Cyanobacterial Siderophores—Physiology, Structure, Biosynthesis, and Applications
Abstract
1. Introduction
2. Hydroxamate Siderophores in Cyanobacteria
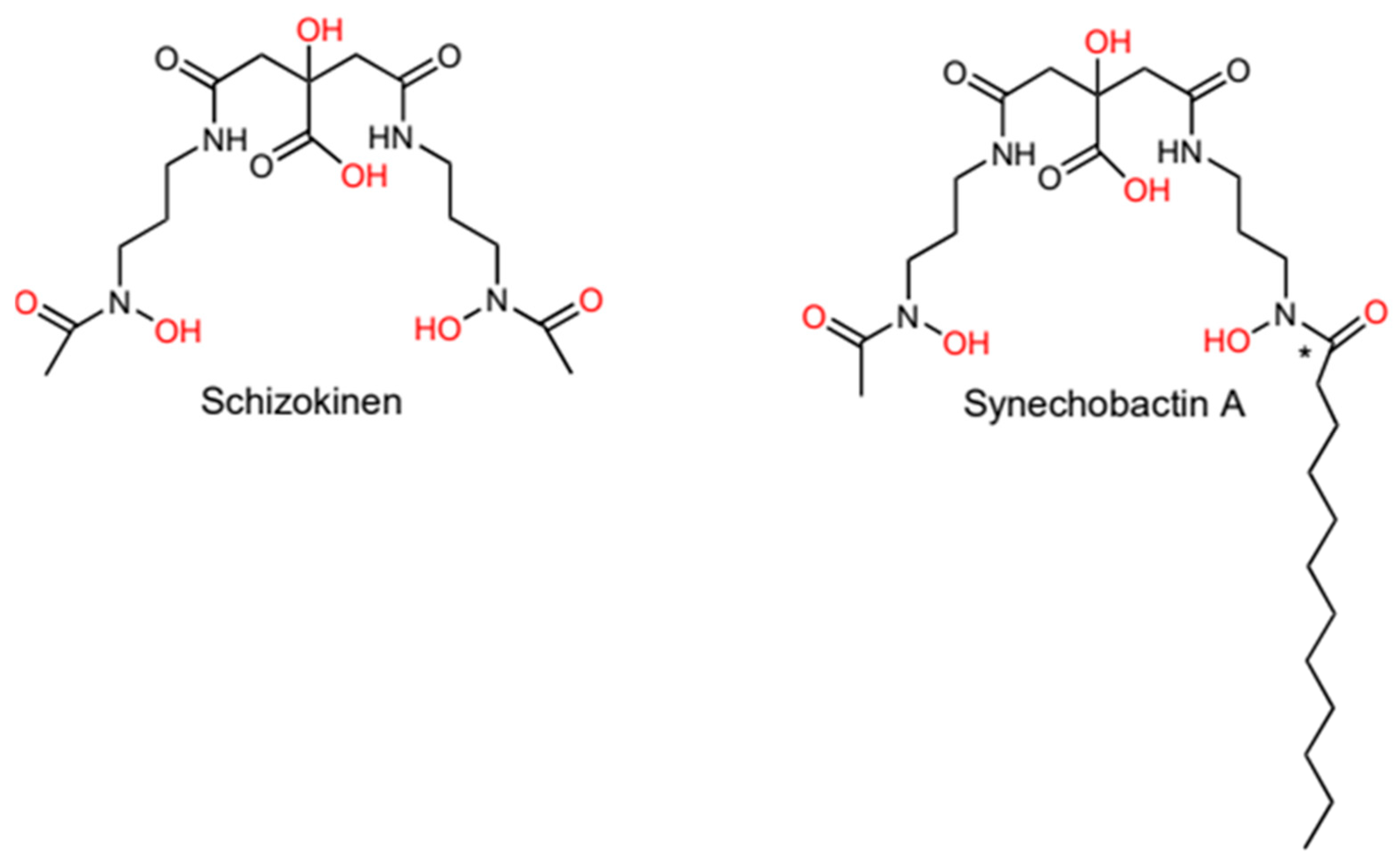
2.1. Synechobactin is an Amphiphilic Siderophore
2.2. Schizokinen and Synechobactins Are Synthesized by NIS-Based Systems
3. (Hydroxamate) Siderophores Can Bind Other Metals
4. Catecholate Siderophores
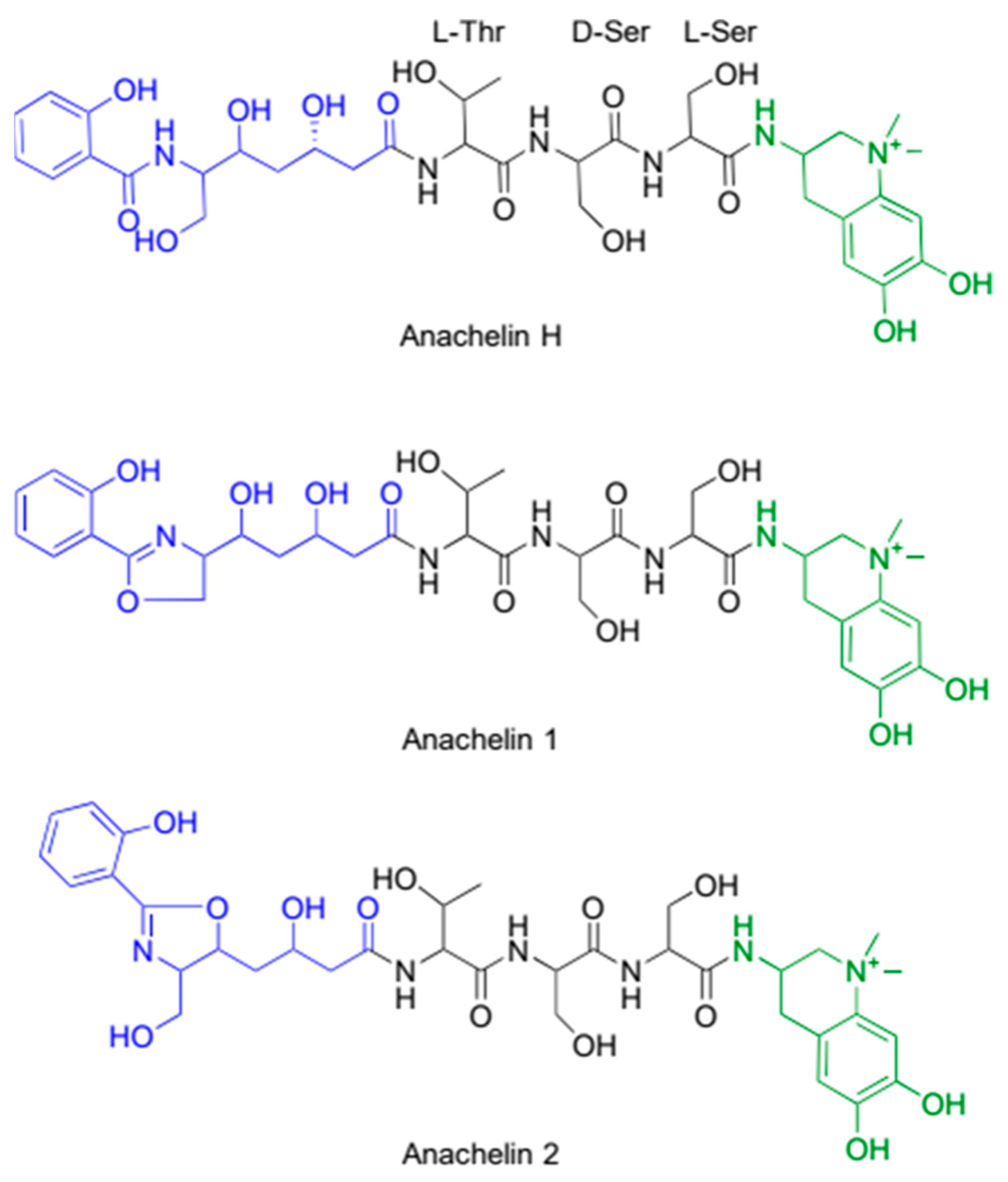
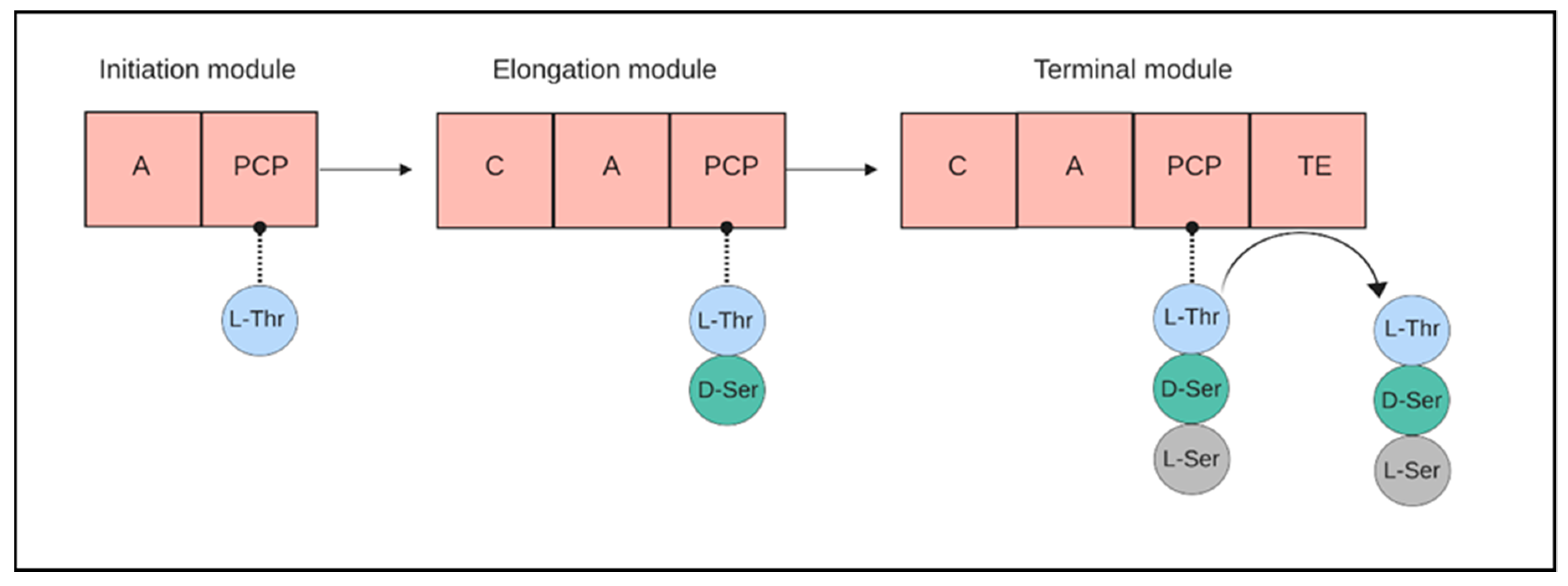
4.1. Anachelin Is Produced by Non-Ribosomal Peptide Synthase Pathway for Siderophores
4.2. Antimicrobial Properties of Anachelin
5. Siderophore Cycling
5.1. Siderophore Export
5.2. Siderophore Import
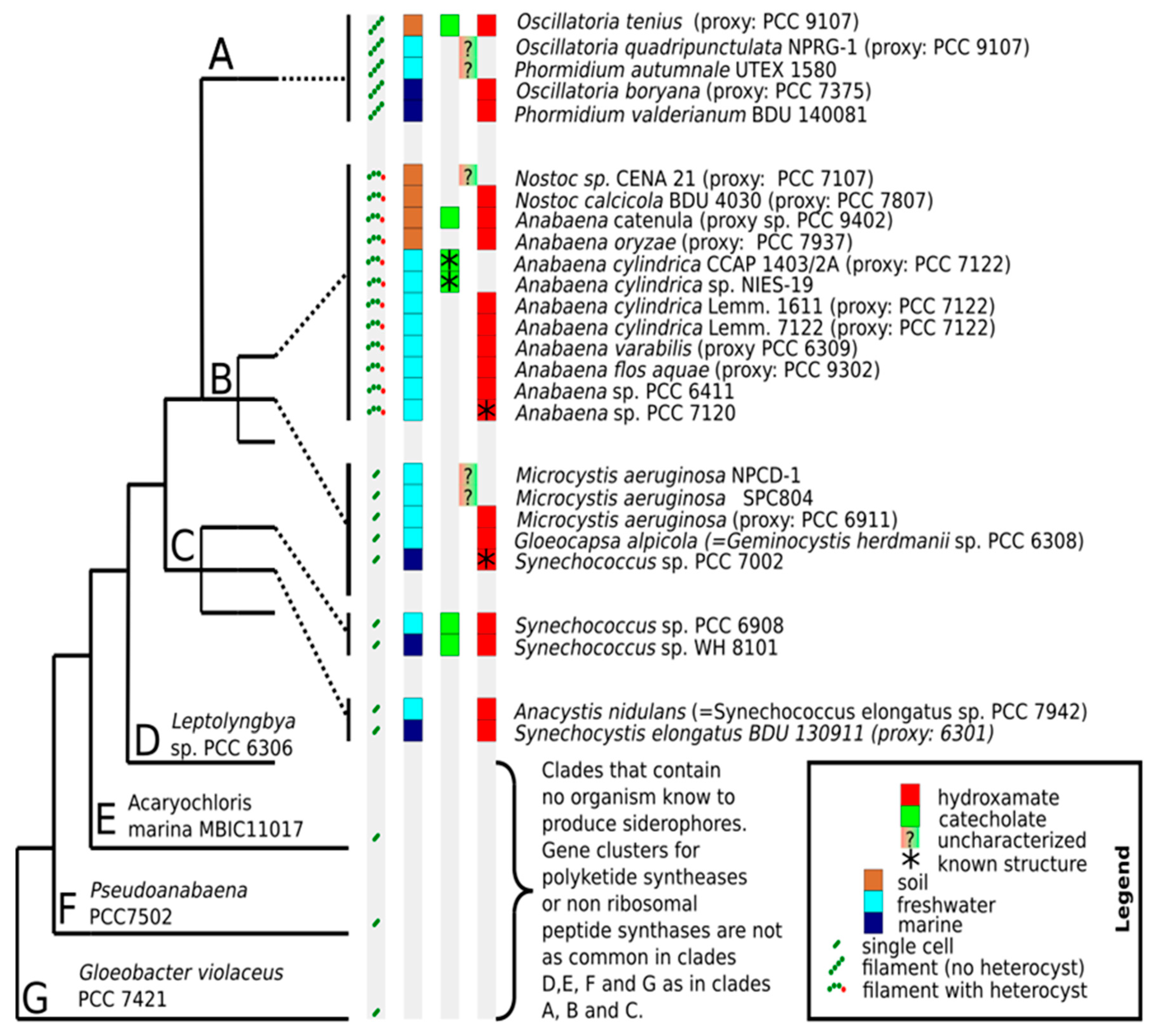
6. The Distribution of Siderophores in Cyanobacteria
7. Identification Methods
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shcolnick, S.; Keren, N. Metal Homeostasis in Cyanobacteria and Chloroplasts. Balancing Benefits and Risks to the Photosynthetic Apparatus. Plant Physiol. 2006, 141, 805–810. [Google Scholar] [CrossRef]
- Shaked, Y.; Lis, H. Disassembling iron availability to phytoplankton. Front. Microbiol. 2012, 3, 123. [Google Scholar] [CrossRef]
- Kranzler, C.; Rudolf, M.; Keren, N.; Schlieff, E. Iron in Cyanobacteria. In Genomics of Cyanobacteria; Chauvat, F., Cassier-Chauvat, C., Eds.; Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-394603-4. [Google Scholar]
- Moore, J.K.; Braucher, O. Observations of dissolved iron concentrations in the World Ocean: Implications and constraints for ocean biogeochemical models. Biogeosciences 2007, 4, 1241–1277. [Google Scholar] [CrossRef]
- Martin, J.H. Glacial-interglacial CO2 change: The Iron Hypothesis. Paleoceanography 1990, 5, 1–13. [Google Scholar] [CrossRef]
- Edwards, A.M.; Platt, T.; Sathyendranath, S. The high-nutrient, low-chlorophyll regime of the ocean: Limits on biomass and nitrate before and after iron enrichment. Ecol. Model. 2004, 171, 103–125. [Google Scholar] [CrossRef]
- González, A.; Fillat, M.F.; Bes, M.-T.; Peleato, M.-L.; Sevilla, E. The Challenge of Iron Stress in Cyanobacteria. In Cyanobacteria; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Keren, N.; Aurora, R.; Pakrasi, H.B. Critical Roles of Bacterioferritins in Iron Storage and Proliferation of Cyanobacteria. Plant Physiol. 2004, 135, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Lis, H.; Kranzler, C.; Keren, N.; Shaked, Y. A Comparative Study of Iron Uptake Rates and Mechanisms amongst Marine and Fresh Water Cyanobacteria: Prevalence of Reductive Iron Uptake. Life 2015, 5, 841–860. [Google Scholar] [CrossRef]
- Lamb, J.J.; Hill, R.E.; Eaton-Rye, J.J.; Hohmann-Marriott, M.F. Functional Role of PilA in Iron Acquisition in the Cyanobacterium Synechocystis sp. PCC 6803. PLoS ONE 2014, 9, e105761. [Google Scholar] [CrossRef]
- Gledhill, M.; Buck, K.N. The Organic Complexation of Iron in the Marine Environment: A Review. Front. Microbiol. 2012, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Guerinot, M.L.; Meidl, E.J.; Plessner, O. Citrate as a siderophore in Bradyrhizobium japonicum. J. Bacteriol. 1990, 172, 3298–3303. [Google Scholar] [CrossRef]
- Silva, A.M.N.; Kong, X.; Parkin, M.C.; Cammack, R.; Hider, R.C. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 2009, 0, 8616–8625. [Google Scholar] [CrossRef]
- Laglera, L.M.; van den Berg, C.M.G. Evidence for geochemical control of iron by humic substances in seawater. Limnol. Oceanogr. 2009, 54, 610–619. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Mende, D.R.; Hawco, N.J.; McIlvin, M.R.; Fitzsimmons, J.N.; Saito, M.A.; Sedwick, P.N.; DeLong, E.F.; Repeta, D.J. Siderophore-based microbial adaptations to iron scarcity across the eastern Pacific Ocean. Proc. Natl. Acad. Sci. USA 2016, 113, 14237–14242. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Garnerin, T.; Dassonville-Klimpt, A.; Sonnet, P. Fungal Hydroxamate Siderophores: Biosynthesis, Chemical Synthesis and Potential Medical Applications. In Antimicrobial Research: Novel Bioknowledge and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2017. [Google Scholar]
- Baakza, A.; Vala, A.K.; Dave, B.P.; Dube, H.C. A comparative study of siderophore production by fungi from marine and terrestrial habitats. J. Exp. Mar. Biol. Ecol. 2004, 311, 1–9. [Google Scholar] [CrossRef]
- McKnight, D.M.; Morel, F.M.M. Release of weak and strong copper-complexing agents by algae. Limnol. Oceanogr. 1979, 24, 823–837. [Google Scholar] [CrossRef]
- McKnight, D.M.; Morel, F.M.M. Copper complexation by siderophores from filamentous blue-green algae. Limnol. Oceanogr. 1980, 25, 62–71. [Google Scholar] [CrossRef]
- Goldman, S.J.; Lammers, P.J.; Berman, M.S.; Sanders-Loehr, J. Siderophore-mediated iron uptake in different strains of Anabaena sp. J. Bacteriol. 1983, 156, 1144–1150. [Google Scholar]
- Wilhelm, S.W.; Trick, C.G. Iron-limited growth of cyanobacteria: Multiple siderophore production is a common response. Limnol. Oceanogr. 1994, 39, 1979–1984. [Google Scholar] [CrossRef]
- Persmark, M.; Pittman, P.; Buyer, J.S.; Schwyn, B.; Gill, P.R.; Neilands, J.B. Isolation and structure of rhizobactin 1021, a siderophore from the alfalfa symbiont Rhizobium meliloti 1021. J. Am. Chem. Soc. 1993, 115, 3950–3956. [Google Scholar] [CrossRef]
- de Lorenzo, V.; Bindereif, A.; Paw, B.H.; Neilands, J.B. Aerobactin biosynthesis and transport genes of plasmid ColV-K30 in Escherichia coli K-12. J. Bacteriol. 1986, 165, 570–578. [Google Scholar] [CrossRef]
- Simpson, F.B.; Neilands, J.B. Siderochromes in cyanophyceae: Isolation and characterization of schizokinen from Anabaena sp.1. J. Phycol. 1976, 12, 44–48. [Google Scholar]
- Mullis, K.B.; Pollack, J.R.; Neilands, J.B. Structure of schizokinen, An iron-transport compound from Bacillus megaterium. Biochemistry 1971, 10, 4894–4898. [Google Scholar] [CrossRef]
- Plowman, J.E.; Loehr, T.M.; Goldman, S.J.; Sanders-Loehr, J. Structure and siderophore activity of ferric schizokinen. J. Inorg. Biochem. 1984, 20, 183–197. [Google Scholar] [CrossRef]
- Raymond, K.N.; Carrano, C.J. Coordination chemistry and microbial iron transport. Acc. Chem. Res. 1979, 12, 183–190. [Google Scholar] [CrossRef]
- Zhang, G.; Amin, S.A.; Küpper, F.C.; Holt, P.D.; Carrano, C.J.; Butler, A. Ferric Stability Constants of Representative Marine Siderophores: Marinobactins, Aquachelins, and Petrobactin. Inorg. Chem. 2009, 48, 11466–11473. [Google Scholar] [CrossRef]
- Armstrong, J.E.; Van Baalen, C. Iron Transport in Microalgae: The Isolation and Biological Activity of a Hydroxamate Siderophore from the Blue-Green Alga Agmenellum quadruplicatum. J. Gen. Microbiol. 1979, 111, 253–262. [Google Scholar] [CrossRef]
- Ito, Y.; Butler, A. Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnol. Oceanogr. 2005, 50, 1918–1923. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Repeta, D.J. An extended siderophore suite from Synechococcus sp. PCC 7002 revealed by LC-ICPMS-ESIMS. Metallomics 2015, 7, 877–884. [Google Scholar] [CrossRef]
- Barbeau, K.; Rue, E.L.; Trick, C.G.; Bruland, K.W.; Butler, A. Photochemical reactivity of siderophores produced by marine heterotrophic bacteria and cyanobacteria based on characteristic Fe(III) binding groups. Limnol. Oceanogr. 2003, 48, 1069–1078. [Google Scholar] [CrossRef]
- Passananti, M.; Vinatier, V.; Delort, A.-M.; Mailhot, G.; Brigante, M. Siderophores in Cloud Waters and Potential Impact on Atmospheric Chemistry: Photoreactivity of Iron Complexes under Sun-Simulated Conditions. Environ. Sci. Technol. 2016, 50, 9324–9332. [Google Scholar] [CrossRef]
- Kranzler, C.; Lis, H.; Shaked, Y.; Keren, N. The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ. Microbiol. 2011, 13, 2990–2999. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carrano, C.J.; Kuhn, J.-U.; Butler, A. Photoreactivity of Iron(III)−Aerobactin: Photoproduct Structure and Iron(III) Coordination. Inorg. Chem. 2006, 45, 6028–6033. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.S.; Zhang, G.P.; Holt, P.D.; Jung, H.-T.; Carrano, C.J.; Haygood, M.G.; Butler, A. Self-Assembling Amphiphilic Siderophores from Marine Bacteria. Science 2000, 287, 1245–1247. [Google Scholar] [CrossRef]
- Martinez, J.S.; Carter-Franklin, J.N.; Mann, E.L.; Martin, J.D.; Haygood, M.G.; Butler, A. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc. Natl. Acad. Sci. USA 2003, 100, 3754–3759. [Google Scholar] [CrossRef]
- Gauglitz, J.M.; Zhou, H.; Butler, A. A suite of citrate-derived siderophores from a marine Vibrio species isolated following the Deepwater Horizon oil spill. J. Inorg. Biochem. 2012, 107, 90–95. [Google Scholar] [CrossRef]
- Kem, M.P.; Zane, H.K.; Springer, S.D.; Gauglitz, J.M.; Butler, A. Amphiphilic siderophore production by oil-associating microbes. Metallomics 2014, 6, 1150. [Google Scholar] [CrossRef]
- Luo, M.; Fadeev, E.A.; Groves, J.T. Membrane Dynamics of the Amphiphilic Siderophore, Acinetoferrin. J. Am. Chem. Soc. 2005, 127, 1726–1736. [Google Scholar] [CrossRef]
- Xu, G.; Martinez, J.S.; Groves, J.T.; Butler, A. Membrane Affinity of the Amphiphilic Marinobactin Siderophores. J. Am. Chem. Soc. 2002, 124, 13408–13415. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.S.; Moore, M.M. Ironing out siderophore biosynthesis: A review of non-ribosomal peptide synthetase (NRPS)-independent siderophore synthetases. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 356–381. [Google Scholar] [CrossRef] [PubMed]
- Oves-Costales, D.; Kadi, N.; Challis, G.L. The long-overlooked enzymology of a nonribosomal peptide synthetase-independent pathway for virulence-conferring siderophore biosynthesis. Chem. Commun. 2009, 43, 6530–6541. [Google Scholar] [CrossRef]
- Lynch, D.; O’Brien, J.; Welch, T.; Clarke, P.; Ó Cuív, P.; Crosa, J.H.; O’Connell, M. Genetic Organization of the Region Encoding Regulation, Biosynthesis, and Transport of Rhizobactin 1021, a Siderophore Produced by Sinorhizobium meliloti. J. Bacteriol. 2001, 183, 2576–2585. [Google Scholar] [CrossRef]
- Nicolaisen, K.; Moslavac, S.; Samborski, A.; Valdebenito, M.; Hantke, K.; Maldener, I.; Muro-Pastor, A.M.; Flores, E.; Schleiff, E. Alr0397 Is an Outer Membrane Transporter for the Siderophore Schizokinen in Anabaena sp. Strain PCC 7120. J. Bacteriol. 2008, 190, 7500–7507. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, M.; Stevanovic, M.; Kranzler, C.; Pernil, R.; Keren, N.; Schleiff, E. Multiplicity and specificity of siderophore uptake in the cyanobacterium Anabaena sp. PCC 7120. Plant Mol. Biol. 2016, 92, 57–69. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Shen, G.; Bryant, D.A.; Golbeck, J.H. Regulatory Roles for IscA and SufA in Iron Homeostasis and Redox Stress Responses in the Cyanobacterium Synechococcus sp. Strain PCC 7002. J. Bacteriol. 2006, 188, 3182–3191. [Google Scholar] [CrossRef]
- Ludwig, M.; Bryant, D.A. Acclimation of the Global Transcriptome of the Cyanobacterium Synechococcus sp. Strain PCC 7002 to Nutrient Limitations and Different Nitrogen Sources. Front. Microbiol. 2012, 3, 145. [Google Scholar] [CrossRef]
- Ludwig, M.; Chua, T.T.; Chew, C.Y.; Bryant, D.A. Fur-type transcriptional repressors and metal homeostasis in the cyanobacterium Synechococcus sp. PCC 7002. Front. Microbiol. 2015, 6, 1217. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Nolan, E.M. Beyond Iron: Non-Classical Biological Functions of Bacterial Siderophores. Dalton Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.E.; Stuart, J.; Sanders-Loehr, J. Induction of siderophore activity in Anabaena spp. and its moderation of copper toxicity. Appl. Environ. Microbiol. 1987, 53, 917–922. [Google Scholar] [PubMed]
- Nicolaisen, K.; Hahn, A.; Valdebenito, M.; Moslavac, S.; Samborski, A.; Maldener, I.; Wilken, C.; Valladares, A.; Flores, E.; Hantke, K.; et al. The interplay between siderophore secretion and coupled iron and copper transport in the heterocyst-forming cyanobacterium Anabaena sp. PCC 7120. Biochim. Biophys. Acta BBA Biomembr. 2010, 1798, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Matz, C.J.; Christensen, M.R.; Bone, A.D.; Gress, C.D.; Widenmaier, S.B.; Weger, H.G. Only iron-limited cells of the cyanobacterium Anabaena flos-aquae inhibit growth of the green alga Chlamydomonas reinhardtii. Can. J. Bot. 2004, 82, 436–442. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, V.; ShylajaNaciyar, M.; Rajalakshmi, R.; D’Souza, S.F.; Prabaharan, D.; Uma, L. Siderophore mediated uranium sequestration by marine cyanobacterium Synechococcus elongatus BDU 130911. Bioresour. Technol. 2013, 130, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaushik, M.S.; Srivastava, M.; Tiwari, D.N.; Mishra, A.K. Siderophore mediated attenuation of cadmium toxicity by paddy field cyanobacterium Anabaena oryzae. Algal Res. 2016, 16, 63–68. [Google Scholar] [CrossRef]
- O’Brien, I.G.; Gibson, F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoyne conjugates from Eschericha coli. Biochim. Biophys. Acta BBA Gen. Subj. 1970, 215, 393–402. [Google Scholar] [CrossRef]
- Pollack, J.R.; Neilands, J.B. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem. Biophys. Res. Commun. 1970, 38, 989–992. [Google Scholar] [CrossRef]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef]
- Zeng, H.; Hwang, D.S.; Israelachvili, J.N.; Waite, J.H. Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water. Proc. Natl. Acad. Sci. USA 2010, 107, 12850–12853. [Google Scholar] [CrossRef]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef] [PubMed]
- Loomis, L.D.; Raymond, K.N. Solution equilibria of enterobactin and metal-enterobactin complexes. Inorg. Chem. 1991, 30, 906–911. [Google Scholar] [CrossRef]
- Beiderbeck, H.; Taraz, K.; Budzikiewicz, H.; Walsby, A.E. Anachelin, the siderophore of the cyanobacterium Anabaena cylindrica CCAP 1403/2A. Z. Naturforsch. C 2000, 55, 681–687. [Google Scholar] [CrossRef]
- Itou, Y.; Okada, S.; Murakami, M. Two structural isomeric siderophores from the freshwater cyanobacterium Anabaena cylindrica (NIES-19). Tetrahedron 2001, 57, 9093–9099. [Google Scholar] [CrossRef]
- Gademann, K.; Portmann, C. Secondary Metabolites from Cyanobacteria: Complex Structures and Powerful Bioactivities. Curr. Org. Chem. 2008, 12, 326–341. [Google Scholar] [CrossRef]
- Bethuel, Y.; Gademann, K. Synthesis and Evaluation of the Bis-Nor-Anachelin Chromophore as Potential Cyanobacterial Ligand. J. Org. Chem. 2005, 70, 6258–6264. [Google Scholar] [CrossRef]
- Barry, S.M.; Challis, G.L. Recent advances in siderophore biosynthesis. Curr. Opin. Chem. Biol. 2009, 13, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.-C.; Gatte Picchi, D.; Dittmann, E. Natural product biosyntheses in cyanobacteria: A treasure trove of unique enzymes. Beilstein J. Org. Chem. 2011, 7, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Calteau, A.; Fewer, D.; Latifi, A.; Coursin, T.; Laurent, T.; Jokela, J.; Kerfeld, C.A.; Sivonen, K.; Piel, J.; Gugger, M. Phylum-wide comparative genomics unravel the diversity of secondary metabolism in Cyanobacteria. BMC Genomics 2014, 15, 977. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural Product Biosynthetic Diversity and Comparative Genomics of the Cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Weissman, K.J. The structural biology of biosynthetic megaenzymes. Nat. Chem. Biol. 2015, 11, 660–670. [Google Scholar] [CrossRef]
- Süssmuth, R.D.; Mainz, A. Nonribosomal Peptide Synthesis—Principles and Prospects. Angew. Chem. Int. Ed. 2017, 56, 3770–3821. [Google Scholar] [CrossRef] [PubMed]
- Ridley, C.P.; Lee, H.Y.; Khosla, C. Evolution of polyketide synthases in bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 4595–4600. [Google Scholar] [CrossRef]
- Perry, R.D.; Balbo, P.B.; Jones, H.A.; Fetherston, J.D.; DeMoll, E. Yersiniabactin from Yersinia pestis: Biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology 1999, 145, 1181–1190. [Google Scholar] [CrossRef]
- Ito, Y.; Ishida, K.; Okada, S.; Murakami, M. The absolute stereochemistry of anachelins, siderophores from the cyanobacterium Anabaena cylindrica. Tetrahedron 2004, 60, 9075–9080. [Google Scholar] [CrossRef]
- Gademann, K.; Bethuel, Y. A Biomimetic Route to the Peptide Alkaloid Anachelin. Angew. Chem. Int. Ed. 2004, 43, 3327–3329. [Google Scholar] [CrossRef] [PubMed]
- De Sarkar, S.; Blom, J.F.; Bethuel, Y.; Jüttner, F.; Gademann, K. Allelopathic Activity of the Iron Chelator Anachelin—A Molecular Hybrid with a Dual Mode of Action. Helv. Chim. Acta 2016, 99, 760–773. [Google Scholar] [CrossRef]
- Gademann, K.; Bethuel, Y.; Locher, H.H.; Hubschwerlen, C. Biomimetic Total Synthesis and Antimicrobial Evaluation of Anachelin H. J. Org. Chem. 2007, 72, 8361–8370. [Google Scholar] [CrossRef]
- McWhirter, M.J.; Bremer, P.J.; Lamont, I.L.; McQuillan, A.J. Siderophore-Mediated Covalent Bonding to Metal (Oxide) Surfaces during Biofilm Initiation by Pseudomonas aeruginosa Bacteria. Langmuir 2003, 19, 3575–3577. [Google Scholar] [CrossRef]
- Upritchard, H.G.; Yang, J.; Bremer, P.J.; Lamont, I.L.; McQuillan, A.J. Adsorption of Enterobactin to Metal Oxides and the Role of Siderophores in Bacterial Adhesion to Metals. Langmuir 2011, 27, 10587–10596. [Google Scholar] [CrossRef] [PubMed]
- Gademann, K. Cyanobacterial Natural Products for the Inhibition of Biofilm Formation and Biofouling. Chimia 2007, 61, 373–377. [Google Scholar] [CrossRef]
- Zürcher, S.; Wäckerlin, D.; Bethuel, Y.; Malisova, B.; Textor, M.; Tosatti, S.; Gademann, K. Biomimetic Surface Modifications Based on the Cyanobacterial Iron Chelator Anachelin. J. Am. Chem. Soc. 2006, 128, 1064–1065. [Google Scholar] [CrossRef]
- Wach, J.-Y.; Bonazzi, S.; Gademann, K. Antimicrobial Surfaces through Natural Product Hybrids. Angew. Chem. Int. Ed. 2008, 47, 7123–7126. [Google Scholar] [CrossRef]
- Stevanovic, M.; Hahn, A.; Nicolaisen, K.; Mirus, O.; Schleiff, E. The components of the putative iron transport system in the cyanobacterium Anabaena sp. PCC 7120. Environ. Microbiol. 2011, 14, 1655–1670. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. MMBR 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Noinaj, N.; Guillier, M.; Barnard, T.J.; Buchanan, S.K. TonB-dependent transporters: Regulation, structure, and function. Annu. Rev. Microbiol. 2010, 64, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pierik, A.J.; Peuckert, F.; Seubert, A.; Marahiel, M.A. Identification and Characterization of a Novel-type Ferric Siderophore Reductase from a Gram-positive Extremophile. J. Biol. Chem. 2011, 286, 2245–2260. [Google Scholar] [CrossRef]
- Mirus, O.; Strauss, S.; Nicolaisen, K.; von Haeseler, A.; Schleiff, E. TonB-dependent transporters and their occurrence in cyanobacteria. BMC Biol. 2009, 7, 68. [Google Scholar] [CrossRef]
- Rudolf, M.; Kranzler, C.; Lis, H.; Margulis, K.; Stevanovic, M.; Keren, N.; Schleiff, E. Multiple modes of iron uptake by the filamentous, siderophore-producing cyanobacterium, Anabaena sp. PCC 7120. Mol. Microbiol. 2015, 97, 577–588. [Google Scholar] [CrossRef]
- Babykin, M.M.; Obando, T.S.A.; Zinchenko, V.V. TonB-Dependent Utilization of Dihydroxamate Xenosiderophores in Synechocystis sp. PCC 6803. Curr. Microbiol. 2018, 75, 117–123. [Google Scholar] [CrossRef]
- Obando, S.T.A.; Babykin, M.M.; Zinchenko, V.V. A Cluster of Five Genes Essential for the Utilization of Dihydroxamate Xenosiderophores in Synechocystis sp. PCC 6803. Curr. Microbiol. 2018, 75, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.M.; Wu, D.; Latifi, A.; Axen, S.D.; Fewer, D.P.; Talla, E.; Calteau, A.; Cai, F.; de Marsac, N.T.; Rippka, R.; et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 1053–1058. [Google Scholar] [CrossRef]
- Hopkinson, B.M.; Morel, F.M.M. The role of siderophores in iron acquisition by photosynthetic marine microorganisms. BioMetals 2009, 22, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Jeanjean, R.; Talla, E.; Latifi, A.; Havaux, M.; Janicki, A.; Zhang, C.-C. A large gene cluster encoding peptide synthetases and polyketide synthases is involved in production of siderophores and oxidative stress response in the cyanobacterium Anabaena sp. strain PCC 7120. Environ. Microbiol. 2008, 10, 2574–2585. [Google Scholar] [CrossRef] [PubMed]
- Silva-Stenico, M.E.; Silva, C.S.P.; Lorenzi, A.S.; Shishido, T.K.; Etchegaray, A.; Lira, S.P.; Moraes, L.A.B.; Fiore, M.F. Non-ribosomal peptides produced by Brazilian cyanobacterial isolates with antimicrobial activity. Microbiol. Res. 2011, 166, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.P.; Lean, D.R.; Nalewajko, C. Blue-green algae: Their excretion of iron-selective chelators enables them to dominate other algae. Science 1976, 192, 900–902. [Google Scholar] [CrossRef]
- Kerry, A.; Laudenbach, D.E.; Trick, C.G. Influence of iron limitation and nitrogen source on growth and siderophore production by cyanobacteria. J. Phycol. 1988, 24, 566–571. [Google Scholar] [CrossRef]
- Trick, C.G.; Kerry, A. Isolation and purification of siderophores produced by cyanobacteria Synechococcus sp. PCC 7942 and Anabaena variabilis ATCC 29413. Curr. Microbiol. 1992, 24, 241–245. [Google Scholar] [CrossRef]
- Brown, C.M.; Trick, C.G. Response of the cyanobacterium, Oscillatoria tenuis, to low iron environments: The effect on growth rate and evidence for siderophore production. Arch. Microbiol. 1992, 157, 349–354. [Google Scholar] [CrossRef]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.S.; Fernández, F.J. O-CAS, a fast and universal method for siderophore detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef]
- Csáky, T.Z. On the Estimation of Bound Hydroxylamine in Biological Materials. Acta Chem. Scand. 1948, 2, 450–454. [Google Scholar] [CrossRef]
- Atkin, C.L.; Neilands, J.B.; Phaff, H.J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J. Bacteriol. 1970, 103, 722–733. [Google Scholar] [PubMed]
- Gillam, A.H.; Lewis, A.G.; Andersen, R.J. Quantitative determination of hydroxamic acids. Anal. Chem. 1981, 53, 841–844. [Google Scholar] [CrossRef]
- Arnow, L.E. Colorimetric determination of the components of 3,4-dihydroxyphenylalaninetyrosine mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Maki, T.; Asano, K.; Ueda, K.; Ueda, K. Detection of Iron(III)-Binding Ligands Originating from Marine Phytoplankton Using Cathodic Stripping Voltammetry. Anal. Sci. 2004, 20, 89–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCormack, P.; Worsfold, P.J.; Gledhill, M. Separation and Detection of Siderophores Produced by Marine Bacterioplankton Using High-Performance Liquid Chromatography with Electrospray Ionization Mass Spectrometry. Anal. Chem. 2003, 75, 2647–2652. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, M.; McCormack, P.; Ussher, S.; Achterberg, E.P.; Mantoura, R.F.C.; Worsfold, P.J. Production of siderophore type chelates by mixed bacterioplankton populations in nutrient enriched seawater incubations. Mar. Chem. 2004, 88, 75–83. [Google Scholar] [CrossRef]
- Mawji, E.; Gledhill, M.; Milton, J.A.; Tarran, G.A.; Ussher, S.; Thompson, A.; Wolff, G.A.; Worsfold, P.J.; Achterberg, E.P. Hydroxamate Siderophores: Occurrence and Importance in the Atlantic Ocean. Environ. Sci. Technol. 2008, 42, 8675–8680. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, L.; Heumann, K.G. Determination of Heavy Metal Interactions with Dissolved Organic Materials in Natural Aquatic Systems by Coupling a High-Performance Liquid Chromatography System with an Inductively Coupled Plasma Mass Spectrometer. Anal. Chem. 1994, 66, 3709–3715. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Fitzsimmons, J.N.; Repeta, D.J.; Boyle, E.A. Detection of Iron Ligands in Seawater and Marine Cyanobacteria Cultures by High-Performance Liquid Chromatography–Inductively Coupled Plasma-Mass Spectrometry. Anal. Chem. 2013, 85, 4357–4362. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.R.; Tfaily, M.M.; Shaw, J.B.; Hess, N.J.; Paša-Tolić, L.; Koppenaal, D.W. Unambiguous identification and discovery of bacterial siderophores by direct injection 21 Tesla Fourier transform ion cyclotron resonance mass spectrometry. Metallomics 2017, 9, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Sandonato, B.B.; Santos, V.G.; Luizete, M.F.; Bronzel, J.L., Jr.; Eberlin, M.N.; Milagre, H.M.S. MALDI Imaging Mass Spectrometry of Fresh Water Cyanobacteria: Spatial Distribution of Toxins and Other Metabolites. J. Braz. Chem. Soc. 2017, 28, 521–528. [Google Scholar] [CrossRef]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.W.; Lomas, M.W.; Veneziano, D.; et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Årstøl, E.; Hohmann-Marriott, M.F. Cyanobacterial Siderophores—Physiology, Structure, Biosynthesis, and Applications. Mar. Drugs 2019, 17, 281. https://doi.org/10.3390/md17050281
Årstøl E, Hohmann-Marriott MF. Cyanobacterial Siderophores—Physiology, Structure, Biosynthesis, and Applications. Marine Drugs. 2019; 17(5):281. https://doi.org/10.3390/md17050281
Chicago/Turabian StyleÅrstøl, Erland, and Martin F. Hohmann-Marriott. 2019. "Cyanobacterial Siderophores—Physiology, Structure, Biosynthesis, and Applications" Marine Drugs 17, no. 5: 281. https://doi.org/10.3390/md17050281
APA StyleÅrstøl, E., & Hohmann-Marriott, M. F. (2019). Cyanobacterial Siderophores—Physiology, Structure, Biosynthesis, and Applications. Marine Drugs, 17(5), 281. https://doi.org/10.3390/md17050281




