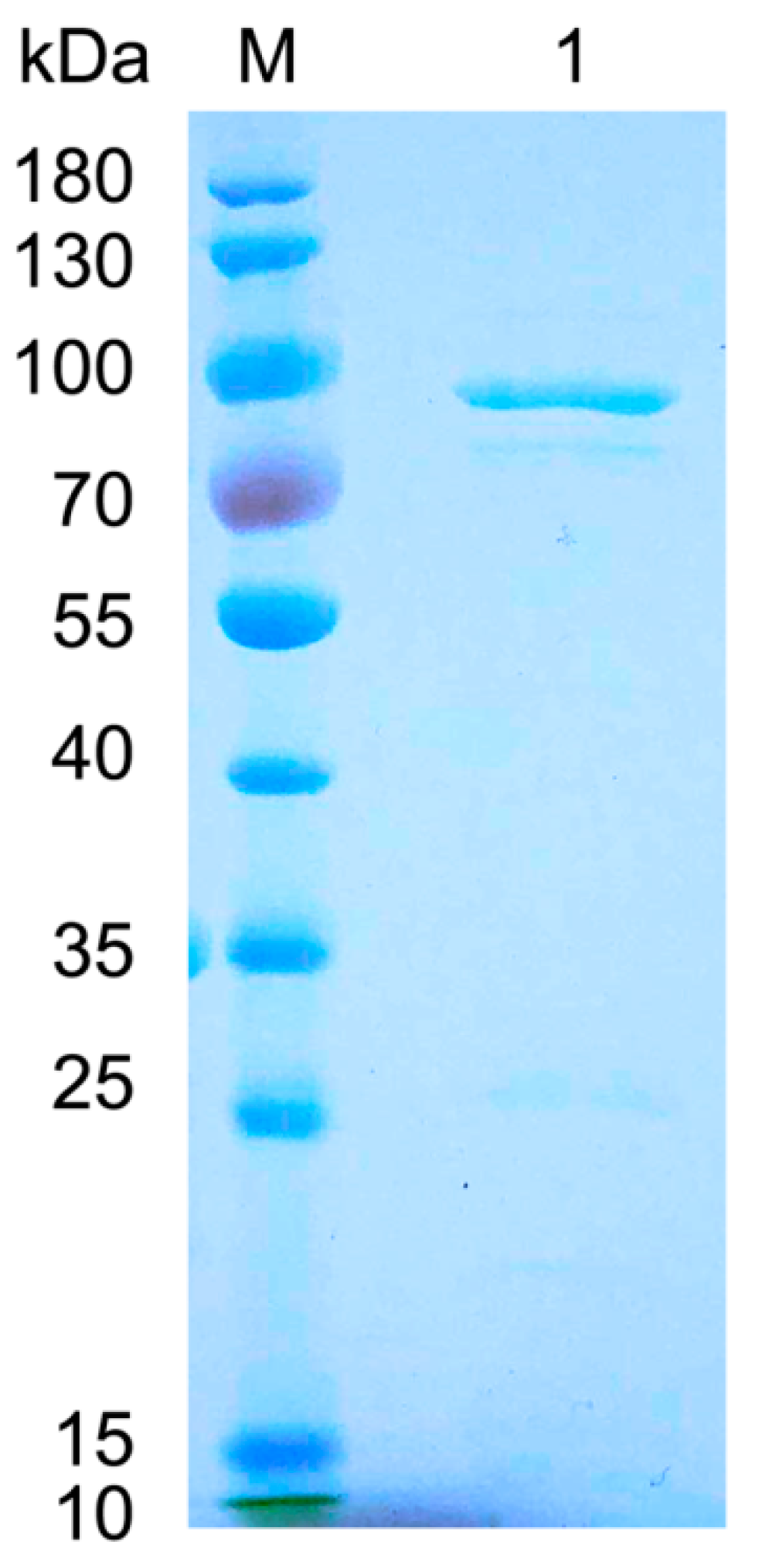
2.2. Expression and Purification of FsAlyPL6
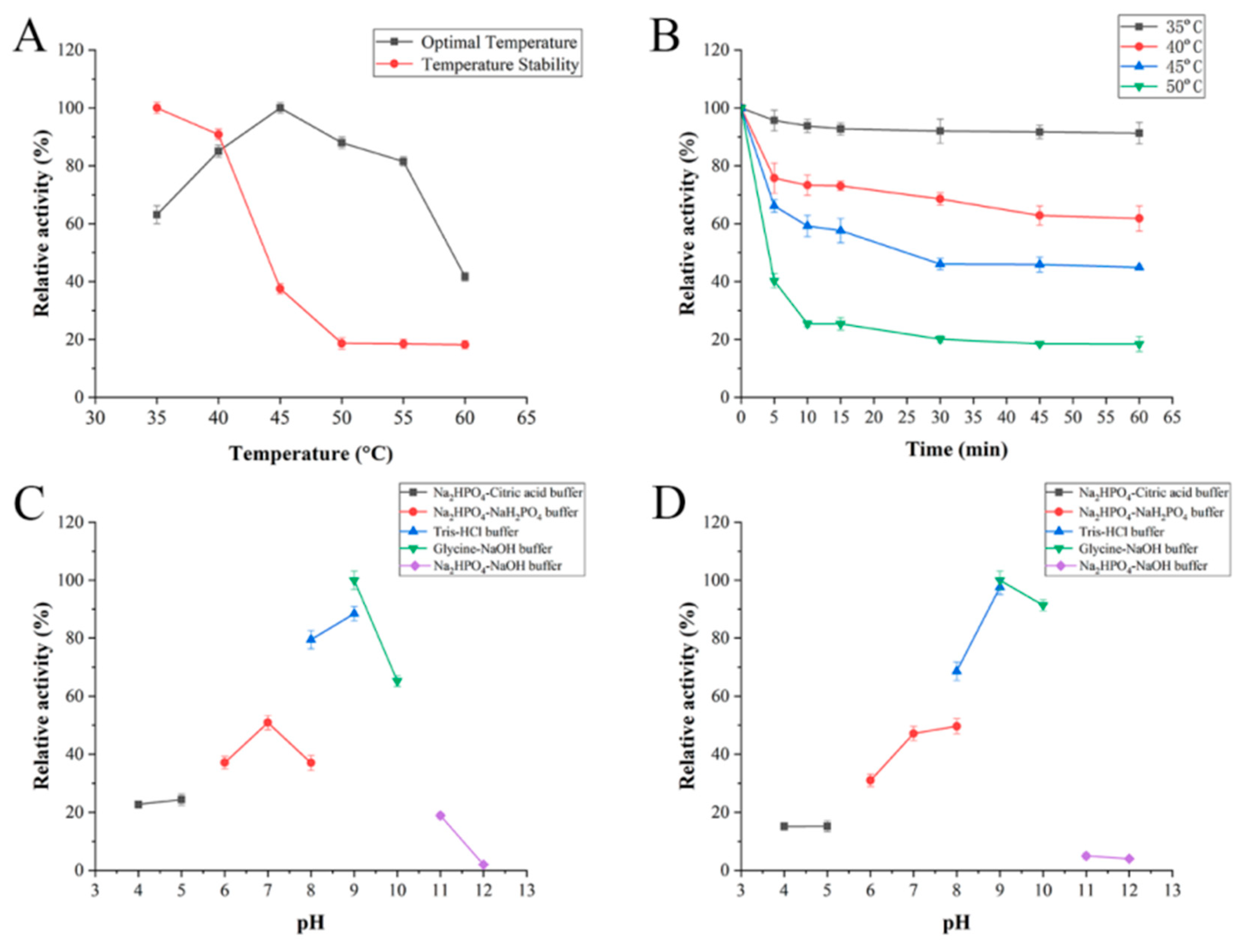
2.3. Biochemical Characterization of FsAlyPL6
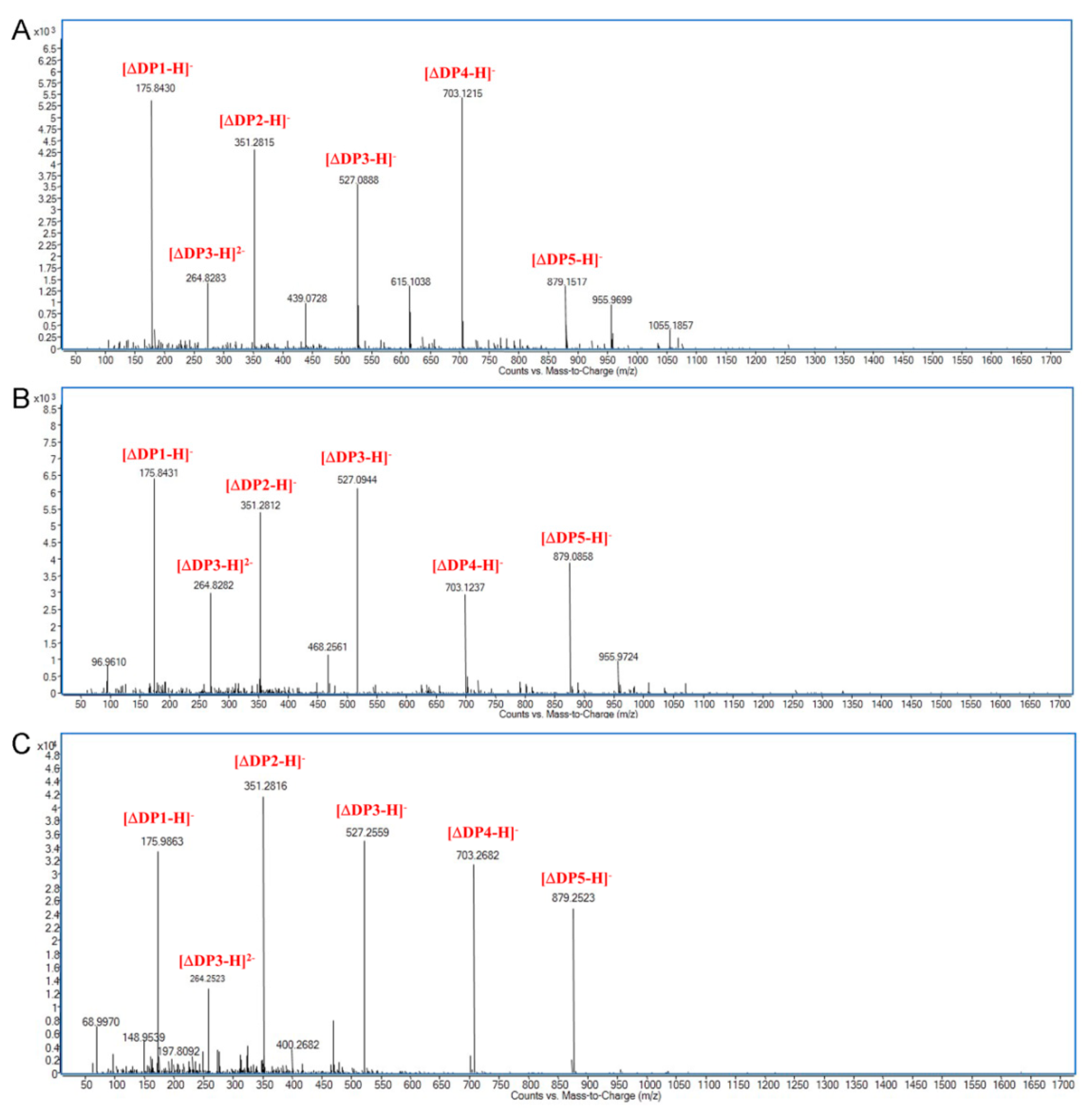
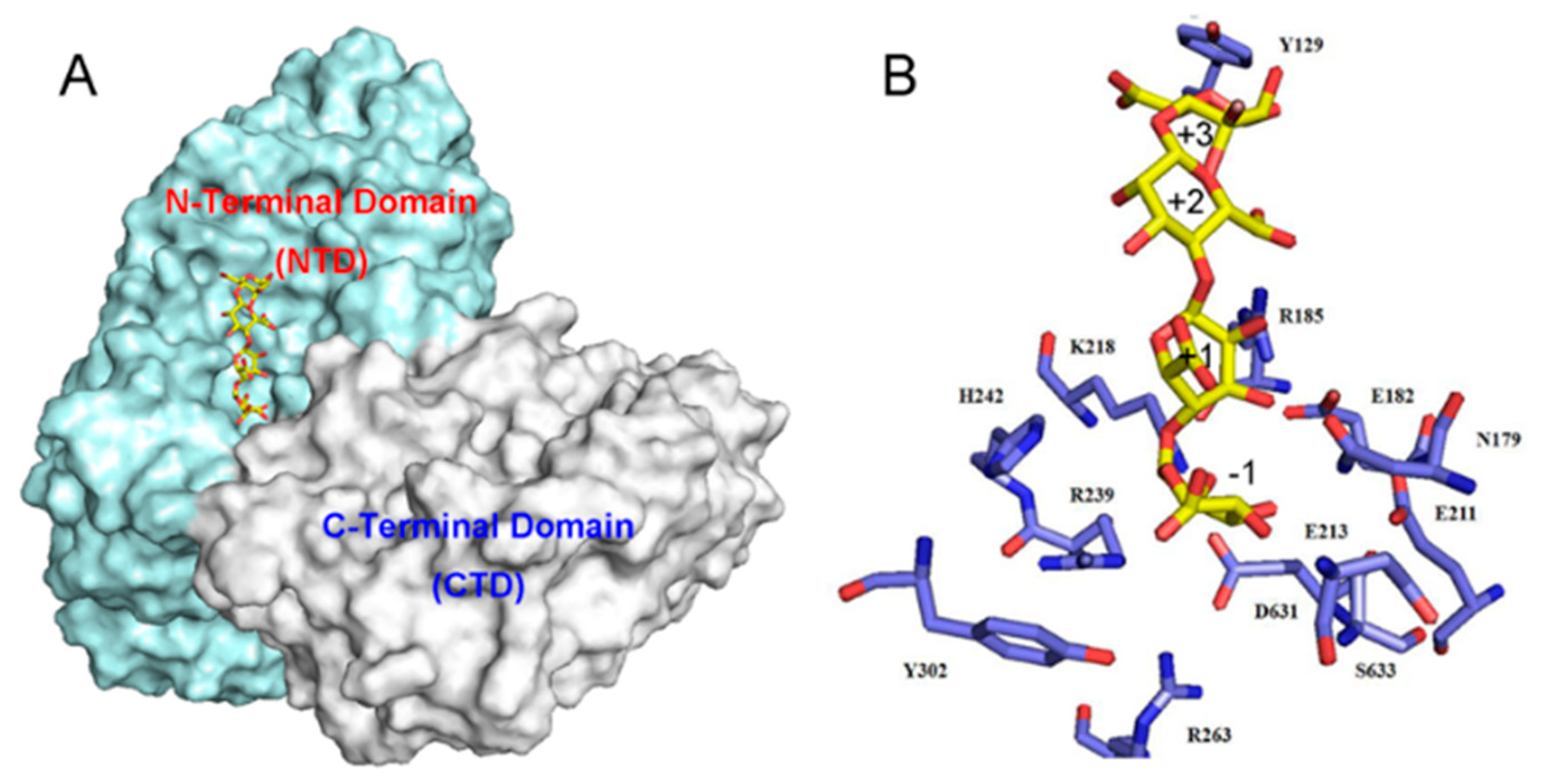
2.4. Action Pattern and Substrate Docking of FsAlyPL6 Product Analysis
3. Materials and Methods
3.1. Materials and Strains
3.2. Cloning and Sequence Analysis of Alginate Lyase
3.3. Hereologous Expression and Purification of the Recombinant Enzyme
3.4. Substrate Specificity and Enzymatic Kinetics
3.5. Biochemical Characterization of the Recombinant Enzyme FsAlyPL6
3.6. Action Pattern and Degradation Product Analysis
3.7. Molecular Modeling and Docking Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gacesa, P. Enzymic degradation of alginates. Int. J. Biochem. 1992, 24, 545–552. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- An, Q.D.; Zhang, G.H.; Zhang, Z.C.; Zheng, G.S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2010, 106, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef] [PubMed]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef] [PubMed]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; And, L.; Schiller, N.L. Alginate Lyase: Review of Major Sources and Enzyme Characteristics, Structure-Function Analysis, Biological Roles, and Applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, M.; Yin, H.; Du, Y.; Ning, L. Enzymatic Hydrolysis of Alginate to Produce Oligosaccharides by a New Purified Endo-Type Alginate Lyase. Mar. Drugs 2016, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yin, H. Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ni, F.; Sun, Y.; Yao, Z. Expression and characterization of a new heat-stable endo-type alginate lyase from deep-sea bacterium Flammeovirga sp. NJ-04. Extremophiles 2017, 21, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Anraku, M.; Nakagawa, S.; Ojima, T. Discovery of a Novel Alginate Lyase from Nitratiruptor sp. SB155-2 Thriving at Deep-sea Hydrothermal Vents and Identification of the Residues Responsible for Its Heat Stability. J. Biol. Chem. 2016, 291, 15551–15563. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dong, F.; Wang, P.; Cao, H.Y.; Li, C.Y.; Li, P.Y.; Pang, X.H.; Zhang, Y.Z.; Chen, X.L. Novel Molecular Insights into the Catalytic Mechanism of Marine Bacterial Alginate Lyase AlyGC from Polysaccharide Lyase Family 6. J. Biol. Chem. 2017, 292, 4457–4468. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; Henrissat, B.; Labre, F.; Skjak-Braek, G.; Helbert, W. Functional Exploration of the Polysaccharide Lyase Family PL6. PLoS ONE 2016, 11, e0159415. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, Z.L.; Li, S.Y.; Su, H.; Tang, L.Y.; Tan, Y.L.; Yu, W.G.; Han, F. Characterization of a new endo-type polysaccharide lyase (PL) family 6 alginate lyase with cold-adapted and metal ions-resisted property. Int. J. Biol. Macromol. 2018, 120, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, L.N.; Han, F.; Gong, Q.H.; Yu, W.G. Cloning and characterization of the first polysaccharide lyase family 6 oligoalginate lyase from marine Shewanella sp Kz7. J. Biochem. 2016, 159, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, L.N.; Chen, X.H.; Zhao, W.W.; Sun, M.; Han, Y.T. Cloning, Expression, and Biochemical Characterization of Two New Oligoalginate Lyases with Synergistic Degradation Capability. Mar. Biotechnol. 2018, 20, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Zhang, K.; Shi, Y.; Li, W.; Diao, X.; Liu, W. Structural insights into a novel Ca2+-independent PL-6 alginate lyase from Vibrio OU02 identify the possible subsites responsible for product distribution. BBA Gen. Subjects 2019, 1863, 1167–1176. [Google Scholar] [CrossRef] [PubMed]


| Substrate | Sodium Alginate | PolyM | PolyG |
|---|---|---|---|
| Activity (U/mg) | 483.95 | 221.5 | 19.35 |
| Km (mg/mL) | 0.50 | 1.52 | 1.62 |
| Vmax (nmol/s) | 1.36 | 0.71 | 0.20 |
| kcat (s−1) | 33.98 | 17.66 | 4.98 |
| kcat/Km (mL·s−1·mg−1) | 62.91 | 11.58 | 3.08 |
| Reagent | Relative Activity (%) |
|---|---|
| Control | 100.00 ± 2.97 |
| K+ | 93.26 ± 2.23 |
| Na+ | 118.57 ± 1.08 |
| Ca2+ | 104.33 ± 1.12 |
| Mg2+ | 102.31 ± 2.78 |
| Co2+ | 22.14 ± 1.32 |
| Zn2+ | 24.88 ± 3.57 |
| Cu2+ | 15.28 ± 1.20 |
| Ni2+ | 50.19 ± 3.93 |
| Mn2+ | 6.46 ± 0.60 |
| Fe3+ | 26.55 ± 1.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Hu, F.; Zhu, B.; Sun, Y.; Yao, Z. Biochemical Characterization and Elucidation of Action Pattern of a Novel Polysaccharide Lyase 6 Family Alginate Lyase from Marine Bacterium Flammeovirga sp. NJ-04. Mar. Drugs 2019, 17, 323. https://doi.org/10.3390/md17060323
Li Q, Hu F, Zhu B, Sun Y, Yao Z. Biochemical Characterization and Elucidation of Action Pattern of a Novel Polysaccharide Lyase 6 Family Alginate Lyase from Marine Bacterium Flammeovirga sp. NJ-04. Marine Drugs. 2019; 17(6):323. https://doi.org/10.3390/md17060323
Chicago/Turabian StyleLi, Qian, Fu Hu, Benwei Zhu, Yun Sun, and Zhong Yao. 2019. "Biochemical Characterization and Elucidation of Action Pattern of a Novel Polysaccharide Lyase 6 Family Alginate Lyase from Marine Bacterium Flammeovirga sp. NJ-04" Marine Drugs 17, no. 6: 323. https://doi.org/10.3390/md17060323
APA StyleLi, Q., Hu, F., Zhu, B., Sun, Y., & Yao, Z. (2019). Biochemical Characterization and Elucidation of Action Pattern of a Novel Polysaccharide Lyase 6 Family Alginate Lyase from Marine Bacterium Flammeovirga sp. NJ-04. Marine Drugs, 17(6), 323. https://doi.org/10.3390/md17060323






