Potential TSPO Ligand and Photooxidation Quencher Isorenieratene from Arctic Ocean Rhodococcus sp. B7740
Abstract
1. Introduction
2. Results and Discussion
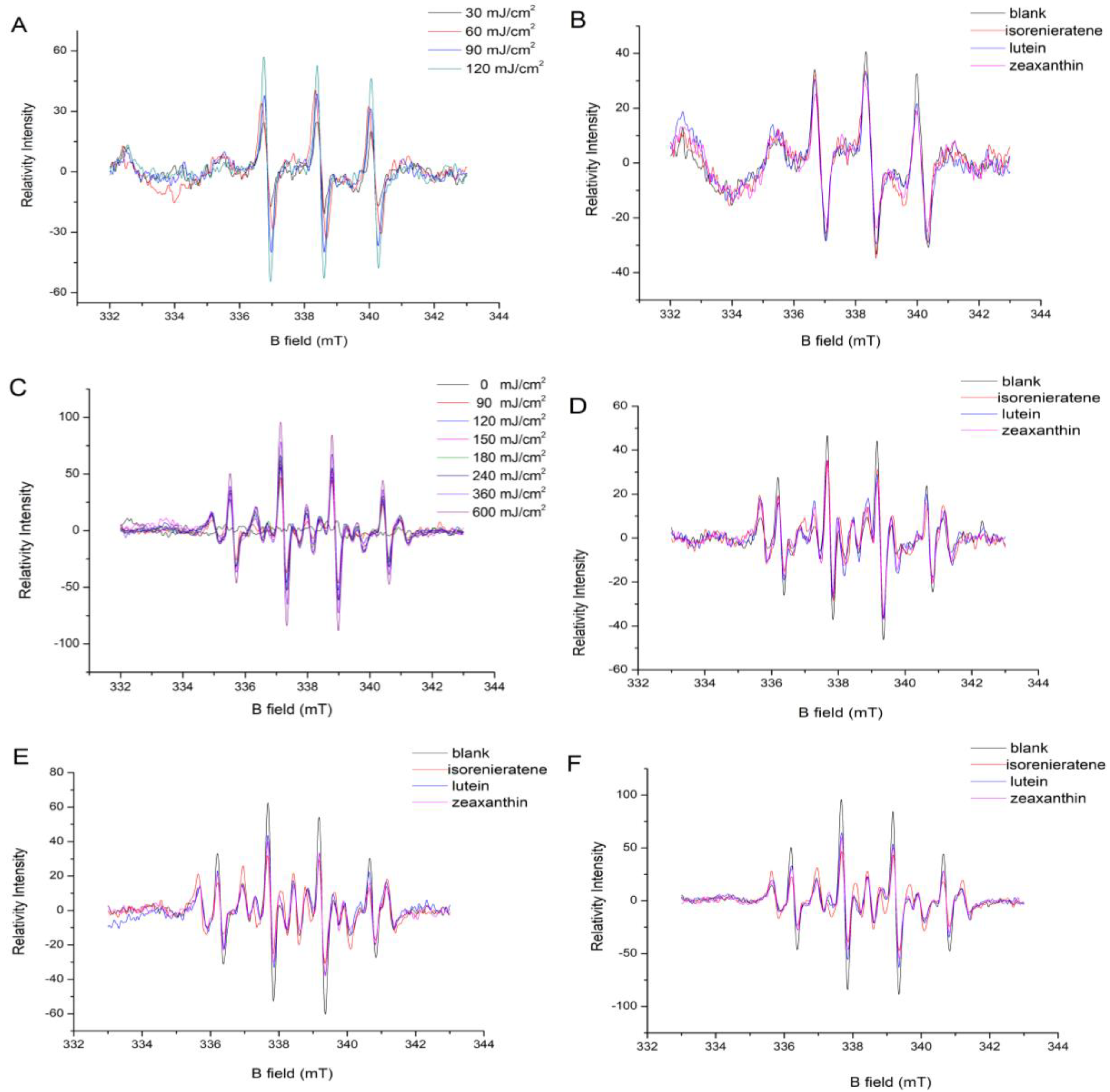
2.1. EPR Analysis
2.1.1. Singlet Oxygen Measurement
2.1.2. Hydroxyl Radical Measurement
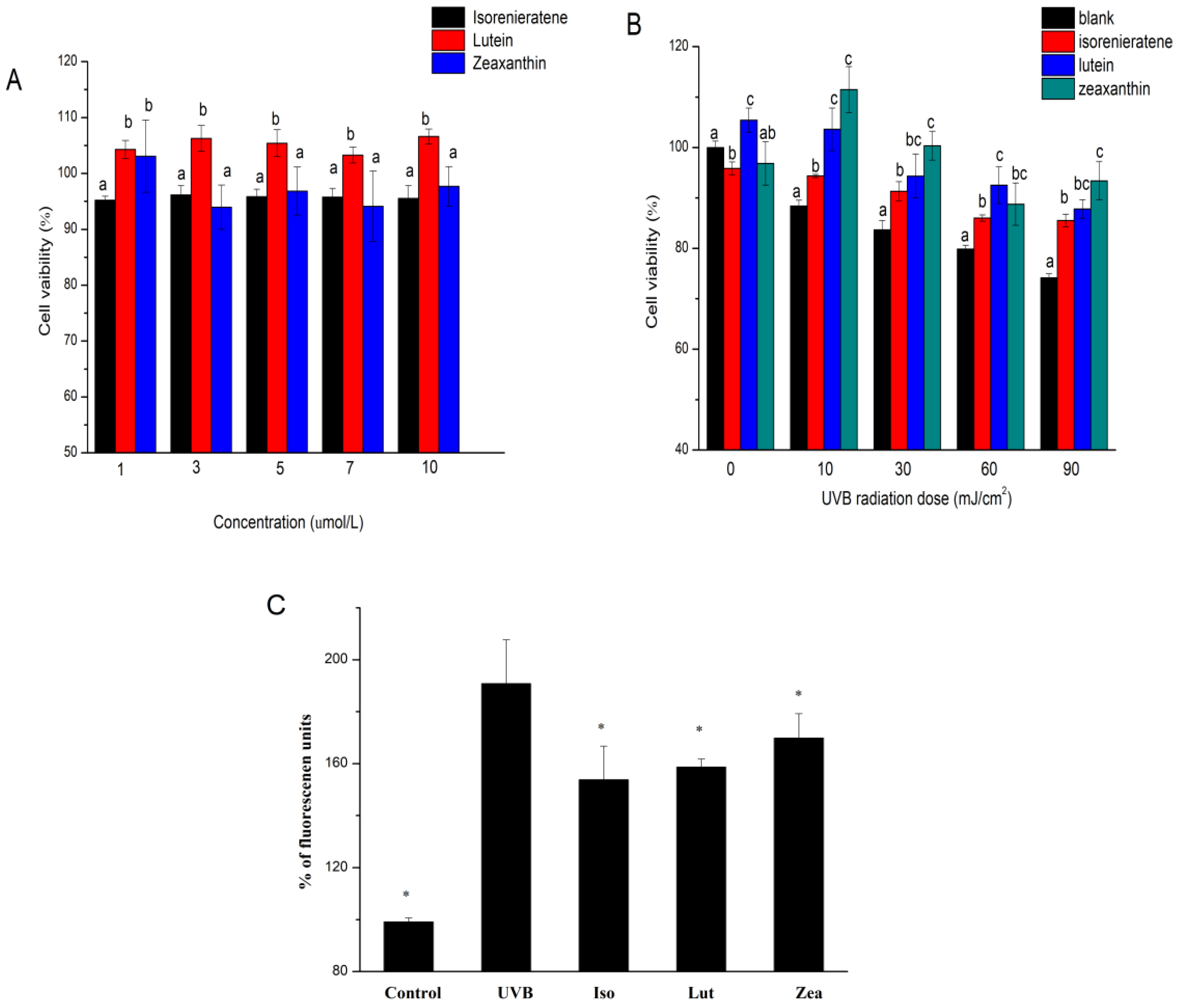
2.2. Cytotoxic Effects of Isorenieratene and Macular Xanthophylls
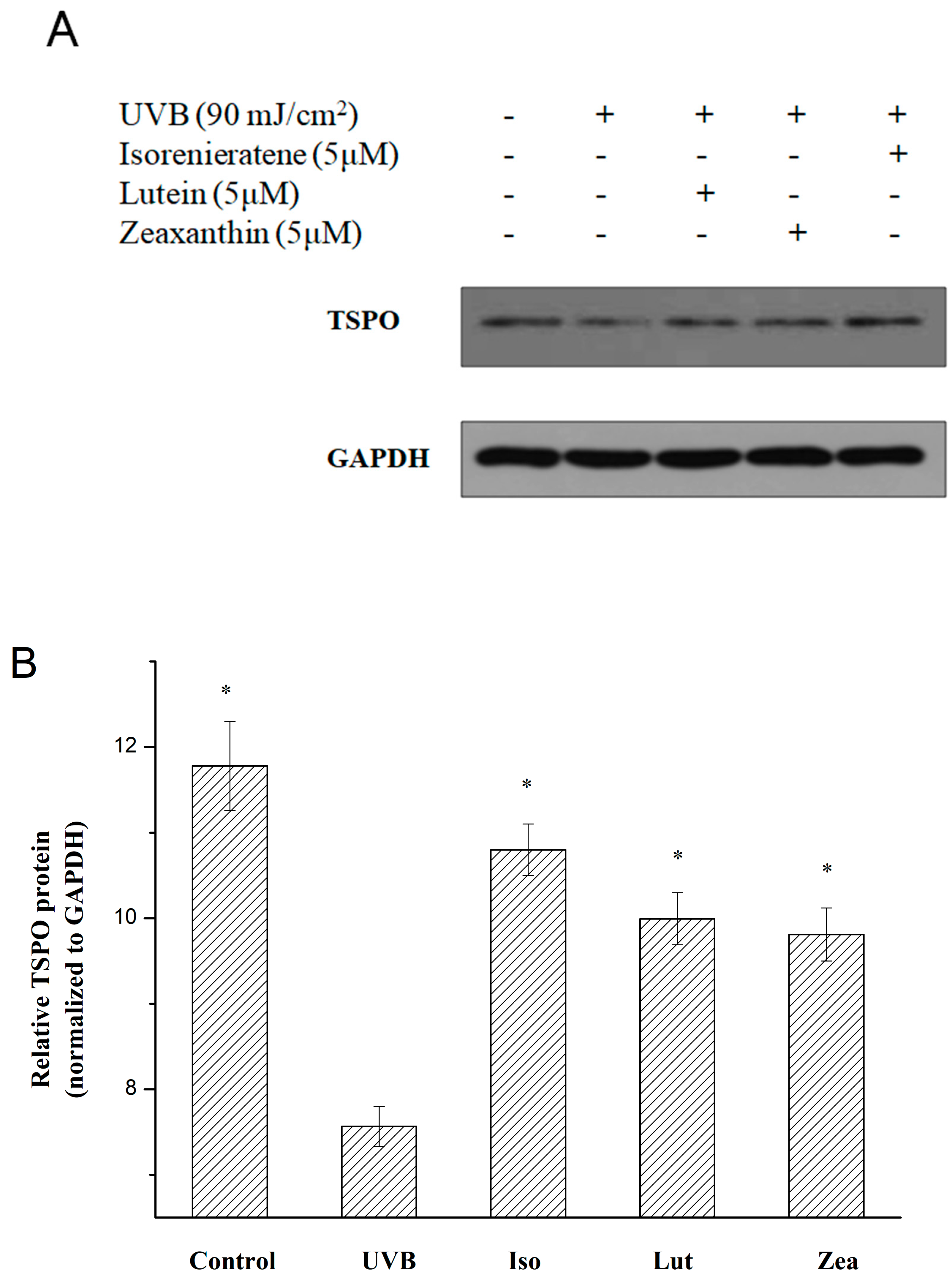
2.3. Isorenieratene Increases ARPE-19 Cell Survive Rate after UVB Radiation
2.4. ROS Production in ARPE-19 Cell
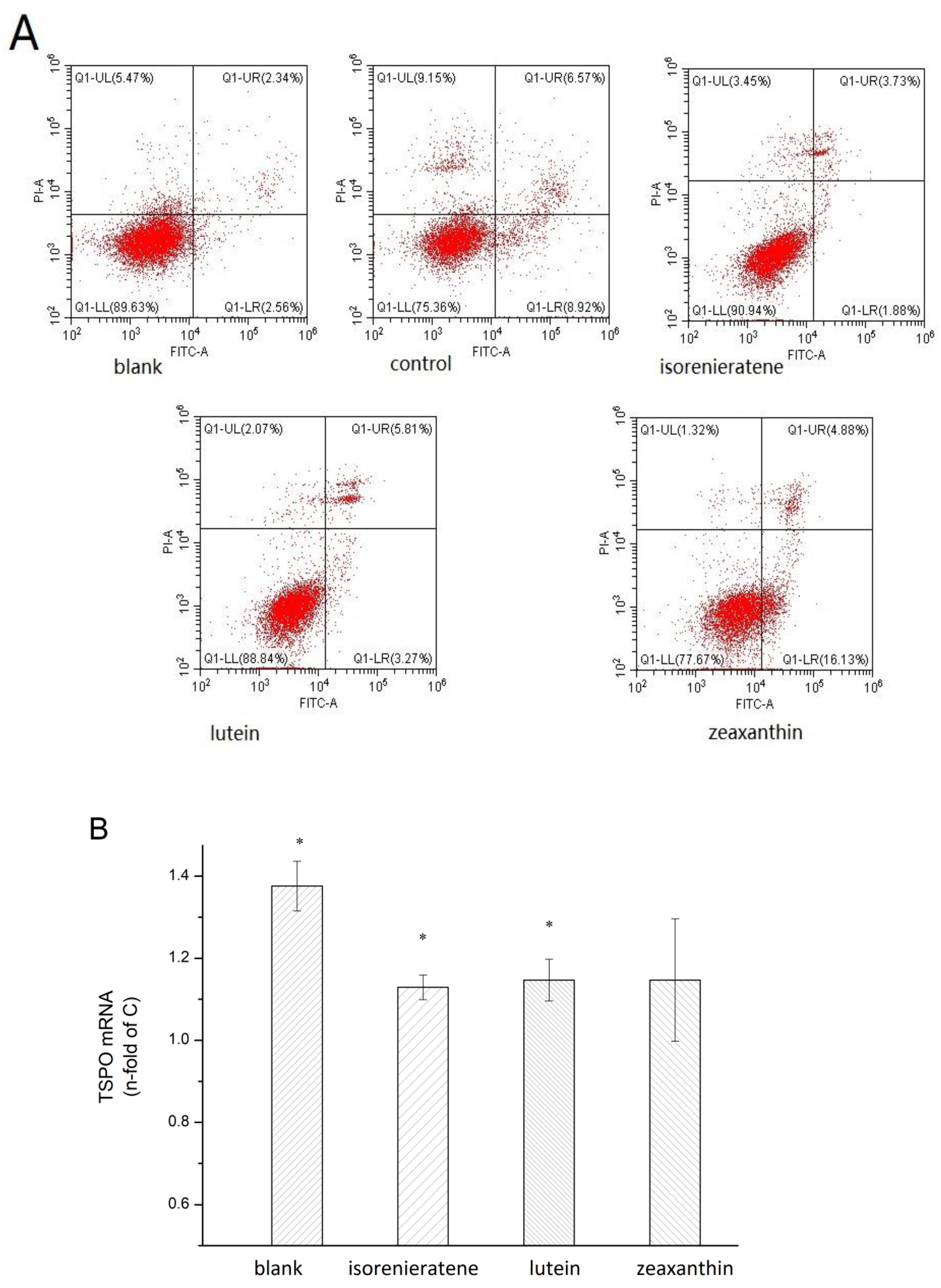
2.5. Cell Apoptosis Measurements
2.6. Real-time Fluorescent Quantitative PCR and Western Blot Analysis
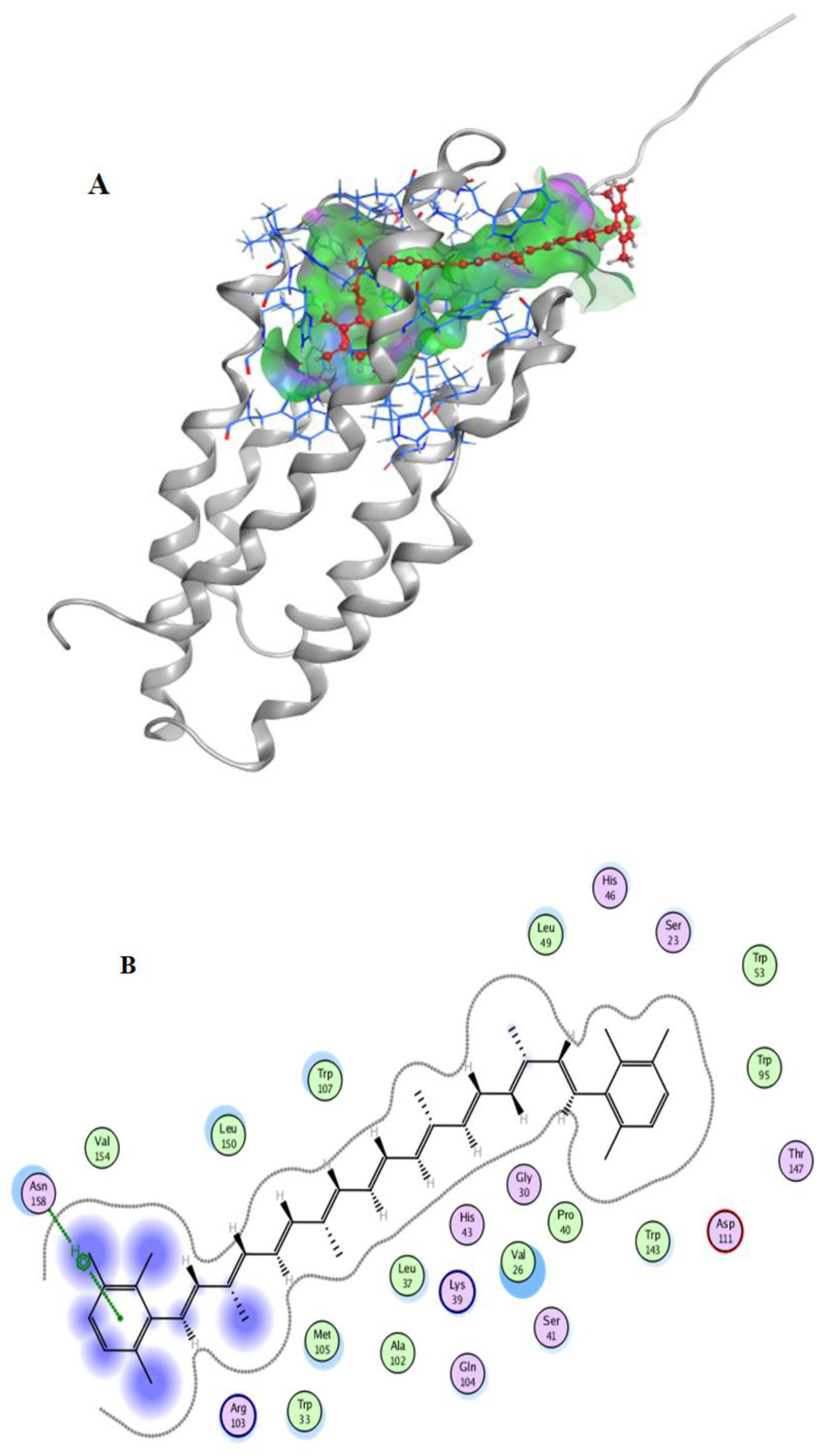
2.7. Molecular Docking
3. Materials and Methods
3.1. Materials and Reagents
3.2. Detection of Free Radicals by EPR Spectroscopy in Model Liposomes System
3.2.1. Model Liposomes Preparation
3.2.2. Detection of Hydroxyl Radicals and Singlet Oxygen by EPR Spectroscopy
3.3. Cell Culture
3.4. Cytotoxicity Assay of Carotenoids
3.5. ARPE-19 Cell Viability after Pre-treatment with Carotenoid and UVB Irradiation
3.6. Flow Cytometry
3.7. Reactive Oxygen Species (ROS) Generation Determination in ARPE 19 Cell
3.8. Real-time PCR Analysis
3.9. Western Blot Analysis
3.10. Docking Simulation
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colijn, J.M.; Den Hollander, A.I.; Demirkan, A.; Cougnard-Gregoire, A.; Verzijden, T.; Kersten, E.; Meester-Smoor, M.A.; Merle, B.M.J.; Papageorgiou, G.; Ahmad, S.; et al. Increased High-Density Lipoprotein Levels Associated with Age-Related Macular Degeneration: Evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology 2019, 126, 393–406. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Sur, A.; Kesarajub, S.; Prenticea, H.; Ayyanathan, K.; Baronas-Lowellb, D.; Zhue, D.; Hinton, D.R.; Blanks, J.; Weissbach, H. Pharmacological Protection of Retinal Pigmented Epithelial Cells by Sulindac Involves PPAR-α. Proc. Natl. Acad. Sci. USA 2014, 111, 16754–16759. [Google Scholar] [CrossRef]
- Kerr, J.B.; McElroy, C.T. Evidence for Large Upward Trends of Ultraviolet-B Radiation Linked to Ozone Depletion. Science 1993, 262, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Dixon, Z.; Chen, Y.; Llerena, C.M. Lutein and Zeaxanthin in the Eyes, Serum and Diet of Human Subjects. Exp. Eye Res. 2000, 71, 239–245. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Widomska, J. Location of Macular Xanthophylls in the Most Vulnerable Regions of Photoreceptor Outer-Segment Membranes. Arch. Biochem. Biophys. 2010, 504, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernandez, J.E.; Romero-Castillo, K.D.; Parra-Arroyo, L.; Aguilar-Aguila-Isaias, M.A.; Garcia-Reyes, I.E.; Ahmed, I.; Parra-Saldivar, R.; Bilal, M.; Iqbal, H.M.N. Mexican Microalgae Biodiversity and State-Of-The-Art Extraction Strategies to Meet Sustainable Circular Economy Challenges: High-Value Compounds and Their Applied Perspectives. Mar. Drugs 2019, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, X.; Ma, Q.; Lu, Y.; Bai, F.; Dai, J.; Wu, Q. Photosynthetic Accumulation of Lutein in Auxenochlorella Protothecoides after Heterotrophic Growth. Mar. Drugs 2018, 16, 283. [Google Scholar] [CrossRef]
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid Profiling of a Red Seaweed Pyropia Yezoensis: Insights into Biosynthetic Pathways in the Order Bangiales. Mar. Drugs 2018, 16, 426. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, B.; Yang, J.; Chen, J.; Sun, Z. Identification of Microbial Carotenoids and Isoprenoid Quinones from Rhodococcus sp. B7740 and Its Stability in the Presence of Iron in Model Gastric Conditions. Food Chem. 2018, 240, 204–211. [Google Scholar] [CrossRef]
- Richer, S.P.; Stiles, W.; Graham-Hoffman, K.; Levin, M.; Ruskin, D.; Wrobel, J.; Park, D.W.; Thomas, C. Randomized, Double-Blind, Placebo-Controlled Study of Zeaxanthin and Visual Function in Patients with Atrophic Age-Related Macular Degeneration: The Zeaxanthin and Visual Function Study (ZVF) FDA IND #78, 973. Optometry 2011, 82, 667–680. [Google Scholar] [PubMed]
- Frede, K.; Ebert, F.; Kipp, A.P.; Schwerdtle, T.; Baldermann, S. Lutein Activates the Transcription Factor Nrf2 in Human Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2017, 65, 5944–5952. [Google Scholar] [CrossRef]
- Silvan, J.M.; Reguero, M.; De Pascual-Teresa, S. A Protective Effect of Anthocyanins and Xanthophylls on UVB-Induced Damage in Retinal Pigment Epithelial Cells. Food Funct. 2016, 7, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mu, Q.; Hu, K.; Chen, M.; Yang, J.; Chen, J.; Xie, B.; Sun, Z. Characterization of MK(8)(H(2)) from Rhodococcus sp. B7740 and Its Potential Antiglycation Capacity Measurements. Mar. Drugs 2018, 16, 391. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.D.; Kock, S.; Scherrers, R.; Lutter, K.; Wagener, T.; Hundsdorfer, C.; Frixel, S.; Schaper, K.; Ernst, H.; Schrader, W.; et al. 3,3′-Dihydroxyisorenieratene, a Natural Carotenoid with Superior Antioxidant and Photoprotective Properties. Angew. Chem. 2009, 48, 400–403. [Google Scholar] [CrossRef]
- Zareba, M.; Widomska, J.; Burke, J.M.; Subczynski, W.K. Nitroxide Free Radicals Protect Macular Carotenoids Against Chemical Destruction (Bleaching) during Lipid Peroxidation. Free Radic. Biol. Med. 2016, 101, 446–454. [Google Scholar] [CrossRef]
- Youn, H.Y.; Bantseev, V.; Bols, N.C.; Cullen, A.P.; Sivak, J.G. In Vitro Assays for Evaluating the Ultraviolet B-Induced Damage in Cultured Human Retinal Pigment Epithelial Cells. J. Photochem. Photobiol. B Biol. 2007, 88, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.W.; Wang, Y.S.; Chen, K.C.; Wu, J.M.; Liang, C.L.; Juo, S.H. Tannic Acid Suppresses Ultraviolet B-Induced Inflammatory Signaling and Complement Factor B on Human Retinal Pigment Epithelial Cells. Cell. Immunol. 2012, 273, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Biswas, L.; Zhou, X.; Dhillon, B.; Graham, A.; Shu, X. Retinal Pigment Epithelium Cholesterol Efflux Mediated by the 18 kDa Translocator Protein, TSPO, a Potential Target for Treating Age-Related Macular Degeneration. Hum. Mol. Genet. 2017, 26, 4327–4339. [Google Scholar] [CrossRef]
- Mages, K.; Grassmann, F.; Jagle, H.; Rupprecht, R.; Weber, B.H.F.; Hauck, S.M.; Grosche, A. The Agonistic TSPO Ligand XBD173 Attenuates the Glial Response Thereby Protecting Inner Retinal Neurons in a Murine Model of Retinal Ischemia. J. Neuroinflamm. 2019, 16, 43. [Google Scholar] [CrossRef]
- Barron, A.M.; Ji, B.; Kito, S.; Suhara, T.; Higuchi, M. Steroidogenic Abnormalities in Translocator Protein Knockout Mice and Significance in the Aging Male. Biochem. J. 2018, 475, 75–85. [Google Scholar] [CrossRef]
- Fuciman, M.; Chabera, P.; Zupcanova, A.; Hribek, P.; Arellano, J.B.; Vacha, F.; Psencik, J.; Polivka, T. Excited State Properties of Aryl Carotenoids. Phys. Chem. Chem. Phys. PCCP 2010, 12, 3112–3120. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Zhang, X.; Pang, H.; Hai, X.; Zhan, G.; Zhou, W.; Song, H.; Zhang, L.; Chen, H.; Ye, J. In Situ Carbon Homogeneous Doping on Ultrathin Bismuth Molybdate: A Dual-Purpose Strategy for Efficient Molecular Oxygen Activation. Adv. Funct. Mater. 2017, 27, 1703923. [Google Scholar] [CrossRef]
- Yin, J.; Andersen, M.L.; Thomsen, M.K.; Skibsted, L.H.; Hedegaard, R.V. Formation of Radicals during Heating Lysine and Glucose in Solution with an Intermediate Water Activity. Free Radic. Res. 2013, 47, 643–650. [Google Scholar] [CrossRef]
- Ebegboni, V.J.; Dickenson, J.M.; Sivasubramaniam, S.D. Antioxidative Effects of Flavonoids and Their Metabolites Against Hypoxia/Reoxygenation-Induced Oxidative Stress in a Human First Trimester Trophoblast Cell Line. Food Chem. 2019, 272, 117–125. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Chen, M.; Wang, J.; Xie, B.; Sun, Z. (-)-Epigallocatechin-3-Gallate (EGCG) Inhibits Starch Digestion and Improves Glucose Homeostasis through Direct or Indirect Activation of PXR/CAR-Mediated Phase II Metabolism in Diabetic Mice. Food Funct. 2018, 9, 4651–4663. [Google Scholar] [CrossRef]
- Jiang, S.; Jia, Y.; Tang, Y.; Zheng, D.; Han, X.; Yu, F.; Chen, Y.; Huang, F.; Yang, Z.; Ding, G. Anti-Proliferation Activity of a Decapeptide from Perinereies Aibuhitensis toward Human Lung Cancer H1299 Cells. Mar. Drugs 2019, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, S.; Li, X.; Sui, Y.; Yang, Y.; Dong, L.; Xie, B.; Sun, Z. Inhibition of Advanced Glycation Endproduct Formation by Lotus Seedpod Oligomeric Procyanidins through RAGE-MAPK Signaling and NF-kappaB Activation in High-Fat-Diet Rats. J. Agric. Food Chem. 2015, 63, 6989–6998. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Jaremko, Ł.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Structure of the Mitochondrial Translocator Protein in Complex with a Diagnostic Ligand. Science 2014, 343, 1363–1366. [Google Scholar] [CrossRef]
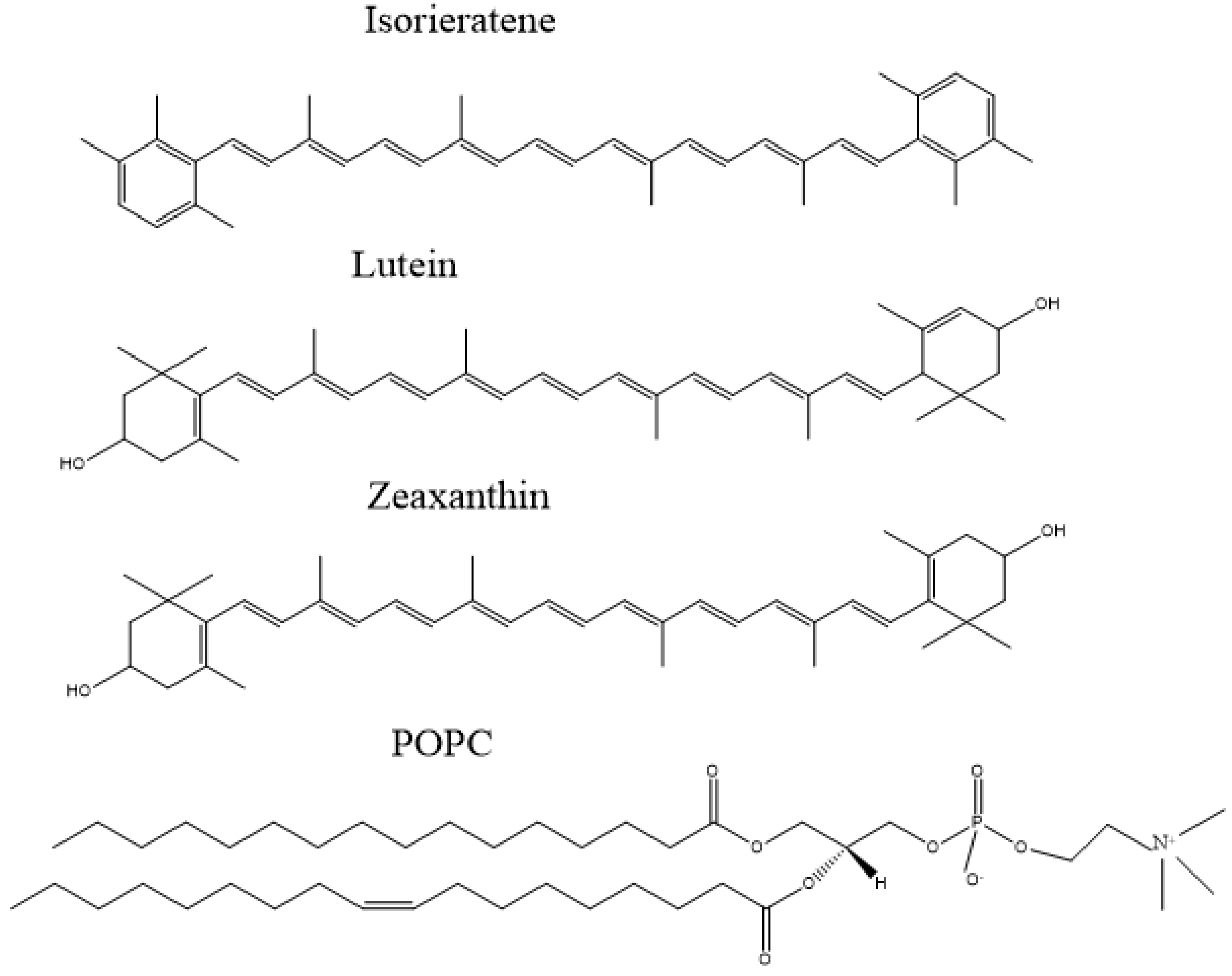
| Name | Primer | Sequence | Size |
|---|---|---|---|
| Homo GAPDH | Forward | 5′-TCAAGAAGGTGGTGAAGCAGG-3′ | 115 bp |
| Reverse | 5′-TCAAAGGTGGAGGAGTGGGT-3′ | ||
| Homo TSPO | Forward | 5′-CATCTTCTTTGGTGCCCGAC-3′ | 163 bp |
| Reverse | 5′-GTTGAGTGTGGTCGTGAAGG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Guo, M.; Yang, J.; Chen, J.; Xie, B.; Sun, Z. Potential TSPO Ligand and Photooxidation Quencher Isorenieratene from Arctic Ocean Rhodococcus sp. B7740. Mar. Drugs 2019, 17, 316. https://doi.org/10.3390/md17060316
Chen Y, Guo M, Yang J, Chen J, Xie B, Sun Z. Potential TSPO Ligand and Photooxidation Quencher Isorenieratene from Arctic Ocean Rhodococcus sp. B7740. Marine Drugs. 2019; 17(6):316. https://doi.org/10.3390/md17060316
Chicago/Turabian StyleChen, Yashu, Mengyao Guo, Jifang Yang, Jigang Chen, Bijun Xie, and Zhida Sun. 2019. "Potential TSPO Ligand and Photooxidation Quencher Isorenieratene from Arctic Ocean Rhodococcus sp. B7740" Marine Drugs 17, no. 6: 316. https://doi.org/10.3390/md17060316
APA StyleChen, Y., Guo, M., Yang, J., Chen, J., Xie, B., & Sun, Z. (2019). Potential TSPO Ligand and Photooxidation Quencher Isorenieratene from Arctic Ocean Rhodococcus sp. B7740. Marine Drugs, 17(6), 316. https://doi.org/10.3390/md17060316





