Abstract
Three novel monomeric naphtho-γ-pyrones, peninaphones A–C (compounds 1–3), along with two known bis-naphtho-γ-pyrones (compounds 4 and 5) were isolated from mangrove rhizosphere soil-derived fungus Penicillium sp. HK1-22. The absolute configurations of compounds 1 and 2 were determined by electronic circular dichroism (ECD) spectra, and the structure of compound 3 was confirmed by single-crystal X-ray diffraction analysis. Compounds 4 and 5 are a pair of hindered rotation isomers. A hypothetical biosynthetic pathway for the isolated monomeric and dimeric naphtho-γ-pyrones is also discussed in this study. Compounds 1–3 showed antibacterial activity against Staphylococcus aureus (ATCC 43300, 33591, 29213, and 25923) with minimum inhibitory concentration (MIC) values in the range of 12.5–50 μg/mL. Compound 3 exhibited significant activity against the rice sheath blight pathogen Rhizoctonia solani.
1. Introduction
Natural marine products have been recognized as a valuable and excellent source of structurally novel pharmaceuticals. Marine-derived microorganisms, especially mangrove-associated microorganisms, have developed distinctive metabolic mechanisms on account of the unique properties of the marine environment and their specific functions in the ecosystem, providing a lot of structurally diverse secondary metabolites with a wide variety of biological activities. In recent decades, mangrove-derived fungi have become one of the research hotspots in the area of natural products and marine drugs [1,2,3,4,5,6], with a steady and continued growth in the number of new metabolites, from 108 in 2014 and 126 in 2015 to 142 in 2016 [7].
Naphtho-γ-pyrones, isolated from multiple fungal genera, including Chaetomium, Guanomyces, Aspergillus, Ustilaginoidea, and Penicillium [8,9,10,11,12,13], are a class with a skeletal structural formula composed of a tricyclic system incorporating naphthalene and fused γ-pyrone rings. Structurally, they are divided into linear and angular shapes according to whether the naphthalene and pyrone rings are in a straight line [14]. Furthermore, they usually occur as dimeric forms, and only a small quantity of monomeric compounds has been reported [9,15,16]. In terms of bioactivities, naphtho-γ-pyrones have been reported for cytotoxicity against human epidermoid carcinoma cells [17], inhibitory effects on HIV-1 integrase [18], triacylglycerol synthesis in mammalian CHO-K1 cells [13], and mouse spleen cell proliferation [19], as well as phytotoxic [11], antitubercular [16], and antimicrobial activities [19].
In our ongoing research on bioactive secondary metabolites produced by mangrove-derived fungi [20,21,22], a strain of Penicillium sp. HK1-22 isolated from mangrove rhizosphere soil attracted our attention, because its culture extract showed antibacterial activity against a panel of pathogenic bacteria. Chemical investigation of this fungus cultivated with potato dextrose broth led to the isolation of three new monomeric naphtho-γ-pyrones, peninaphones A–C (Figure 1, compounds 1–3), along with two known bis-naphtho-γ-pyrones (Figure 1, compounds 4 and 5). In this study, the isolation, structure elucidation, and biological activities of compounds 1–5 are described. A hypothetical biosynthetic pathway for the isolated naphtho-γ-pyrones (compounds 1–5) is also discussed.
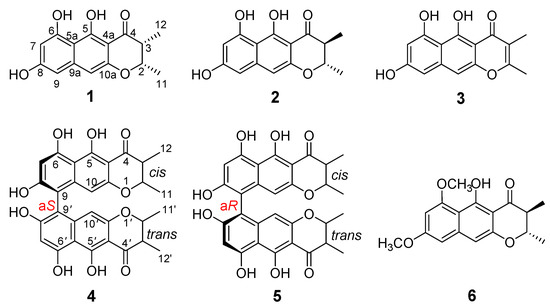
Figure 1.
Structures of compounds 1–6.
2. Results and Discussion
Peninaphone A (compound 1) was isolated as a yellow powder and exhibited the molecular formula of C15H14O5 (nine degrees of unsaturation) based on high resolution electrospray ionization mass spectroscopy (HRESIMS) ([M + H]+ 275.0857 (calculated for C15H15O5 275.0860)) and NMR spectroscopic data. The IR spectrum of compound 1 revealed the presence of hydroxyl (3379 cm−1) and carbonyl (1642 cm−1). Its 1H NMR (Table 1) spectrum revealed two methyl doublet signals at δH 1.20 (3 H, d, J = 6.6 Hz) and 1.38 (3 H, d, J = 6.6 Hz), two methine groups at δH 2.82 (1 H, dq, J = 3.0, 6.6 Hz) and 4.67 (1 H, dq, J = 3.0, 6.6 Hz), and three aromatic signals at δH 6.28 (1 H, brs), 6.46 (1 H, s), and 6.52 (1 H, brs), as well as three hydroxyl protons at δH 9.15 (1 H, s), 9.48 (1 H, s), and 15.59 (1 H, s). The 13C NMR (Table 1) and distortionless enhancement by polarization transfer (DEPT) spectrum displayed 15 carbon resonance signals, which were assigned to one carbonyl (δC 203.2), 10 aromatic carbon atoms, two methines, and two methyl groups. These fragments accounted for six of the nine degrees of unsaturation, requiring three additional rings to be present in compound 1. The above NMR spectroscopic data showed that compound 1 was very similar to (2S,3S)-5-hydroxy-6,8-dimethoxy-2,3-dimethyl-4H-2,3- dihydronaphtho[2,3-b]-pyran-4-one (Figure 1, compound 6), a naphtho-γ-pyrone equipped with a linear tricyclic system isolated from coprophilous fungus Guanomyces polythrix [9]. The obvious difference in the 1H NMR spectrum was the disappearance of two oxygenated methyl groups with the concomitant presence of an additional two hydroxyl signals, suggestive of three hydroxyl groups in compound 1. In the heteronuclear multiple bond correlation (HMBC) spectra (Figure 2), correlations from OH-6 (δH 9.48) to C-5a (δC 105.0), C-6 (δC 160.8), and C-7 (δC 100.7) and from OH-5 to C-4a (δC 101.8) and C-5a (δC 105.0) enabled two hydroxyl groups to be placed at C-6 and C-5, respectively. The remaining hydroxyl group could be deduced at C-8 according to a comparison of the 13C NMR chemical shift of C-8 (δC 162.9 in compound 1 vs. δC 162.3 in compound 6), although the HMBC connection from OH-8 (δH 9.15) to C-8 (δC 162.9) could not be observed for compound 1. Detailed assignments for proton and carbon signals (Table 1) for compound 1 were unambiguously accomplished by analysis of its one-dimensional (1D) and two-dimensional (2D) NMR data.

Table 1.
NMR spectroscopic data (600 MHz, acetone-d6) for compounds 1–3.
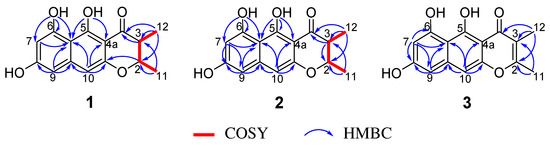
Figure 2.
COSY and key HMBC correlations for compounds 1–3.
The relative configuration of compound 1 was determined by the analysis of its coupling constant. The small coupling constant (J = 3.0 Hz) between the two methine protons (H-2 and H-3) in the dihydro-γ-pyrone ring suggested the cis orientation of 2-H and 3-H. The absolute configuration of compound 1 was established by its electronic circular dichroism (ECD) spectrum (Figure S7) according to previous reports [9,23]. The ECD spectrum of compound 1 displayed negative Cotton effect around 314 nm, suggesting the 2S configuration for compound 1. Automatically, the configuration at C-3 was assigned as R in compound 1 due to the established cis relationship of the methyl groups. Therefore, the absolute configuration of compound 1 was assigned as 2S,3R.
Peninaphone B (compound 2) exhibited the same molecular formula as compound 1 according to its HRESIMS data ([M + H] + 275.0907 (calculated for C15H15O5 275.0914)). The 1D and 2D NMR data of compound 2 (Table 1) were almost identical to those of compound 1, indicating that these two compounds were isomers sharing the same planar structure. It is worth noting that their NMR data changed slightly in the γ-pyrone part. Firstly, differences were found for the chemical shifts of the methines H-2/C-2 (δH/δC 4.67/76.4 in compound 1 vs. 4.29/79.1 in compound 2) and H-3/C-3 (δH/δC 2.82/44.9 in compound 1 vs. 2.80/46.7 in compound 2) and the methyls CH3-11 (δH/δC 1.38/16.6 in compound 1 vs. 1.50/19.9 in compound 2) and CH3-12 (δH/δC 1.20/9.8 in compound 1 vs. 1.25/10.3 in compound 2). Secondly, the coupling constant values of H-2 and H-3 in compound 2 were significantly changed (J = 3.0 Hz in compound 1 vs. J = 10.8 Hz in compound 2), clearly indicating that H-2 and H-3 in compound 2 were in a trans relationship. The ECD spectrum of compound 2 displayed negative Cotton effect around 314 nm (Figure S14), suggesting the same 2S absolute configuration for compound 2. Combined with the trans configuration of H-2 and H-3, the absolute configuration of compound 2 was established as 2S,3S.
Peninaphone C (compound 3) was also obtained as a yellow powder, giving the molecular formula C15H12O5 with 10 degrees of unsaturation by the analysis of its HRESIMS, revealing two fewer H than compounds 1 and 2. The presence of two nonprotonated vinylic signals (δc 165.2 and 111.8) in the 13C NMR spectrum of compound 3 suggested that it was an unsaturated analogue of compound 1. Comprehensive analysis of 1D and 2D NMR data of compounds 1 and 3 revealed that the CH–CH moiety of compound 1 was replaced by a double bond at C-2(3) in compound 3. Furthermore, by slow crystallization from CH3OH, single crystals of compound 3 were obtained, which were suitable for X-ray analysis. Subsequently, by X-ray crystallography, the structure of compound 3 was unequivocally determined, and its Oak Ridge thermal ellipsoid plot (ORTEP)-like view is shown in Figure 3.
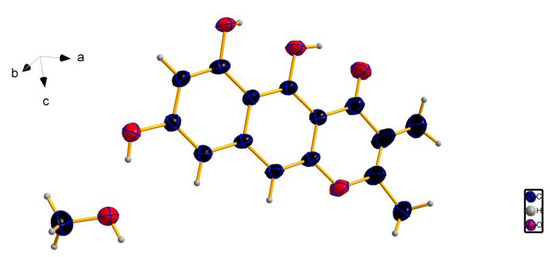
Figure 3.
Oak Ridge thermal ellipsoid plot (ORTEP)-like view of the molecular structure of compound 3.
Compounds 4 and 5 were elucidated as two isomers of bis (naphthodihydropyran-4-one) with the same planar structure. The 1H NMR data showed that they were asymmetrical dimers having one cis-2,3-dimethyl group and one trans-2,3-dimethyl group in dihydro-γ-pyrone rings, respectively. The ECD spectra of compounds 4 and 5 (Figure S20) suggested that the structural difference between them comes from the 9/9’ chiral axis (aS or aR) of the two monomers. The ECD curve of compound 4 showed positive Cotton effect at 295 nm and negative Cotton effect at 265 nm, indicating that compound 4 possesses an aS 9,9’-axial chirality [24,25]. On the contrary, compound 5 displayed a mirror image ECD spectra relative to compound 4; therefore, an aR 9,9’-axial stereochemistry was assigned to compound 5. Therefore, compounds 4 and 5 were identified as a pair of hindered rotation isomers isochaetochromins B1 and B2, respectively, by the comparison of their ECD, NMR, and electrospray ionization-mass spectrometry (ESIMS) data with those reported in the previous literature [13].
A research of the literature revealed that, in general, the mono- and bis-naphtho-γ-pyrones were rarely obtained in the same strain simultaneously. For example, 10 new monomeric naphtho-γ-pyrones were isolated from Guanomyces polythrix [9,15], but no bis-naphtho-γ-pyrones appeared. Again, Zhou’s research group obtained 15 new bis-naphtho-γ-pyrones, ustilaginoidins K–T, from Ustilaginoidea virens (Villosiclava virens) [11,12], but no monomeric naphtho-γ-pyrones were isolated. However, in the present study, three monomeric (compounds 1–3) and two related bis-naphtho-γ-pyrones (compounds 4 and 5) were isolated from strain HK1-22 at the same time.
A plausible biosynthetic pathway for the isolated naphtho-γ-pyrones (compounds 1–5) was proposed (Scheme 1), where the monomeric metabolites were produced via the polyketide pathway and the dimers formed through oxidative coupling [11]. The biosynthesis was started from Claisen condensation of one acetyl-CoA starter unit, six malonyl-CoA extender units, and one methyl unit donated by S-adenosylmethionine (SAM); then, compound 3 was produced and further reduced to form its dihydro derivatives, compounds 1 and 2. The oxidative coupling of compounds 1 and 2 yielded the dimeric compounds 4 and 5 with different axial chirality (Scheme 1). In the previous literature [11], the oxidative coupling between monomeric naphtho-γ-pyrone radicals was proposed to yield the dimeric compounds, but no monomer was isolated from the fungal strain. Noteworthy in this paper, the monomeric naphtho-γ-pyrones and related bis-naphtho-γ-pyrones were obtained simultaneously for the first time, which confirmed the rationality of the proposed biosynthetic pathway.
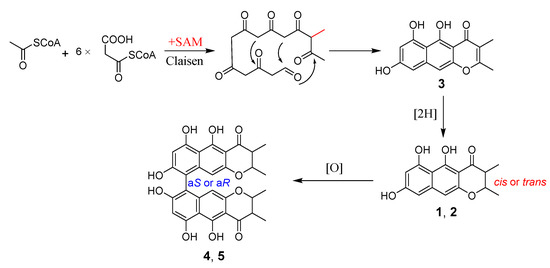
Scheme 1.
Proposed biosynthetic pathway of compounds 1–5. SAM: S-adenosylmethionine.
Naphtho-γ-pyrones are a large class of fungal secondary metabolites possessing a wide range of biological activities [11,12,13,14,15,16,17,18,19]. In this study, the antibacterial effects of the isolated compounds (1–5) are tested against seven bacterial strains, including gram-positive Staphylococcus aureus (ATCC 43300, 33591, 29213, and 25923), Enterococcus faecalis (ATCC 51299), Enterococcus faecium (ATCC 35667), and gram-negative Escherichia coli (ATCC 25922). The results (Table 2) indicate that monomeric compounds 1–3 showed similar antibacterial activities against the four strains of S. aureus with minimum inhibitory concentration (MIC) values in the range of 12.5–50 μg/mL. It is worth noting that compounds 1–3 exhibited moderate antibacterial activities against methicillin-resistant S. aureus (ATCC 43300 and ATCC 33591) with the MIC values of 12.5 and 25 μg/mL, respectively. Neither of the two dimers (compounds 4 or 5) showed activity against the tested bacterial strains. Comparing the antibacterial activities of the monomeric compounds (1–3) and the two dimers (compounds 4 and 5), the results suggest that the dimeric forms decrease the antibacterial activity.

Table 2.
Antibacterial activities of compounds 1–5 a.
Phytopathogens can cause significant crop failure and financial loss; therefore, the screening of microbial metabolites for agricultural fungicide has become a hot topic in recent years [26,27,28]. In an attempt to discover a potential new fungicide, the antifungal activities in vitro of compound 3 were screened by the mycelium linear growth rate method against four crop pathogenic fungi, including Rhizoctonia solani, Rhizoctonia cerealis, Gaeumannomyces graminis, and Alternaria alternata at 50, 10, and 2 μg/mL. The results showed that compound 3 exhibited significant antifungal activities against the four tested crop pathogens (Table 3), especially against the rice sheath blight pathogen R. solani.

Table 3.
Antifungal activities of compound 3.
Meanwhile, compounds 1–3 were screened for their cytotoxic activities against the human hepatocellular carcinoma cell line SMMC-7721. However, none of these monomeric compounds were proved to be active.
3. Materials and Methods
3.1. General Experimental Procedures
IR spectra were recorded on a Cary 610/670 spectrometer (Varian, Salt Lake City, UT, USA) using KBr pellets. UV spectra were measured on a Beckman DU 640 spectrophotometer (Beckman Coulter, Kraemer Boulevard Brea, CA, USA). Optical rotations were acquired on an Anton Paar MCP300 automatic polarimeter (Anton Paar, Graz, Austria). 1D and 2D NMR spectra were recorded on an Avance 600 NMR spectrometer (Bruker, Karlsruhe, Germany)(600 MHz for 1H and 150 MHz for 13C), using TMS (tetramethylsilane) as an internal standard. HRESIMS spectra were obtained from a maXis spectrometer (Bruker, Karlsruhe, Germany). Single-crystal data were determined on a Bruker D8 Quest diffractometer (Mo Kα radiation) (Bruker, Karlsruhe, Germany). Semi-preparative HPLC was performed on a Hitachi system (Hitachi, Tokyo, Japan) using a semi-preparative C18 (Kromasil, 5 μm, 10 × 250 mm) column coupled with a 2400 UV detector. Silica gel (200–300 mesh; Qing Dao Hai Yang Chemical Group Co., Qing Dao, China), Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden), and octadecylsilyl silica gel (45–60 μm; Unicorn) were used for column chromatography (CC). Precoated silica gel plates (G60, F-254; Yan Tai Zi Fu Chemical Group Co., Yan Tai, China) were used for thin layer chromatography (TLC).
3.2. Fungal Material
Penicillium sp. HK1-22 was isolated from mangrove rhizosphere soil, which was collected from the Dongzhaigang mangrove natural reserve in Hainan Island in September 2015. The strain was preserved at the Marine Science & Technology Institute, College of Environmental Science & Engineering, Yangzhou University, Yangzhou, China and identified according to its morphological traits and molecular protocol by amplification and sequencing of the DNA of the internal transcribed spacer (ITS) region of the rRNA gene as described previously [29]. The 593 base pair ITS sequence was submitted to GenBank with the accession number MK790264, and 99% sequence identity resulted with Penicillium javanicum CBS 129771 (MH877078.1).
3.3. Fermentation, Extraction, and Isolation
The Penicillium sp. HK1-22 strain was statically fermented in 75 × 1 L Erlenmeyer flasks containing 400 mL of potato dextrose broth (20 g of dextrose and 30 g of natural sea salt in 1 L of potato infusion) for 4 weeks at room temperature. The culture (30 L) was filtered to separate the broth from the mycelia. The former was exhaustively extracted three times with an equal volume of EtOAc, while the latter was extracted three times with MeOH. The organic extracts were combined and concentrated in vacuo to afford 51.0 g of the total extract, which was subjected to vacuum liquid chromatography (VLC) on silica gel using solvents in a gradient of increasing polarity—petroleum ether–EtOAc–MeOH—to yield a total of five fractions (Fr.1–Fr.5). Fr.3 was subjected to Sephadex LH-20 CC with mixtures of CHCl2–MeOH (1:1, v/v) to obtain subfractions Fr.3-1–Fr.3-3. Fr.3-2 was further purified by semi-preparative HPLC, eluting with MeOH–H2O (70:30, v/v) to yield compound 1 (50.0 mg) and compound 2 (40.0 mg). Recrystallization of Fr.3-3 obtained compound 3 (260.0 mg). Fr.5 was repeatedly subjected to silica gel CC, purified by octadecylsilyl (ODS) CC, and, finally, purified by semipreparative HPLC eluting with MeOH–H2O (65:35, v/v) to give compound 4 (2.0 mg) and compound 5 (3.0 mg).
Peninaphone A (compound 1): yellow amorphous powder; [α]20D +89 (c, 0.05, MeOH); UV (MeOH) λmax (log ε) 332 (0.5), 282 (2.5), 232 (1.8) nm; ECD (1.24 mM, MeOH) λmax (Δε) 339 (−2.35), 325 (+0.04), 313 (−2.10), 296 (+2.73), 280 (−11.67), 247 (+4.51), 231 (−2.07) nm; IR (KBr) νmax 3379, 2948, 2843, 1642, 1454, 1361, 1054, 1032 cm–1; 1H and 13C NMR data (Table 1); HRESIMS m/z 275.0857 [M + H]+ (calculated for C15H15O5, 275.0860).
Peninaphone B (compound 2): yellow amorphous powder; [α]20D +86 (c, 0.06, MeOH); UV (MeOH) λmax (log ε) 328 (0.4), 280 (2.2), 232 (1.5) nm; ECD (1.24 mM, MeOH) λmax (Δε) 336 (−1.69), 325 (−0.21), 294 (−11.81), 256 (5.01), 237 (−3.34) nm; IR (KBr) νmax 3377, 2948, 2833, 1641, 1455, 1419, 1032 cm–1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 275.0907 [M + H]+ (calculated for C15H15O5, 275.0914).
Peninaphone C (compound 3): yellow amorphous powder; UV (MeOH) λmax (log ε) 310 (0.05), 278 (0.3), 216 (0.11) nm; IR (KBr) νmax 3382, 2948, 2843, 1641, 1454, 1054, 1032 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 273.0747 [M + H]+ (calculated for C15H13O5, 273.0757).
3.4. X-ray Crystallographic Analysis of Compound 3
A colorless crystal of compound 3 was obtained by the slow evaporation of a methanol solution. All crystallographic data were collected at 296 K on a Bruker D8 Quest diffractometer with Mo Kα radiation (λ = 0.71073 Å). The structure was elucidated by direct methods using a SHELXS-97 and refined by means of full-matrix least-squares on F2. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were located by geometrical calculations, and their positions and thermal parameters were determined during the structure refinement. Crystallographic data for compound 3 have been deposited in the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 1909893.
Crystal data for compound 3: C15H12O5·CH3OH, Mr = 304.29, monoclinic, a = 9.2406(14) Å, b = 12.9091(19) Å, c = 12.6168(17) Å, α = 90°, β = 91.827(5)°, γ = 90°, V = 1504.3(4) Å3, space group P21/c, Z = 4, Dx = 1.344 mg/mm3, μ(Mo Kα) = 0.103 mm−1, and F(000) = 640. Crystal dimensions: 0.150 × 0.120 × 0.080 mm3. Reflections collected: 19,440. Independent reflections: 2634 (Rint = 0.0522). The final R1 values were 0.0987, wR2 = 0.2363 (I > 2σ(I)), R indices for all data R1 = 0.1201, and wR2 = 0.2497.
3.5. Biological Assays
3.5.1. Antibacterial Assay
The antibacterial assay was carried out as described previously [30].
3.5.2. Anti-Phytopathogenic Assay
The antifungal activities of four crop pathogenic fungi (R. Solani, R. cerealis, G. graminis, and A. alternata) were assayed in vitro by the mycelium linear growth rate method according to [31].
3.5.3. Cytotoxic Assay
The cytotoxic activity was evaluated against the human hepatocellular carcinoma cell line SMMC-7721 by the MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, thiazolyl blue tetrazolium bromide) method as described in [32]. Cisplatin was used as a positive control.
4. Conclusions
In summary, three new monomeric naphtho-γ-pyrones (compounds 1–3) and two known bis-naphtho-γ-pyrones (compounds 4 and 5) were isolated from mangrove rhizosphere soil-derived fungus Penicillium sp. HK1-22. Compounds 1 and 2 were a pair of monomeric isomers, whereas compounds 4 and 5 were a pair of hindered rotation isomers. Monomeric naphtho-γ-pyrones and related bis-naphtho-γ-pyrones were simultaneously obtained from a fungal strain for the first time. Compounds 1–3 showed moderate antibacterial activities against four strains of S. aureus with MIC values in the range of 12.5–50 μg/mL. Compound 3 was also found to exhibit significant antifungal activities against the four tested crop pathogens.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/6/322/s1, Figures S1–S20: The ECD spectra of compounds 1, 2, 4, and 5; HRESIMS; and 1D and 2D NMR spectra of compounds 1–3.
Author Contributions
Extraction, isolation, and manuscript preparation, Y.-Y.Z. and Z.-Y.L.; fungal fermentation, N.-X.S.; X-ray crystallographic analysis, W.L.-L.; antimicrobial bioassays, X.-J.Z.; NMR analysis, X.-M.F.; project leaders guiding the experiments and manuscript writing, M.C. and C.-Y.W. All authors approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81703411; 41830535; U1606403; 41776156), financially supported by the Marine S & T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018SDKJ0406-5), the National Science and Technology Major Project for Significant New Drugs Development (No. 2018ZX09735-004), the Program of Open Studio for Druggability Research of Marine Natural Products, the Pilot National Laboratory for Marine Science and Technology (Qingdao, China) directed by Kai-Xian Chen and Yue-Wei Guo, and the Taishan Scholars Program, China.
Acknowledgments
The Testing Center of Yangzhou University is acknowledged for its assistance with the testing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Swathi, J.; Narendra, K.; Sowjanya, K.M.; Satya, A.K. Marine fungal metabolites as a rich source of bioactive compounds. Afr. J. Biochem. Res. 2013, 7, 184–196. [Google Scholar] [CrossRef]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.Y.; Zhang, J.Y.; Liang, Y.J.; Chen, L.M.; Zhen, L.S.; Wang, F.; Mi, Y.J.; She, Z.G.; To, K.K.W.; Lin, Y.C.; et al. Anticancer effect and structure-activity analysis of marine products isolated from metabolites of mangrove fungi in the South China Sea. Mar. Drugs 2010, 8, 1094–1105. [Google Scholar] [CrossRef]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Deng, C.M.; Liu, S.X.; Huang, C.H.; Pang, J.Y.; Lin, Y.C. Secondary metabolites of a mangrove endophytic fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616–2624. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Koyama, K.; Aida, S.; Natori, S. Supplemental observations on atropisomerism of fungal bis(naphtho-γ-pyrone)s. Chem. Pharm. Bull. 1990, 38, 2259–2261. [Google Scholar] [CrossRef]
- Macías, M.; Ulloa, M.; Gamboa, A.; Mata, R. Phytotoxic compounds from the new coprophilous fungus Guanomyces polythrix. J. Nat. Prod. 2000, 63, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Biswas, K.; Chakrabarti, D.K. Toxic naphtho-γ-pyrones from Aspergillus niger. J. Agric. Food Chem. 1979, 27, 1347–1351. [Google Scholar] [CrossRef]
- Lu, S.Q.; Sun, W.B.; Meng, J.J.; Wang, A.L.; Wang, X.H.; Tian, J.; Fu, X.X.; Dai, J.G.; Liu, Y.; Lai, D.W.; et al. Bioactive bis-naphtho-γ-pyrones from rice false smut pathogen Ustilaginoidea virens. J. Agric. Food Chem. 2015, 63, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.B.; Wang, A.L.; Xu, D.; Wang, W.X.; Meng, J.J.; Dai, J.G.; Liu, Y.; Lai, D.W.; Zhou, L.G. New ustilaginoidins from rice false smut balls caused by Villosiclava virens and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2017, 65, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Ugaki, N.; Matsuda, D.; Yamazaki, H.; Nonaka, K.; Masuma, R.; Ōmura, S.; Tomoda, H. New isochaetochromin, an inhibitor of triacylglycerol synthesis in mammalian cells, produced by Penicillium sp. FKI-4942: I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 2012, 65, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Donner, C.D. Naphthopyranones–isolation, bioactivity, biosynthesis and synthesis. Nat. Prod. Rep. 2015, 32, 578–604. [Google Scholar] [CrossRef] [PubMed]
- Macías, M.; Gamboa, A.; Ulloa, M.; Toscano, R.A.; Mata, R. Phytotoxic naphthopyranone derivatives from the coprophilous fungus Guanomyces polythrix. Phytochemistry 2001, 58, 751–758. [Google Scholar] [CrossRef]
- Kong, X.L.; Ma, X.H.; Xie, Y.Y.; Cai, S.X.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Aromatic polyketides from a sponge-derived fungus Metarhizium anisopliae mxh-99 and their antitubercular activities. Arch. Pharm. Res. 2013, 36, 739–744. [Google Scholar] [CrossRef]
- Koyama, K.; Ominato, K.; Natori, S.; Tashiro, T.; Tsuruo, T. Cytotoxicity and antitumor activities of fungal bis (naphtho-γ-pyrone) derivatives. J. Pharmacobio-Dyn. 1998, 11, 630–635. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Bills, G.F.; Teran, A.; Silverman, K.C.; Lingham, R.B.; Felock, P.; Hazuda, D.J. Four novel bis-(naphtho-γ-pyrones) isolated from Fusarium species as inhibitors of HIV-1 integrase. Bioorg. Med. Chem. Lett. 2003, 13, 713–717. [Google Scholar] [CrossRef]
- Xu, G.B.; Yang, T.; Bao, J.K.; Fang, D.M.; Li, G.Y. Isochaetomium A2, a new bis(naphthodihydropyran-4-one) with antimicrobial and immunological activities from fungus Chaetomium microcephalum. Arch. Pharm. Res. 2014, 37, 575–579. [Google Scholar] [CrossRef]
- Chen, M.; Shen, N.X.; Chen, Z.Q.; Zhang, F.M.; Chen, Y. Penicilones A–D, anti-MRSA azaphilones from the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2017, 80, 1081–1086. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Y.Y.; Chen, Z.Q.; Shen, N.X.; Shen, L.; Zhang, F.M.; Zhou, X.J.; Wang, C.Y. NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2019, 82, 368–374. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Shen, N.X.; Liang, Z.Y.; Shen, L.; Chen, M.; Wang, C.Y. Paraherquamide J, a new prenylated indole alkaloid from the marine-derived fungus Penicillium janthinellum HK1-6. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Gaffield, W. Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides: Determination of aglycone chirality in flavanone glycosides. Tetrahedron 1970, 26, 4093–4108. [Google Scholar] [CrossRef]
- Ugaki, N.; Yamazaki, H.; Uchida, R.; Tomoda, H. New isochaetochromin, an inhibitor of triacylglycerol synthesis in mammalian cells, produced by Penicillium sp. FKI-4942: II. structure elucidation. J. Antibiot. 2012, 65, 21–24. [Google Scholar] [CrossRef]
- Koyama, K.; Natori, S. Further characterization of seven bis(naphtho-γ-pyrones) congeners of ustilaginoidins, coloring matters of Claviceps virens (Ustilaginoidea virens). Chem. Pharm. Bull. 1998, 36, 146–152. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef]
- Boukaew, S.; Prasertsan, P. Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi RM-1-138. Crop Prot. 2014, 61, 1–10. [Google Scholar] [CrossRef]
- Suprapta, D.N. Potential of microbial antagonists as biocontrol agents against plant fungal pathogens. J. ISSAAS 2012, 18, 1–8. [Google Scholar]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Yang, R.; Gao, Z.F.; Zhao, J.Y.; Li, W.B.; Zhou, L.; Miao, F. New class of 2-aryl-6-chloro-3,4-dihydroisoquinolinium salts as potential antifungal agents for plant protection: Synthesis, bioactivity and structure-activity relationships. J. Agric. Food Chem. 2015, 63, 1906–1914. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).