Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides
Abstract
1. Introduction
2. Brief Overview of the Immune System
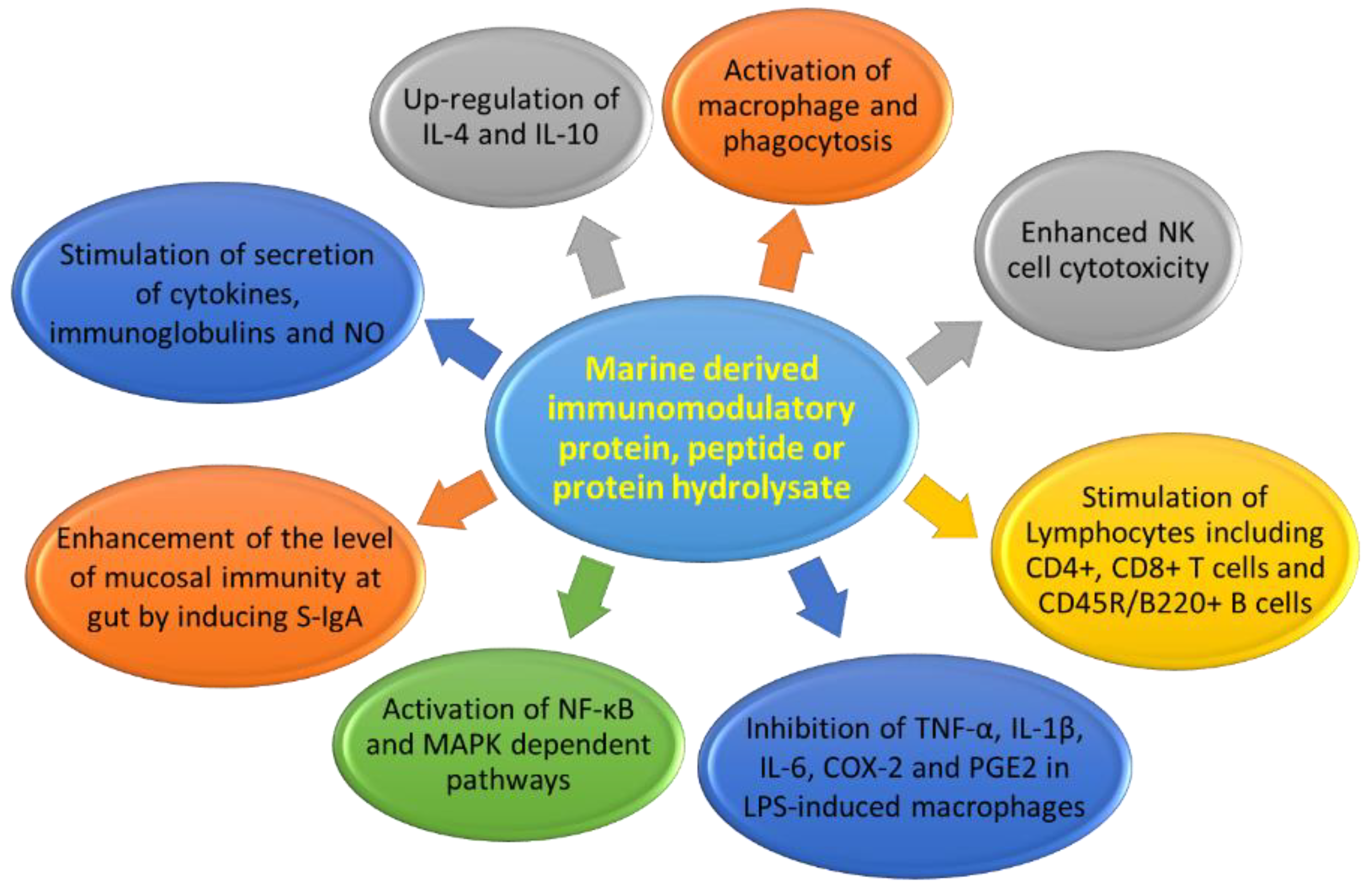
3. Immunomodulatory Compounds from Marine Organisms
3.1. Immunomodulatory Proteins and Amino Acid
3.1.1. Hemocyanins
3.1.2. Lectins
3.1.3. Taurine
3.2. Antimicrobial and Immunomodulatory Peptides
3.2.1. Callinectin
3.2.2. Clavanin A and Clavanin-MO
3.2.3. Crustin
3.2.4. Defensin
3.2.5. Myticin
3.2.6. Mytilin
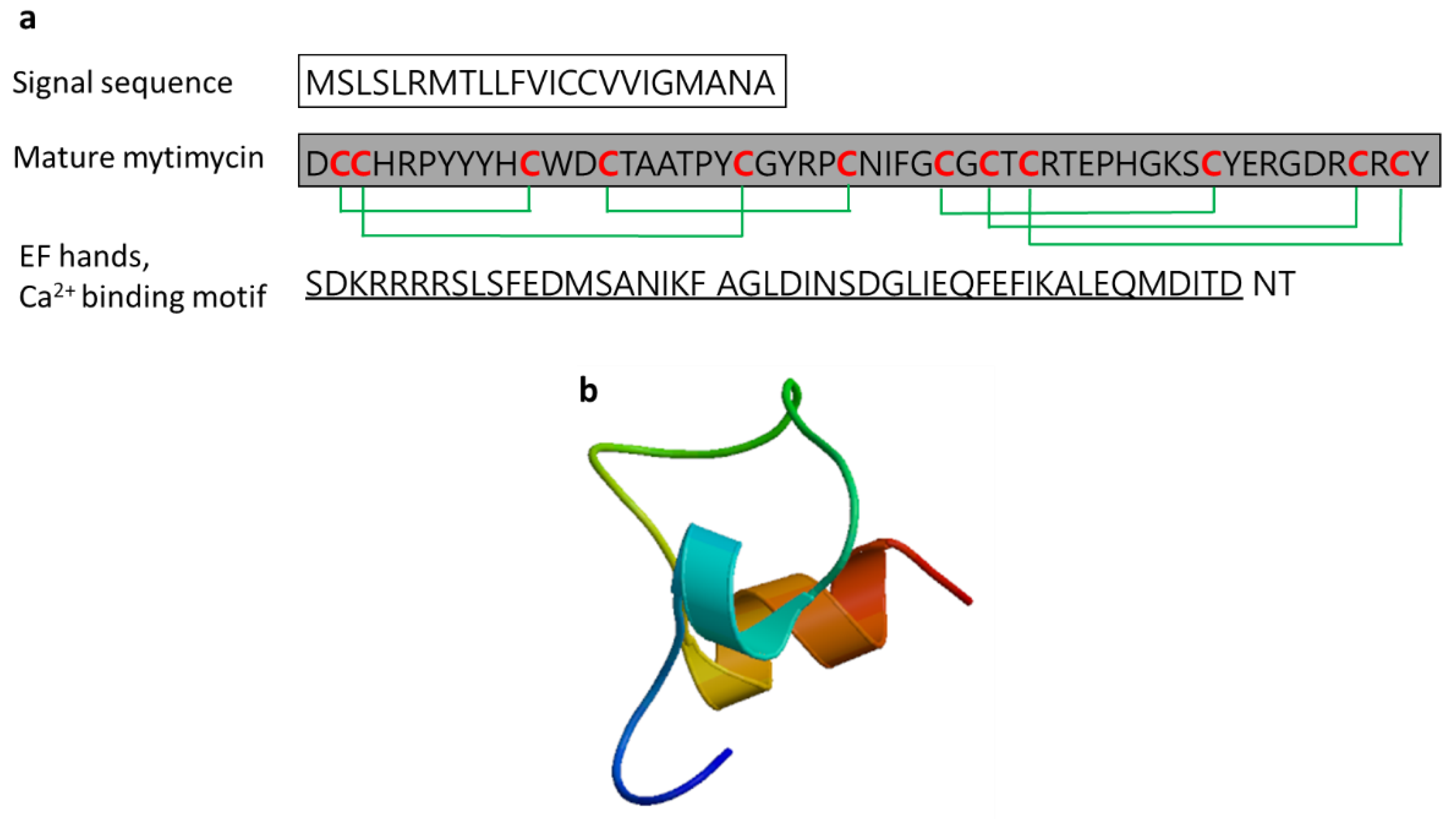
3.2.7. Mytimycin
3.2.8. Phosvitin-Derived Peptide (Pt5)
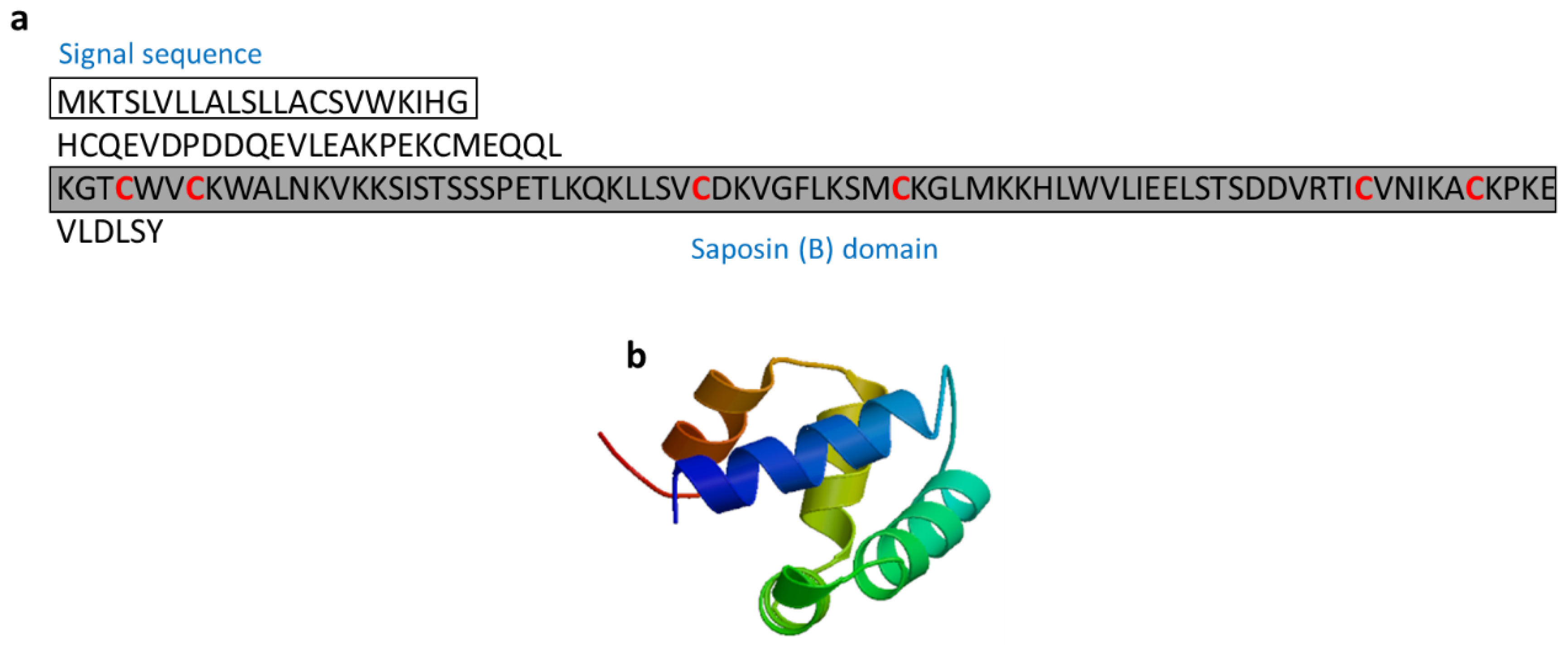
3.2.9. Salmo Salar NK-Lysin-Derived Peptides
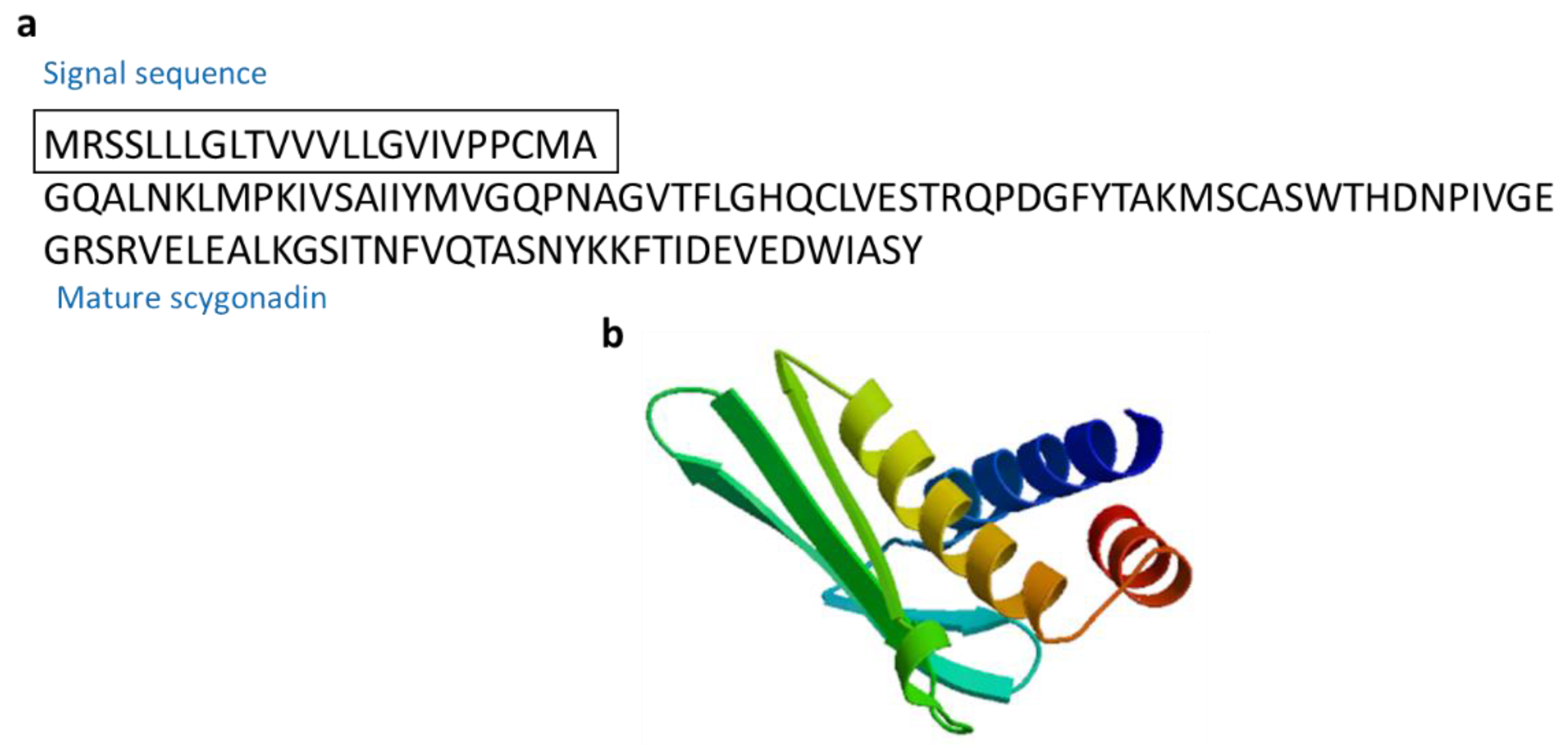
3.2.10. Scygonadin
3.2.11. Thalassospiramides A and D
3.2.12. Tilapia Piscidin 3 (TP3) and Tilapia Piscidin 4 (TP4)
3.3. Immunomodulatory Protein Hydrolysates
3.3.1. Chlorella Protein Hydrolysate
3.3.2. Ecklonia Protein Hydrolysate
3.3.3. Porphyra Protein Hydrolysate
3.3.4. Porphyra columbina Protein Hydrolysate
3.3.5. Edible Red Algae Protein Hydrolysate
3.3.6. Edible Microalgae Spirulina Protein Hydrolysate
3.3.7. Oyster Peptide-Based Enteral Nutrition Formula
3.3.8. Oyster Protein Hydrolysate
3.3.9. Paphia Undulata Meat Protein Hydrolysate
3.3.10. Cyclina sinensis Protein Hydrolysate (Novel Pentadecapeptide)
3.3.11. Ruditapes Protein Hydrolysate
3.3.12. Shellfish Mytilus Protein Hydrolysate
3.3.13. Alaska Pollock Protein Hydrolysate
3.3.14. Alaska Pollock Frame Protein Hydrolysate
3.3.15. Fermented Pacific Whiting Protein
3.3.16. Chum Salmon Oligopeptide Preparation
3.3.17. Salmon Fish Protein Hydrolysate
3.3.18. Salmon Byproduct Protein Hydrolysate
3.3.19. Salmon Pectoral Fin Byproduct Protein Hydrolysate
3.3.20. Shark-Derived Protein Hydrolysate
3.3.21. Sweetfish-Derived Protein Hydrolysate
3.3.22. Common Carp Egg Protein Hydrolysate
3.3.23. Rohu Egg Protein Hydrolysate
4. Marine Immunomodulatory Peptide-Based Drug Therapeutics and Future Prospects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Villani, A.C.; Sarkizova, S.; Hacohen, N. Systems immunology: Learning the rules of the immune system. Annu. Rev. Immunol. 2018, 36, 813–842. [Google Scholar] [CrossRef] [PubMed]
- Routy, J.P.; Mehraj, V.; Cao, W. HIV immunotherapy comes of age: Implications for prevention, treatment and cure. Expert Rev. Clin. Immunol. 2016, 12, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Page, D.B.; Wolchok, J.D. Immune modulation for cancer therapy. Br. J. Cancer 2014, 111, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Havla, J.; Kumpfel, T.; Hohlfeld, R. Immunotherapies for multiple sclerosis: Review and update. Internist 2015, 56, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Gruppen, M.P.; Bouts, A.H.; Jansen-van der Weide, M.C.; Merkus, M.P.; Zurowska, A.; Maternik, M.; Massella, L.; Emma, F.; Niaudet, P.; Cornelissen, E.A.M.; et al. A randomized clinical trial indicates that levamisole increases the time to relapse in children with steroid-sensitive idiopathic nephrotic syndrome. Kidney Int. 2018, 93, 510–518. [Google Scholar] [CrossRef]
- Ulrich, C.; Bichel, J.; Euvrard, S.; Guidi, B.; Proby, C.M.; van de Kerkhof, P.C.M.; Amerio, P.; Rønnevig, J.; Slade, H.B.; Stockfleth, E. Topical immunomodulation under systemic immunosuppression: Results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br. J. Dermatol. 2007, 157, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Trabattoni, D.; Clerici, M.; Centanni, S.; Mantero, M.; Garziano, M.; Blasi, F. Immunomodulatory effects of pidotimod in adults with community-acquired pneumonia undergoing standard antibiotic therapy. Pulm. Pharmacol. Ther. 2017, 44, 24–29. [Google Scholar] [CrossRef]
- Ekins, S.; Lingerfelt, M.A.; Comer, J.E.; Freiberg, A.N.; Mirsalis, J.C.; O’Loughlin, K.; Harutyunyan, A.; McFarlane, C.; Green, C.E.; Madrid, P.B. Efficacy of tilorone dihydrochloride against ebola virus infection. Antimicrob. Agents Chemother. 2018, 62, e01711–e01717. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Cornejo-García, J.A.; Perkins, J.R.; Jurado-Escobar, R.; García-Martín, E.; Agúndez, J.A.; Viguera, E.; Pérez-Sánchez, N.; Blanca-López, N. Pharmacogenomics of prostaglandin and leukotriene receptors. Front. Pharmacol. 2016, 7, e316. [Google Scholar] [CrossRef]
- Flores, C.; Fouquet, G.; Moura, I.C.; Maciel, T.T.; Hermine, O. Lessons to learn from low-dose cyclosporin-A: A new approach for unexpected clinical applications. Front. Immunol. 2019, 10, e00588. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, e00655. [Google Scholar] [CrossRef]
- Ozkan, M.C.; Tombuloglu, M.; Sahin, F.; Saydam, G. Evaluation of immunomodulatory drugs in multiple myeloma: Single center experience. Am. J. Blood Res. 2015, 5, 95–100. [Google Scholar]
- Gong, Y.; Frederiksen, S.L.; Gluud, C. D-penicillamine for primary biliary cirrhosis. Cochrane Datab. Syst. Rev. 2004, 18, CD004789. [Google Scholar] [CrossRef]
- Wang, Y.K.; He, H.L.; Wang, G.F.; Wu, H.; Zhou, B.C.; Chen, X.L.; Zhang, Y.Z. Oyster (Crassostrea gigas) Hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar. Drugs 2010, 8, 255–268. [Google Scholar] [CrossRef]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, F.; Mancera-Andrade, E.I.; Iqbal, H.M.N. Marine-derived bioactive peptides for biomedical sectors: A review. Protein Peptide Lett. 2017, 24, 109–117. [Google Scholar] [CrossRef]
- Page, D.B.; Bourla, A.B.; Daniyan, A.; Naidoo, J.; Smith, E.; Smith, M.; Friedman, C.; Khalil, D.N.; Funt, S.; Shoushtari, A.N.; et al. Tumor immunology and cancer immunotherapy: Summary of the 2014 SITC primer. J. Immunother. Cancer 2015, 3, 25. [Google Scholar] [CrossRef][Green Version]
- Nijkamp, F.P.; Parnham, M.J. T Cell Subsets and T Cell-Mediated Immunity in Principles of Immunopharmacology: 3rd Revised and Extended Edition; Springer: Basel, Switzerland, 2011. [Google Scholar]
- Kiewiet, M.B.G.; Faas, M.M.; de Vos, P. Immunomodulatory protein hydrolysates and their application. Nutrients 2018, 10, E904. [Google Scholar] [CrossRef]
- Okolie, C.L.; Rajendran, S.R.C.K.; Udenigwe, C.C.; Aryee, A.N.A.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41, e12392. [Google Scholar] [CrossRef]
- Miccadei, S.; Masella, R.; Mileo, A.M.; Gessani, S. ω3 Polyunsaturated fatty acids as immunomodulators in colorectal cancer: new potential role in adjuvant therapies. Front. Immunol. 2016, 7, e00486. [Google Scholar] [CrossRef]
- Dolashka-Angelova, P.; Stefanova, T.; Livaniou, E.; Velkova, L.; Klimentzou, P.; Stevanovic, S.; Salvato, B.; Neychev, H.; Voelter, W. Immunological potential of Helix vulgaris and Rapana venosa hemocyanins. Immunol. Investig. 2008, 37, 822–840. [Google Scholar] [CrossRef]
- Del Campo, M.; Arancibia, S.; Nova, E.; Salazar, F.; Gonzalez, A.; Moltedo, B.; de Ioannes, P.; Ferreira, J.; Manubens, A.; Becker, M.I. Hemocyanins as immunostimulants. Rev. Med. Chile 2011, 139, 236–246. [Google Scholar]
- Lammers, R.J.; Witjes, W.P.; Janzing-Pastors, M.H.; Caris, C.T.; Witjes, J.A. Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: Results from a prospective randomized phase III trial. J. Clin. Oncol. 2012, 30, 2273–2279. [Google Scholar] [CrossRef]
- McFadden, D.W.; Riggs, D.R.; Jackson, B.J.; Vona-Davis, L. Keyhole limpet hemocyanin, a novel immune stimulant with promising anticancer activity in Barrett’s esophageal adenocarcinoma. Am. J. Surg. 2003, 186, 552–555. [Google Scholar] [CrossRef]
- Mora Roman, J.J.; Del Campo, M.; Villar, J.; Paolini, F.; Curzio, G.; Venuti, A.; Jara, L.; Ferreira, J.; Murgas, P.; Lladser, A.; et al. Immunotherapeutic potential of mollusk hemocyanins in combination with human vaccine adjuvants in murine models of oral cancer. J. Immunol. Res. 2019, 2019, 7076942. [Google Scholar] [CrossRef]
- Arancibia, S.; Espinoza, C.; Salazar, F.; Del Campo, M.; Tampe, R.; Zhong, T.Y.; De Ioannes, P.; Moltedo, B.; Ferreira, J.; Lavelle, E.C.; et al. A novel immunomodulatory hemocyanin from the limpet Fissurella latimarginata promotes potent anti-tumor activity in melanoma. PLoS ONE 2014, 9, e87240. [Google Scholar] [CrossRef]
- Sharon, N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007, 282, 2753–2764. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C., Jr. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef]
- Ma, Y.G.; Cho, M.Y.; Zhao, M.; Park, J.W.; Matsushita, M.; Fujita, T.; Lee, B.L. Human mannose-binding lectin and L-ficolin function as specific pattern recognition proteins in the lectin activation pathway of complement. J. Biol. Chem. 2004, 279, 25307–25312. [Google Scholar] [CrossRef]
- Chikalovets, I.V.; Kondrashina, A.S.; Chernikov, O.V.; Molchanova, V.I.; Luk’yanov, P.A. Isolation and general characteristics of lectin from the mussel Mytilus trossulus. Chem. Nat. Compd. 2013, 48, 1058–1061. [Google Scholar] [CrossRef]
- Fredrick, W.S.; Ravichandran, S. Hemolymph proteins in marine crustaceans. Asian Pac. J. Trop. Biomed. 2012, 2, 496–502. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef]
- Schuller-Levis, G.B.; Park, E. Taurine and its chloramine: Modulators of immunity. Neurochem. Res. 2004, 29, 117–126. [Google Scholar] [CrossRef]
- Song, M.K.; Salam, N.K.; Roufogalis, B.D.; Huang, T.H. Lycium barbarum (Goji Berry) extracts and its taurine component inhibit PPAR-gamma-dependent gene transcription in human retinal pigment epithelial cells: Possible implications for diabetic retinopathy treatment. Biochem. Pharmacol. 2011, 82, 1209–1218. [Google Scholar] [CrossRef]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Kong, D.; Xu, P. Production and functionality of food-derived bioactive peptides: A review. Mini Rev. Med. Chem. 2018, 18, 1524–1535. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Wang, G.L.; Yuan, H.W.; Chai, Y.; Xiao, Z.L. cDNA sequence and expression analysis of an antimicrobial peptide, theromacin, in the triangle-shell pearl mussel Hyriopsis cumingii. Comp. Biochem. Phys. B 2010, 157, 119–126. [Google Scholar] [CrossRef]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef]
- Noga, E.J.; Stone, K.L.; Wood, A.; Gordon, W.L.; Robinette, D. Primary structure and cellular localization of callinectin, an antimicrobial peptide from the blue crab. Dev. Comp. Immunol. 2011, 35, 409–415. [Google Scholar] [CrossRef]
- Li, C.; Haug, T.; Moe, M.K.; Styrvold, O.B.; Stensvag, K. Centrocins: Isolation and characterization of novel dimeric antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Dev. Comp. Immunol. 2010, 34, 959–968. [Google Scholar] [CrossRef]
- Lee, I.H.; Cho, Y.; Lehrer, R.I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 1997, 65, 2898–2903. [Google Scholar]
- Silva, O.N.; de la Fuente-Nunez, C.; Haney, E.F.; Fensterseifer, I.C.; Ribeiro, S.M.; Porto, W.F.; Brown, P.; Faria-Junior, C.; Rezende, T.M.; Moreno, S.E.; et al. An anti-infective synthetic peptide with dual antimicrobial and immunomodulatory activities. Sci. Rep. 2016, 6, 35465. [Google Scholar] [CrossRef]
- Smith, V.J.; Fernandes, J.M.; Kemp, G.D.; Hauton, C. Crustins: Enigmatic WAP domain-containing antibacterial proteins from crustaceans. Dev. Comp. Immunol. 2008, 32, 758–772. [Google Scholar] [CrossRef]
- Donpudsa, S.; Rimphanitchayakit, V.; Tassanakajon, A.; Soderhall, I.; Soderhall, K. Characterization of two crustin antimicrobial peptides from the freshwater crayfish Pacifastacus leniusculus. J. Invertebr. Pathol. 2010, 104, 234–238. [Google Scholar] [CrossRef]
- Suleiman, S.; Smith, V.J.; Dyrynda, E.A. Unusual tissue distribution of carcinin, an antibacterial crustin, in the crab, Carcinus maenas, reveals its multi-functionality. Dev. Comp. Immunol. 2017, 76, 274–284. [Google Scholar] [CrossRef]
- Imjongjirak, C.; Amparyup, P.; Tassanakajon, A.; Sittipraneed, S. Molecular cloning and characterization of crustin from mud crab Scylla paramamosain. Mol. Biol. Rep. 2009, 36, 841–850. [Google Scholar] [CrossRef]
- Vatanavicharn, T.; Supungul, P.; Puanglarp, N.; Yingvilasprasert, W.; Tassanakajon, A. Genomic structure, expression pattern and functional characterization of crustin Pm5, a unique isoform of crustin from Penaeus monodon. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 244–252. [Google Scholar] [CrossRef]
- Yang, R.; Murillo, F.M.; Cui, H.; Blosser, R.; Uematsu, S.; Takeda, K.; Akira, S.; Viscidi, R.P.; Roden, R.B. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J. Virol. 2004, 78, 11152–11160. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Gao, B. Evolutionary origin of beta-defensins. Dev. Comp. Immunol. 2013, 39, 79–84. [Google Scholar] [CrossRef]
- Seo, J.K.; Crawford, J.M.; Stone, K.L.; Noga, E.J. Purification of a novel arthropod defensin from the American oyster, Crassostrea virginica. Biochem. Biophys. Res. Commun. 2005, 338, 1998–2004. [Google Scholar] [CrossRef]
- Hubert, F.; Noel, T.; Roch, P. A member of the arthropod defensin family from edible Mediterranean mussels (Mytilus galloprovincialis). Eur. J. Biochem. 1996, 240, 302–306. [Google Scholar] [CrossRef]
- Mitta, G.; Hubert, F.; Noel, T.; Roch, P. Myticin, a novel cysteine-rich antimicrobial peptide isolated from haemocytes and plasma of the mussel Mytilus galloprovincialis. Eur. J. Biochem. 1999, 265, 71–78. [Google Scholar] [CrossRef]
- Balseiro, P.; Falco, A.; Romero, A.; Dios, S.; Martinez-Lopez, A.; Figueras, A.; Estepa, A.; Novoa, B. Mytilus galloprovincialis myticin C: A chemotactic molecule with antiviral activity and immunoregulatory properties. PLoS ONE 2011, 6, e23140. [Google Scholar] [CrossRef]
- Domeneghetti, S.; Franzoi, M.; Damiano, N.; Norante, R.; El Halfawy, N.M.; Mammi, S.; Marin, O.; Bellanda, M.; Venier, P. Structural and antimicrobial Features of peptides related to Myticin C, a special defense molecule from the Mediterranean Mussel Mytilus galloprovincialis. J. Agric. Food Chem. 2015, 63, 9251–9259. [Google Scholar] [CrossRef]
- Charlet, M.; Chernysh, S.; Philippe, H.; Hetru, C.; Hoffmann, J.A.; Bulet, P. Innate immunity. Isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J. Biol. Chem. 1996, 271, 21808–21813. [Google Scholar] [CrossRef]
- Mitta, G.; Hubert, F.; Dyrynda, E.A.; Boudry, P.; Roch, P. Mytilin B and MGD2, two antimicrobial peptides of marine mussels: Gene structure and expression analysis. Dev. Comp. Immunol. 2000, 24, 381–393. [Google Scholar] [CrossRef]
- Mitta, G.; Vandenbulcke, F.; Roch, P. Original involvement of antimicrobial peptides in mussel innate immunity. FEBS Lett. 2000, 486, 185–190. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, X.; Bu, L.; Li, H.; Zhang, S. Antimicrobial-immunomodulatory activities of zebrafish phosvitin-derived peptide Pt5. Peptides 2012, 37, 309–313. [Google Scholar] [CrossRef]
- Andersson, M.; Holmgren, A.; Spyrou, G. NK-lysin, a disulfide-containing effector peptide of T-lymphocytes, is reduced and inactivated by human thioredoxin reductase. Implication for a protective mechanism against NK-lysin cytotoxicity. J. Biol. Chem. 1996, 271, 10116–10120. [Google Scholar] [CrossRef]
- Acosta, J.; Roa, F.; Gonzalez-Chavarria, I.; Astuya, A.; Maura, R.; Montesino, R.; Munoz, C.; Camacho, F.; Saavedra, P.; Valenzuela, A.; et al. In vitro immunomodulatory activities of peptides derived from Salmo salar NK-lysin and cathelicidin in fish cells. Fish Shellfish Immunol. 2019, 88, 587–594. [Google Scholar] [CrossRef]
- Huang, W.S.; Wang, K.J.; Yang, M.; Cai, J.J.; Li, S.J.; Wang, G.Z. Purification and part characterization of a novel antibacterial protein Scygonadin, isolated from the seminal plasma of mud crab, Scylla serrata (Forskal, 1775). J. Exp. Mar. Biol. Ecol. 2006, 339, 37–42. [Google Scholar] [CrossRef]
- Yedery, R.D.; Reddy, K.V. Purification and characterization of antibacterial proteins from granular hemocytes of Indian mud crab, Scylla serrata. Acta Biochim. Pol. 2009, 56, 71–82. [Google Scholar] [CrossRef]
- Oh, D.C.; Strangman, W.K.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Thalassospiramides A and B, immunosuppressive peptides from the marine bacterium Thalassospira sp. Org. Lett. 2007, 9, 1525–1528. [Google Scholar]
- Um, S.; Pyee, Y.; Kim, E.H.; Lee, S.K.; Shin, J.; Oh, D.C. Thalassospiramide G, a new gamma-amino-acid-bearing peptide from the marine bacterium Thalassospira sp. Mar. Drugs 2013, 11, 611–622. [Google Scholar] [CrossRef]
- Lin, W.C.; Chang, H.Y.; Chen, J.Y. Electrotransfer of the tilapia piscidin 3 and tilapia piscidin 4 genes into skeletal muscle enhances the antibacterial and immunomodulatory functions of Oreochromis niloticus. Fish Shellfish Immunol. 2016, 50, 200–209. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.L.; Wu, J.P. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Gros, M.; van Neerven, R.J.J.; Faas, M.M.; de Vos, P. Immunomodulating properties of protein hydrolysates for application in cow’s milk allergy. Pediat. Allerg. Imm. 2015, 26, 206–217. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. Targeted separation of antibacterial peptide from protein hydrolysate of anchovy cooking wastewater by equilibrium dialysis. Food Chem. 2015, 168, 115–123. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.-B.; Luo, H.Y.; Wang, D.F. Isolation and characterization of an antibacterial peptide fraction from the pepsin hydrolysate of half-fin anchovy (Setipinna taty). Molecules 2012, 17, 2980–2991. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bonnet, C.; Bryl, P.; Carbonneau, M.E. Detection of antibacterial activity in an enzymatic hydrolysate fraction obtained from processing of Atlantic rock crab (Cancer irroratus) by-products. Pharma Nutr. 2013, 1, 149–157. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Prasad, B.; Rai, A.K.; Velappan, S.P.; Subbanna, M.N.; Narayan, B. In vitro antioxidant and antibacterial properties of hydrolysed proteins of delimed tannery fleshings: Comparison of acid hydrolysis and fermentation methods. Biodegradation 2011, 22, 287–295. [Google Scholar] [CrossRef]
- Morris, H.J.; Carrillo, O.; Almarales, A.; Bermudez, R.C.; Lebeque, Y.; Fontaine, R.; Llaurado, G.; Beltran, Y. Immunostimulant activity of an enzymatic protein hydrolysate from green microalga Chlorella vulgaris on undernourished mice. Enzym. Microb. Technol. 2007, 40, 456–460. [Google Scholar] [CrossRef]
- Ahn, G.; Hwang, I.; Park, E.; Kim, J.; Jeon, Y.J.; Lee, J.; Park, J.W.; Jee, Y. Immunomodulatory effects of an enzymatic extract from Ecklonia cava on murine splenocytes. Mar. Biotechnol. 2008, 10, 278–289. [Google Scholar] [CrossRef]
- Cian, R.E.; Martinez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Cian, R.E.; Lopez-Posadas, R.; Drago, S.R.; de Medina, F.S.; Martinez-Augustin, O. A Porphyra columbina hydrolysate upregulates IL-10 production in rat macrophages and lymphocytes through an NF-kappa B, and p38 and JNK dependent mechanism. Food Chem. 2012, 134, 1982–1990. [Google Scholar] [CrossRef]
- Senevirathne, M.; Ahn, C.B.; Je, J.Y. Enzymatic extracts from edible red algae, Porphyra tenera, and their antioxidant, anti-acetylcholinesterase, and anti-inflammatory activities. Food Sci. Biotechnol. 2010, 19, 1551–1557. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kang, K.H.; Park, S.J.; Kim, S.K. The role of peptides derived from Spirulina maxima in downregulation of Fcepsilon RI-mediated allergic responses. Mol. Nutr. Food Res. 2014, 58, 2226–2234. [Google Scholar] [CrossRef]
- Cai, B.N.; Pan, J.Y.; Wu, Y.T.; Wan, P.; Sun, H.L. Immune functional impacts of oyster peptide-based enteral nutrition formula (OPENF) on mice: A pilot study. Chin. J. Oceanol. Limn. 2013, 31, 813–820. [Google Scholar] [CrossRef]
- He, X.Q.; Cao, W.H.; Pan, G.K.; Yang, L.; Zhang, C.H. Enzymatic hydrolysis optimization of Paphia undulata and lymphocyte proliferation activity of the isolated peptide fractions. J. Sci. Food Agric. 2015, 95, 1544–1553. [Google Scholar] [CrossRef]
- Li, W.; Ye, S.; Zhang, Z.; Tang, J.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G.; et al. Purification and characterization of a novel pentadecapeptide from protein hydrolysates of Cyclina sinensis and its immunomodulatory effects on RAW264.7 cells. Mar. Drugs 2019, 17, E30. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, E.K.; Kim, Y.S.; Hwang, J.W.; Lee, K.H.; Choi, D.K.; Kang, H.; Moon, S.H.; Jeon, B.T.; Park, P.J. Purification and characterization of a nitric oxide inhibitory peptide from Ruditapes philippinarum. Food Chem. Toxicol. 2012, 50, 1660–1666. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, Y.S.; Hwang, J.W.; Kang, S.H.; Choi, D.K.; Lee, K.H.; Lee, J.S.; Moon, S.H.; Jeon, B.T.; Park, P.J. Purification of a novel nitric oxide inhibitory peptide derived from enzymatic hydrolysates of Mytilus coruscus. Fish Shellfish Immunol. 2013, 34, 1416–1420. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Wang, S.K.; Si, L.L.; Li, B.F. Immunomodulatory activity of Alaska pollock hydrolysates obtained by glutamic acid biosensor—Artificial neural network and the identification of its active central fragment. J. Funct. Foods 2016, 24, 37–47. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Li, B.F.; Xue, C.H.; Yu, G.L. Preparation of immunomodulatory hydrolysates from Alaska pollock frame. J. Sci. Food Agric. 2012, 92, 3029–3038. [Google Scholar] [CrossRef]
- Duarte, J.; Vinderola, G.; Ritz, B.; Perdigon, G.; Matar, C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 2006, 211, 341–350. [Google Scholar] [CrossRef]
- Yang, R.Y.; Zhang, Z.F.; Pei, X.R.; Han, X.L.; Wang, J.B.; Wang, L.L.; Long, Z.; Shen, X.Y.; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470. [Google Scholar] [CrossRef]
- Nesse, K.O.; Nagalakshmi, A.P.; Marimuthu, P.; Singh, M. Efficacy of a fish protein hydrolysate in malnourished children. Ind. J. Clin. Biochem. 2011, 26, 360–365. [Google Scholar] [CrossRef]
- Ahn, C.B.; Je, J.Y.; Cho, Y.S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Ahn, C.B.; Cho, Y.S.; Je, J.Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef]
- Mallet, J.F.; Duarte, J.; Vinderola, G.; Anguenot, R.; Beaulieu, M.; Matar, C. The immunopotentiating effects of shark-derived protein hydrolysate. Nutrition 2014, 30, 706–712. [Google Scholar] [CrossRef]
- Sung, N.Y.; Jung, P.M.; Yoon, M.; Kim, J.S.; Choi, J.I.; Jeong, H.G.; Lee, J.W.; Kim, J.H. Anti-inflammatory effect of sweetfish-derived protein and its enzymatic hydrolysate on LPS-induced RAW264.7 cells via inhibition of NF-kappa B transcription. Fish. Sci. 2012, 78, 381–390. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T.; Diwan, P.V.; Bhaskarachary, K.; Vajreswari, A.; Ramesh Kumar, R.; Dinesh Kumar, B. Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg. Nutrition 2015, 31, 388–398. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T.; Diwan, P.V.; Kumar, P.U.; Nimgulkar, C.; Kumar, B.D. Immunomodulatory effects of protein hydrolysates from rohu (Labeo rohita) egg (roe) in BALB/c mice. Food Res. Int. 2014, 62, 1054–1061. [Google Scholar] [CrossRef]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Plowden, J.; Renshaw-Hoelscher, M.; Engleman, C.; Katz, J.; Sambhara, S. Innate immunity in aging: Impact on macrophage function. Aging Cell 2004, 3, 161–167. [Google Scholar] [CrossRef]
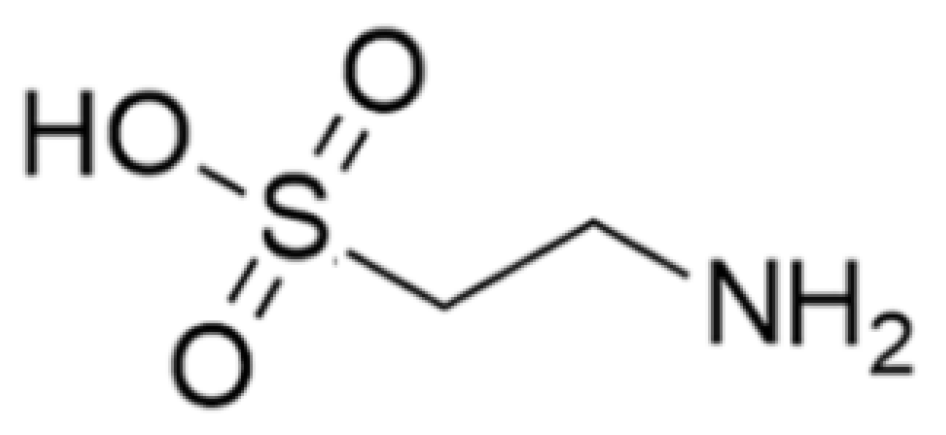


| Name of Protein | Source | Protein Type | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Hemocyanin | Mollusk: Concholepas concholepas, Megathura crenulata, Fissurella latimarginata | Oxygen carrying metalloprotein | Immunostimulatory activities against certain cancers without side effects; interact with T cells, monocytes, macrophages, and polymorphonuclear lymphocytes to improve the host immune response | [23,24,25,26,27,28] |
| Lectin | Clam: Crenomytilus grayanus, Mytilus trossulus, Fissurella latimarginata | Glycoprotein | C-type lectins recognize carbohydrates during the immune response. Tachylectins recognize pathogen associated molecules via phagocytosis or the lectin pathway of the complement system. The C-type lectins play a key role in carbohydrate recognition during immune response. Lectins have been reported as pathogenic recognizing receptors from marine invertebrates. MTL stimulates the expression of proinflammatory cytokines (TNF-α and IFN-γ), but reduces the hyper-expressions of anti-inflammatory cytokine (IL-10). | [29,30,31,32,33,34] |
| Taurine | Clam: Tapes philippinarum | 2-Amino ethane sulfonic acid | Cytoprotective and immunomodulatory effects in immune cells including lymphocytes, monocytes, and neutrophils; accumulation of phagocytes, contact with pathogens, activated cells (neutral and macrophages) produce toxic oxidants and various antibacterial substances using the peroxidase system and destroy the pathogens; scavenger to remove unwanted or harmful substances from the cells and protect them from oxidative stress; modulation of the immune system by activating NF-κB and activation PPAR-g. | [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] |
| Name of peptide | Source | Mechanism of action | Ref. | ||
|---|---|---|---|---|---|
| Callinectin | Blue crab: Callinectes sapidus, Mediterranean mussel: Mytilus galloprovincialis | Antibacterial activity against gram-negative bacteria, binding to anti-callinectin-like peptides antibodies in blue crab hemocytes | [43,44] | ||
| Clavanin A, clavanin-MO | Tunicate: Styela clava | Antimicrobial activity against Gram-negative, Gram-positive drug-resistant bacteria and fungi; immunomodulation by inhibiting the inflammatory response that causes sepsis and destroys certain biofilms; affect components of the immune system and influence inflammatory response; cytokine modulations (down-regulation of IL-12 and TNF-α, up-regulation of IL-1) in mice | [45,46] | ||
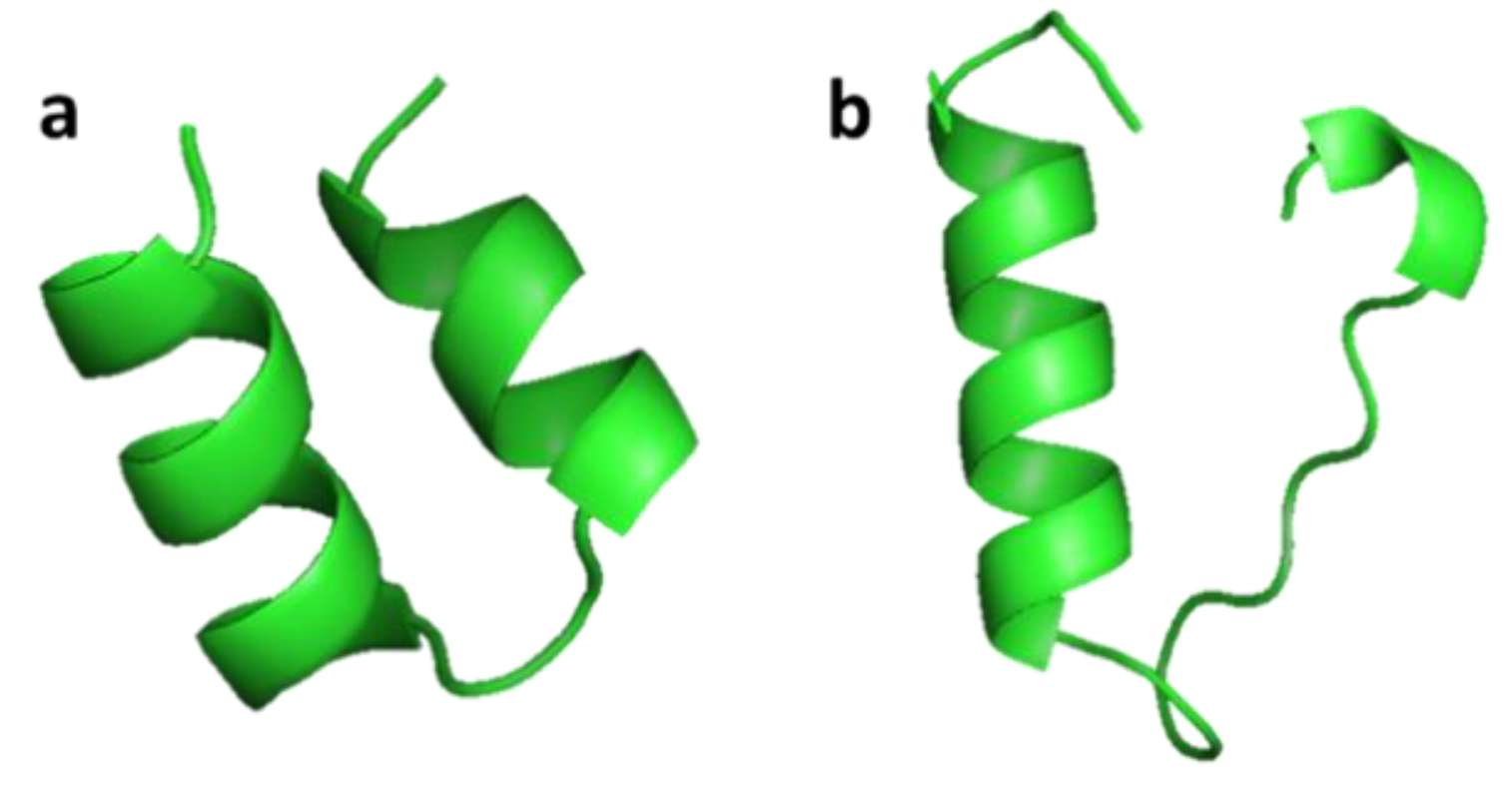
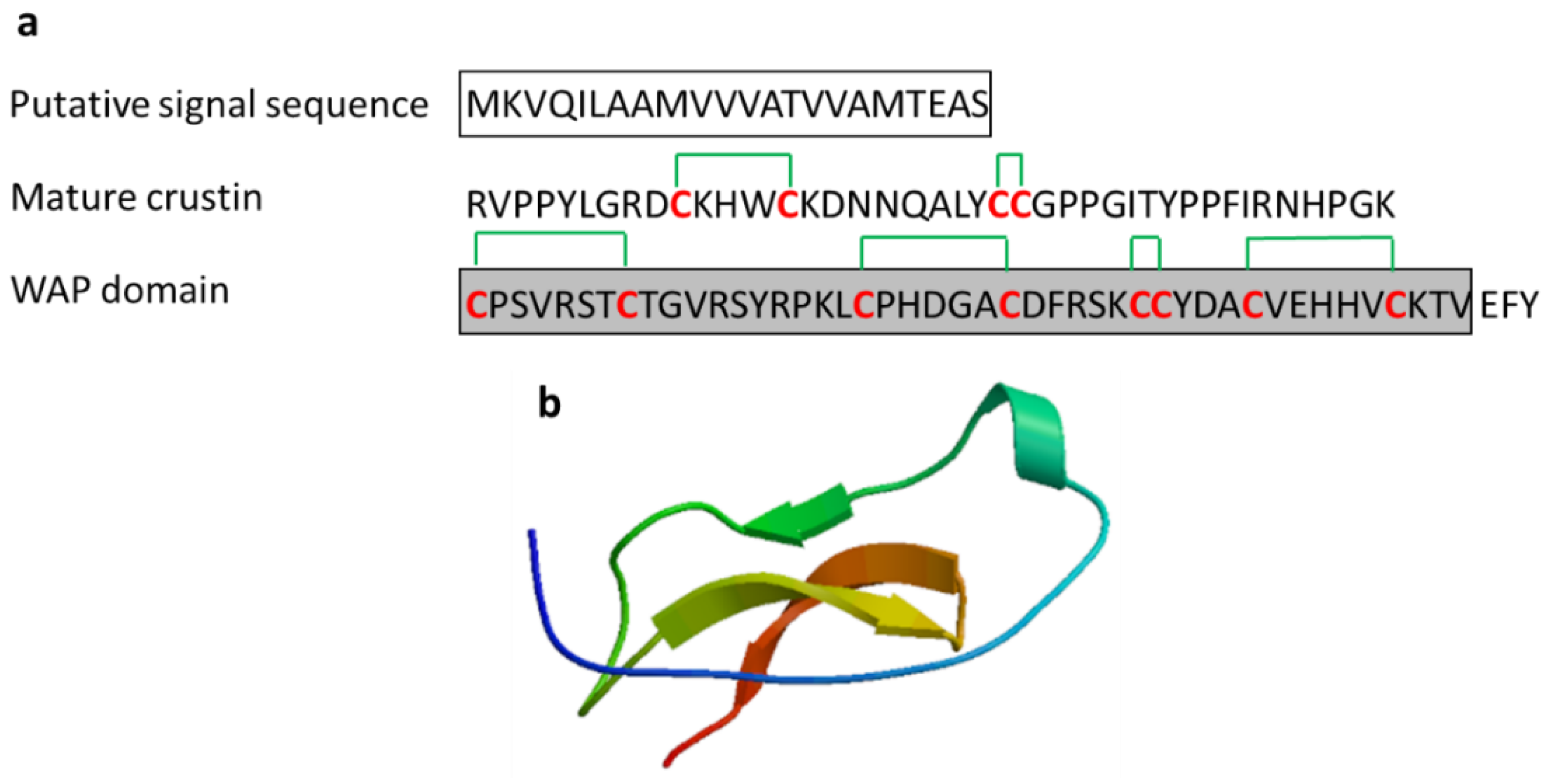
| Crustin | Crustacean: Carcinus maenas, Pacifastacus leniusculus, Fenneropenaeus chinensis, Scylla serrata, Scylla paramamosain, Penaeus monodon | Antimicrobial activity against marine Gram-positive bacteria; release from the hemocytes of crustacean by exocytosis | [47,48,49,50,51] | ||
| Defensin | Oyster: Crassostrea virginica. Crassostrea gigas Mediterranean mussel: Mytilus galloprovincialis M. edulis | Antimicrobial peptides (AMPs) acting as host defense peptides that disrupt the membrane of microbial pathogens, and play a major role in immunomodulation by acting in the innate and adaptive immune response; after bacterial infection, increased MGD-1 in M. galloprovincialis plasma and MGD-2 stimulates release from hemocytes | [52,53,54,55] | ||
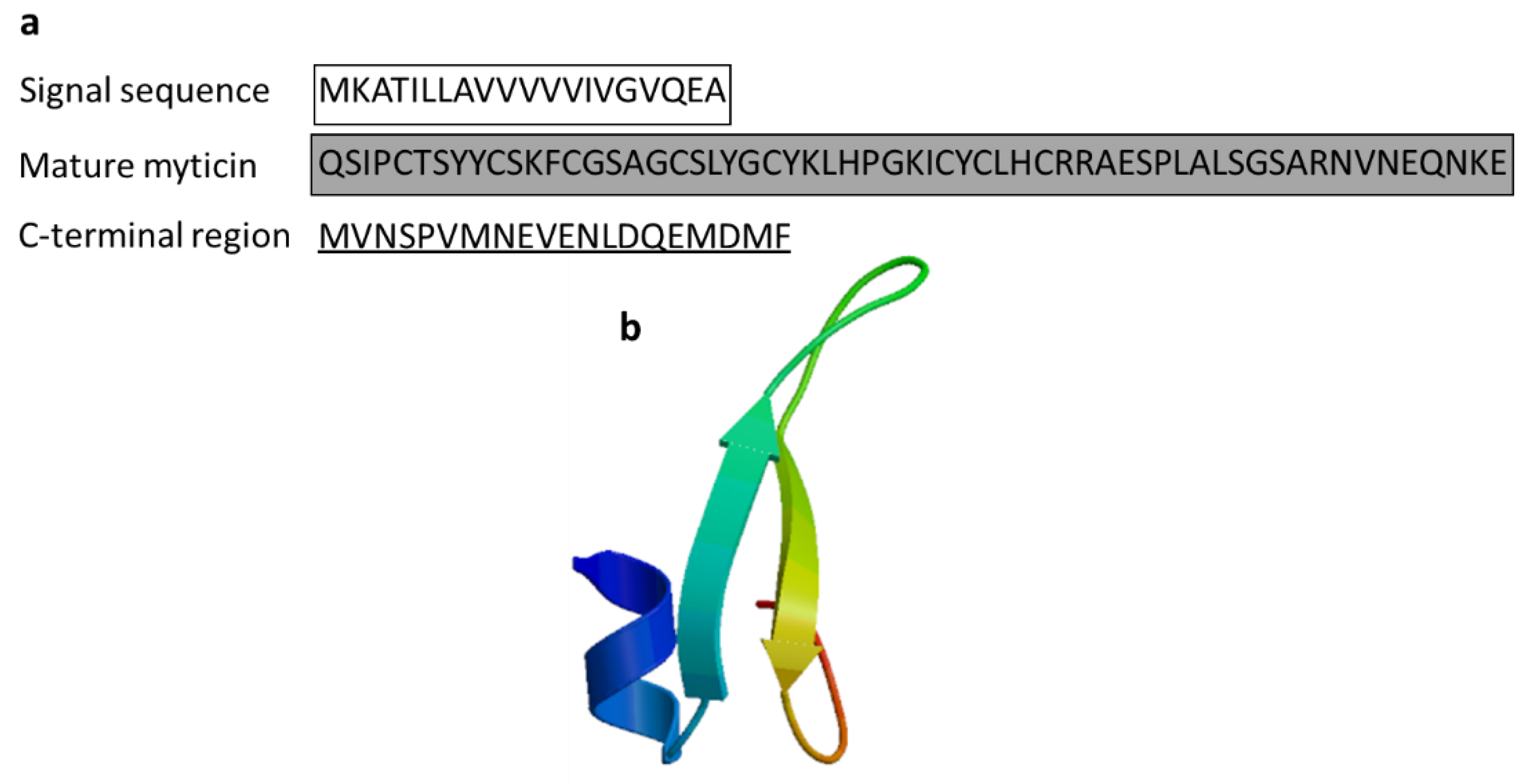
| Myticin | Mediterranean mussel: Mytilus galloprovincialis | Reached in the bacteria by transportation through hemocytes; antibacterial activity against Gram-positive bacteria (myticin A, myticin B, myticin C) and the fungus Fusarium oxysporum and E. coli (myticin C) and acts as immunomodulator in vivo; immune-related gene expression following in vivo immunostimulation in mussels | [56,57,58] | ||
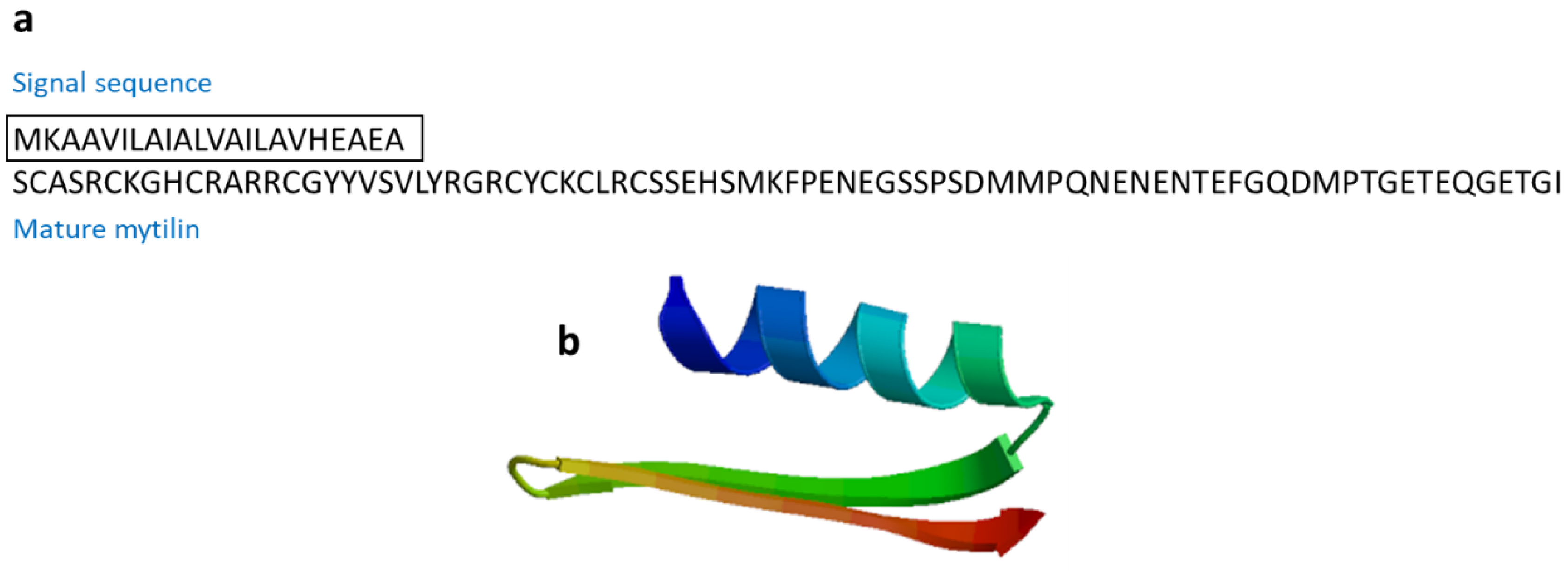
| Mytilin | Mollusk: Mytilus edulis (mytilin A and mytilin B), M. galloprovincialis (mytilin C, mytilin D, mytilin G1) | Antimicrobial activities; transported through hemocytes to reach bacteria, and cells containing mytilin act as phagocytosing bacteria to prevent microbes from entering the circulatory system | [59,60] | ||
| Mytimycin | Blue mussel: Mytilus edulis, Mediterranean mussel: M. galloprovincialis | Antifungal activity; defense against invading pathogenic microbes; the gene responsible for mytomycin is mainly expressed in circulatory hemocytes | [55,59,61] | ||
| Phosvitin-derived peptide Pt5 | Fish: Danio rerio | Antimicrobial activity and immunomodulatory activity; increase the survival rate of zebrafish infected by Aeromonas hydrophila, decrease the number of A. hydrophila in the blood, spleen, kidneys, liver, and muscles; inhibition expression of IL-1β, IL-6, TNF-α, and IFN-γ within the spleen and head kidneys of A. hydrophila-infected zebrafish, but increased the expression of IL-10 and IL-14 | [62] | ||
| Salmo salar natural killer (NK)-lysin | Fish: Salmo salar | Antimicrobial activity; Salmo salar NK-lysin-derived peptides induce expression of IL-1β and IL-8 in Salmo salar head kidney leukocytes | [63,64] | ||
| Scygonadin | Mud crab: Scylla serrata | AMPs for host defense to protect the reproductive system of organisms | [65,66] | ||
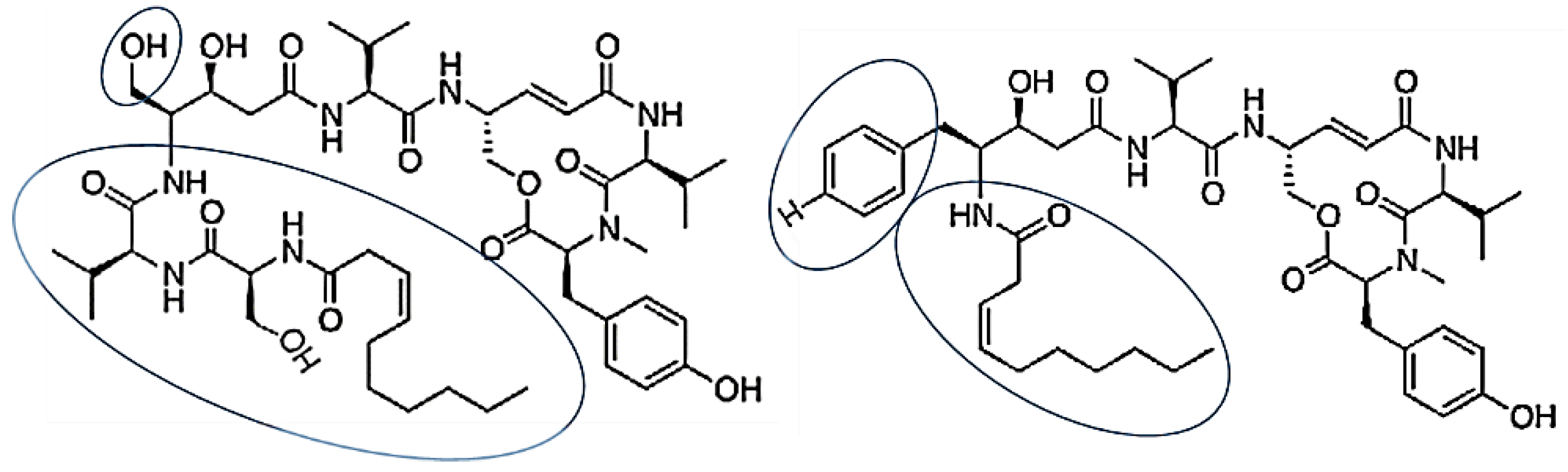
| Thalassospiramides A and D | Bacteria: Thalassospira sp. | Suppression of LPS-induced NO production in RAW 264.7 macrophages; inhibition of IL-5 expression in TH-2-mediated inflammatory diseases such as asthma | [67,68] | ||
| Tilapia piscidin 3 (TP3) and tilapia piscidin 4 (TP4) | Fish: Oreochromis niloticus | Antimicrobial, wound-healing, and antitumor activity; increased expression of several immune-related genes in O. niloticus muscle (IL-1β, IL-6, IL-8, TGF-β, IκB), decreased expression of TLR5 after Vibrio vulnificus infection, down-regulation of IL-1β, IL-8 TLR5, TGF-β, and IκB after Streptococcus agalactiae infection | [69] | ||
| Peptide | Source | Amino acid sequences | Ref./Genbank |
|---|---|---|---|
| Callinectin | Callinectes sapidus | WNSNRRFRVGRPPVVGRPGCVCFRAPCPCSNY-NH2 | [43] |
| Clavanin-A, clavanin-MO | Styela clava | Clavanin A: VFQFLGKIIHHVGNFVHGFSHVF-NH2 Clavanin-MO: FLPIIVFQFLGKIIHHVGNFVHGFSHVF-NH2 | [45,46] |
| Crustin | Scylla serrata | EASRVPPYLGRDCKHWCKDNNQALYCCGPPGITYPPFIRNHPGKCPSVRSTCTGVRSYRPKLCPHDGACDFRSKCCYDACVEHHVCKTV | [47] ADW11096.1 |
| Defensin | Crassostrea gigas | GFGCPGNQSKCNNHCKSISCRAGYCDAATLWLRCTCTDCNGKK | [52] ACQ76262.1 |
| Myticin C | Mytilus galloprovincialis | QSIPCTSYYCSKFCGSAGCSLYGCYKLHPGKICYCLHCRRAESPLALSGSARNVNEQNKE | [58] AEZ79080.1 |
| Mytilin B | Mytilus galloprovincialis | SCASRCKGHCRARRCGYYVSVLYRGRCYCKCLRCSSEHSMKFPENEGSSPSDMMPQNENENTEFGQDMPTGETEQGETGI | [59] AAD45013.1 |
| Mytomycin | Mytilus edulis | DCCHRPYYYHCWDCTAATPYCGYRPCNIFGCGCTCRTEPHGKSCYERGDRCRCYT | [61] AET85056.1 |
| Phosvitin-derived peptide Pt5 | Danio rerio | SRMSKTATIIEPFRKFHKDRYLAHHSATKDTSSGSAAASFEQMQKQNRFLGNDIP | [62] |
| Salmo salar NK-lysin | Salmo salar | KGTCWVCKWALNKVKKSISTSSSPETLKQKLLSVCDKVGFLKSMCKGLMKKHLWVLIEELSTSDDVRTICVNIKACKPKE | [63] XP_013985382 |
| Scygonadin | Scylla serrata | GQALNKLMPKIVSAIIYMVGQPNAGVTFLGHQCLVESTRQPDGFYTAKMSCASWTHDNPIVGEGRSRVELEALKGSITNFVQTASNYKKFTIDEVEDWIASY | [65] AAW57403.1 |
| Thalassospiramides A and D | Thalassospira sp. | cyclic lipopeptides contained rigid 12-membered ring containing an α,β-unsaturated carbonyl moiety | [67] |
| TP3 and TP4 | Oreochromis niloticus | TP3: FIHHIIGGLFSVGKHIHSLIHGH, TP4: FIHHIIGGLFSAGKAIHRLIRRRRR | [69] |
| Name of hydrolysate | Source/amino acid sequence, MW | Treated enzymes | Mechanism of action | Ref. |
|---|---|---|---|---|
| Chlorella protein hydrolysate | Algae: Chlorella vulgaris (<5000 Da) | Pancreatin | Enhanced hemopoiesis, leukocyte count, peritoneal exudate cells, macrophage activity; stimulation of both humoral and cell-mediated immune functions (T-dependent antibody response and reconstitution of delayed-type hypersensitivity response) in BALB/c mice | [77] |
| Ecklonia protein hydrolysate | Algae: Ecklonia cava | KojizymeTM | Increases in lymphocytes, monocytes, and granulocytes; increase in numbers of CD4+ T cells, CD8+ T cells, and CD45R/B220+ B cells; down-regulation of TNF-α and IFN-γ, up-regulation of IL-4 and IL-10 in ICR mice | [78] |
| Porphyra protein hydrolysate | Algae: Porphyra columbina | Alcalase®, trypsin, combination of both protease | Cytokine modulations (inhibition of TNF-α and IFN-γ, increase of IL-10) in rat splenocytes | [79] |
| Porphyra columbina protein hydrolysate | Algae: Porphyra columbina | Flavourzyme® and fungal protease concentrate | Immunomodulatory effects on rat macrophages and lymphocytes, activates NF-κB- and MAPK-dependent pathways, and mainly induces IL-10 production; inhibition of TNF-α, IL-1β, and IL-6 | [80] |
| Edible red algae protein hydrolysate | Algae: Porphyra tenera | Alcalase®, Flavourzyme®, Neutrase®, ProtamexTM, amyloglucosidase (AMG), Celluclast®, Dextrozyme®, Maltogenase, Promozyme, Termamyl®, Viscozyme® | Inhibition of LPS-induced NO production by murine macrophage RAW 264.7 cells | [81] |
| Edible microalgae Spirulina protein hydrolysate | Algae: Spirulina maxima LDAVNR (686 Da), MMLDF (655 Da) | Trypsin, pepsin, α-chymotrypsin | Inhibited histamine release and production from RBL-2H3 mast cells; interference with signaling pathways dependent on Ca2+ and microtubules (LDAVNR); inhibition of phospholipase Cγ activation and reactive oxygen species formation (MMLDF); NF-κB translocation and formation of IL-4 | [82] |
| Oyster peptide-based enteral nutrition formula | Oyster: Crassostrea hongkongensis | Bromelain, pepsin, trypsin | Enhanced spleen lymphocyte proliferation and activity of NK cells in BALB/c mice | [83] |
| Oyster protein hydrolysate | Oyster: Crassostrea gigas (<3 kDa) | Protease from Bacillus sp. SM98011 | Enhanced spleen lymphocyte proliferation; macrophage phagocytosis and NK cell cytotoxicity in BALB/c mice | [15] |
| Paphia undulata meat protein hydrolysate | Mollusk: Paphia undulata PHTC, VGYT, EF, LF and EGAL, WI, or WL | Protease from Bacillus subtilis | Enhanced mice spleen lymphocyte proliferation ability ex vivo | [84] |
| Cyclina sinensis protein hydrolysate | Venus clam: Cyclina sinensis RVAPEEHPVEGRYLV (1750.81 Da) | Pepsin | Enhanced macrophage phagocytosis, increased production of NO, TNF-α, IL-6, and IL-1β, and up-regulated protein levels of iNOS, NF-κB, and NLRP3 in RAW 264.7 cells; down-regulation of the expression of inhibitor of IκB-α; stimulation of macrophage activities by activating the NF-κB signaling pathway | [85] |
| Rudi tapes protein hydrolysate | Short-necked clam: Ruditapes philippinarum QCQQAVQSAV (876 Da) | Alcalase®, Flavourzyme®, Neutrase®, ProtamexTM, α-chymotrypsin, papain, pepsin, trypsin | NO inhibitory activity in LPS-stimulated RAW 264.7 macrophages | [86] |
| Shellfish Mytilus protein hydrolysate | Shellfish: Mytilus coruscus GVSLLQQFFL (1151.37 Da) | Alcalase®, Flavourzyme®, Neutrase®, α-chymotrypsin, papain, pepsin, trypsin | Inhibited LPS-induced NO production in RAW264.7 macrophages | [87] |
| Alaska pollock protein hydrolysate | Alaska pollock: Theragra chalcogramma PYGADY (622 Da) | Trypsin | Enhanced humoral, cellular, and non-specific immunity in immunosuppressed mice | [88] |
| Alaska pollock frame protein hydrolysate | Alaska Pollock: Theragra chalcogramma NGMTY (584 MW), NGLAP (470 MW), and WY (305 MW) | Trypsin | Enhanced mice spleen lymphocyte proliferation activity | [89] |
| Fermented pacific whiting protein | Fish: Merluccius merluccius (<1 kDa) | Yeast | Enhanced phagocytic activity of peritoneal macrophages, increased number of IgA+ cells, and increased IL-4, IL-6, IL-10, IFN-γ, and TNF-α levels in the small intestine lamina propria in mice | [90] |
| Chum salmon oligopeptide preparation | Fish: Oncorhynchus keta (300–860 Da) | Complex protease | Enhanced lymphocyte proliferation capacity increased number of plaque-forming cells, increased NK cell activity, increased percentage of CD4+ TH cells in spleen and secretion of TH1 (IL-2, IFNγ) and TH2 (IL-5, IL-6)-type cell cytokines in ICR mice | [91] |
| Salmon fish protein hydrolysate | Fish: Atlantic salmon fish (Contained 60–70% di/tri peptides of < 10 kDa) | Endogenous hydrolyzing agents | Changes of IgM, IgG, and IgA and CD4/CD8 ratios were observed in malnourished Indian children | [92] |
| Salmon byproduct protein | Salmon fish byproduct from pectoral fin (1000–2000 Da) | Alcalase®, Flavourzyme®, Neutrase®, ProtamexTM, pepsin, trypsin | Inhibited TNF-α, IL-6, and IL-1β in LPS-induced RAW264.7 macrophages | [93] |
| Salmon pectoral fin byproductprotein | Salmon fish byproduct from pectoral fin PAY (349.15 Da) | Pepsin | Inhibited production of NO and prostaglandin E2; production of pro-inflammatory cytokines, TNF-α, IL-6, and IL-1β in LPS-stimulated RAW264.7 cells | [94] |
| Shark-derived protein hydrolysate | PeptiBalTM, (innoVactiv, Inc.) (<10 kDa) | Trypsin, α-chymotrypsin | Enhanced gut barrier function via up-regulation of IgA-producing cells and intestinal cytokine production, including IL-6 and TNF-α in mice; inhibited production of TGF-β and IL-10 caused by infection with enterotoxigenic E. coli H10407 | [95] |
| Sweetfish-derived protein hydrolysate | Sweetfish | Pepsin, trypsin, α-chymotrypsin | Inhibited production of NO, cytokines (TNF-α and IL-6), and PGE2 in LPS-induced RAW264.7 macrophages | [96] |
| Common carp egg protein hydrolysate | Fish: Cyprinus carpio egg (5-90 KDa) | Alcalase®, pepsin, trypsin | Enhanced proliferation of spleen lymphocytes, NK cell cytotoxicity, macrophage phagocytosis, level of mucosal immunity (S-IgA), and percentages of CD4+ and CD8+ cells in BALB/c mice | [97] |
| Rohu egg protein hydrolysate | Fish: Labeo rohita egg (<10 kDa) | Alcalase®, pepsin, trypsin | Significantly enhanced macrophage phagocytosis, NK cell cytotoxicity, mucosal immunity (S-IgA), splenic CD4+ & CD8+ T cells, and level of serum IgA in mice | [98] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. https://doi.org/10.3390/md17060350
Kang HK, Lee HH, Seo CH, Park Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Marine Drugs. 2019; 17(6):350. https://doi.org/10.3390/md17060350
Chicago/Turabian StyleKang, Hee Kyoung, Hyung Ho Lee, Chang Ho Seo, and Yoonkyung Park. 2019. "Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides" Marine Drugs 17, no. 6: 350. https://doi.org/10.3390/md17060350
APA StyleKang, H. K., Lee, H. H., Seo, C. H., & Park, Y. (2019). Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Marine Drugs, 17(6), 350. https://doi.org/10.3390/md17060350





